Abstract
The RBP sorbin and SH3 domain-containing 2 (SORBS2) has been reported to be a tumor suppressor and is dysregulated in several cancer types. Nonetheless, the exact function and mechanism of action of SORBS2 in hepatocellular carcinoma (HCC) remain unclear. In this study, we found that expression levels of SORBS2 were significantly lower in HCC tissues than that in normal tissue samples, and underexpression of SORBS2 was associated with lower overall survival tates of patients with HCC. In HCC cell lines, SORBS2 overexpression inhibited cell migration, invasion, and epithelial-mesenchymal transition, whereas SORBS2 inhibition yielded the opposite results. In vivo metastasis assays confirmed that overexpression of SORBS2 markedly inhibited HCC metastasis. Mechanistically, SORBS2 exerted tumor-suppressive effects on HCC by inhibiting the c-Abl/ERK signaling pathway. Furthermore, MEF2D, which binds to the promoter of SORBS2, was identified as an upstream regulator of SORBS2 and reduced SORBS2 expression. Our data suggest that SORBS2, downregulated by MEF2D, suppresses HCC metastasis through the c-Abl/ERK signaling pathway and has the potential to serve as a novel prognostic marker or therapeutic target in HCC.
Keywords: SORBS2, HCC, MEF2D, metastasis, c-Abl-ERK signaling pathway
Introduction
Hepatocellular carcinoma (HCC) originates from hepatocytes and accounts for approximately 80% of liver cancer cases. Moreover, HCC is the third most common cause of cancer-related deaths in the world. Approximately 1% of deaths every year are associated with HCC [1]. Marked progress in HCC treatment has been made in recent years owing to the combination of surgical resection and chemotherapy [1]. Nonetheless, HCC recurrence and metastasis are common after primary treatment and are the primary causes of HCC-related deaths [2,3]. Thus, the molecular mechanism regulating the HCC initiation, progression, and metastasis needs to be further explored to develop effective therapies.
SORBS2 (sorbin and SH3 domain-containing 2, also known as ArgBP2) is located in the 4q35 region of the human genome. At the cellular level, SORBS2 is localized at actin stress fibers, focal adhesions, and the apical junction complex in association with ZO-1, occludin, E-cadherin, and perijunctional actin. It participates in the regulation of actin dynamics, signal transduction, and cytoskeleton establishment [4,5]. Decreased SORBS2 expression enhances cell migration by inducing pseudopodia elongation and the detachment of actin from focal adhesions [6]. Reconstitution of SORBS2 expression in cervical cancer cell lines significantly inhibits cell proliferation, colony formation, and anchorage-independent growth, indicating that SORBS2 plays a role as a tumor suppressor in cervical carcinogenesis [7]. SORBS2 expression is repressed during pancreatic oncogenic transformation, and the tumor-suppressing function of SORBS2 in pancreatic cancer occurs through the regulation of cell adhesion and migration, and at least partly via by controlling the formation of the WAVE/PTP-PEST/c-Abl signaling complex [8]. In addition, decreased SORBS2 levels are seen in gastric and breast cancers [9-11]. More recently, Zhao et al. reported that SORBS2 suppresses metastatic colonization of ovarian cancer by stabilizing the tumor-suppressive immunomodulatory transcripts of WFDC1 or IL-17D [12]. These findings point to the involvement of SORBS2 in HCC progression. Nevertheless, the expression levels and biological roles of SORBS2 in HCC remain unclear.
In this study, we found that SORBS2 expression was significantly lower in HCC tissues compared with normal tissues, and the underexpression of SORBS2 was associated with shorter overall survival of HCC patients. Functional assays showed that SORBS2 inhibited HCC cell migration, invasion, and epithelial-mesenchymal transition (EMT) in vitro, and attenuated metastasis in vivo. Furthermore, SORBS2 expression was repressed by myocyte enhancer factor 2D (MEF2D), which promotes HCC cell migration and invasiveness. The biological functions of SORBS2 in HCC were found to be mediated by inhibition of the c-Abl/ERK signaling pathway. These results suggest that SORBS2, when not downregulated by MEF2D, functions as a metastasis suppressor in HCC through c-Abl-ERK signaling.
Materials and methods
Clinical samples and cell lines
Twelve fresh HCC tissue samples and matched normal tissue samples were collected from the Qingpu Branch of Zhongshan Hospital (Fudan University, Shanghai, China). Tissue samples were stored at -80°C for RNA isolation and protein extraction. Paraffin-embedded pathological samples from 102 patients with HCC and matched normal tissue samples were obtained from this hospital between 2012 and 2016. The clinical parameters are shown in Table 1. The protocols used in this study were approved by the Institutional Review Board of Qingpu Branch of Zhongshan Hospital, Fudan University (Shanghai, China), and written informed consent was obtained from each patient. The normal human hepatic cell line LO2 and five HCC cell lines Huh7, PLC, HepG2, SMMC-7721, and HCCLM3 were purchased from the Cell Resource Center, Chinese Academy of Science Committee (Shanghai, China). These cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS; GIBCO, Grand Island, USA), 100 U/mL penicillin, and 100 g/mL streptomycin in a humidified incubator containing 5% (v/v) of CO2, at 37°C.
Table 1.
Correlative analysis of SORBS2 levels with clinicopathological features
| Variable | SORBS2 expression | * P-value | |
|---|---|---|---|
|
| |||
| High expression | Low expression | ||
| All cases | 44 | 58 | |
| Age (years) | |||
| > 60 | 9 | 11 | 1.000 |
| ≤ 60 | 35 | 47 | |
| Gender | |||
| Male | 43 | 47 | 0.011 |
| Female | 1 | 11 | |
| HBs antigen | |||
| Positive | 39 | 54 | 0.494 |
| Negative | 5 | 4 | |
| Liver function | |||
| Child A | 39 | 53 | 1.000 |
| Child B | 5 | 5 | |
| AFP (μg/L) | |||
| > 20 | 26 | 40 | 0.403 |
| ≤ 20 | 18 | 18 | |
| Tumor size (cm) | |||
| > 5 | 21 | 25 | 0.691 |
| ≤ 5 | 23 | 33 | |
| Stage | |||
| I-II | 37 | 37 | 0.026 |
| III-IV | 7 | 21 | |
| Tumor number | |||
| Single | 36 | 47 | 1.000 |
| Multiple | 8 | 11 | |
| Differentiation | |||
| I-II | 3 | 1 | 0.313 |
| III-IV | 41 | 57 | |
| Satellite nodules | |||
| Present | 38 | 48 | 0.785 |
| Absent | 6 | 10 | |
| Recurrence | |||
| Present | 27 | 27 | 0.164 |
| Absent | 17 | 31 | |
P < 0.05 by χ2 test.
