Abstract
Macrophage migration inhibitory factor (MIF) is an inflammatory cytokine that serves many roles in inflammation and immunity; however, it is also involved in carcinogenesis. This is a review of the clinical and experimental data published on MIF and its role in various types of cancers such as glioblastomas, lung cancer, breast cancer, gastric cancer, melanoma, bladder cancer, and head and neck cancers. The goal of this review is to show MIFs role in various types of cancers. Data show that MIF is overexpressed in these malignancies in humans, and contributes to the deregulation of the cell cycle, angiogenesis, and metastasis. Clinical studies show that MIF overexpression in these types of tumors significantly decreases survival rate, and increases tumor aggression. There are multiple anti-MIF molecules that are currently being explored and investigations should be continued.
Keywords: MIF, CD74, cancer
Introduction
Macrophage migration inhibitory factor (MIF) is a pluripotent and pleiotropic cytokine expressed in numerous human malignancies such as glioblastomas, lung cancer, breast cancer, gastric cancer, bladder cancer, and melanoma. MIF is heavily involved in the development inflammation and cancer; therefore, inhibitors of MIF should be further investigated as these molecules may have the capability to decrease the rate at which tumors proliferate and metastasize.
Structure and genetics of MIF
Macrophage migration inhibitory factor (MIF) was originally identified as a cytokine released from active T cells to inhibit the random movement of macrophages [1]. It is secreted by epithelial cells, endothelial cells, lymphocytes, monocytes, and macrophages, showing that it has a role in innate and acquired immunity. MIF also plays a role in sepsis, inflammation, tissue damage, and a relationship between inflammation and cancer [2]. In humans, the MIF gene is found on chromosome 22q11.2 and codes for an evolutionarily conserved protein consisting of 115 amino acids [3]. The MIF gene has two polymorphic sites located in the promoter region. The first site is at CATT repeat starting at the -794 position, and the second is at a single nucleotide polymorphism at the -173 position [4]. The MIF protein has a molecular weight of 12.5 kD in its monomeric form. When active, MIF forms a trimer composed of three identical subunits, with each monomer containing two antiparallel alpha-helices that pack against a four-stranded beta-sheet [3].
Roles of MIF
MIF has various biological roles, with the most significant being inflammation and immunity. MIF counter-regulates the actions of glucocorticoids, which are natural steroid hormones produced by the adrenal glands during cellular stress that possess anti-inflammatory effects [5]. MIF may stimulate the expression of other cytokines involved in inflammation. Inflammation is needed for the survival of organisms, but when it is incorrectly regulated, it may contribute to tumorigenesis [6]. In a study by Hagemann et al. (2007), a MIF knockout in a murine epithelial ovarian cancer cell line (ID8) showed a reduction in tumor growth preceded by modulating the expression of inflammatory mediators such as TNF-α, IL-6, and VEGF. MIF, therefore, attracts tumor-associated macrophages and promotes the tumor microenvironment [7].
MIF demonstrates chemokine-like function and was identified as a ligand of both CXCR2 and CXCR4. Binding of MIF to these receptors enhances monocyte recruitment and leukocyte chemotaxis (Figure 1). In human chondrosarcoma cells, this recruitment is mediated by Gαi proteins and PI3K in T cell adhesion through upregulation of the transcription of the αvβ3 integrin through PI3K/AKT/NF-κB signaling in a CXCR2- and CXCR4-mediated way. However, molecular mechanisms underlying MIF-mediated receptor signaling still needs to be delineated [8]. In another study, it was reported that MIF directly interacts with CXCR2 and CXCR4 to promote the recruitment of inflammatory cells [9]. The inflammatory cascade relies on the activation of CXCR2 and CD74, suggesting that MIF operates via a functional CXCR2/CD74 complex. To further understand this mechanism, MIF deficient mice that showed a deficiency in monocyte adhesion to the arterial wall were used. As a consequence of MIF blockage in mice, plaque regression, reduced monocyte count, and reduced T-cell levels were recorded. When CXCR2 and CXCR4 were activated, MIF displayed a chemokine function and acted as a major regulator of inflammatory cell recruitment [9], confirming that MIF interacts with CXCR2/CXCR4 complexes to recruit inflammatory cells.
Figure 1.
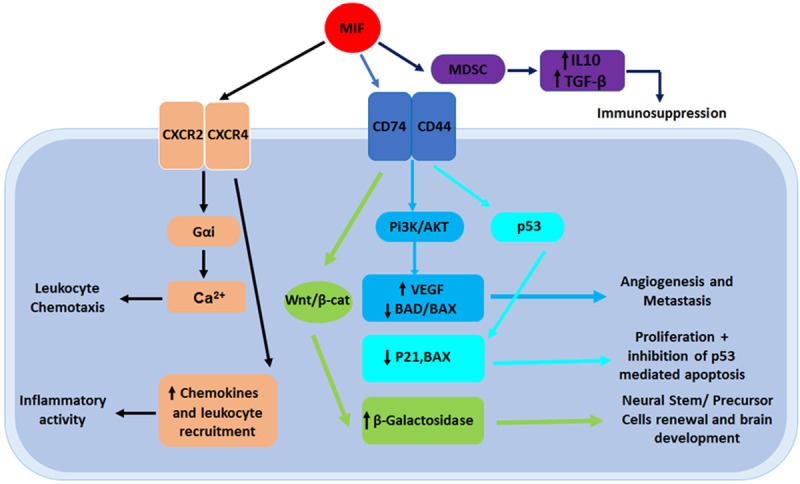
An overview of MIF signaling pathways: MIFs interactions can contribute to the formation of cancer and neural development. When MIF targets the Wnt/β-catenin signaling pathway, β-galactosidase is upregulated, resulting in an increase of NSPCs. MIFs interaction with the PI3K/AKT pathway results in an increase of VEGF and a decrease of the pro-apoptotic factors BAD and BAX, leading to both angiogenesis and metastasis. MIF also has the capability to interact with p53, decreasing the expression of p21 and BAX, which results in crucial cell proliferation. MIF can also directly interact with CXCR2 and CXCR4, which result in inflammatory activity and leukocyte chemotaxis.
