Abstract
Our previous studies indicated that tumor invasion and 5-flurouracil (5-FU) resistance in colorectal cancer (CRC) was more affected by cytoplasmic localization of expressed Nrf2 (cNrf2) than by nuclear localization (nNrf2), indicating a need for novel antitumor agents to overcome 5-FU resistance and improve outcomes in patients with CRC. In the present study, 20 nitrogen-substituted anthra[1,2-c][1,2,5] thiadiazole-6,11-dione derivatives were collected to verify the compound most able to suppress cell growth in nuclear location sequence (NLS)-mutated Nrf2-transfected shNrf2-HCT116 stable clones that have high cNrf2 expression. The MTT assay indicated that these high-cNrf2-expressing shNrf2-HCT116 stable clones exhibited the lowest percentage survival when treated with RV-59 than with the other 19 compounds. As expected, the high-cNrf2-expressing cells also showed a higher value for the inhibitory concentration of 50% cell survival (IC50) for 5-FU when compared with Nrf2-knockdown HCT116 stable clones (17.74 μM vs. 5.34 μM). Interestingly, a lower RV-59 IC50 value was seen in the high-cNrf2-expressing stable clones than in the Nrf2-knockdown stable clones (3.55 μM vs. 16.81 μM). A similar low RV-59 IC50 value was observed in high-cNrf2-expressing NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones and p53 null (-/-) HCT116 cells (4.2 μM vs. 4.4 μM), whereas the IC50 value was 17.6 μM in normal colon FHC epithelial cells. Colony-forming assays confirmed that RV-59 treatment inhibited colony formation in NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones and in p53-/- HCT116 cells. Annexin-V/PI staining showed an involvement of apoptosis in the inhibitory effect of RV-59 on cell viability. A nude mouse xenograft tumor model showed that RV-59 efficiently suppressed tumor growth induced by transplanted NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones without affecting the body weight of the nude mice over the 37 day experimental period. These results strongly suggest that RV-59 may be a novel antitumor agent for suppression of 5-FU resistance and may have therapeutic potential for improving outcomes in patients with cNrf2-expressing tumors.
Keywords: Nrf2, 5-fluorouracil, and colorectal cancer
Introduction
Colorectal cancer (CRC) has been the leading cancer in Taiwan for the past two decades [1]. Surgery and chemotherapy are two major treatment options for patients with CRC, with 5-fluorouracil (5-FU)-based chemotherapy as the main chemotherapy in these patients. However, 5-FU resistance occurs frequently and results in treatment failure in patients with CRC, particularly in patients with metastatic disease [2-4]. Therefore, novel agents are needed to overcome 5-FU resistance and, in turn, to increase the treatment efficacy.
Our previous studies indicated that Nrf2 expression in a cytoplasmic localization (cNrf2) is a greater contributor to CRC tumor invasion and 5-FU resistance than is Nrf2 expression in a nuclear localization (nNrf2) [5,6]. Moreover, CRC patients with cNrf2-expressing tumors had poorer prognosis and more unfavorable response to 5-FU-based chemotherapy when compared with patients with nNrf2-expressing tumors [5,6]. The mechanisms of cNrf2-mediated 5-FU resistance were predominately through activation of the NF-κB/AKT/β-catenin/ZEB1 cascades by cNrf2-induced PSMD4 expression [5].
The 5-FU resistance mediated by cNrf2-induced PSMD4 expression in colorectal cancer may occur mainly through effects on the epithelial-to-mesenchymal transition (EMT). This is consistent with a large body of literature that implicates NF-κB, β-catenin, and ZEB1 in EMT-mediated chemoresistance [6-10]. Unfortunately, no inhibitors targeting NF-κB, β-catenin, or ZEB1 are yet available for clinical use. Therefore, a novel agent is urgently needed to overcome cNrf2-mediated 5-FU resistance and, consequently, to improve the therapeutic response and clinical outcomes in patients with cNrf2-expressing CRC tumors.