Western blotting
RIPA buffer (Sigma-Aldrich Chemie, Steinheim, Germany) containing a protease inhibitor was used to lyse tissues and cells. The protein amounts were determined using the BCA Protein Assay Kit (Pierce, Rockford, USA). Lysates were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Merck-Millipore, Darmstadt, Germany). The membranes were blocked with 10% bovine serum albumin (BSA) and incubated with primary antibodies against SORBS2 (Abcam, #ab73444, Cambridge, USA), E-cadherin (Proteintech, #20874, Wuhan, China), Vimentin (Proteintech, #10366), Snail (Proteintech, #13099), c-Abl (Cell Signaling Technology [CST], #2862, USA), Slug (CST, #9585), phospho-(p-)ERK1/2 (CST, #4370), ERK1/2 (CST, #4695), MEF2D (CST, #56830), and β-actin (Proteintech, #60008) at 4°C overnight. The membranes were then incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h. Signals were detected after a chemiluminescent reaction with an HRP substrate (Merck Millipore).
RNA extraction and quantitative reverse-transcription PCR (qRT-PCR)
Total mRNA from tissues and cell lines was isolated using TRIzol Reagent (Life Technologies, Carlsbad, USA). The PrimeScript RT Reagent Kit (TaKaRa, Dalian, China) was used to synthesize cDNA. The mRNA expression levels of SORBS2, E-cadherin, Vimentin, Slug and Snail were determined using SYBR Premix Ex Taq (TaKaRa) and gene-specific primers. The primers used were as follows: SORBS2: forward 5’-CAACCCACCCTACAGTGCTCA-3’, reverse 5’-TCCTTGGCTCAGACCGAAAT-3’; E-cadherin: forward 5’-GAGTGCCAACTGGACCATTC-3’, reverse 5’-ACCCACCTCTAAGGCCATCT-3’; Vimentin: forward 5’-AGATGGCCCTTGACATTGAG-3’, reverse 5’-CCAGAGGGAGTGAATCCAGA-3’; Slug: forward 5’-GACCCTGGTTGCTTCAAGGA-3’, reverse 5’-TGTTGCAGTGAGGGCAAGAA-3’; Snail: forward 5’-CACTATGCCGCGCTCTTTC-3’, reverse 5’-GGTCGTAGGGCTGCTGGAA-3’; and MEF2D: forward 5’-CGTGCTATGTGACTGCGAGAT-3’, reverse 5’-GCGTCGGTACTTGTCCTCC-3’. The mRNA expression level of GAPDH was used for normalization. Relative expression levels of target genes were analyzed using the 2-ΔΔCT method. All of the reactions were run in triplicate.
Immunohistochemistry (IHC)
The 5-µm-thick paraffin-embedded tissue slices were first subjected to deparaffinization and hydration, and the endogenous peroxidase activity was then quenched in 3% H2O2 in methanol. Next, the tissue sections were blocked with 10% BSA at room temperature for 60 min, followed by incubation with primary antibodies at 4°C overnight. HRP-conjugated secondary antibodies were incubated with the tissue slides after three washes in PBS. The signals in the tissue sections were visualized with the DAB chromogen (Dako, Glostrup, Denmark). Quantitative analysis of the immunostained images was performed after color segmentation on the basis of fixed threshold values of hue, saturation, and intensity.
Lentivirus infection and oligonucleotide transfection
The cDNA sequences of SORBS2 (GenBank accession number NM_021069.4) and MEF2D (GenBank accession number NM_005920.3) were cloned into the lentiviral vector pCDH-CMV-MCS-EF1-coGFP (System Biosciences, USA) to generate pCDH-CMV-SORBS2 and pCDH-CMV-MEF2D vectors, respectively. Short hairpin RNAs (shRNAs) targeting SORBS2 and MEF2D were obtained from Hanbio (Shanghai, China), and their DNA sequences were inserted into the lentiviral vector pLKO.1 to knock down SORBS2 and MEF2D. Lentiviruses were produced in HEK293T cells, and then purified, concentrated, and titered. Successfully infected cells were selected with puromycin. The small interfering RNA (siRNA) that targeted c-Abl (5’-GGAAGAGUUCUUGAAAGAATT-3’) was designed as described elsewhere [13]. Target cells were transfected with c-Abl siRNA or the negative control using Lipofectamine 2000. Cells were collected 48 h after transfection.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
Cell proliferation was evaluated by the MTT assay. Briefly, cells were seeded (2 × 103 cells/well) in 96-well plates. Next, 100 μL of sterile MTT dye (0.5 mg/mL, Sigma) was added into each well followed by incubation at 37°C for 4 h. Three parallel wells were set up for each group. The supernatants were discarded, and 150 μL of dimethyl sulfoxide was added into each well. The absorbance of each well was measured at 490 nm on a microplate reader.
Migration and invasion assays
A Transwell system (24-well plates, a polycarbonate membrane with 8 µm pore size) was employed to perform the cell migration assay. Cells were seeded (5 × 104 cells/well) into the upper chamber of plates with serum-free medium, while the medium with 10% FBS was added into the lower chamber. After incubation for 48 h, cells remaining in the upper chamber were scraped out, fixed in methanol, and stained with 0.1% crystal violet solution. Five random visual fields were selected to count the cells that migrated to the lower side. For the cell invasion assay, 105 cells were seeded into each upper chamber that was coated with Matrigel (BD Biosciences, Bedford, USA). The cell invasion assay then followed the same protocol as the migration assay. The average values of the results of three independent experiments were recorded.
Animals and intrasplenic injection
The animal experiment was approved by the Animal Research Committee of Fudan University (Shanghai, China). Nude mice were kept on a 12 h light/12 h dark cycle at 21-24°C in an animal room. The mice were subdivided into groups randomly (n = 6). For intrasplenic injection, 5 × 105 lentivirus-infected HCC cells in 20 μL of PBS were injected into the spleen of nude mice. Three weeks later, the mice were euthanized to extract the liver. The number of tumors on the liver surface was determined, and liver tissues were fixed for hematoxylin and eosin (HE) staining.