MIF serves a role in both innate and adaptive immunity and is constitutively expressed by monocytes, macrophages, blood dendritic cells, B cells, neutrophils, eosinophils, mast cells, and basophils. It promotes the stimulation and proliferation of T cells in response to foreign agents and acts as a regulator of responses to infections by increasing the expression of TLR4 (the transduction molecule for endotoxins) [10]. Activated T cells release MIF to inhibit glucocorticoid-mediated interleukin 2 and interferon γ production. Since circulating glucocorticoid levels are increased during infection and inflammation, MIF exerts its immunosuppressive effects, which enables the primary immune response and reduces the need for steroid therapy [11]. The results indicate that MIF expression is instrumental for a proper immune response, including the release of T cell and IL-2. MIF must nullify the inhibitory effect of glucocorticoids on T cell activators for T cells to be released. MIF is also reported to possess enzymatic activity, and it converts D-dopachrome in 5,6-dihydroxy-2-carboxylic acid (DHICA). Although identification of DHICA as a true biological MIF substrate sheds light on this mechanism of action, the role of MIFs enzymatic activity is not fully understood [12].
In addition to MIFs immunogenic role, it has also been reported to play an important role during neural embryonic development. Shen et al. (2013) demonstrated that the MIF pathway is required for the survival of statoacoustic ganglia and sensory hair cells in a zebrafish model. Using knockdown with antisense oligonucleotide morpholinos (MOs) and/or with a biochemical MIF inhibitor, 4-IPP, there was a significant reduction in the size of the SAG, the number of sensory HC, and the size of the brain in zebrafish. These perturbations are partly due to dependency on p53 [13]. This study shows that MIF serves a role in nervous system development, although the exact nature of this role is still undefined.
MIF also has the ability to interact with embryonic stem cells (ESC). Wang et al. (2012) shed light on this phenomenon by injecting undifferentiated ESC into the spinal cord of wild-type mice and MIF knockout mice. Hind limb function was measured using the Basso Mouse Scale (BMS), which was initially normal for both groups of mice. After ten days, the BMS score rapidly decreased to zero (indicating paralysis) in the WT mice. However, the BMS score of the MIF knockout mice slightly declined, with only one mouse being paralyzed by day 17 [14]. This study suggests that MIF interferes with and affects the pluripotency of ESCs by promoting embryonic stem cell to proliferation.
MIF activates the proliferation and differentiation of neural stem and progenitor cells (NSPC) through the well-known Wnt/β-catenin signal pathway (Figure 1). NSPCs are self-renewing cells that are responsible for the growth and development of nervous tissue along with neural plasticity. A study by Zhang et al., (2013) suggests that NSPCs proliferate more rapidly with than without MIF stimulation. Immunostaining and Western blots were also performed, and they showed a higher expression of β-catenin in MIF stimulated mice compared to the control (P<0.05), thereby suggesting that MIF activates the Wnt/β-catenin signal pathway. Cells were also treated with IWR-1, an inhibitor of the Wnt/β-catenin pathway. IWR-1 caused decreased proliferation in the control cells in comparison to the MIF cells, which shows that MIF enhances the proliferation of NSPC through the Wnt/β-catenin pathway [15]. However, the exact effect of MIF on NSPC differentiation is still unknown.
MIF carcinogenic processes
MIFs cell cycle interactions
MIF has been shown to contribute to many different forms of cancer in multiple studies. MIF is a regulator of the p53 signal pathway and can physically interact with p53. MIF suppresses the activity of p53, which leads to the deregulation of the normal cell cycle [16]. In one study conducted by Jung et al. (2008), it was demonstrated that MIF can physically bind to p53 in vivo. The study demonstrated that MIF was present in the p53 immunoprecipitation from three cell lines (293T, MCF7, and HCT116). The cysteine residues present in each protein interacted with one other, leading to the conclusion that MIF negatively regulates p53 by stabilizing the association between p53 and Mdm2. Further data from this study showed that the overexpression of MIF leads to a decreased expression of p21, BAX, and p53, further supporting that MIF negatively regulates p53 [16]. MIF stabilize the bond between p53 and Mdm2, which renders p53 inactive and does not allow it to perform its usual role. Since MIF functionally inactivates p53, cell cycle arrest and apoptosis do not occur and mutations build up that can lead to the progression of tumors [17].