We sought a novel agent against cNrf2-mediated 5-FU resistance by screening a series of nitrogen-substituted anthra[1,2-c][1,2,5] thiadiazole-6,11-dione derivatives that showed promise as antitumor agents in p53 null (-/-) HCT116 cells that have high cNrf2 expression and a HCT116 colon cancer cell line that showed low cNrf2 expression. We established a high cNrf2-expressing HCT116 cell line by transfecting a mutated nuclear location sequence (NLS) of the Nrf2 expression vector into shNrf2-HCT116 stable clones and used these clones to explore which compound(s) could best inhibit cell growth in 5-FU resistant NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones. Of the 20 compounds tested, RV-59 showed the highest inhibitory effects on cell growth in the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones.
Materials and methods
Cell lines
The HCT116 and p53-/- HCT116 cell lines were kindly provided by Dr. C. C. Chang (Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan). Both cell lines were maintained in RPMI-1640 (HyClone Logan, UT, USA). The medium contained 10% fetal bovine serum (FBS) supplemented with penicillin (100 U/mL) and streptomycin (100 mg/mL). Cells were cultured and stored according to the suppliers’ instructions and used at passages 5 to 20.
Plasmid construction
The procedures of plasmid construction were according to our previous report [5]. Nrf2 cDNA was cloned into pcDNA3.1 Zeo(+) (Invitrogen, Carlsbad, CA, USA) by PCR amplification with newly created XhoI and BamHI sites attached onto the 5’ends of the forward and reverse Nrf2 primers, using H116 cDNA as a template. The NLS-mutated Nrf2 was generated using the QuickChange site-directed mutagenesis system (Stratagene, San Diego, CA, USA). Mutant NLS primers were based on a previous report [5]. The shRNA was purchased from the National RNAi Core Facility, Academia Sinica, Taiwan.
Plasmid transfection reaction
Different concentrations of expression plasmids were transiently transfected into HCT116 colon cancer cells (1 × 106) using the Turbofect transfection reagents (Thermo, Waltham, MA, USA). After 48 h, the cells were harvested and whole-cell extracts were assayed in subsequent experiments.
Selection of HCT116 stable clone
The selection of shNrf2-HCT116 and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones was based on our previous report [5]. The Nrf2 shRNA plasmids (10 μg) were mixed with Turbofect transfection reagent (Thermo, Waltham, MA, USA) and added to 1 × 105 HCT116 cells. After 48 h, stable transfectants for Nrf2 shRNA were selected from using 1 μg/ml puromycin (Sigma-Aldrich, St. Louis, MO, USA). The selection medium was replaced every three days for three weeks.
The MTT assay
The cell lines were cultured in 96-well flat-bottomed microtiter plates supplemented with RPMI 1640 and DMEM containing 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 100 units/mL streptomycin. The cells were incubated in a humidified atmosphere containing 95% air and 5% CO2 at 37°C until they reached the exponential growth phase. The cells pretreated with shNrf2 or NLS-mutated Nrf2 expression vectors for 24 h, followed by treatment of 5-FU or RV-59. After a 48 h incubation, the in vitro cytotoxic effects of these treatments were determined by MTT assays (at 570 nm).
Annexin-V/PI staining
The cells were collected by trypsinization and centrifugation at 1,000 g for 5 minutes. Following resuspension at a final cell density of 1 to 2 × 106 cells/mL in binding buffer (10 mmol/L HEPES-NaOH, 140 mmol/L NaCl, 2.5 mmol/L CaCl2), 100 μL of a single-cell suspension (1-2 × 105 cells) was incubated with 5 μL Annexin-V-FITC and 5 μL propidium iodide (PI) for 15 minutes at room temperature in the dark. After addition of 400 µL of binding buffer, the samples were analyzed with a BD FACS Calibur flow cytometer (BD Biosciences) within 1 h. For each sample, 10,000 events were counted.