Chromatin immunoprecipitation (ChIP) assay
This assay was performed on Huh7 cells as previously described [14]. Briefly, cells were harvested and then fixed with 1% formaldehyde. Lysates were sonicated and subjected to chromatin immunoprecipitation with the anti-MEF2D antibody. The primers specific for the detection of MREs-containing regions are as follows: region 1: forward 5’-TGGTGAAACCCCGTCTCTAC-3’, reverse 5’-TGCTCTGAATCCTATTTTGA-3’; region 2: forward 5’-GGTGAAAGTGTTCTCAAATCATCCA-3’, reverse 5’-AAATACACACAACACACATAGGCAC-3’.
Reporter vector construction and luciferase reporter assay
Two fragments of the SORBS2 promoter sequence were amplified and named Luc-2000 and Luc-1000. They were then inserted into the pGL-enhancer firefly luciferase vector (Promega, Madison, USA). These plasmids were co-transfected with the pRL-SV40 Renilla luciferase plasmid into cells. After 48 h, the cells were harvested and lysed, and luciferase activity was measured with a Dual-Luciferase Reporter Assay System.
Statistical analysis
Experimental data were presented as the means ± SD of three independent experiments, and analyzed in GraphPad Prism 5 software by Student’s t-test when comparing only two groups or one-way analysis of variance when more than two groups were compared. Overall survival of HCC patients was evaluated by the log-rank (Mantel-Cox) test; P < 0.05 indicated a statistically significant difference.
Results
SORBS2 is downregulated in HCC and serves as a prognostic factor in patients with HCC
To determine the clinical significance of SORBS2 in HCC, we first analyzed multiple microarray datasets in the oncomine database. As depicted in Figure 1A, SORBS2 mRNA levels were significantly lower in HCC tissues than that in matched normal liver tissues [15,16]. The underexpression of SORBS2 was strongly associated with lower survival rates of patients with HCC (Figure 1B). To verify these results, we performed western blotting and qRT-PCR assays on 12 pairs of human HCCs and normal tissues. Eight of the 12 HCC tissues showed lower amounts of SORBS2 as compared with their respective normal tissues (Figure 1C). qRT-PCR also revealed the downregulation of SORBS2 mRNA in HCC tissues (Figure 1D). Consistently, the SORBS2 protein level was significantly downregulated in the four established HCC cell lines relative to LO2. To further analyze the correlation of SORBS2 expression with HCC progression, we performed IHC analysis on a tissue microarray that contained 102 HCC tissues. In agreement with the above observations, a strong positive expression of SORBS2 was observed in normal tissues, whereas only negligible expression of SORBS2 was detected in HCCs (Figure 1F). SORBS2 downregulation was associated with patient gender and tumor stage but not with other clinical parameters (Table 1). Furthermore, the log-rank test revealed that HCC patients with low SORBS2 expression had low overall survival rates (Figure 1G). These results suggest that SORBS2 could be critically involved in HCC development.
Figure 1.
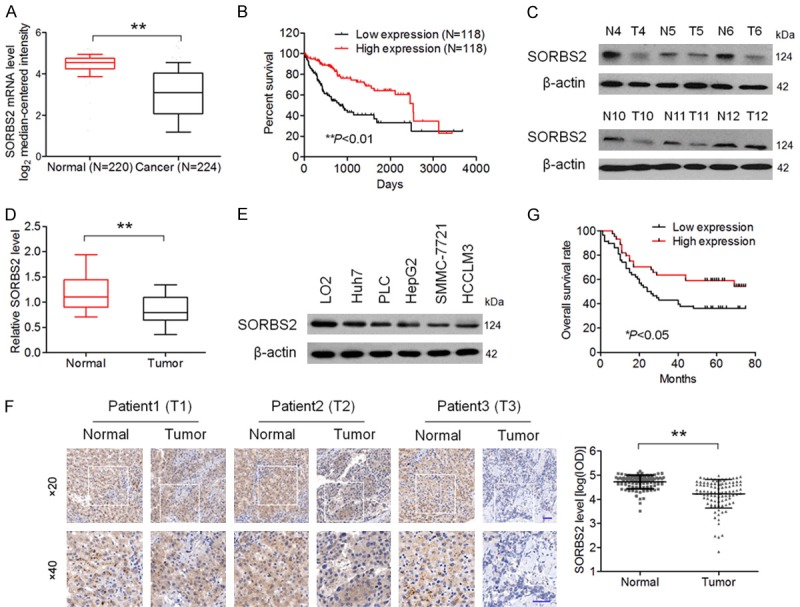
SORBS2 expression is related to HCC progression. A. Analysis based on the oncomine database indicates that SORBS2 mRNA expression was significantly lower in HCC tissue samples compared with normal tissue samples. Data were pooled from two published studies on HCC gene expression [15,16]. B. Relationship between SORBS2 mRNA expression and the overall survival rates of patients with HCC in the TCGA database. C. SORBS2 protein expression was examined in 12 paired tumor (T) samples and normal (N) tissues. D. qRT-PCR analysis of SORBS2 mRNA expression in 12 paired tumor (T) and normal (N) tissues. E. Western blotting analysis of SORBS2 expression in five HCC cell lines and LO2 cells. F. IHC staining of clinical tissue samples with an anti-SORBS2 antibody (samples from 102 patients with HCC). Representative clinical samples of HCC stages I, II, and III are presented (Left). Scale bar, 50 μm. Quantitative analysis of SORBS2 staining revealed a significantly lower staining intensity in HCC samples compared with normal tissue samples (Right). The integrated optical density (IOD) at the same level (× 20) from three sections per sample was measured using Image-ProPlus 6.0 software. G. Low intensity of SORBS2 immunostaining was strongly associated with poor survival rates of patients with HCC (n = 44 in the SORBS2 high-expression group, n = 58 in the SORBS2 low-expression group). **P < 0.01.