MIF is also involved in the phosphoinositide-3-kinase (PI3K)/Akt pathway, which plays a key role in the development of tumors [18]. Activation of this pathway allows crucial cells to withstand apoptosis. Upon AKT phosphorylation, BAD cannot inhibit the actions of Bcl-2, which promotes cell survival. Lue et al. (2007) investigated MIFs function and found that it enhances Akt phosphorylation 3-6-fold. To see if MIF was linked to PI3K, Ly294002 was used to inhibit PI3K. The phosphorylation of Akt induced by MIF was completely suppressed, indicating that PI3K is necessary for activation. Next, the interaction between MIF and its main receptor, CD74, was studied. When mouse embryonic fibroblast expressing high concentrations of CD74 were treated with recombinant MIF, the Akt phosphorylation was enhanced up to eightfold [18]. This study demonstrates that MIF and CD74 initiate Akt activation and when MIF is overexpressed, it causes crucial cells to progress through the cell cycle via the PI3K/Akt pathway.
MIF and angiogenesis
Tumor cell migration and angiogenic factors have also been reported when MIF is highly expressed. Vascular endothelial growth factor (VEGF), hypoxia inducible factor 1 (HIF-1), and other angiogenic factors are responsible for the creation of new blood vessels. When cells are exposed to hypoxic conditions, HIF-1 is upregulated, which leads to a higher expression of VEGF and other angiogenic factors. HIF-1 also increases the production of MIF, which contributes to angiogenesis [19]. The study by Oda et al. showed that the overexpression of MIF leads to an increase in production of HIF-1α expression [17]. In a study by Veillat et al. (2010), RT-PCR showed that levels of VEGF, IL-8, and MCP-1 mRNAs were all increased after exposure to MIF [20]. Both studies suggest that MIF exerts an effect on angiogenic factors. Amin et al. (2003) was able to stimulate angiogenesis with MIF in vivo. In order to do so, a matrigel plug angiogenesis assay was performed in the corneas of mice. The hemoglobin content was 4 times higher with MIF compared to the control mice [21]. The plugs treated with MIF were reported to have an increase in neovascularization. The purpose of the mouse corneal bioassay was to further define MIFs role in angiogenesis. In the presence of MIF, the angiogenic response was similar to the positive control of basic fibroblast growth factor (bFGF) and was substantially higher than the negative control [21]. Amin et al. demonstrated the pathway of how MIF contributes to angiogenesis by inhibiting the PI3K pathway. When this pathway was inhibited angiogenesis did not occur, demonstrating that MIF requires the PI3K pathway to contribute to angiogenesis [21]. Though it does in most cases, it is also of note that the MIF required to induce neovascularization does not have to come from the tumor itself. In the MIF-negative 38C13 line of B-cell lymphoma, tumor cells were found to be siphoning MIF from CD31+ endothelial cells to support angiogenesis in the cancer [22].
MIF and metastasis
MIF leads to the metastasis of tumor cells by decreasing the expression of E-cadherin and increasing the expression of N-cadherin. Funamizu et al. 2014 used mice overexpressing MIF to further investigate the role of metastasis in pancreatic cancer. The mice cells with overexpressed MIF showed a significant (P<0.001) increase in tumor growth compared to the control mice cells, which indicates that MIF accelerates the growth and metastasis of tumors in pancreatic cancer [23]. E-cadherin (a cell adhesion molecule) keeps cells in contact with the basal membrane, and the downregulation of E-cadherin can contribute to metastasis through the disruption of cell to cell junctions [24]. The decreased expression of E-cadherin also promotes epithelial mesenchymal transition (EMT) and can lead to the establishment of secondary tumors. EMT is a process that molds epithelial cells to acquire characteristics of mesenchymal cells, which in turn leads to invasion and metastasis. In EMT, epithelial cells lose their intercellular connections, separate from their epithelial sheets, take on select functions of a mesenchymal cell such as enhanced migration, invasion, and resistance to apoptosis, and increased expression of matrix metalloproteinases [25]. Cancer cells take advantage of EMT because it allows the cells to progress to different areas and commence cancer metastasis [26].
MIF in specific cancers
Glioblastoma
In glioblastomas, MIF is expressed near areas of necrosis. In a study conducted by Bacher et al., (2003), MIF showed immunoreactivity in 17 out of 49 (35%) glioblastomas. MIF was frequently observed in the cytoplasm of the large tumor cells. In 16 samples, the p53 protein was observed within the nucleus of the tumor. Furthermore, within the group of the MIF positive tumors, 65% showed a co-localization with p53. In normal brain tissue, MIF was either only localized in scattered cells or not present at all, suggesting that MIF is upregulated in GBM [27]. Indeed, it appears that MIF expression is highly correlated with GBM, as was shown by Ha et al. (2019) in their own study, in which they examined the expression of MIF in an independent cohort of 168 samples of human GBM using IHC. They found that, of the 168 patients, 113 (67%) expressed MIF. Furthermore, the health outcomes and survival of the members of the independent cohort were tracked, and they showed that MIF expression in GBM led to significantly diminished survival. More specifically, the median survival time of individuals in the independent cohort with MIF expression was found to be 11.0 months, compared to only 19.0 months for those that did not express MIF (P<0.0001) [28]. These findings corroborate the idea that not only are GBMs tied to MIF expression, but that they also produce worse outcomes in the presence of MIF.