Xenograft tumor models
The nude mouse study was approved by the Institutional Animal Care and Use Committee at Taipei Medical University. All mice were maintained in individual ventilated cages according to the guidelines established in “Guide For The Care and Use of Laboratory Animals” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources Commission on Life Sciences, National Research Council, U.S.A. (1985). The use of nude mice has been approved by the Institutional Animal Care and Use Committee of Taipei Medical University, Taipei, Taiwan (LAC-2014-0257). The models of colorectal adenocarcinoma were 4-week-old female BALB/c nude mice (n = 20; supplied by the National Laboratory Animal Center, Taiwan) that were acclimated for 1 week while caged in groups of 5. The mice were housed in SPF conditions and fed a diet of animal chow and water throughout the experiment. Therapeutic experiments on tumor growth were initiated by injecting NLS-mutated Nrf2 HCT116 cells (106 cells in 0.1 mL of PBS) subcutaneously into the backs of 5-week-old female BALB/c nude mice. The xenograft size was measured every three days and the tumor volume was determined as (length × width2)/2. When tumors had grown to 0.5 cm3, mice were randomized to the following groups: vehicle control (DMSO), RV-59 (2 mg/kg), RV-59 (5 mg/kg), and 5-FU (20 mg/kg). Drugs were administered by intraperitoneal injection every 7 days.
Statistical analysis
Statistical analysis was conducted using the SPSS statistical software program (Version 15.0; SPSS Inc.). Student’s t test was used to analyze the data. Results are given as mean ± SD unless otherwise indicated. P < 0.05 was considered statistically significant.
Results
Discovery of RV-59
We screened our in-house small molecule library (> 300 compounds with structural diversity) and found a series of nitrogen-substituted anthra[1,2-c][1,2,5] thiadiazole-6,11-dione derivatives that exhibited potent and promising antitumor activity (Figure 1A, 1B). In recent years, small-molecule targeted therapies have shown spectacular progress as cancer treatments, and the HCT116 human colon cancer cell line has been instrumental in conducting therapeutic research and drug screenings. We initiated a preliminary investigation to determine whether the substitution of a terminal aliphatic group of the small molecule RV-59 with electron-withdrawing groups and electron-donating groups would affect its potency. We synthesized and evaluated the 19 compounds listed in Figure 1A: 5d, B1, D1, CL24, SJ-3, SJ-10, RV-59, TC-N2, TC-N7, TC-N14, TC-N19, TC-N25, CC-12, LCC-01, LCC-02, LCC-03, LCC-10, J4-1, and J3-6. Notably, the introduction of a dimethylamino-ethyl-amino group to the 6,6,6,5-tetraheterocyclic ring system scaffold at the 4 position increased the inhibitory activity significantly when compared to other modifications. Another study revealed that sulfur-substituted anthra[1,2-c][1,2,5] thiadiazole-6,11-dione derivatives were promising antitumor agents [11]. Screening tests against HCT116 colon cancer cells led to the optimization of small molecules that targeted colon cancer. Small molecules with an N-substituted aliphatic substituent were examined by the MTT assay in these screenings as the most potent leads against HCT116 colon cancer cells. The overall chemical structure is presented in Figure 1B. The results indicated that the length of the N-substituted aliphatic side chain greatly affected the inhibitory activity of the small molecules.
Figure 1.
