Abstract
The secreted fibroblast growth factor (FGF) binding protein (FGF-BP), which is an extracellular chaperone molecule for FGFs, has been demonstrated to enhance the biological and biochemical activities of FGFs and to be closely related to the growth of several cancers. However, the role of FGFBP1 in pancreatic adenocarcinoma (PDAC) has not been studied extensively. We previously reported that decreased FBW7 could induce pancreatic cancer proliferation and progression. In the present study, we investigated whether FBW7 inhibited cell proliferation and metastasis by decreasing the expression of FGFBP1 in pancreatic cancer. We initially confirmed that pancreatic cancer patients with higher FGFBP1 expression had a worse prognosis. Next, we demonstrated that FGFBP1 silencing inhibited the proliferation and metastasis of PANC-1 and Mia PaCa-2 cells. Mechanistically, FGFBP1 was negatively correlated with FBW7 but positively correlated with c-Myc in PDAC tissue samples, and FBW7 regulated FGFBP1 in a c-Myc-dependent manner. We also found that FBW7 silencing could partly reverse the effect of FGFBP1 silencing on proliferation and metastasis. In summary, FGFBP1 is a prognostic marker for overall survival and is required for pancreatic cancer cell proliferation and metastasis, which is mediated by FBW7 in a c-Myc-dependent manner. Thus, targeting the FBW7/c-Myc/FGFBP1 axis might suppress recurrence and metastasis and provide novel treatment strategies for PDAC.
Keywords: Pancreatic adenocarcinoma, FGFBP1, FBW7, c-Myc, proliferation, metastasis
Introduction
Pancreatic cancer is one of the most malignant tumors of the digestive system, with a five-year survival rate of only 9% [1]. The low survival rate is due to several factors, the most important of which is the diagnosis that most patients receive, namely, late-stage disease with loco-regional spread and distant metastases [2]. Surgery is currently the most effective treatment for pancreatic cancer, but only approximately 20% of patients have surgical opportunities in the clinic [2]. Moreover, metastatic relapse occurs in most patients undergoing resection, and their 5-year survival rate is still between 20 and 30% [3]. Autopsy cases have also revealed that ~90% of patients with pancreatic cancer have distant metastases. Both the metastatic behavior and progression of PDAC contribute to the poor clinical outcome of the malignancy [4]. It may be impossible to overcome the disease without new therapeutic strategies targeting the proliferation and metastasis of pancreatic cancer cells. The tumor biology of pancreatic cancer plays an important role in early recurrence and metastasis [5]. Hence, a better understanding of the novel mechanisms underlying malignant biological activities may yield new knowledge for therapeutic purposes.
As a secreted protein, fibroblast growth factor-binding protein 1 (FGFBP1) binds and releases immobilized fibroblast growth factor (FGF) from the extracellular matrix. In addition, FGFBP1 enhances the activities of FGFs by chaperoning them to their receptors [6]. FGFBP1 is conducive to proliferation and differentiation during embryonic development and wound healing [7]. Compared to its low expression in normal adult tissues, FGFBP1 expression is significantly higher in several cancers [8]. In addition to its important factor in tumor angiogenesis, upregulation of FGFBP1 facilitates cancer growth and metastasis [9,10]. FGFBP1 is precisely regulated by different transcription factors including β-catenin/TCF4, C/EBP, and KLF5 that bind to several promoter elements. Correspondingly, Wnt/β-catenin and KLF5-induced tumorigenesis and metastasis are decreased by knocking down FGFBP1 [11-13]. However, the mechanisms of these FGFBP1-mediated effects in PDAC have not been fully elucidated. The promoter region of FGFBP1 has been shown to be directly bound by c-Myc [14]. In addition, a previous study demonstrated that c-Myc is a crucial substrate of F-box and WD repeat domain-containing 7 (FBW7) in PDAC [15].
FBW7 acts as the substrate recognition component of an ubiquitin ligase complex, which regulates cell apoptosis, proliferation and metabolism [16]. Previous screenings have suggested that FBXW7 mutations occur in approximately 6% of all cancers [17]. However, in pancreatic cancer, FBW7 mutations are rare, and activation of the Ras pathway leads to the substantially downregulated protein level of FBW7 [15]. FBW7 markedly promotes apoptosis and suppresses proliferation through promoting degradation of proto-oncogenes [18]. Our previous studies indicated that downregulated FBW7 results in enhanced pancreatic cancer progression due to a corresponding reduction in c-Myc degradation [15]. In addition, downregulated FBW7 induces the aerobic glycolysis of pancreatic cancer cells via a c-Myc-mediated decrease in thioredoxin-interacting protein [19]. Moreover, downregulated FBW7 has been shown to enhance Muc16 expression in pancreatic cancer cells in a c-Myc-dependent manner [20]. These studies demonstrate that decreased FBW7 and increased c-Myc can promote PDAC progression.
In this study, we observed that FGFBP1 is highly expressed in pancreatic cancer tissues compared with adjacent tissues and that high expression of FGFBP1 is associated with a poor outcome for PDAC patients. We also found that FGFBP1 silencing inhibited the proliferation and metastasis capacity of pancreatic cancer cells. Mechanistically, decreased FBW7 may induce the upregulation of FGFBP1 in a c-Myc-dependent manner. Collectively, our results provide promising treatment targets for improving the prognosis of pancreatic cancer patients.
Materials and methods
Tissue specimens and cell line
The tissue specimens were obtained from patients diagnosed with PDAC at Fudan University Shanghai Cancer Center (FUSCC) in 2012. The pathological diagnoses were conducted by two independent pathologists. The clinical characteristics of the samples are summarized in Table 1. We have gained the informed consent of patients and approval from the Clinical Research Ethics Committee of FUSCC, the human PDAC cell lines PANC-1, MiaPaCa-2 were obtained from the American Type Culture Collection (ATCC, USA). The cells were cultured following the standard protocols provided by ATCC. Shortly, PANC-1 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% foetal bovine serum (FBS) with 100 U/mL penicillin and 0.1 mg/mL streptomycin and MIA PaCa-2 cells were cultured in DMEM supplemented with an additional 2.5% horse serum in addition to 10% FBS. The cells were kept in a 37°C humidified incubator with 5% CO2.
Table 1.