In the study by Bacher et al. a northern blot analysis was also utilized in order to show that MIF mRNA was drastically increased under hypoxic and hypoglycemic conditions, which are both activators of angiogenesis. MIF is found in necrotic areas and in close proximity to blood vessels in GBM, which are regions associated with hypoxia [27]. This finding was corroborated by Guo et al. (2017), who conducted double immunofluorescence staining in samples of GBM in order to visualize the co-localization of HIF1α (an indicator of hypoxic conditions) and MIF. Ultimately, it was found that MIF was positively correlated with HIF1α expression in GBM in general (P<0.0001) and was positively correlated with HIF1α within the low- and high-grade GBM subclasses (P<0.001) as well [29]. These findings strengthen the idea that the presence of MIF is tied to hypoxic conditions, and this may imply that MIF expression can be regulated by hypoxia in GBM. Thus, we can see that MIF contributes to angiogenesis and inactivates p53 in GBM.
In an experimental study by Munaut et al. (2002) the expression of MIF was seen in 35 out of 35 GBM, and this finding of a correlation between MIF and GBM is supported by the Ha et al. study, in which the presence of MIF was verified in 113 of 168 GBM samples. In the RT-PCR results of the Manaut et al. study, MIF mRNA expression was at least twice as high compared to normal brain tissue in 25% of the samples. It was also demonstrated that the expression of vascular endothelial growth factor mRNA was elevated in GBM compared to a normal brain. The variance in expression of VEGF mRNA in GBMs was similar to that of normal brains, up to a 15-fold increase. 70% of the cases of GBM showed double MIF mRNA levels of normal samples, and qRT-PCR showed a strong correlation (P<0.001) between the expression of MIF and VEGF mRNAs. From this study, there is a clear correlation between MIF and VEGF, demonstrating that MIF is involved in angiogenesis in GBM. Furthermore, MIF and VEGF may share a regulatory pathway that inactivates p53, which is common for GBMs, and increase the expression of VEGF [30].
Recombinant MIF has also been shown to enhance the autophagy, migration, and colony formation of glioblastomas in three cell lines by Xu et al. 2016. The three GBM cell lines were treated with rMIF, which strongly enhanced actin polymerization in GBM cells. Next, Xu et al. used Y27632, an inhibitor of ROCK1 activity, to confirm that autophagy via ROCK1 is enhanced by MIF. The increased expression of LC3B-II that was induced by MIF was reverted by the ROCK1 knockdown. The migration of the three cell lines (U87, U251, and T98) was measured by the Transwell assay. This study showed that rMIF promoted migration in all three cell lines, and that Y27632 suppressed the migration induced by MIF [31]. These results demonstrated that MIF interacts with ROCK1 in the tumorigenesis process.
In GBM, MIF binds to CD74, an HLA class II histocompatibility antigen gamma chain. When MIF is bound to CD74 and the complex becomes phosphorylated, CD44 is recruited. This complex can activate the AKT pathway, which delivers a signal allowing cells to resist apoptosis [32]. This occurs due to inactivation of the pro-apoptotic proteins BAD and BAX. In a study conducted by Presti et al. (2018), microarray analysis showed that the expression of MIF was upregulated in GBM compared to lower grade gliomas. This study also showed higher levels of CD74 and the co-receptor, CD44, associated with MIF in GBM compared to lower grade gliomas [32]. These results show the association between MIF and CD74 in GBM. In GBM, temozolomide (TMZ) is the most used and effective chemotherapeutic drugs, and the upregulation of CD74 in GBM may be responsible for the resistance to TMZ. In an experimental study performed by Kitange et al. (2011) CD74 and MIF mRNA demonstrate an inverse relationship between CD74 mRNA expression levels and TMZ responsiveness from a qRT-PCR analysis. Mice with xenograft lines showing low (% expression <10; n=105) and high (% expression ≥10; n=70) CD74 expression were also used. The nude mice with lower expression survived significantly longer than the mice with higher expression when treated with TMZ and, on average, the mice with lower expression survived for 122 days while the mice with higher expression survived for 62.5 days [33]. This study shows MIF-CD74 signaling may possibly contribute to the TMZ resistance.
Lung cancer
Lung cancer was the most common cancer in 2018 and has an estimated 5-year survival rate of <16% [34]. MIF is a key regulator of tumor growth and is also correlated with lung cancers. The high expression of MIF promotes lung tumor growth, and it should be looked at as a therapeutic target in lung cancer due to its poor prognosis and low survival rate. White et al. (2003) showed that MIF levels are increased in non-small cell lung cancer compared to normal lung tissue. The level of MIF in 87 patients with lung cancer was measured, and it was found that 42 of the tumors had normal levels of MIF compared to healthy lungs while 45 of the tumors had elevated levels. This study also involved a follow-up 16 months later, and 27 of the 87 patients had a recurrence of lung cancer. Upon further analysis, it was seen that when both VEGF and MIF were elevated, survival rate was significantly worse. Furthermore, for tumors in which MIF levels were normal, there was no correlation between MIF and vessel density. Comparatively, the tumors with high levels of MIF were correlated with significant strengthening of vessel density, and this indicates that lung tumors highly expressing MIF are more detrimental than tumors that do not [35]. These findings, specifically the idea that higher MIF expression is tied to worse survival rate was corroborated by Huang et al. (2019), who analyzed a public database of 2437 non-small cell lung cancer samples for MIF expression and outcomes. Their findings indicated that levels of MIF were significantly higher in patients that exhibited poor responsiveness to chemotherapeutic drugs and experienced worse outcomes (P<0.0001). The results also found similar patterns for other markers, specifically Src and CD155, and concluded that MIF, SRC, and CD155 must interact cooperatively in the promotion of tumor progression [36].