SORBS2 inhibits the migration, invasiveness, and EMT of HCC cells in vitro
To determine the biological role of SORBS2 in HCC, we overexpressed SORBS2 in HCC cell lines SMMC-7721 and HCC-LM3, and confirmed the results through western blotting (Figure 2A). We found that overexpression of SORBS2 had no appatent influence on the proliferation of SMMC-7721 and HCC-LM3 cells (Figure 2B). In contrast, SORBS2 overexpression significantly inhibited cell migration and invasion (Figure 2C and 2D). Furthermore, SORBS2 overexpression increased E-cadherin (epithelial marker) mRNA expression levels and decreased mRNA expression levels of Vimentin and Slug (mesenchymal marker) but had no apparent influence on Snail mRNA levels (Figure 2E). Similar effects were observed on the protein levels of the molecular indicators of SORBS2 (Figure 2F). SORBS2 was also knocked down using shRNA in PLC and Huh7 cells, and the successful downregulation of SORBS2 was verified by western blotting (Figure 3A). Functional assays suggested that SORBS2 inhibition significantly promoted cell migration, invasion, and EMT (Figure 3B-E). These results implied that SORBS2 negatively regulated HCC cell migration, invasion and EMT in vitro.
Figure 2.
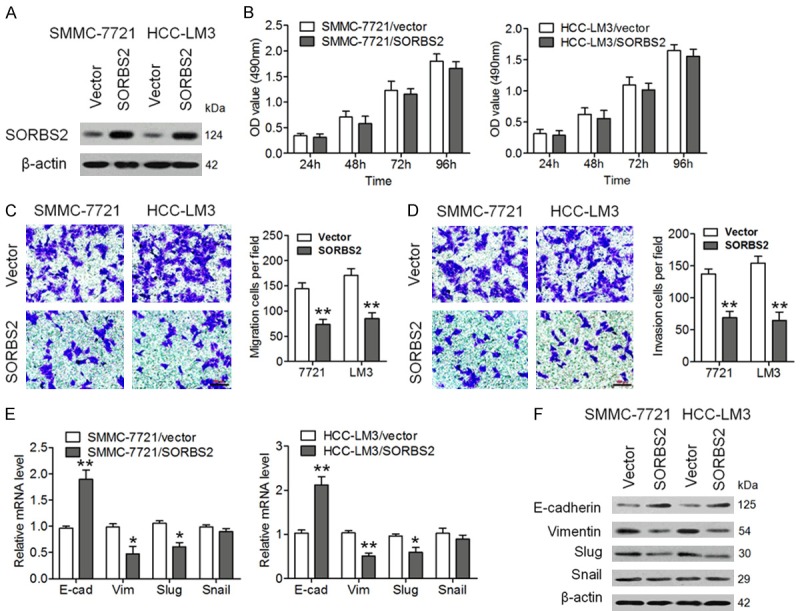
SORBS2 overexpression inhibits HCC cell migration, invasion, and EMT. (A) SMMC-7721 and HCC-LM3 cells were infected with SORBS2-overexpressing lentivirus or controllentivirus (indicated as “Vector”). SORBS2 protein expression was determined using western blotting. (B) Cell proliferation at different time points was assessed by the MTT assay. Representative cell migration (C) and invasion (D) patterns of SMMC-7721 and HCC-LM3 cells, as determined by Transwell migration and invasion assays. Scale bar, 100 μm. (E) mRNA levels of E-cadherin, vimentin, Slug and Snail were determined by qRT-PCR. (F) Protein amounts of E-cadherin, Vimentin, Slug, and Snail were evaluated by western blotting. β-actin protein levels served as an internal control. *P < 0.05, **P < 0.01.
Figure 3.
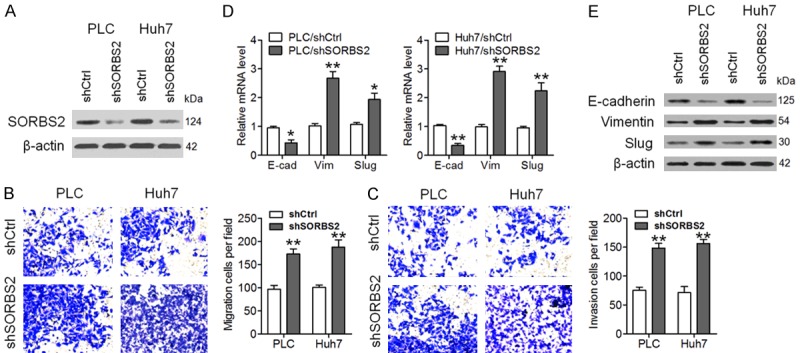
SORBS2 knockdown promoted HCC cell migration, invasion, and EMT. (A) PLC and Huh7 cells were infected with a lentivirus expressing SORBS2 shRNA (shSORBS2) or shCtrl (control shRNA). SORBS2 protein expression was determined via western blotting. Migration (B) and invasiveness (C) of PLC and Huh7 cells are presented. The SORBS2 knockdown significantly promoted the EMT in PLC and Huh7 cells as determined by the analysis of mRNA (D) and protein (E) levels of E-cadherin, Vimentin, Slug and Snail. *P < 0.05, **P < 0.01.
SORBS2 inhibits liver metastasis in vivo
In order to explore the biological function of SORBS2 in vivo, we performed an intrasplenic injection of SMMC-7721 or HCC-LM3 cells overexpressing SORBS2 into nude mice. As shown in Figure 4A, SORBS2 overexpression inhibited liver metastasis of HCC cells. HE staining revealed the presence of smaller liver tumors after SORBS2 overexpression as compared to the controls (Figure 4B). Moreover, the number of tumors on the liver surface significantly decreased after SORBS2 overexpression (Figure 4C). These results suggested that SORBS2 serves as a metastasis suppressor in HCC.
Figure 4.