Relationship between FGFBP1 expression and clinicopathological features of pancreatic cancer
| Variables | Patients number (n) | FGFBP1 expression in tumor tissue of pancreatic cancer | ||
|---|---|---|---|---|
|
| ||||
| Low (n=45) | High (n=64) | P value | ||
| Age (years) | 0.507 | |||
| ≤60 | 54 | 24 | 30 | |
| >60 | 55 | 21 | 34 | |
| Gender | 0.581 | |||
| Male | 62 | 27 | 35 | |
| Female | 47 | 18 | 29 | |
| Tumor size (cm) | 0.903 | |||
| ≤4 | 83 | 34 | 49 | |
| >4 | 26 | 11 | 15 | |
| TNM stage | 0.234 | |||
| I, IIa | 58 | 27 | 31 | |
| IIb, III, IV | 51 | 18 | 33 | |
| Histological grade | 0.310 | |||
| Grade 1, 2 | 69 | 31 | 38 | |
| Grade 3 | 40 | 14 | 26 | |
| Lymph node status | 0.345 | |||
| Negative | 62 | 28 | 34 | |
| Positive | 47 | 17 | 30 | |
| Vascular emboli | 0.936 | |||
| Negative | 90 | 37 | 53 | |
| Positive | 19 | 8 | 11 | |
Vectors and siRNA targets
In order to silence FGFBP1 expression in pancreatic cancer cells, pLKO.1-TRC cloning vector was used. 21 bp targets against FGFBP1 were GAGCTCTCTCTGCACATTCTT and GCAAAGTGGTCTCAGAACAAA respectively. The promoter region of FGFBP1 covering from -2000 to +200 was amplified from genomic DNA of PANC-1 cells and ligated into pGL3-Basic vector. siRNA targets against cMyc were GTCAAGAGGCGAACACACA and CGGTGCAGCCGTATTTCTA respectively. siRNA targets against FBW7 were ACCTTCTCTGGAGAGAGAAATGC and GGAATGCAGAGACTGGAGA respectively.
Cell viability assay
Cell viability was measured using a Cell Counting Kit-8 (Dojindo, Japan). In brief, cells maintained in 200 μL of medium were seeded in each well of 96-well plate and cultured for the indicated times. After CCK-8 solution was added into each well and incubated for 2 hours, the absorbance of each well at 450 nm was measured using a microplate reader.
Colony formation assay
PANC-1 and MiaPaCa-2 cells (500/well) was seeded and cultivated for indicated times. After being fixed by 4% paraformaldehyde, the cells were stained with 0.1% crystal violet (Sigma, USA). The colonies were counted under microscope subsequently.
Transwell migration assay
A 24-transwell chamber was used to perform migration assays. The lower chamber contained 800 μL of media supplemented with 10% FBS. 200 μL of serum-free medium containing cells (~6×104 cells) was added to the upper chamber. After cultivation for 24 h at 37°C with 5% CO2, the non-migrating cells were removed, migrating cells were fixed, stained and imaged. Finally, the number of migrating cells was quantified in random six fields.
Immunohistochemistry
IHC staining with primary antibodies against FGFBP1 (1:200; Affinity, China), FBW7 (1:200; Bethyl, USA), c-Myc (1:200; Abcam, UK) were conducted using standard protocols as shown previously [21,22]. The score scare includes the multiplying the positivity (0, <10%; 1, 10%-25%; 2, 25%-50%; 3, 50%-75%; 4, >75%) and intensity scores (0, negative; 1, low; 2, moderate; 3, strong). The product of the two scores represents the protein expression levels (negative (0, -); weakly positive (1-3, +); moderately positive (4-6, ++); and strongly positive (>6, +++). The overall staining scores >6 was considered a high expression, while a score ≤6 was defined as low expression.
Western blotting
Western blotting was performed as shown in a previous study15. The antibodies chosen in this assay were Tubulin (1:10000; Abcam, UK), FGFBP1 (1:1000; Affinity, China), FBW7 (1:1000; Bethyl, USA) and c-Myc (1:10000; Abcam, UK).
Quantitative real-time PCR
Total RNA was extracted from cells using TRIzol reagent from Invitrogen of USA. cDNA was obtained through reverse transcription using a commercially available TaKaRa PrimeScript RT reagent kit. The expression levels of candidate genes were detected using an ABI 7900HT Real-time PCR system from Applied Biosystems of USA. The primer sequences are shown in Table 2.
Table 2.
Primer sequences used in this study
| β-actin | Forward: 5’-CTACGTCGCCCTGGACTTCGAGC-3’ |
| β-actin | Reverse: 5’-GATGGAGCCGCCGATCCACACGG-3’ |
| c-Myc | Forward: 5’-AACACACAACGTCTTGGAGCGCCA-3’ |
| c-Myc | Reverse: 5’-TCCTCTGCTTGGACGGACAGGATG-3’ |
| FGFBP1 | Forward: 5’-CTTCACAGCAAAGTGGTCTCA-3’ |
| FGFBP1 | Reverse: 5’-GACACAGGAAAATTCATGGTCCA-3’ |
| FBW7 | Forward: 5’-CCACTGGGCTTGTACCATGTT-3’ |
| FBW7 | Reverse: 5’-CAGATGTAATTCGGCGTCGTT-3’ |
Promoter activity evaluation by dual-luciferase assay
The FGFBP1 promoter region, which spans from -2000 to 200 of the transcription starting site, was firstly amplified from human genomic DNA and then cloned into a pGL3-Basic vector. After seeded in 96-well culture plates, HEK-293T cells were transfected with the pGL3 constructs together with Renilla luciferase expression vectors obtained from Lipofectamine 2000 (Invitrogen, USA). Finally, Firefly and Renilla luciferase activities were measured using a dual-luciferase system (Promega, USA) as described in the manufacturer’s protocol.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed following the protocols of an available Magna ChIP™ A/G Chromatin Immunoprecipitation Kit from Merck Millipore Corporation. Two pairs of primers to detect FGFBP1 promoter occupancy were: ChIPFGFBP1F1: 5’-AAGAGCAAAGCAGACGCTGGGATG-3’; ChIPFGFBP1R1: 5’-CTCCACGAACAGGCTCTCTCATGC-3’; ChIPFGFBP1F2: 5’-AGACCCTGTCATGAGGCAAAACG-3’; ChIPFGFBP1R2: 5’-CTACCAGGCTCTGAGAGGCTGGTA-3’.
Statistics
The results were processed using SPSS 24.0 software from Abbott Laboratories, USA and presented as the mean ± SD. The data between groups were statistically analyzed with One-way ANOVA and Student’s test. A Chi square test was used to analyze the relationships between FGFBP1 expression and clinicopathologic characteristics and log-rank test and Cox regression were adopted to perform survival analysis for pancreatic cancer patients. The correlation between the FGFBP1 and either FBW7 or c-Myc expression was analyzed with Spearman correlation analysis. P<0.05 was defined as significant. *Represented significant differences (P<0.05, compared with group control); **Represented with significant differences (P<0.01, compared with group control); ***Represented with significant differences (P<0.001, compared with group control); ****Represented with significant differences (P<0.0001, compared with group control).
Results
FGFBP1 expression is negatively correlated with PDAC prognosis
First, FGFBP1 expression in 35 paired-patient specimens of PDAC and adjacent normal tissues was measured using IHC (Figure 1A and 1B). Next, the IHC results of FGFBP1 expression were further confirmed in tissue micro arrays containing 109 pairs of patient specimens (Figure 1C). Additionally, analysis by log-rank testing indicated that high FGFBP1 expression significantly correlates with a poor prognosis for patients with PDAC (P<0.01; Figure 1D). Then, the correlation between FGFBP1 expression and survival time was validated using GEPIA analysis (Figure 1E). Based on a Cox regression analysis, FGFBP1 was an independent prognostic marker of PDAC (Table 3).
Figure 1.