The study by Li et al. (2018) demonstrated that MIF promotes cell proliferation and the Warburg effect in lung cancer. The expression of MIF was detected by RT-qPCR in seven lung cancer cell lines, and the overexpression of MIF was analyzed in the H524 cells, leading to findings that MIF significantly promoted the Warburg effect (the phenomenon in which cancer cells utilize aerobic glycolysis instead of oxidative phosphorylation) while the knockout of MIF in the H358 cells inhibited the Warburg effect. These results support the conclusion that MIF contributes to the promotion of the Warburg effect in lung cancer. The activation of HIF-1α by MIF was studied to determine how MIF regulates the Warburg effect. From a Western blot analysis, it was shown that MIF overexpression promotes the upregulation of HIF-1α [34]. This indicates that MIF promotes the Warburg effect and the upregulation of HIF-1α, which promotes angiogenesis in lung cancer.
Potential methods of treatment for MIF-mediated non-small cell lung cancers have also been explored. In their study, Goto et al. (2017) explored the utility of miR-451 (a tumor suppressive micro RNA). They cultured cell lines with MIF and without miR-451 and, as expected, observed a direct relationship between MIF expression and cell proliferation and migration. However, upon induction of miR-451, they observed significant reductions in cell proliferation and migration in cell lines that expressed MIF (P<0.005) [37]. As such, it can be seen that miR-451 represents a potential agent to target and inhibit MIF function in non-small cell lung cancers.
Other inhibitors of MIF have also been explored as potential avenues for development of treatments for lung cancer. Mawhinney et al. (2015) studied SCD-19 and determined it to be the most effective inhibitor of MIFs tautomeric enzymatic activity. As it was found that MIF directly promoted proliferation in Lewis lung carcinoma (LLC), LLC cells were treated with SCD-19 in vitro. It was found that the rate of cell growth was reduced by 47% compared to the control. SCD-19 treatment was then used in vivo, and mice were treated with 35 mg/kg of SCD-19 intraperitoneally twice a week, which resulted in a 90% reduction of tumor volume compared to the control [38]. This experiment shows that MIF enhances tumor growth, and the inhibition of MIF may be a future treatment for lung cancer.
Breast cancer
Breast cancer was the second most common cancer in 2018 and is also characterized by high MIF expression. As a result, MIF (or, more specifically, particular MIF variants) have been shown to function as high-fidelity predictors of increased breast cancer risk. Lin et al. (2016) studied samples from 560 breast cancer patients and sequenced the DNA of the samples. As a result, they observed that in individuals with the rs755622 MIF variant (the most common MIF variant), individuals with three specific genotypes: C/G, C/C, and C/G-C/C exhibited a significantly higher likelihood of developing breast cancer than other genotypes (P=0.004) [39].
The function of MIF as an agent in breast cancer tumor progression has also been studied. Verjans et al. (2009) used qPCR and Western blots to study the levels of MIF in non-cancerous epithelial breast cells (MCF-12A), invasive (MDA-MB-231), and non-invasive (MDA-MB-468 and ZR-75-1) breast cancer cell lines [40]. Surprisingly, MIF was upregulated in non-invasive cell lines, whereas the invasive cell line exhibited significantly lower levels of MIF. Constitutive expression of MIF was not found in the non-cancerous cells. Next, expression levels of the MIF receptor (CD74) were tested. The non-invasive cell lines showed a weak expression of CD74, while the invasive cell line exhibited the highest level of expression [40]. The high expression of CD74 in the invasive cell line may be due to their intracellular location. Overall, this data indicates that invasive breast cancer cells may be prone to stimulation with MIF. Next, the authors compared the proliferation rates of breast tumors. The invasive breast cancer cells proliferated at the highest rate, 7.8 times the non-cancerous proliferation rate, while the non-invasive cells proliferated at 2.1 times the rate of the non-cancerous cells [40]. The invasive cells were then treated with recombinant MIF (rMIF), which promoted the migration and invasion of breast cancer. The results from Verjans et al. show that MIF contributes to migration and invasion.
Wang et al. (2019) observed variation of MIF expression levels in luminal vs. triple negative (basal-like) breast cancer. Through an analysis of MIF expression in the UALCAN database, it was found that levels of MIF expression are higher in triple negative breast cancer samples (n=166) than in luminal breast cancer samples (n=566), (P<0.05). This research also corroborated the study by Verejans et al., elucidating the relationship between MIF expression and cancer survival. Through the analysis of MIF mRNA expression levels in microarray data from 3951 patients and the development of Kaplan-Meier survival curves, it was found that increased levels of MIF expression are associated with reduced surival rate (P<0.01) [41].