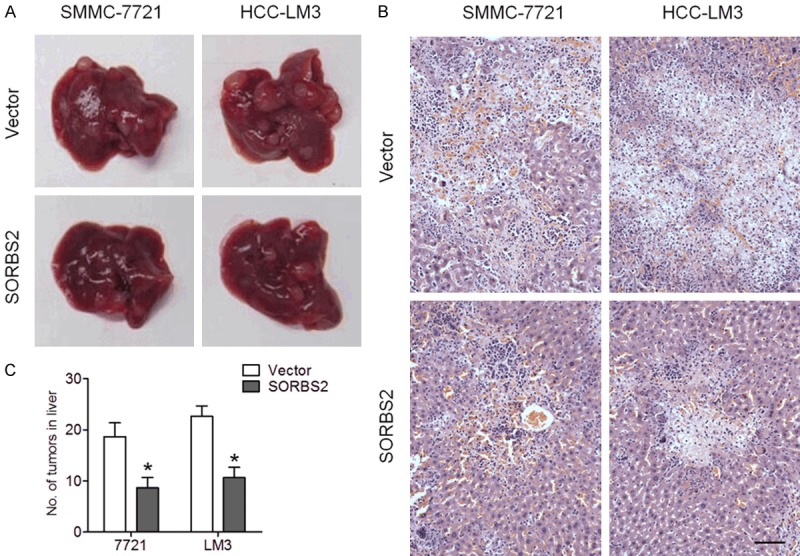
SORBS2 inhibited liver metastasis. A total of 5 × 105 SMMC-7721 or HCC-LM3 cells infected with an empty lentivirus (indicated as “Vector”) or SORBS2-expressing lentivirus were intrasplenically injected into nude mice. Three weeks later, the mice were euthanized for liver extraction. A. Representative livers after intrasplenic injection. B. Representative images of HE stained liver tissues after intrasplenic injection. Scale bar, 100 μm. C. Analysis of tumor numbers on the liver surface. *P < 0.05.
SORBS2 inhibits HCC cell migration, invasiveness, and EMT through the c-Abl-ERK signaling pathway
SORBS2 has been known to negatively regulate c-Abl function by promoting its degradation [17]. In order to determine the mechanism of how SORBS2 affects HCC metastasis, we first measured c-Abl expression in HCC cell lines after SORBS2 overexpression or knockdown. Western blotting analysis showed that SORBS2 overexpression reduced the expression of c-Abl (Figure 5A), whereas SORBS2 knockdown enhanced c-Abl expression in HCC cells (Figure 5B). Furthermore, we observed that SORBS2 overexpression decreased ERK1/2 phosphorylation, whereas SORBS2 knockdown increased ERK1/2 phosphorylation in HCC cells compared to the control cells (Figure 5A and 5B). Based on these results, we hypothesize that SORBS2 affects HCC metastasis by regulating c-Abl-ERK signaling. This hypothesis was tested in two ways, first by suppressing c-Abl expression with siRNAs (si-c-Abl), and second by reducing ERK1/2 phosphorylation with an inhibitor (U0126), in SORBS2 knockdown HCC cells (PLC-shSORBS2 and Huh7-shSORBS2 cell lines) (Figure 5C). As illustrated in Figure 5D and 5E, cell migration and invasion significantly decreased after downregulation of c-Abl and inhibitory treatment using U0126. In addition, the expression level of E-cadherin increased, while Vimentin and Slug expression levels decreased after both c-Abl and p-ERK1/2 downregulation (Figure 5F). Taken together, these results imply that SORBS2 inhibits HCC cell migration, invasion, and EMT through the c-Abl-ERK signaling pathway.
Figure 5.
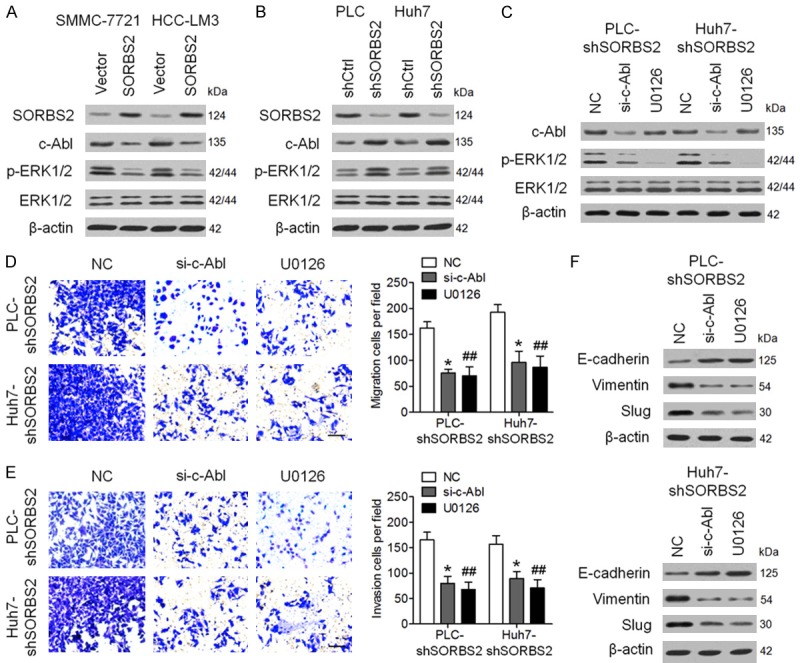
SORBS2 inhibits HCC cell migration, invasion, and EMT through the c-Abl-ERK signaling pathway. (A) The protein amounts of SORBS2, c-Abl, p-ERK1/2, and ERK1/2 were determined by western blotting in SMMC-7721 and HCC-LM3 cells infected with the empty lentiviral vector (indicated as “Vector”) or the lentiviral vector expressing SORBS2. (B) Western blotting analysis of the amounts of SORBS2, c-Abl, p-ERK1/2, and ERK1/2 in PLC and Huh7 cells infected with a lentivirus expressing shCtrl or shSORBS2. (C) PLC-shSORBS2 or Huh7-shSORBS2 cells were transfected with negative control (NC) siRNA or si-c-Abl, or were treated with the MEK inhibitor U0126 (10 μM). Next, the protein levels of c-Abl, p-ERK1/2, and ERK1/2 were evaluated. Cell migration (D) and invasion (E) were determined. Quantitative analysis showed significantly weaker cell migration and invasion after transfection with si-c-Abl or treatment with U0126. (F) The protein levels of E-cadherin, vimentin, and Slug were determined in the indicated cells. *P < 0.05, **P < 0.01.