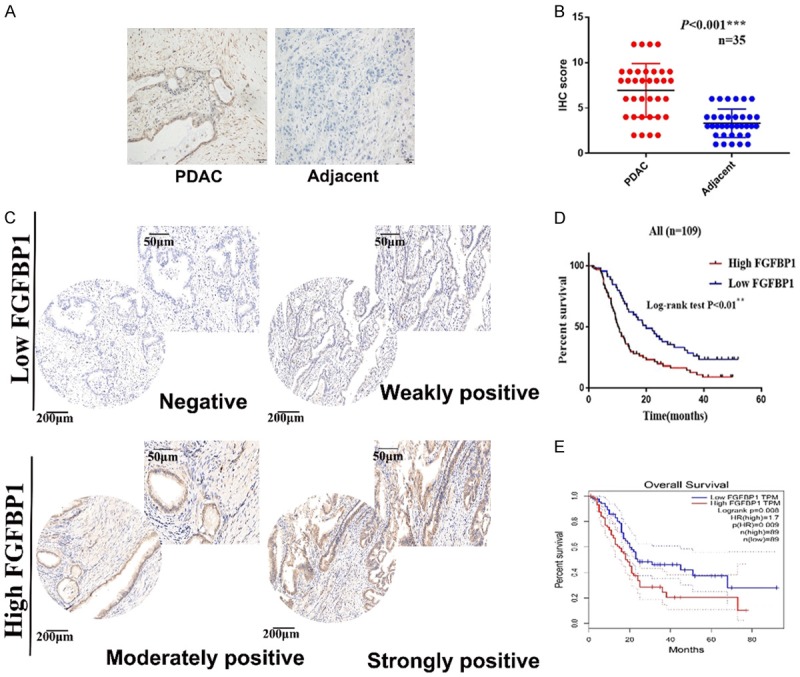
FGFBP1 expression is increased in PDAC tissues. A. Representative images of IHC staining for FGFBP1 in PDAC and adjacent normal tissue (scale bar, 50 μm). B. FGFBP1 expression in PDAC and adjacent normal tissues, as determined by the IHC score (n=35, ***P<0.001). C. Representative images of IHC staining for FGFBP1 in tissue microarrays (scale bar, 200 µm; inset scale bar, 50 µm). D. The overall survival of patients with PDAC was analyzed using the Kaplan-Meier analysis on the basis of FGFBP1 expression in the tissues from our center (n=109, **P<0.01). E. The overall survival of patients with PDAC was analyzed using the Kaplan-Meier analysis on the basis of FGFBP1 expression in the tissues from GEPIA (n=178, **P=0.008).
Table 3.
Univariate and multivariate Cox regression of overall survival for patients with PDAC
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | ||||||
| >60 | 1.216 | 0.805 to 1.837 | 0.352 | - | ||
| ≤60 | ||||||
| Gender | ||||||
| Male | 0.902 | 0.594 to 1.370 | 0.630 | - | ||
| Female | ||||||
| Tumor size (cm) | ||||||
| >4 | 2.134 | 1.325 to 3.437 | 0.002** | 1.899 | 1.164 to 3.097 | 0.010* |
| ≤4 | ||||||
| TNM stage | ||||||
| IIb, III, IV | 1.751 | 1.158 to 2.648 | 0.008** | - | ||
| I, IIa | ||||||
| Histological grade | ||||||
| Grade 3 | 1.805 | 1.175 to 2.773 | 0.007** | 1.857 | 1.182 to 2.918 | 0.007** |
| Grade 1, 2 | ||||||
| Lymph node status | ||||||
| Positive | 2.027 | 1.337 to 3.073 | 0.001** | - | ||
| Negative | ||||||
| Vascular emboli | ||||||
| Positive | 1.130 | 0.649 to 1.966 | 0.666 | - | ||
| Negative | ||||||
| FGFBP1 expression | ||||||
| High | 1.908 | 1.243 to 2.929 | 0.003** | 2.047 | 1.32 to 3.174 | 0.001** |
| Low | ||||||
P<0.05;
P<0.01.
FGFBP1 promotes the proliferation and metastasis of PDAC
Based on the crucial role of FGFBP1 in the PDAC prognosis, we subsequently explored the effect of FGFBP1 on the biological behavior of pancreatic cancer cells. We began by constructing stable FGFBP1-silenced cell lines by transfecting PANC-1 and MiaPaCa-2 cells with an FGFBP1-specific shRNA and then screening the cells by puromycin selection. We next validated the knockdown efficiency using qPCR and Western blotting (Figure 2A and 2B). The results of CCK-8 assays suggested that FGFBP1 knockdown markedly reduced the viability of PANC-1 and MiaPaCa-2 cells (Figure 2C and 2D). Furthermore, FGFBP1 silencing substantially decreased the colony formation capacity of PANC-1 and MiaPaCa-2 cells (Figure 2E and 2F). In addition, the results of transwell assays suggested that FGFBP1 silencing in PANC-1 and MiaPaCa-2 cells led to weakened migration (Figure 2G and 2H). Collectively, these data indicate that FGFBP1 may be a proto-oncogene and promote PDAC cell proliferation and metastasis.
Figure 2.
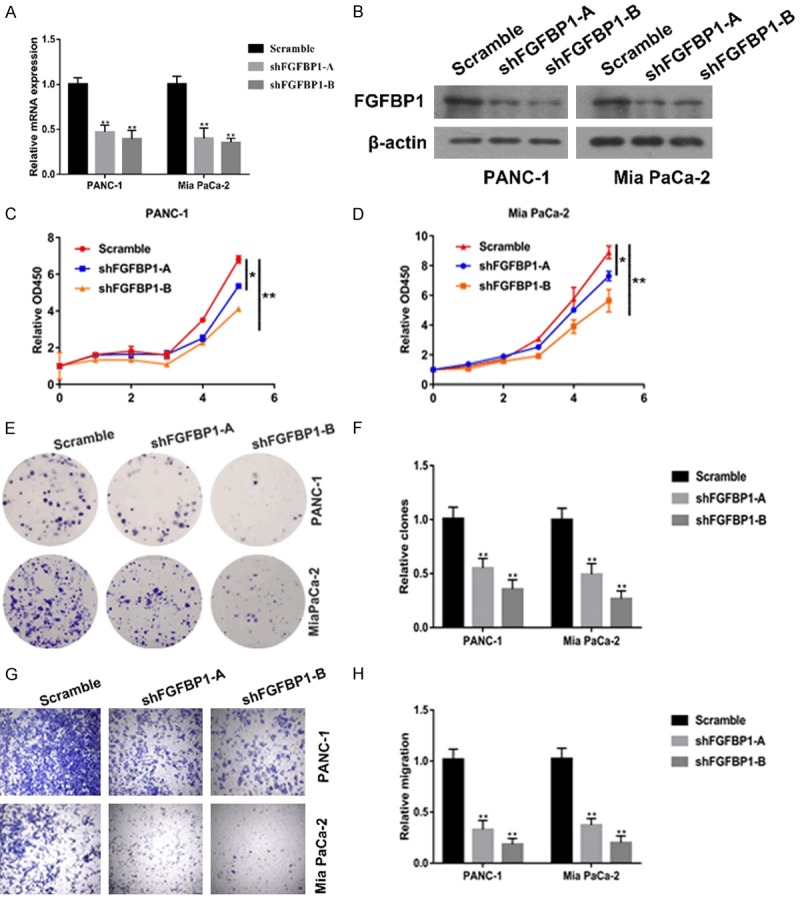
Specific silencing of FGFBP1 decreases the proliferation and metastasis of pancreatic cancer cells. A. Analysis of relative gene expression data of FGFBP1 using real-time quantitative PCR and the 2-ΔΔCT method. B. Analysis of protein expression of FGFBP1 using Western blotting assay. C, D. CCK-8 assays were used to test the proliferation of PDAC cells transfected with the FGFBP1 shRNA. E, F. Colony formation assays were conducted to confirm the effect of FGFBP1 silencing on pancreatic cancer cell lines; G, H. Transwell assays were performed to assess the effect of FGFBP1 silencing on pancreatic cancer cell lines.