Xu et al. (2008) showed that MIF also induces angiogenesis in human breast cancer cells [42]. In this study, 20 normal breast tissues and 121 cancerous breast tissue were obtained. Out of the 121 cancerous tissues, 36 samples (29.8%) showed an overexpression of MIF. The study found that patients with MIF positive tumors exhibited a lower mean survival (115.6 months versus 108.2 months), significantly worse disease-free survival (P=0.029), and increased IL-8 levels compared to patients with MIF negative tumors [42]. Increased IL-8, along with VEGF, may contribute to angiogenesis and tumor growth. Richard et al. (2014) looked at the interactions between MIF and CD74 to show their involvement in tumorigenesis [43]. ELISA results had shown that MIF levels in the serum of 36 breast cancer patients were four-fold higher than in healthy individuals. Next, immunohistochemistry showed that stromal CD74 expression correlated with triple-negative receptor status and the absence of estrogen receptors [43]. This study concludes by suggesting that MIF/CD74 could be targeted with anti-angiogenic drugs in the treatment of triple-negative breast cancer. MIF over expression is partly due to its stabilization by HSP90 and HIF1α [44].
Gastric cancer
He et al. (2006) showed that MIF is expressed in 12% of normal mucosa, 52% of gastritis, 66% of intestinal metaplasia, and 96% of gastric cancers [45]. He et al. (2015) also showed that MIF is highly expressed in gastric tumors from a microarray containing 117 samples of gastric cancer and adjacent non-cancerous normal tissue [46]. This was further confirmed by Western blot analysis, which showed that MIF was expressed in all five gastric cell lines (AGS, MKN-28, MKN-45, SGC-7901 and BCG-823). The elevated level of MIF expression in gastric cancer was supported by Yoon et al., who analyzed gastric tissue samples from 371 individuals, in which 206 individuals had gastric cancer and 165 did not. Upon study of MIF levels in the tissue, it was observed that the tissue from individuals with gastric cancer had more than double the level of MIF expression when compared to tissue from individuals without cancer (P=0.001) [47]. As such, it can be seen that MIF expression is a major differentiating factor between samples with and without gastric cancer. MIF is also a poor prognosis factor in gastric cancer; when overexpressed the mean survival time is only 24.2 months as compared to 47 months in cancers with low MIF expression. Additionally, MIF knockout showed inhibited proliferation of gastric cancer cells, a finding that furthers the importance of MIF inhibitors in cancer treatment [46].
Zheng et al. (2012) collected 120 samples of gastric cancer tissue to find the relation between MIF and CD74 in gastric cancer. CD74 was observed in 100 of the 120 samples (81%), while MIF was observed in 97 of the 120 samples (81%) [48]. Zheng et al. incubated rMIF with MKN-45 cells (gastric cancer cell line). This was immunoprecipitated by the MIF antibody and a Western blot of this revealed that CD74 was coprecipitated with MIF [47]. This study suggests that CD74 and MIF can form a complex that promotes cell proliferation. Kong et al. (2018), through the use of western blots of gastric cancer cell lines along with either anti-MIF antibodies or anti-p53 antibodies, not only determined that MIF physically binds to p53 in gastric cancer cells, but also found that increases and decreases in MIF expression produced a parallel affect in p53 expression. The role of the ZFPM2-AS1 gene was also studied using a western blot and RT-PCR analysis, and the findings indicated that in gastric cancer cells, deregulation of ZFPM2-AS1 led to decreased mRNA and protein expression of molecules downstream of p53. As such, a potential mechanism through which MIF affects gastric oncogenesis was proposed: that an increase in ZFPM2-AS1 expression increases MIF expression, which binds to and suppresses the ability of p53 to translocate into the nucleus and thereby causes increased growth and proliferation of gastric cancer cells [49].
Melanoma
MIF has also been observed in the most common type of skin cancer, melanoma. Using MIF knockdowns in melanoma cell lines, Oliveira et al. (2014) showed a substantial reduction of MIF in the cell lysates compared to a negative control. Both cell lines gave an equal biological response, which shows MIF can increase the proliferation rate of cells, a finding corroborated by the fact that MIF knockdown showed a 2-3 fold increase in apoptosis [50]. Higher levels of MIF did not affect patient outcome; however, elevated levels of MIF did correspond to faster recurrence in melanoma [51].
Yaddanapudi et al. (2017) collected peripheral blood from 27 patients with stage III or IV metastatic melanoma and from 12 healthy individuals. The frequency and phenotypes of monocytic myeloid-derived suppressor cells (MDSCs) were tested for using FACS analysis. MDSCs were found to be significantly elevated in the cancerous cells compared to the control. Next, the effects of the inhibition of MIF with 4-IPP = was tested in MDSCs. Addition of 4-IPP to the A375 melanoma cell line resulted in the reduction of CD14, CD33, and PD-L1, an increase in DC-SIGN expression, as well as a reduction of MDSCs suppressive effects on T cell activation [52]. The inhibition of MIF in the A375 cell line showed a reversion of gene production comparable to tumor free cells [52]. Yaddanapudi et al. results show that MIF is pro-tumorigenic, and inhibition of MIF may be a therapeutic target to look at in melanomas.