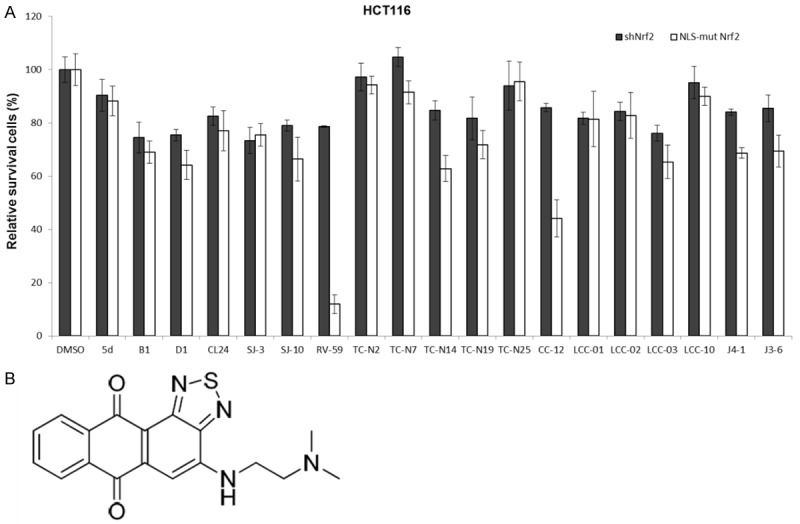
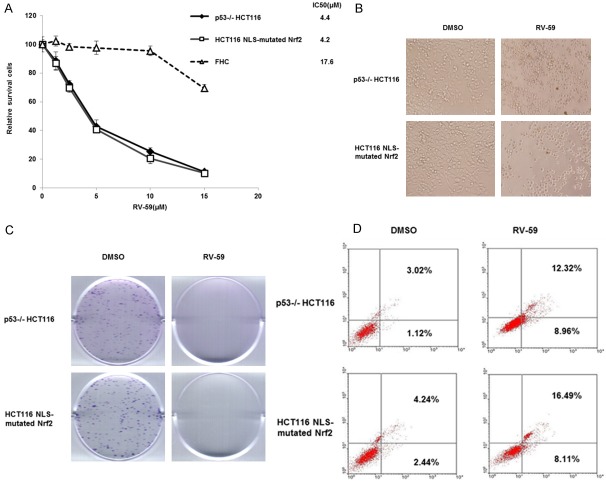
RV-59 inhibits cell growth of NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones. A. shNrf2-HCT116 stable clones and NLS-mutated Nrf2 shNrf2-HCT116 stable clones were treated with 19 novel drugs (10 μM) for 24 h, and the cell viability was evaluated by the MTT assay. B. Chemical structure of RV-59.
RV-59 potentially inhibits cell growth of higher cNrf2-expressing HCT116 colon cancer cells
Nrf2-knockdown HCT116 cells (which have no Nrf2 expression) and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones (which have high cNrf2 expression) were used to examine whether cell survival determined by the MTT assay could be changed by treatment with RV-59. Figure 1A shows that the lowest survival was observed in the high-cNrf2-expressing NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones after treatment with RV-59 (10 μM) when compared to treatment with the other 19 compounds. By contrast, the shNrf2-HCT116 stable clones treated with all 20 compounds showed > 70% cell survival. These results suggest that RV-59 may potentially inhibit the growth of cNrf2-expressing HCT116 colon cancer cells but is relatively ineffective in suppressing the growth of Nrf2-silenced HCT116 colon cancer cells.
RV-59 efficiently inhibits cell growth and overcomes cNrf2-mediated 5-FU resistance in HCT116 colon cancer cells
The MTT assay was also conducted to calculate the 50% inhibitory concentration of cell survival (IC50) value from survival curves based on five concentrations of RV-59 and 5-FU (0-20 μM) in shNrf2-HCT116 stable clones and in the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones. The IC50 value of 5-FU was 5.34 μM for the shNrf2-HCT116 stable clones and 17.74 μM for the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones (Figure 2A). The IC50 value of RV-59 was 16.81 μM for the shNrf2-HCT116 stable clones and 3.55 μM for the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones (Figure 2B). These results strongly suggest that RV-59 may predominately kill and overcome cNrf2-mediated resistance in 5-FU resistant cells, but does not affect Nrf2-negative colon cancer cells.
Figure 2.