SORBS2 is transcriptionally regulated by MEF2D in human HCC
In silico analysis was performed using the jaspar and PROMO databases to determine possible upstream transcription factors associated with the downregulation of SORBS2 in HCC. MEF2D was predicted to be an upstream regulator of SORBS2 (Figure 6A) and selected for further study because of its positive roles in cancer cell proliferation and invasion [14,18]. As presented in Figure 6B, MEF2D showed relatively low expression in Huh7 cells and relatively high expression in HCC-LM3 cells; this opposite to the SORBS2 expression pattern in these cell lines (Figure 1E), indicating an antagonistic relation between SORBS2 and MEF2D. In vitro experiments showed that the downregulation of MEF2D (shMEF2D) in SMMC-7721 and HCC-LM3 cells increased SORBS2 expression both at the mRNA and protein levels, whereas the upregulation of MEF2D inhibited SORBS2 expression in both PLC and Huh7 cells (Figure 6C). Luciferase assay confirmed the antagonistic relationship between SORBS2 and MEF2D because the luciferase activity of SORBS2 (Luc-2000) increased after MEF2D downregulation and decreased after MEF2D upregulation; there was no difference in the luciferase activity after downregulation or upregulation of MEF2D when Luc-1000 was used (Figure 6D). A ChIP assay was also conducted to determine the interaction between SORBS2 and MEF2D. As expected, DNA fragments of the two SORBS2 promoter regions were enriched by the anti-MEF2D antibody (Figure 6E). In 12 pairs of HCC and normal tissue samples, it was found that the mRNA expression of MEF2D was much higher in HCC tissues than that in normal tissues (Figure 6F). A negative correlation was observed between SORBS2 and MEF2D mRNA levels in HCC tissues (Figure 6G). Additionally, we determined the effects of MEF2D on the migration and invasion of HCC cells. As depicted in Figure 7, downregulation of MEF2D inhibited the migration, invasiveness, and EMT of both SMMC-7721 and HCC-LM3 cells. Taken together, these results suggest that MEF2D mediates the suppression of SORBS2 during HCC metastasis.
Figure 6.
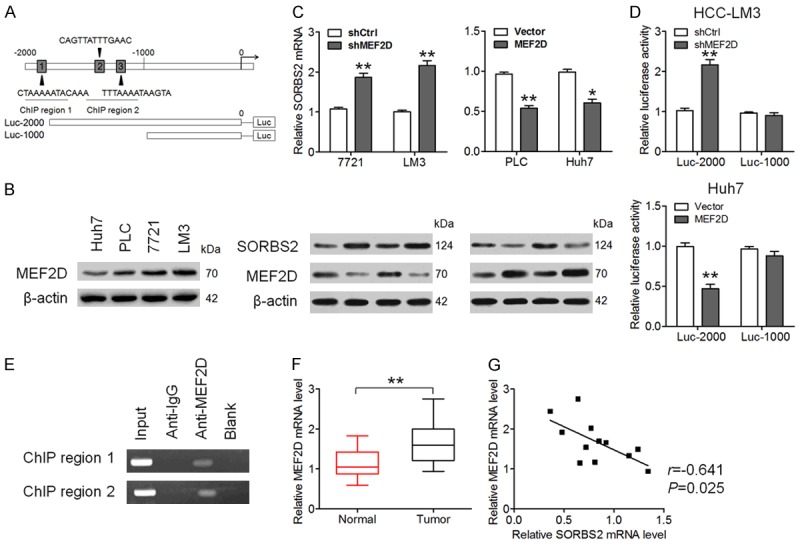
SORBS2 is inversely related to MEF2D in HCC. A. A diagram of the promoter region of the SORBS2 gene and putative MEF2D-binding sites. pGL3-enhancer reporter vectors with or without the putative MEF2D binding sites are illustrated below. B. Western blotting analysis of MEF2D expression in four HCC cell lines. C. qRT-PCR and western blotting analyses of SORBS2 and MEF2D expression in SMMC-7721 and HCC-LM3 cells with MEF2D knockdown and in PLC or Huh7 cells with MEF2D overexpression. D. The relative luciferase activity of plasmids with different versions of the SORBS2 promoter was detected in MEF2D knockdown HCC-LM3 cells and MEF2D-overexpressing Huh7 cells. E. The ChIP assay was performed to detect the binding of MEF2D to the SORBS2 promoter. The IgG-treated and blank groups were regarded as negative controls, whereas the input fraction as a positive control. F. MEF2D mRNA levels in 12 pairs of HCC tissue samples and normal tissue samples. G. Pearson analysis of the correlation between SORBS2 and MEF2D mRNA levels in HCC tissue samples. *P < 0.05, **P < 0.01.
Figure 7.
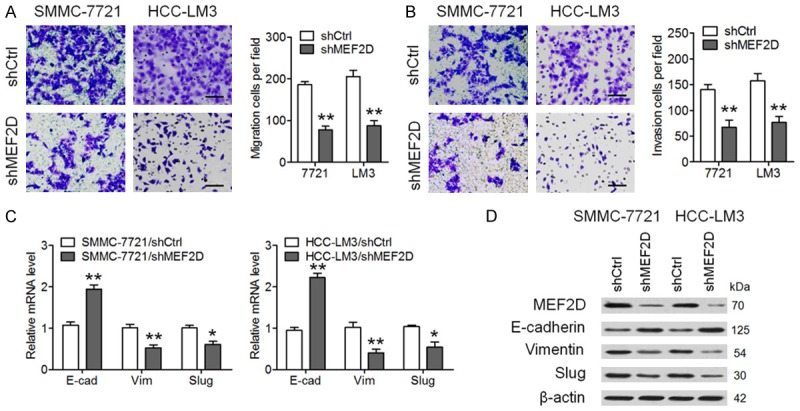
MEF2D promotes the migration, invasiveness, and EMT of HCC cells. SMMC-7721 and HCC-LM3 cells were infected with a lentivirus expressing shCtrl or shMEF2D. Cell migration (A) and invasion (B) are shown. Quantitative analysis revealed significantly weaker cell migration and invasion after the MEF2D knockdown. Scale bar, 100 μm. (C) The mRNA levels of E-cadherin, Vimentin and Slug. (D) Protein amounts of E-cadherin, Vimentin and Slug. *P < 0.05, **P < 0.01.