FBW7 negatively regulates FGFBP1 expression
Considering the significant role of FBW7/c-Myc in PDAC progression and the results of the previous study showing that c-Myc could bind to the promoter region of FGFBP1, we hypothesized that FGFBP1 may be regulated by FBW7/c-Myc. We first explored whether FBW7 regulated FGFBP1 expression. The results of qPCR and Western blot analyses indicated increased expression of FGFBP1 after knockdown of FBW7 in the PANC-1 and MiaPaCa-2 cell lines (Figure 3A-D). To further confirm the findings of the in vitro experiments, we analyzed the correlation between FGFBP1 and FBW7 in PDAC patient tissue samples. The IHC results revealed that FGFBP1 expression is negatively correlated with FBW7 levels in PDAC patients (Figure 3E and 3F). Additionally, we found decreased expression of FGFBP1 mRNA and protein in PANC-1 and Mia PaCa-2 cells overexpressing FBW7 (Figure S1A and S1B). Moreover, the results of an ELISA analysis suggested that the amount of FGFBP1 secreted into the extracellular space was significantly reduced in the culture media of FBW7-overexpressing PANC-1 and Mia PaCa-2 cells (Figure S1C).
Figure 3.
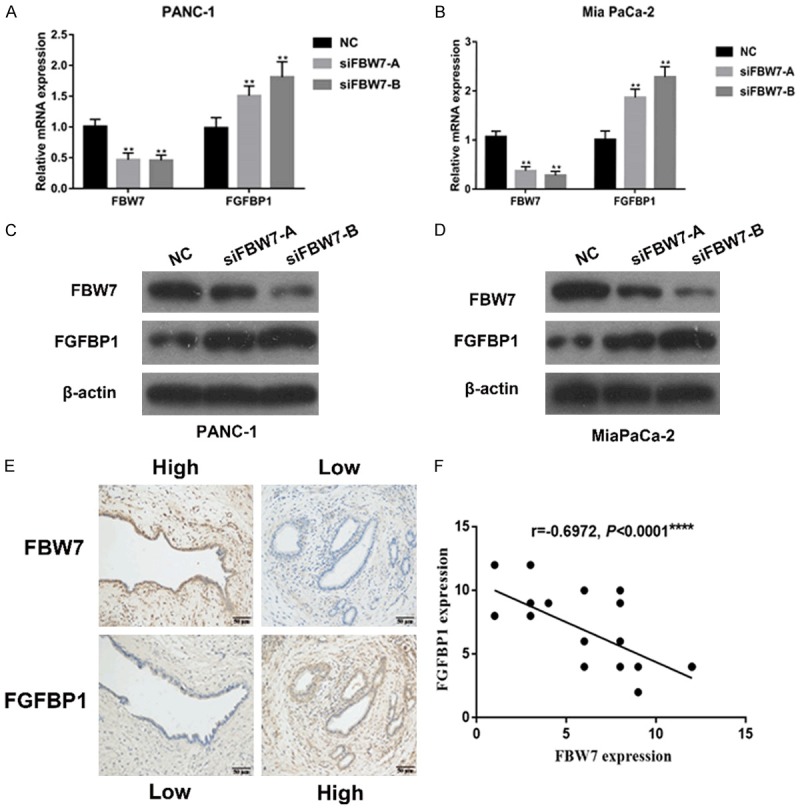
Correlation between FGFBP1 and FBW7 expression. A, B. The mRNA levels of FGFBP1 in the pancreatic cancer cell lines were measured by real-time PCR. C, D. The protein levels of FGFBP1 were analyzed by Western blotting following treatment. E. Representative images of IHC staining for FGFBP1 and FBW7 in PDAC tissues (scale bar, 50 μm). F. Correlation analysis of FGFBP1 expression and FBW7 expression in PDAC tissues, as determined by the IHC score (n=30, ****P<0.0001).
c-Myc positively regulates FGFBP1 expression
Based on the observation that FBW7 downregulates FGFBP1 at the transcriptional level and that two potential c-Myc binding elements exist in the promoter of FGFBP1, we hypothesized that FBW7 downregulates FGFBP1 by targeting c-Myc degradation. We initially investigated FGFBP1 expression in c-Myc-silenced pancreatic cancer cells. The subsequent qPCR analysis revealed that FGFBP1 was downregulated in response to c-Myc silencing (Figure 4A and 4B). FGFBP1 protein expression was also significantly decreased in these PANC-1 and Mia PaCa-2 cells (Figure 4C and 4D). Moreover, the IHC results revealed that FGFBP1 expression is positively correlated with c-Myc levels in PDAC patients (Figure 4E and 4F).
Figure 4.
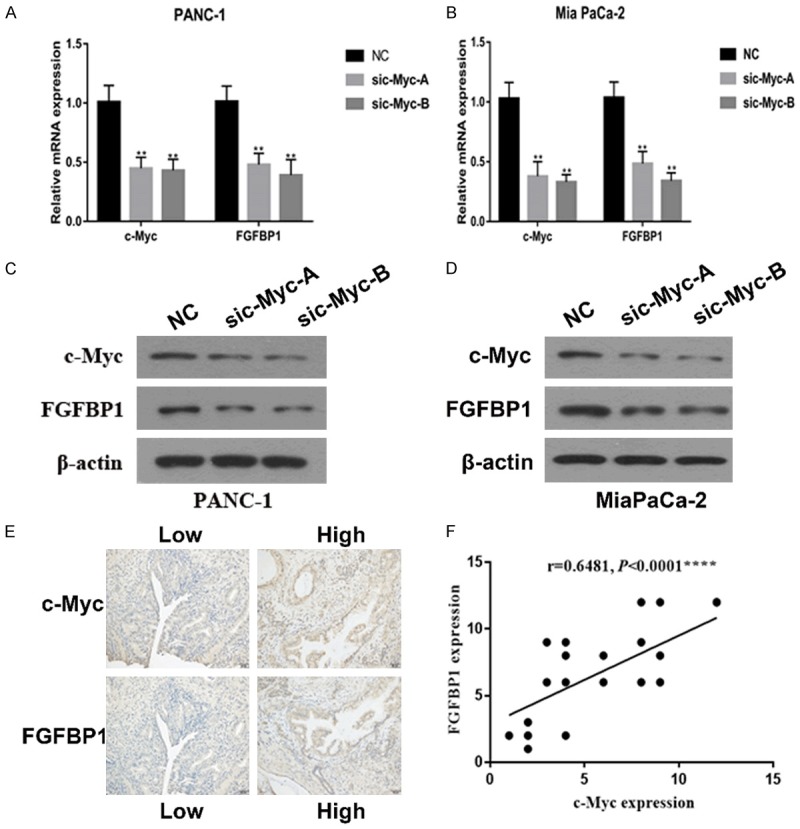
Correlation between the expression of FGFBP1 and c-Myc. A, B. The mRNA levels of FGFBP1 in pancreatic cancer cell lines were measured by real-time PCR. C, D. The protein levels of FGFBP1 were analyzed by Western blotting following treatment. E. Representative images of IHC staining for FGFBP1 and c-Myc in PDAC tissues (scale bar, 50 μm). F. Correlation analysis of FGFBP1 expression and c-Myc expression in PDAC tissues, as determined by the IHC score (n=30 ****P<0.0001).