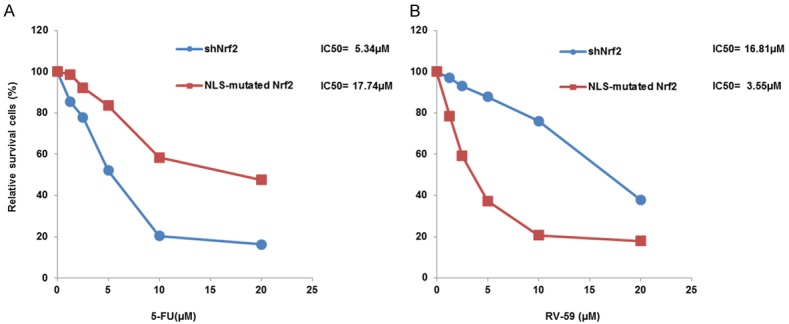
The IC50 value of shNRf2-HCT116 and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones after treatment of 5-FU or RV-59. A. Both stable clones were treated with five concentrations of 5-FU for 48 h and the IC50 value for both clones were calculated from their dose-response survival curves. B. Both stable clones were treated with five concentrations of RV59 for 48 h and the IC50 value for both clones were calculated from their dose-response survival curves.
RV-59 kills cNrf2-mediated 5-FU resistant NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones by apoptosis
We next examined whether RV-59 could kill cNrf2-mediated 5-FU resistant cells by an apoptotic pathway. The MTT assay was used to verify whether RV-59 could efficiently kill cNrf2-mediated 5-FU resistant cells without having adverse effects on normal FHC colon epithelial cells. Figure 3A shows that the cell viability was gradually decreased by increasing doses of RV-59 (0-15 μM) in the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones. The IC50 of RV-59 was 4.20 μM for NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones and 17.6 μM for FHC cells. As expected, the IC50 of RV-59 in NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones (4.2 μM) was similar to that observed in p53-/- HCT116 cells (4.4 μM), which had an even higher cNrf2 expression (Figure 3A). The MTT and colony formation assays indicated that treatment with RV-59 almost completely inhibited cell survival and colony formation in both the p53-/- HCT116 cells and the NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones when compared to vehicle controls (DMSO) (Figure 3B and 3C). Annexin-V/PI staining showed that the percentages of apoptotic cells were significantly higher when the p53-/- HCT116 cells and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones were treated with RV-59 than with a vehicle control (12.32% vs. 3.02% for p53-/- HCT116 cells; 16.49% vs. 4.24% for NLS-mutated Nrf2-transfected HCT116 cells; Figure 3D). These results suggest that RV-59 may kill cNrf2-mediated 5-FU resistant colon cancer cells by an apoptotic pathway, but it is ineffective at killing normal colon epithelial cells.
Figure 3.
RV59 kills cNrf2-mediated 5-FU resistance in colon cancer cells via apoptotic pathway. A. The IC50 value of RV-59 for p53-/- HCT116 cells, NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones and normal FHC colon epithelial cells was calculated from their dose response survival curves. Each cells were treated with RV-59 for 48 h. B. The MTT assay was used to evaluated the cell viability when p53-/- HCT116 and NLS-mutated Nrf2-transfected HCT116 cells were treated with RV-59 or DMSO for 48 h. C. The colony forming ability was determined by colony formation assay when p53-/- HCT116 cells and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones were treated with RV-59 or DMSO for 48 h. D. Annexin V analysis was conducted to evaluate the percentage of apoptotic cells when p53-/- HCT116 and NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones were treated with RV-59 or DMSO for 48 h.