Additionally, MIF overexpression in melanoma appears to play a significant role in angiogenesis. This finding was supported both in vivo using a melanoma-bearing rodent model, where treatment with anti-MIF antibodies led to a reduction in angiogenesis, as well as in the B16-F10 cell line where the introduction of interfering MIF RNA significantly decreased tumor vascularization [53,54]. Though molecular alterations of MIF have only been found in 1% of melanomas, these alterations (most of which were amplifications) may be a key driver of metastatic disease as they were associated with significantly lower overall and relapse-free survival in a 2019 study by Soumov et al. [55]. The role of MIF in promoting metastasis is furthered by Yaddanapudi et al., who observed that MIF knockout in rodent models protected against the development of lung metastasis in melanomas [56].
Bladder cancer
Bladder cancer is the 9th most common human malignancy and 13th most common cause of cancer death, leading to approximately 16,000 deaths in 2015 [55]. In 2004, Meyer-Siegler first discovered MIF in bladder cancer localized throughout the urothelial cytoplasm [57]. HT-1376 cells were treated with Hyaluronan or anti-MIF antibodies. The anti-MIF treatment resulted in a significant decrease in cell proliferation (P<0.01), showing that MIF promotes proliferation in bladder cancer [57]. This finding is corroborated by Gai et al. (2018), who studied the effects of CD74 knockdown in HT-1376 cells. Knockdown of CD74 resulted in decreased cell proliferation, tumor volume, and angiogenesis compared to MIF-positive controls [58]. It is hypothesized that when active, MIF and CD74 promote tumor growth and angiogenesis through upregulation of the ERK1/2 and PI3K/AKT pathways.
Another hallmark of MIF in bladder cancers is an increase in the levels of products that mediate decreases in apoptosis and, as discussed earlier, increased angiogenesis. To determine the role of MIF in bladder cancer, Taylor et al. gave MIF knockout and wild-type mice a known carcinogen and studied tumor progression [46]. They found MIF knockout mice to exhibit lower tumor staging with no invasion into the muscles, while the MIF wild-type mice had higher staging and muscle invasion. It has been determined that MIF increases stromal vascularity, leading to the development of muscle invasive bladder cancers as seen in Taylor et al. study. Further, MIF activation also recruits the accumulation of myeloid-derived suppressor cells (MDSCs) via activation of CXCL2/MIF-CXCR2 by simultaneous activation of mitogen-activated protein kinase and nuclear factor kappa B pathways [59].
As in other cancers, MIF is also thought to promote the development of bladder cancers in part through the induction of chronic inflammation. In healthy individuals, anti-thrombin III (ATIII) complexes with MIF, a process known to reduce MIF’s biological activity and attenuate the cytokine’s pro-inflammatory effects [60]. In a 2013 study, it was found that despite increased serum levels of MIF in patients with bladder cancer (n=50) as compared to controls (n=50), the level of MIF-ATIII complexes was significantly decreased [60]. It can then be observed that overexpression of MIF contributes to tumor aggression in a number of ways: through inducing angiogenesis, promoting systemic inflammation, and increasing cell proliferation.
Head and neck cancer
Head and neck squamous cell carcinoma (HNSCC) accounts for 90% of head and neck cancers and is the sixth most common cancer worldwide [61]. The five-year disease-free survival is only about 50%, therefore new biomarkers to detect HNSCC, such as MIF, are needed. Kindt el al. (2019) looked into MIFs involvement with HNSCC and its relationship to human papillomavirus (HPV), as. MIF is expressed three times greater in HPV-positive cell lines than in HPV-negative cell lines [62]. Western blotting of from HPV-positive and HPV-negative cell lines demonstrated that HPV-positive cell lines expressed more HIF-1α, which in turn leads to an increase of MIF secretion and tumor angiogenesis in HPV positive cell lines [62]. Regardless of HPV status, however, elevated MIF expression has been uniformly observed as a biomarker in HNSCC [63].
In a clinical study by Kindt et al. (2013) involving HNSCC patients (n=66) and healthy individuals (n=16), it was found that elevated MIF levels lead to an unfavorable prognosis as patients with high MIF expression showed recurrence, nodal metastasis, and an overall lower survival time [64]. After looking at the clinical data, Kindt et al. looked at MIF experimentally. They knocked down MIF in the SCCVII cell line, which was shown to decrease the rate at which cells proliferate and compared to the control. Syngeneic mice were then used to study MIFs effect in vivo. The mice with decreased MIF expression had delayed tumor appearance, better survival rates, and were more responsive to chemotherapy [64]. MIF is also reported to be involved in the progression, invasion and proliferation of HNSCC, and upon treatment with milatuzumab and inhibitors of Src-1, the tumors showed a reduction in growth [63]. A study by Lo et al. (2013) further implicates MIFs role in tumor progression, as inoculation of nasopharyngeal carcinoma tumor spheres with MIF siRNAs also led to a marked reduction in growth [65].
MIF inhibitors
Due to MIFs aggressive role in cancer, inhibitors may potentially provide a therapeutic benefit. The most studied MIF inhibitors will be reviewed here and are shown in Table 1. Isoxazoline inhibitor, ISO-1, inhibits MIFs tautomerase activity by binding to the active site of MIF [66]. Through this mechanism ISO-1 has been shown to significantly reduce prostate cancer, colon cancer, and melanoma cell growth and proliferation [54]. Another Isoxazoline MIF inhibitor, ISO-66, suppresses tumor growth in colon cancer and melanomas by enhancing the cytotoxicity of lymphocytes [61].
Table 1.