FBW7 regulates FGFBP1 expression in a c-Myc-dependent fashion
Given these results, the promoter region of FGFBP1 harbors two possible c-Myc binding elements (Figure 5A). We performed a ChIP assay to evaluate whether c-Myc could occupy the E-boxes in the FGFBP1 promoter region. As shown, c-Myc was merely enriched in E-box2 instead of E-box1 in the promoter region (Figure 5B), which further supports its regulation of FGFBP1. Subsequent luciferase assays suggested that the manipulation of c-Myc expression increased FGFBP1 promoter activity, which was confirmed by the mutation of FGFBP1 binding sites. When the sequence was mutated from CACATG into AATATG to generate a mutant, c-Myc lost its promoting function for the mutated luciferase construct (Figure 5C).
Figure 5.
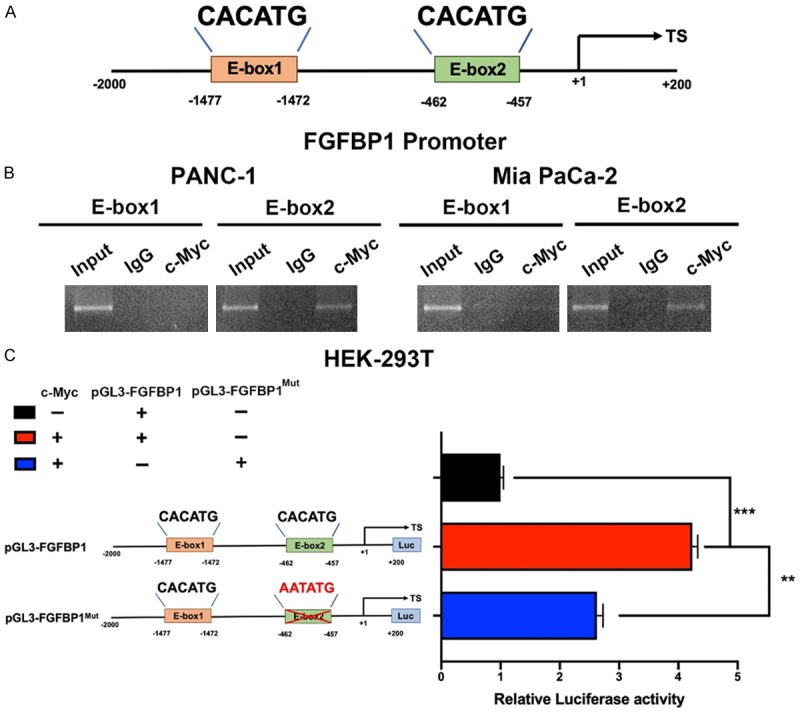
c-Myc is involved in FGFBP1 transcriptional expression in PDAC. A. The position of the c-Myc binding sites in the FGFBP1 promoter. B. c-Myc occupies the binding sites of the FGFBP1 promoter region in PANC-1 and MiaPaCa-2 cells, as measured by ChIP assay. C. c-Myc affected FGFBP1 promoter activity in HEK-293T cells.
c-Myc reversed the inhibitory effects of FBW7 on FGFBP1 expression
To further confirm the role of c-Myc in the effect of FBW7 on FGFBP1, we targeted both FBW7 and c-Myc in PANC-1 and Mia PaCa 2 cells. As expected, FGFBP1 mRNA levels were substantially decreased, while c-Myc was silenced in pancreatic cancer cells, even when FBW7 was also silenced (Figure 6A and 6B). Subsequent Western blotting analysis demonstrated that FBW7 silencing significantly promoted FGFBP1 protein expression, which could be reversed by c-Myc silencing (Figure 6C and 6D).
Figure 6.
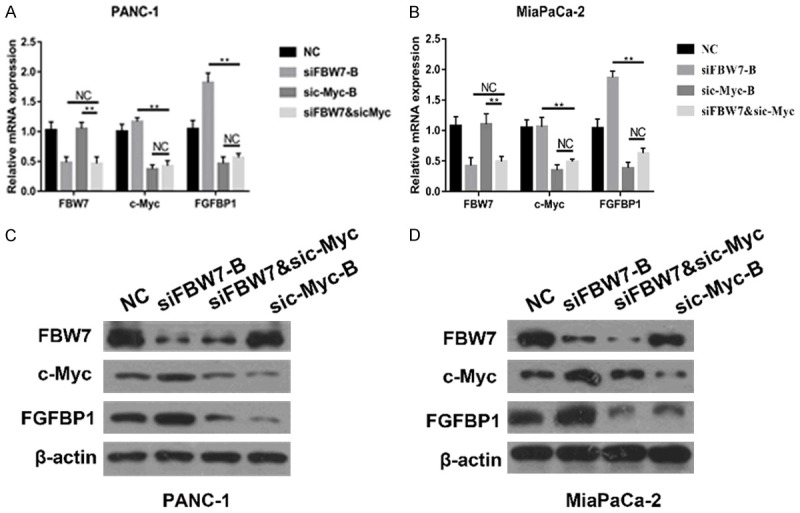
The inhibition of FGFBP1 by FBW7 could be reversed by c-Myc. A, B. The mRNA levels of FGFBP1 in the pancreatic cancer cell lines were measured by real-time PCR. C, D. The protein levels of FGFBP1 were analyzed by Western blotting following treatment.
FBW7 can weaken the promoting effect of FGFBP1 on cell proliferation and metastasis in PDAC
Consistent with the above results, the results of CCK-8 assays demonstrated that FGFBP1 knockdown markedly reduced the viability of PANC-1 and MiaPaCa-2 cells and FBW7 knockdown substantially increased the viability but that silencing FBW7 could weaken the inhibitory influence of FGFBP1 silencing (Figure 7A and 7B). Additionally, FGFBP1 silencing inhibited pancreatic cancer proliferation and abrogation of FBW7 promoted pancreatic cancer proliferation and FBW7 silencing resulted in partial recovery of the proliferation capacity of the FGFBP1-silenced PANC-1 and MiaPaCa-2 cell lines (Figure 7C and 7D). FBW7 silencing could also partly reverse the inhibition of FGFBP1 knockdown on the migration capacity of PANC-1 and MiaPaCa-2 cells (Figure 7E and 7F).