RV-59 nearly completely suppresses xenograft tumor growth induced by NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones
We conducted a xenograft tumor study in nude mice to explore whether RV-59 could suppress tumor growth induced by NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones. Five nude mice were randomly distributed into each group. All mice were injected with NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones (1 × 106 cells/0.1 mL) and every 3 days they were injected with RV-59 (either 2.5 or 5.0 mg/kg) or DMSO. Representative xenograft tumors from each group are shown in Figure 4A. The body weights of the mice in the two RV-59 treated groups were unchanged when compared with the vehicle control group (Figure 4B). The tumor volume of the control mice was markedly increased during the 37 days; however, the tumor volume of the mice in the other two groups was significantly decreased by RV-59 (2.5 mg/kg) when compared to the vehicle controls. More surprisingly, the tumor growth was almost completely suppressed by a high concentration of RV-59 (5.0 mg/kg) (Figure 4C). These results strongly suggest that RV-59 may efficiently suppress tumor growth induced by colon cancer cells with cNrf2-mediated 5-FU resistance.
Figure 4.
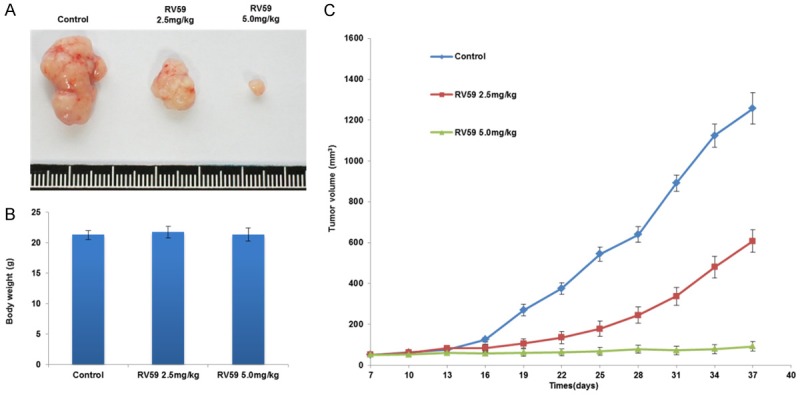
RV-59 suppresses tumor growth induced by NLS-mutated Nrf2-transfected shNrf2-HCT116 stable clones in nude mice. A. The representative tumor burdens of each group are presented. B. The body weights of the mice were measured on day 37. The body weights of the mice were measured on day 37. C. The nude mice were subcutaneously injected with NLS-mutated Nf2-transfected shNrf2-HCT116 stable clones (1 × 106 cells/0.1 mL) on day 0 and then given a peritoneal injection of RV-59 (2.5 mg/kg or 5.0 mg/kg) on day 7, 14, 21, 28, and 35. All mice were sacrificed on day 37 and their tumors were removed to measure tumor volumes. C. The tumor volume of all mice was measured by 3-day intervals from day 10 to day 37. Mean ± S.E.M. values (cm3) were calculated from the tumor volume of five nude mice in each group.
Discussion
The results of this study, obtained from both cell and animal models, provide evidence to support RV-59 as an effective agent that can overcome cNrf2-mediated 5-FU resistance in colon cancer cells (Figures 2, 3 and 4). To confirm the hypothesis, two colon cancer cell lines, HCT-15 and HT-29, the former having higher cNrf2 expression and the latter having lower cNrf2 expression, were collected to treat with RV-59 (1-5 μM). As expected, the MTT assay indicated that a lower IC50 value was observed in HCT-15 cells when compared with HT-29 cells (1.8 μM vs. 3.5 μM, Figure S1). More importantly, the growth of cNrf2-induced xenograft tumors in nude mice can be completely suppressed by RV-59, even when used alone as a single agent (Figure 4). The potential of RV-59 for suppressing cNrf2-induced tumor growth was similar to that of a combination treatment of carfilzomib plus 5-FU [6]. To the best our knowledge, this is the first report of a single agent that can nearly completely suppress tumor growth induced by cNrf2-mediated 5-FU resistant colon cancer cells.