Discussion
Accumulating evidence now links SORBS2 with carcinogenesis and tumor progression [3]. However, only a recent study by Zhao and colleagues addressed the clinical and prognostic significance of SORBS2 levels in the tumors of cancer patients, by proving that SORBS2 is significantly downregulated in ovarian cancer and its expression is closely associated with the clinical outcomes of patients with ovarian cancer [12]. To broaden our knowledge regarding the involvement of SORBS2 in HCC, we measured SORBS2 expression in HCC tissues and cell lines. We found that SORBS2 expression levels are significantly lower in patients with HCC, and the underexpression of SORBS2 was strongly associated with lower survival rates of patients with HCC. We subsequently confirmed that SORBS2 significantly suppressed HCC cell invasion and metastasis both in vitro and in vivo. These data were similar to the findings on cervical, pancreatic, and gastric cancers, where SORBS2 acted as a supposed tumor suppressor gene involved in either carcinogenesis or metastasis [7-9].
In the experiments to determine the mechanism of SORBS2 action, we principally focused on understanding how SORBS2 suppresses HCC metastasis since local invasion and metastasis at distant sites are the cause of 90% of cancer-related deaths in humans [20]. Some studies indicate that the biological functions of SORBS2 are partly mediated by interactions with other proteins, such as c-Abl, c-Cbl, PYK2, PKB, PAK1, and WAVE. Notably, SORBS2 may cause ubiquitination-linked degradation of c-Abl [4,21]. c-Abl tyrosine kinase has been reported to regulate the invasive activity of aggressive breast cancer [22]. Similarly, c-Abl is activated in liver cells overexpressing CLD1 and is associated with the CLD1-dependent acquisition of cellular invasive capacity [23]. In the present study, we demonstrated that SORBS2 reduces the expression of c-Abl and phosphorylation of ERK in HCC cells. Downregulation of c-Abl in HCC cells or treatment with ERK inhibitor U0126 decreased cell migration, invasion, and EMT. These results indicate that SORBS2 inhibits cell migration, invasion, and EMT through the c-Abl-ERK signaling pathway.
MEF2D is a transcription factor from the MEF2 family that has four members in mammals: MEF2A, MEF2B, MEF2C, and MEF2D [14,24]. B-cell development is blocked in mice deficient in MEF2C and MEF2D; MEF2 transcription factors are activated by the pre-B-cell receptor through phosphorylation by ERK5 mitogen-activated kinase. This indicates the crucial function of the MEF family in B-cell development [25]. In colorectal cancer tissues, MEF2D expression levels are positively associated with CD31 microvascular density, and it promotes tumor angiogenesis in vitro and in vivo with enhanced proangiogenic cytokine levels [26]. A similar study also showed that overexpressed MEF2D in colorectal cancer enhances tumor cell invasion and EMT by directly regulating ZEB1 transcription [18]. The upregulation of MEF2D has also been detected in HCC samples and is associated with poor prognosis, and silencing of MEF2D inhibits HCC tumorigenicity in xenograft models by triggering G2-M arrest of the cell cycle [14]. In the present study, we found that MEF2D mRNA levels are much lower in HCC tissue samples than in normal tissue samples. MEF2D promotes migration, invasion, and EMT of HCC cells. SORBS2 expression was found to be repressed by MEF2D. Moreover, there was a negative correlation between SORBS2 and MEF2D expression levels in the HCC tissue samples. These results suggest that MEF2D inhibits the SORBS2-mediated suppression of HCC metastasis.
In conclusion, our study demonstrates that SORBS2, which transcriptionally regulated by MEF2D, inhibited HCC cell migration and invasion in vitro and metastasis in vivo by regulating the c-Abl/ERK signaling pathway. Thus, our study underscores the important role of SORBS2 in HCC metastasis, and we believe that our findings on the MEF2D/SORBS2/c-Abl/ERK signaling axis will provide useful information for the development of more promising and effective therapies for HCC.
Acknowledgements
This work was supported in part by the Shanghai Municipal Commission of Health and Family Planning (201440608).
Disclosure of conflict of interest
None.
References
- 1.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the asia-pacific region. Gut Liver. 2016;10:332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen SK, Brown RS, Siegel AB. Hepatocellular carcinoma: review of current treatment with a focus on targeted molecular therapies. Therap Adv Gastroenterol. 2010;3:55–66. doi: 10.1177/1756283X09346669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang ZY. Hepatocellular carcinoma--cause, treatment and metastasis. World J Gastroenterol. 2001;7:445–454. doi: 10.3748/wjg.v7.i4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanger JM, Wang J, Gleason LM, Chowrashi P, Dube DK, Mittal B, Zhukareva V, Sanger JW. Arg/Abl-binding protein, a Z-body and Z-band protein, binds sarcomeric, costameric, and signaling molecules. Cytoskeleton (Hoboken) 2010;67:808–823. doi: 10.1002/cm.20490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kioka N. A novel adaptor protein family regulating cytoskeletal organization and signal transduction--Vinexin, CAP/ponsin, ArgBP2. Seikagaku. 2002;74:1356–1360. [PubMed] [Google Scholar]
- 6.Kimura A, Baumann CA, Chiang SH, Saltiel AR. The sorbin homology domain: a motif for the targeting of proteins to lipid rafts. Proc Natl Acad Sci U S A. 2001;98:9098–9103. doi: 10.1073/pnas.151252898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backsch C, Rudolph B, Steinbach D, Scheungraber C, Liesenfeld M, Hafner N, Hildner M, Habenicht A, Runnebaum IB, Durst M. An integrative functional genomic and gene expression approach revealed SORBS2 as a putative tumour suppressor gene involved in cervical carcinogenesis. Carcinogenesis. 2011;32:1100–1106. doi: 10.1093/carcin/bgr093. [DOI] [PubMed] [Google Scholar]