Figure 7.
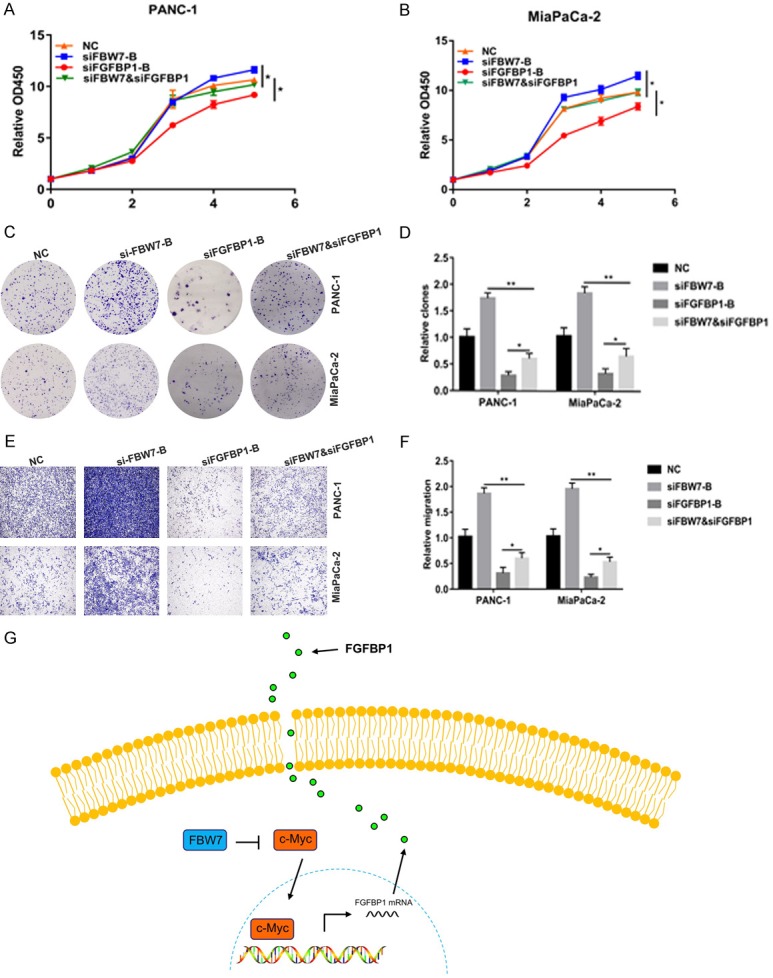
FBW7 could weaken the promoting impact of FGFBP1 on proliferation and migration. A, B. CCK-8 assays were used to test the proliferation of PDAC cells following treatment. C, D. Colony formation assays were conducted to confirm the effects of FGFBP1 and FBW7 silencing on the pancreatic cancer cell lines. E, F. Transwell assays were performed to assess the effects of FGFBP1 and FBW7 silencing on the pancreatic cancer cell lines. G. Schematic representation of the model showing that FBW7 inhibits FGFBP1 expression and secretion in a c-Myc-dependent manner.
Based on these data, we identified FGFBP1 as a novel prognostic marker for overall survival by using the FUSCC cohort and a TCGA dataset. Additionally, in vitro experiments performed with in vitro cell lines suggested that FGFBP1 promoted the proliferation and migration of pancreatic cancer cells. Mechanistically, FBW7 inhibited the expression and secretion of FGFBP1 in a c-Myc-dependent manner (Figure 7G).
Discussion
The biological characteristics of pancreatic cancer cells place a crucial role in tumor progression and the prognosis of patients with PDAC. Thus, a better understanding of the mechanisms underlying cell proliferation and metastasis may provide new targets for treatment. In our study, we explored the role of FGFBP1 in PDAC. Our findings demonstrated that the proliferation and metastasis of pancreatic cancer cells can be enhanced by FGFBP1, which is regulated via FBW7-mediated c-Myc. Furthermore, FGFBP1 expression acts as a powerful prognostic factor for pancreatic cancer patients.
The FGFBP family includes two murine and three human members; these are secreted chaperone proteins that release FGFs that are anchored in the extracellular matrix and facilitate FGF binding to receptors [23,24]. FGFBP1 is the best-characterized member of the FGF family [25]. In addition to being a carrier protein, FGFBP1 has a specific physiological function [26]. The results of a Northern blot analysis suggested that FGFBP1 is expressed at low levels in normal adult human tissues but markedly upregulated in various cancers, including pancreatic cancers, colon adenocarcinomas and squamous cell carcinomas [10,11,27]. Czubayko F et al. reported that FGFBP1 functions as a rate-limiting factor for angiogenesis and tumor growth in FGF-BP-negative cell lines of a nude mouse model [28]. Moreover, FGFBP1 acts as an angiogenic switch in human cancer [29-31]. In addition, upregulation of FGFBP1 in colorectal and pancreatic cancer promotes angiogenesis and growth, which correlates with tumor progression and a poor outcome [23], while FGFBP1 silencing substantially reduces angiogenesis and tumor growth in human colorectal cancer and squamous cell carcinoma cell lines [29]. However, the exact role of FGFBP1 in pancreatic cancer requires further investigation.
In the present study, the results revealed that the expression of FGFBP1 is higher in human PDAC specimens than in adjacent control tissues and that overexpression of FGFBP1 is substantially associated with microvascular invasion. PDAC patients with high FGFBP1 expression have poorer outcomes than patients with low FGFBP1 expression. A multivariate analysis indicated that FGFBP1 expression serves as a crucial risk factor for cancer metastasis and shortens patient overall survival times following curative resection. These clinical results strongly demonstrate that FGFBP1 plays an important role in promoting PDAC progression. Furthermore, we found that FGFBP1 silencing inhibits pancreatic cancer cell proliferation and metastasis. Our results suggested that FGFBP1 may promote the proliferation and metastasis of PDAC cell lines. However, the oncogenic role of FGFBP1 in PDAC needs to be further confirmed be means of in vivo experiments in the future.
Various findings have demonstrated that FGFBP expression is strictly controlled by several mechanisms and promoter elements to which transcription factors including KLF5, C/EBP and β-catenin/TCF4 can directly bind and activate FGFBP transcriptional expression [31]. Ray et al. found that β-catenin upregulates FGFBP during colon carcinogenesis [11]. Harris proposed that the FGFBP promoter can be activated by EGF through AP-1 and C/EBP sites [12]. H-Q Zheng et al. reported that breast cell proliferation can be promoted by KLF5-mediated upregulation of FGFBP1 transcription [13]. Moreover, KLF5 is targeted by FBW7 for ubiquitin-mediated degradation, which inhibits breast cell proliferation [32,33]. Although our previous study revealed that other known FBW7 substrates, including KLF5, were barely affected by the overexpression of FBW7 in PDAC, we analyzed the promoter region of FGFBP1 and found two potential c-Myc binding sites. Furthermore, c-Myc has been reported to bind to the promoter region of FGFBP1 and transregulate its expression [14]. In addition, we found that FBW7 tumor suppressor inhibited pancreatic cancer growth and progression by targeting c-Myc for degradation [34]. Based on these observations, we considered FGFBP1 to be a downstream target of c-Myc, which transregulates FGFBP1. Thus, we initially investigated whether FBW7 regulates FGFBP1. We found that FBW7 silencing upregulated FGFBP1 expression at the transcriptional level. Moreover, our IHC results indicated that FGFBP1 is negatively correlated with FBW7 in human PDAC tissues. Next, we explored whether FGFBP1 expression was altered in response to c-Myc downregulation. The results showed that FGFBP1 promoter activity and expression could be increased by c-Myc. Taken together, these results demonstrate that FGFBP1 expression is regulated by FBW7/c-Myc.