A survey of 160 patients with CRC revealed that more than 50% patients had cNrf2-expressing tumors, but only 5% had nNrf2-expressing tumors and 25% had c/nNrf2 expressing tumors [5]. Of these 160 patients with CRC, 59 were available for a retrospective study to examine the association with the tumor response to 5-FU-based chemotherapy [6]. Patients with cNrf2-expressing tumors had a higher prevalence of unfavorable response to 5-FU-based chemotherapy when compared to patients with c/nNrf2 or Nrf2-negative expressing tumors [6]. Mechanistic studies on a cell model have demonstrated that cNrf2 expression may induce PSMD4 expression and in turn promote tumor invasion and 5-FU resistance via the NF-κB/AKT/ß-catenin/ZEB1 cascades [5,6].
In the present study, we have provided evidence that a single agent, RV-59, on its own, without combination with 5-FU and/or other natural compounds, can efficiently overcome cNrf2-mediated 5-FU resistance and suppress tumor growth in colon cancer cells [6,12-18]. Moreover, RV-59 had no adverse effects on normal FHC colon epithelial cells, nor did it affect the body weights of nude mice during the experimental period, suggesting that RV-59 might not be toxic to normal cells or to nude mice (Figures 3 and 4). However, further pharmacokinetics and toxicological studies should be performed to confirm these possibilities.
In summary, a nitrogen-substituted anthra[1,2-c][1,2,5] thiadiazole-6,11-dione derivative, RV-59, may effectively suppress cNrf2-mediated 5-FU resistance and tumor growth in CRC. These findings strongly support the potential for development of RV-59 as a new drug for clinical therapy in CRC, particularly for patients with cNrf2-expressing tumors.
Acknowledgements
This work was supported by grants from the National Cheng Kung University Hospital, Tainan, Taiwan, and the Chi Mei Medical Center, Tainan, Taiwan (CMNCKU10706) and Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (KMUH-107-7R38).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Cancer Registry Annual Report. Health Promotion Administration, Ministry of Health and Welfare, the Executive Yuan, Republic of China. 2016
- 2.Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N, Cartenì G, Agostara B, Pezzella G, Manzione L, Borsellino N, Misino A, Romito S, Durini E, Cordio S, Di Seri M, Lopez M, Maiello E, Montemurro S, Cramarossa A, Lorusso V, Di Bisceglie M, Chiarenza M, Valerio MR, Guida T, Leonardi V, Pisconti S, Rosati G, Carrozza F, Nettis G, Valdesi M, Filippelli G, Fortunato S, Mancarella S, Brunetti C Gruppo Oncologico Dell’Italia Meridionale. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the gruppo oncologico dell’italia meridionale. J. Clin. Oncol. 2005;23:4866–75. doi: 10.1200/JCO.2005.07.113. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Hielscher J, Scholz M, Müller S, Link H, Niederle N, Rost A, Höffkes HG, Moehler M, Lindig RU, Modest DP, Rossius L, Kirchner T, Jung A, Stintzing S. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 4.Wen C, Chen J, Zhang D, Wang H, Che J, Qin Q, He L, Cai Z, Lin M, Lou Q, Huang L, Chen D, Iwamoto A, Ren D, Wang L, Lan P, Wang J, Liu H, Yang X. Pseudolaric acid B induces mitotic arrest and apoptosis in both 5-fluorouracil-sensitive and -resistant colorectal cancer cells. Cancer Lett. 2016;383:295–308. doi: 10.1016/j.canlet.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Lin PL, Chang JT, Wu DW, Huang CC, Lee H. Cytoplasmic localization of Nrf2 promotes colorectal cancer with more aggressive tumors via upregulation of PSMD4. Free Radic Biol Med. 2016;95:121–32. doi: 10.1016/j.freeradbiomed.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Cheng YM, Lin PL, Wu DW, Wang L, Huang CC, Lee H. PSMD4 is a novel therapeutic target in chemoresistant colorectal cancer activated by cytoplasmic localization of Nrf2. Oncotarget. 