- 8.Taieb D, Roignot J, Andre F, Garcia S, Masson B, Pierres A, Iovanna JL, Soubeyran P. ArgBP2-dependent signaling regulates pancreatic cell migration, adhesion, and tumorigenicity. Cancer Res. 2008;68:4588–4596. doi: 10.1158/0008-5472.CAN-08-0958. [DOI] [PubMed] [Google Scholar]
- 9.Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y, Li F. HSF1, in association with MORC2, downregulates ArgBP2 via the PRC2 family in gastric cancer cells. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1104–1114. doi: 10.1016/j.bbadis.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Tong Y, Li Y, Gu H, Wang C, Liu F, Shao Y, Li J, Cao L, Li F. Microchidia protein 2, MORC2, downregulates the cytoskeleton adapter protein, ArgBP2, via histone methylation in gastric cancer cells. Biochem Biophys Res Commun. 2015;467:821–827. doi: 10.1016/j.bbrc.2015.10.059. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez-Ardila DE, Ruigrok-Ritstier K, Helmijr JC, Look MP, van Laere S, Dirix L, Berns EM, Jansen MP. LRG1 mRNA expression in breast cancer associates with PIK3CA genotype and with aromatase inhibitor therapy outcome. Mol Oncol. 2016;10:1363–1373. doi: 10.1016/j.molonc.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao L, Wang W, Huang S, Yang Z, Xu L, Yang Q, Zhou X, Wang J, Shen Q, Wang C, Le X, Feng M, Zhou N, Lau WB, Lau B, Yao S, Yi T, Wang X, Zhao X, Wei Y, Zhou S. The RNA binding protein SORBS2 suppresses metastatic colonization of ovarian cancer by stabilizing tumor-suppressive immunomodulatory transcripts. Genome Biol. 2018;19:35. doi: 10.1186/s13059-018-1412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh Y, Yoon CH, Kim RK, Lim EJ, Oh YS, Hwang SG, An S, Yoon G, Gye MC, Yi JM, Kim MJ, Lee SJ. Claudin-1 induces epithelial-mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene. 2013;32:4873–4882. doi: 10.1038/onc.2012.505. [DOI] [PubMed] [Google Scholar]
- 14.Ma L, Liu J, Liu L, Duan G, Wang Q, Xu Y, Xia F, Shan J, Shen J, Yang Z, Bie P, Cui Y, Bian XW, Prieto J, Avila MA, Qian C. Overexpression of the transcription factor MEF2D in hepatocellular carcinoma sustains malignant character by suppressing G2-M transition genes. Cancer Res. 2014;74:1452–1462. doi: 10.1158/0008-5472.CAN-13-2171. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, Nevens F, Roskams T, Thorgeirsson SS. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 16.Roessler S, Jia HL, Budhu A, Forgues M, Ye QH, Lee JS, Thorgeirsson SS, Sun Z, Tang ZY, Qin LX, Wang XW. A unique metastasis gene signature enables prediction of tumor relapse in early-stage hepatocellular carcinoma patients. Cancer Res. 2010;70:10202–10212. doi: 10.1158/0008-5472.CAN-10-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin M, Geudens I, Bruyr J, Potente M, Bleuart A, Lebrun M, Simonis N, Deroanne C, Twizere JC, Soubeyran P, Peixoto P, Mottet D, Janssens V, Hofmann WK, Claes F, Carmeliet P, Kettmann R, Gerhardt H, Dequiedt F. PP2A regulatory subunit balpha controls endothelial contractility and vessel lumen integrity via regulation of HDAC7. EMBO J. 2013;32:2491–2503. doi: 10.1038/emboj.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L, Luo Y, Yang Z, Yang J, Yao C, Cheng F, Shan J, Chen J, Li F, Liu L, Liu C, Xu Y, Jiang L, Guo D, Prieto J, Avila MA, Shen J, Qian C. MEF2D transduces microenvironment stimuli to ZEB1 to promote epithelial-mesenchymal transition and metastasis in colorectal cancer. Cancer Res. 2016;76:5054–5067. doi: 10.1158/0008-5472.CAN-16-0246. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Guo P, He Y, Chen Z, Luo Y, Qi L, Liu Y, Wu Q, Cui Y, Fang F, Zhang X, Song T, Guo H. HCC-derived exosomes elicit HCC progression and recurrence by epithelial-mesenchymal transition through MAPK/ERK signalling pathway. Cell Death Dis. 2018;9:513. doi: 10.1038/s41419-018-0534-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu TY, Chen J, Shang CL, Shen HW, Huang JM, Liang YC, Wang W, Zhao YH, Liu D, Shu M, Guo LY, Hu Z, Yao SZ. Tripartite motif containing 62 is a novel prognostic marker and suppresses tumor metastasis via c-Jun/slug signaling-mediated epithelial-mesenchymal transition in cervical cancer. J Exp Clin Cancer Res. 2016;35:170. doi: 10.1186/s13046-016-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soubeyran P, Barac A, Szymkiewicz I, Dikic I. Cbl-ArgBP2 complex mediates ubiquitination and degradation of c-Abl. Biochem J. 2003;370:29–34. doi: 10.1042/BJ20021539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung AC, Lo S, Hou MF, Lee YC, Tsai CH, Chen YY, Liu W, Su YH, Lo YH, Wang CH, Wu SC, Hsieh YC, Hu SC, Tai MH, Wang YM, Yuan SS. Extracellular visfatin-promoted malignant behavior in breast cancer is mediated through c-Abl and STAT3 activation. Clin Cancer Res. 2016;22:4478–4490. doi: 10.1158/1078-0432.CCR-15-2704. [DOI] [PubMed] [Google Scholar]
- 23.Yoon CH, Kim MJ, Park MJ, Park IC, Hwang SG, An S, Choi YH, Yoon G, Lee SJ. Claudin-1 acts through c-Abl-protein kinase Cdelta (PKCdelta) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J Biol Chem. 2010;285:226–233. doi: 10.1074/jbc.M109.054189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pon JR, Marra MA. MEF2 transcription factors: developmental regulators and emerging cancer genes. Oncotarget. 2016;7:2297–2312. doi: 10.18632/oncotarget.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herglotz J, Unrau L, Hauschildt F, Fischer M, Kriebitzsch N, Alawi M, Indenbirken D, Spohn M, Muller U, Ziegler M, Schuh W, Jack HM, Stocking C. Essential control of early B-cell development by Mef2 transcription factors. Blood. 2016;127:572–581. doi: 10.1182/blood-2015-04-643270. [DOI] [PubMed] [Google Scholar]
- 26.Xiang J, Sun H, Su L, Liu L, Shan J, Shen J, Yang Z, Chen J, Zhong X, Avila MA, Yan X, Liu C, Qian C. Myocyte enhancer factor 2D promotes colorectal cancer angiogenesis downstream of hypoxia-inducible factor 1alpha. Cancer Lett. 2017;400:117–126. doi: 10.1016/j.canlet.2017.04.037. [DOI] [PubMed] [Google Scholar]