Mounting evidence has revealed that FBW7 plays a crucial role in suppressing the progression of cancers, including pancreatic cancer [35,36]. However, the contribution of FBW7 to PDAC stroma and the corresponding mechanisms have rarely been investigated. PDAC is characterized by dense desmoplastic stroma including extracellular matrix, leukocytes and fibroblasts [37]. Previous studies have considered the stroma as a physical barrier, which hinders the transport of chemotherapeutic agents to the tumor [38]. In addition, Hanahan and Weinberg et al. summarized that the tumor stroma plays an important role in supporting and promoting the growth of cancer [39]. FGFBP functions to chaperone FGFs in ECM and subsequently activates downstream signaling of FGFs. The FGF pathway is significantly associated with the proliferation, invasion, migration, apoptosis and metastasis of PDAC [40,41]. To date, more than 20 subtypes of FGF have been identified, of which FGF-7 is usually overexpressed in PDAC [42]. FGF-7 can promote the invasion and metastasis of pancreatic cancer cells by activated NF-κB-mediated upregulation of MMP-9 and VEGF [43]. This result confirmed that FGF is a crucial malignancy factor in the tumor stroma. Moreover, FGF10, a member of the FGF-7 subfamily, has been found in stromal cells in pancreatic cancer tissues. It has been reported that FGF-10 can promote cell invasion and migration by interacting with FGFR2 IIIb and upregulating MT1-MMP and TGF-β1 in PDAC [32]. Collectively, these results demonstrated that FBW7 may play a crucial role in the effects of the stroma on pancreatic cancer cells, but further research is required to validate the hypothesis.
In summary, we confirmed that FGFBP1, which is frequently overexpressed in pancreatic cancer, acts as a tumor promoter gene. Moreover, we observed that overexpression of FGFBP1 is associated with a poor outcome for PDAC patients. Additionally, FGFBP1 promotes PDAC cell proliferation and metastasis. Mechanistically, we discovered that FBW7 can downregulate FGFBP1 in a c-Myc-dependent manner. In addition, FBW7 may contribute toward inhibiting the promoting and supporting effects of the stroma on pancreatic cancer cells via the FGFBP1-mediated FGF signaling pathway. In conclusion, these observations reveal that FGFBP1 may serve as a candidate predictor and attractive therapeutic target for pancreatic cancer and suggest a potential novel anticancer mechanism for FBW7 as a factor that targets the stroma.
Acknowledgements
This work was jointly supported by National Science Foundation of China (No., 81871950, 81702871 and 81602085), Shanghai Municipal Commission of Health and Family Planning (No. 20154Y0090 and 2018YQ06) and Shanghai Sailing Program (16YF1401800).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 3.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 4.Giovannetti E, van der Borden CL, Frampton AE, Ali A, Firuzi O, Peters GJ. Never let it go: stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin Cancer Biol. 2017;44:43–59. doi: 10.1016/j.semcancer.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16:553–565. doi: 10.1038/nrc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tassi E, Al-Attar A, Aigner A, Swift MR, McDonnell K, Karavanov A, Wellstein A. Enhancement of fibroblast growth factor (FGF) activity by an FGF-binding protein. J Biol Chem. 2001;276:40247–40253. doi: 10.1074/jbc.M104933200. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt MO, Garman KA, Lee YG, Zuo C, Beck PJ, Tan M, Aguilar-Pimentel JA, Ollert M, Schmidt-Weber C, Fuchs H, Gailus-Durner V, Hrabe de Angelis M German Mouse Clinic Consortium. Tassi E, Riegel AT, Wellstein A. The role of fibroblast growth factor-binding protein 1 in skin carcinogenesis and inflammation. J Invest Dermatol. 2018;138:179–188. doi: 10.1016/j.jid.2017.07.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Chen Z, Shang X, Tian D, Wang D, Wu K, Fan D, Xia L. Sox12, a direct target of FoxQ1, promotes hepatocellular carcinoma metastasis through up-regulating Twist1 and FGFBP1. Hepatology. 2015;61:1920–1933. doi: 10.1002/hep.27756. [DOI] [PubMed] [Google Scholar]
- 9.Schulze D, Plohmann P, Hobel S, Aigner A. Anti-tumor effects of fibroblast growth factor-binding protein (FGF-BP) knockdown in colon carcinoma. Mol Cancer. 2011;10:144. doi: 10.1186/1476-4598-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tassi E, Henke RT, Bowden ET, Swift MR, Kodack DP, Kuo AH, Maitra A, Wellstein A. Expression of a fibroblast growth factor-binding protein during the development of adenocarcinoma of the pancreas and colon. Cancer Res. 2006;66:1191–1198. doi: 10.1158/0008-5472.CAN-05-2926. [DOI] [PubMed] [Google Scholar]
- 11.Ray R, Cabal-Manzano R, Moser AR, Waldman T, Zipper LM, Aigner A, Byers SW, Riegel AT, Wellstein A. Up-regulation of fibroblast growth factor-binding protein, by beta-catenin during colon carcinogenesis. Cancer Res. 2003;63:8085–8089. [PubMed] [Google Scholar]
- 12.Harris VK, Kagan BL, Ray R, Coticchia CM, Liaudet-Coopman ED, Wellstein A, Tate Riegel A. Serum induction of the fibroblast growth factor-binding protein (FGF-BP) is mediated through ERK and p38 MAP kinase activation and C/EBP-regulated transcription. Oncogene. 2001;20:1730–1738. doi: 10.1038/sj.onc.1204249. [DOI] [PubMed] [Google Scholar]
- 13.Zheng HQ, Zhou Z, Huang J, Chaudhury L, Dong JT, Chen C. Kruppel-like factor 5 promotes breast cell proliferation partially through upregulating the transcription of fibroblast growth factor binding protein 1. Oncogene. 2009;28:3702–3713. doi: 10.1038/onc.2009.235. [DOI] [PubMed] [Google Scholar]
- 14.Harris VK, Coticchia CM, List HJ, Wellstein A, Riegel AT. Mitogen-induced expression of the fibroblast growth factor-binding protein is transcriptionally repressed through a non-canonical E-box element. J Biol Chem. 2000;275:28539–48. doi: 10.1074/jbc.M001677200. [DOI] [PubMed] [Google Scholar]