2018;9:26342–52. doi: 10.18632/oncotarget.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Tillo E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. beta-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Proc Natl Acad Sci U S A. 2011;108:19204–9. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sánchez-Tilló E, Fanlo L, Siles L, Montes-Moreno S, Moros A, Chiva-Blanch G, Estruch R, Martinez A, Colomer D, Győrffy B, Roué G, Postigo A. The EMT activator ZEB1 promotes tumor growth and determines differential response to chemotherapy in mantle cell lymphoma. Cell Death Differ. 2014;21:247–57. doi: 10.1038/cdd.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W, Su G, Li J, Liao J, Chen S, Huang C, Liu F, Chen Q, Ye Y. Enhanced anti-colorectal cancer effects of carfilzomib combined with CPT-11 via downregulation of nuclear factor-kappaB in vitro and in vivo. Int J Oncol. 2014;45:995–1010. doi: 10.3892/ijo.2014.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, MacNaughton WK. Overexpressed beta-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells. Cancer Res. 2005;65:8604–7. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- 11.Lee YR, Chen TC, Lee CC, Chen CL, Ahmed Ali AA, Tikhomirov A, Guh JH, Yu DS, Huang HS. Ring fusion strategy for synthesis and lead optimization of sulfur-substituted anthra[1,2-c][1,2,5]thiadiazole-6,11-dione derivatives as promising scaffold of antitumor agents. Eur J Med Chem. 2015;102:661–76. doi: 10.1016/j.ejmech.2015.07.052. [DOI] [PubMed] [Google Scholar]
- 12.Afrin S, Giampieri F, Forbes-Hernández TY, Gasparrini M, Amici A, Cianciosi D, Quiles JL, Battino M. Manuka honey synergistically enhances the chemopreventive effect of 5-fluorouracil on human colon cancer cells by inducing oxidative stress and apoptosis, altering metabolic phenotypes and suppressing metastasis ability. Free Radic Biol Med. 2018;126:41–54. doi: 10.1016/j.freeradbiomed.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Das D, Preet R, Mohapatra P, Satapathy SR, Kundu CN. 1,3-Bis(2-chloroethyl)-1-nitrosourea enhances the inhibitory effect of resveratrol on 5-fluorouracil sensitive/resistant colon cancer cells. World J Gastroenterol. 2013;19:7374–88. doi: 10.3748/wjg.v19.i42.7374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La X, Zhang L, Li Z, Li H, Yang Y. (-)-epigallocatechin gallate (EGCG) enhances the sensitivity of colorectal cancer cells to 5-FU by inhibiting GRP78/NF-kappaB/miR-155-5p/MDR1 pathway. J Agric Food Chem. 2019;67:2510–8. doi: 10.1021/acs.jafc.8b06665. [DOI] [PubMed] [Google Scholar]
- 15.Riahi-Chebbi I, Souid S, Othman H, Haoues M, Karoui H, Morel A, Srairi-Abid N, Essafi M, Essafi-Benkhadir K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci Rep. 2019;9:195. doi: 10.1038/s41598-018-36808-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang AD, Fan F, Camp ER, van Buren G, Liu W, Somcio R, Gray MJ, Cheng H, Hoff PM, Ellis LM. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147–53. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 17.Yu C, Liu SL, Qi MH, Zou X, Wu J, Zhang J. Herbal medicine Guan Chang Fu Fang enhances 5-fluorouracil cytotoxicity and affects drug-associated genes in human colorectal carcinoma cells. Oncol Lett. 2015;9:701–8. doi: 10.3892/ol.2014.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang C, He LJ, Ye HZ, Liu DF, Zhu YB, Miao DD, Zhang SP, Chen YY, Jia YW, Shen J, Liu XP. Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT8/5Fu human colorectal cancer cell line. Mol Med Rep. 2018;18:5409–16. doi: 10.3892/mmr.2018.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.