- 15.Ji S, Qin Y, Shi S, Liu X, Hu H, Zhou H, Gao J, Zhang B, Xu W, Liu J, Liang D, Liu L, Liu C, Long J, Zhou H, Chiao PJ, Xu J, Ni Q, Gao D, Yu X. ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer. Cell Res. 2015;25:561–73. doi: 10.1038/cr.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu K, Nihira NT, Inuzuka H, Wei W. Physiological functions of FBW7 in cancer and metabolism. Cell Signal. 2018;46:15–22. doi: 10.1016/j.cellsig.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 18.Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26:455–464. doi: 10.1016/j.ccell.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, Jin K, Liang D, Xu W, Zhang B, Liu L, Liu C, Xu J, Ni Q, Chiao PJ, Li M, Yu X. FBW7 (F-box and WD repeat domain-containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (thioredoxin-binding protein) axis in pancreatic cancer. Clin Cancer Res. 2016;22:3950–3960. doi: 10.1158/1078-0432.CCR-15-2380. [DOI] [PubMed] [Google Scholar]
- 20.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, Liu J, Xiang J, Liang D, Hu Q, Ni Q, Xu J, Yu X. Oncogenic KRAS targets MUC16/CA125 in pancreatic ductal adenocarcinoma. Mol Cancer Res. 2017;15:201–212. doi: 10.1158/1541-7786.MCR-16-0296. [DOI] [PubMed] [Google Scholar]
- 21.Hu Q, Qin Y, Xiang J, Liu W, Xu W, Sun Q, Ji S, Liu J, Zhang Z, Ni Q, Xu J, Yu X, Zhang B. dCK negatively regulates the NRF2/ARE axis and ROS production in pancreatic cancer. Cell Prolif. 2018;51:e12456. doi: 10.1111/cpr.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Q, Shi S, Liang C, Liang D, Hua J, Zhang B, Xu J, Yu X. Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3beta/Snail signaling. Oncogene. 2018;37:5843–5857. doi: 10.1038/s41388-018-0392-z. [DOI] [PubMed] [Google Scholar]
- 23.Tassi E, Wellstein A. The angiogenic switch molecule, secreted FGF-binding protein, an indicator of early stages of pancreatic and colorectal adenocarcinoma. Semin Oncol. 2006;33(Suppl 11):S50–6. doi: 10.1053/j.seminoncol.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taetzsch T, Brayman VL, Valdez G. FGF binding proteins (FGFBPs): modulators of FGF signaling in the developing, adult, and stressed nervous system. Biochim Biophys Acta Mol Basis Dis. 2018;1864:2983–2991. doi: 10.1016/j.bbadis.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beer HD, Bittner M, Niklaus G, Munding C, Max N, Goppelt A, Werner S. The fibroblast growth factor binding protein is a novel interaction partner of FGF-7, FGF-10 and FGF-22 and regulates FGF activity: implications for epithelial repair. Oncogene. 2005;24:5269–5277. doi: 10.1038/sj.onc.1208560. [DOI] [PubMed] [Google Scholar]
- 26.Chen JH, Wang XC, Sato JD. Bifunctional effects of heparin-binding protein HBp17 on DNA synthesis in cells. Cell Biol Int. 2001;25:567–570. doi: 10.1006/cbir.2000.0688. [DOI] [PubMed] [Google Scholar]
- 27.Rosli SN, Shintani T, Toratani S, Usui E, Okamoto T. 1α,25(OH)2D3 inhibits FGF-2 release from oral squamous cell carcinoma cells through down-regulation of HBp17/FGFBP-1. In Vitro Cell Dev Biol Anim. 2014;50:802–6. doi: 10.1007/s11626-014-9787-5. [DOI] [PubMed] [Google Scholar]
- 28.Czubayko F, Smith RV, Chung HC, Wellstein A. Tumor growth and angiogenesis induced by a secreted binding protein for fibroblast growth factors. J Biol Chem. 1994;269:28243–8. [PubMed] [Google Scholar]
- 29.Czubayko F, Liaudet-Coopman ED, Aigner A, Tuveson AT, Berchem GJ, Wellstein A. A secreted FGF-binding protein can serve as the angiogenic switch in human cancer. Nat Med. 1997;3:1137–1140. doi: 10.1038/nm1097-1137. [DOI] [PubMed] [Google Scholar]
- 30.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abuharbeid S, Czubayko F, Aigner A. The fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell Biol. 2006;38:1463–1468. doi: 10.1016/j.biocel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Zhao D, Zheng HQ, Zhou Z, Chen C. The Fbw7 tumor suppressor targets KLF5 for ubiquitin-mediated degradation and suppresses breast cell proliferation. Cancer Res. 2010;70:4728–4738. doi: 10.1158/0008-5472.CAN-10-0040. [DOI] [PubMed] [Google Scholar]
- 33.Liu N, Li H, Li S, Shen M, Xiao N, Chen Y, Wang Y, Wang W, Wang R, Wang Q, Sun J, Wang P. The Fbw7/human CDC4 tumor suppressor targets proproliferative factor KLF5 for ubiquitination and degradation through multiple phosphodegron motifs. J Biol Chem. 2010;285:18858–67. doi: 10.1074/jbc.M109.099440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hessmann E, Schneider G, Ellenrieder V, Siveke JT. MYC in pancreatic cancer: novel mechanistic insights and their translation into therapeutic strategies. Oncogene. 2016;35:1609–18. doi: 10.1038/onc.2015.216. [DOI] [PubMed] [Google Scholar]
- 35.Jin X, Yang C, Fan P, Xiao J, Zhang W, Zhan S, Liu T, Wang D, Wu H. CDK5/FBW7-dependent ubiquitination and degradation of EZH2 inhibits pancreatic cancer cell migration and invasion. J Biol Chem. 2017;292:6269–6280. doi: 10.1074/jbc.M116.764407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao J, Ge MH, Ling ZQ. Fbxw7 tumor suppressor: a vital regulator contributes to human tumorigenesis. Medicine (Baltimore) 2016;95:e2496. doi: 10.1097/MD.0000000000002496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Ruckert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–61. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266–76. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Kang X, Lin Z, Xu M, Pan J, Wang ZW. Deciphering role of FGFR signalling pathway in pancreatic cancer. Cell Prolif. 2019;52:e12605. doi: 10.1111/cpr.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, Geissler EK, Stoeltzing O, Lang SA. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10:2157–2167. doi: 10.1158/1535-7163.MCT-11-0312. [DOI] [PubMed] [Google Scholar]
- 42.Haines E, Chen T, Kommajosyula N, Chen Z, Herter-Sprie GS, Cornell L, Wong KK, Shapiro GI. Palbociclib resistance confers dependence on an FGFR-MAP kinase-mTOR-driven pathway in KRAS-mutant non-small cell lung cancer. Oncotarget. 2018;9:31572–31589. doi: 10.18632/oncotarget.25803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nomura S, Yoshitomi H, Takano S, Shida T, Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Miyazaki M. FGF10/FGFR2 signal induces cell migration and invasion in pancreatic cancer. Br J Cancer. 2008;99:305–13. doi: 10.1038/sj.bjc.6604473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