Overview of the most studied MIF inhibitors
| Class | Compound | Function | IC50 Value |
|---|---|---|---|
| Phenyl-pyrimidine | 4-IPP | Reduces AKT phosphorylation allowing for cell death [54,66] | 0.2-0.5 |
| Isocoumarin | SCD-19 | Decreased size of LLC tumor by 90% & capable of 100% MIF inhibition [33] | N/A |
| Isoxazoline | ISO-1 | 40% inhibition of MIF & reduction of cell growth in melanoma, prostate, and colon cancer [66] | 24 |
| ISO-66 | Enhances lymphocyte cell cytotoxicity and suppresses tumor growth [54,66] | 1.5 | |
| CPSI-1306 | Increases p53 expression and decreases cell proliferation [66] | N/A | |
| CPSI-2705 | Decreases growth of tumors and progression of bladder cancer [66] | N/A |
Additionally, the inhibitor 4-IPP works by inhibiting the MIF/CD74 pathway and reduces AKT phosphorylation, leading to increased cell death in tumors [62]. In 2015, Varinelli et al. experimentally determined that 4-IPP inhibits the cell growth of thyroid cancer by blocking MIF/CD74 internalization and activating JNK, inducing apoptosis [63]. A second study was also able to demonstrate 4-IPP’s effectiveness, showing that 4-IPP inhibited the proliferation and migration of SCCVII cancer cells by arresting them in the G2/M phase of the cell cycle [64]. CPSI 1306 and 2705 are another group of MIF inhibitors that may hold promise. In 2013, Choudhary et al. orally administered CPSI 1306 and 2705 to mice with bladder cancer [63]. The drugs were found to decrease the growth and progression of bladder cancer in vivo. When compared to ISO-1, CPSI 1306 and 2705 are 100 times and 10 times more potent, respectively, a finding that may indicate these to be superior treatment options [63]. Further studies by Nagarajan et al. 2014 used CPSI-1306 to study MIF in squamous cell carcinoma in mice. When the mice were treated with CPSI-1306, skin thickness and cell proliferation decreased, and p53 expression increased. In 2015, Mawhinney et al. treated LLC cells with SCD-19, which demonstrated its effectiveness by showing a 90% reduction in the tumor compared to controls [31]. Whether it is through inducing apoptosis, reducing proliferation, or inhibiting enzymatic activity, experimental studies have shown that MIF inhibitors attenuate cancer in many ways. Utilizing these inhibitors in future clinical trials may provide further insight into their viability as cancer therapies.
Conclusions and future perspectives
In conclusion, MIF is a cytokine secreted by many different types of cells that exhibits a variety of biological functions. It is a key regulator of the immune system, controls inflammation, and its overexpression can contribute to the development of many different types of cancers. MIF promotes the proliferation, migration, and invasion of nearly all cancers. High expression of MIF also leads to angiogenesis. Additionally, MIF defines the association between chronic inflammation and cancer, as high MIF expression in cancer cells leads to a worse patient survival time and a more aggressive cancer. With regards to its viability as a treatment option, experimental studies on MIF inhibitors look promising, and may lead to a longer patient survival time and a better prognosis for certain cancers. The continued investigation of existing MIF inhibitors will be crucial toward determining their effectiveness in treating cancer.
Acknowledgements
The authors thank the University of Illinois College of Medicine Peoria, The Mark Linder Walk for the Mind, KBstrong Foundation, Washington, IL, The Illinois Neurological Institute and OSF Foundation for support. The authors thank Erika Sung for help in the formatting of the manuscript.
Disclosure of conflict of interest
None.
Abbreviations
- MIF
Macrophage migration inhibitory factor
- ISO-1
Isoxazoline
- 4-IPP
4-iodo-6-phenylpyrimidine
- TNF-α
Tumor Necrosis Factor alpha
- ESC
Embryonic stem cells
- BMS
Basso mouse scale
- NPC
Neural stem and progenitor cells
- PI3K
Phosphoinositide-3-kinase
- VEGF
Vascular endothelial growth factor
- HIF1-α
Hypoxia inducible factor 1 alpha
- EMT
Epithelial mesenchymal transition
- mRNA
Messenger RNA
- GBM
Glioblastoma
- TMZ
Temozolomide
- LLC
Lewis lung carcinoma
- rMIF
Recombinant MIF
- MDSC
Myeloid-derived suppressor cells
- HNSCC
Head and neck squamous cell carcinoma
References
- 1.Conroy H, Mawhinney L, Donnelly SC. Inflammation and cancer: macrophage migration inhibitory factor (MIF)-the potential missing link. QJM. 2010;103:831–836. doi: 10.1093/qjmed/hcq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishihira J, Sakaue S. Overview of macrophage migration inhibitory factor (MIF) as a potential biomarker relevant to adiposity. J Tradit Complement Med. 2012;2:186–191. doi: 10.1016/s2225-4110(16)30098-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun HW, Bernhagen J, Bucala R, Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1996;93:5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baugh JA, Chitnis S, Donnelly SC, Monteiro J, Lin X, Plant BJ, Wolfe F, Gregersen PK, Bucala R. A functional promoter polymorphism in the macrophage migration inhibitory factor (MIF) gene associated with disease severity in rheumatoid arthritis. Genes Immun. 2002;3:170–176. doi: 10.1038/sj.gene.6363867. [DOI] [PubMed] [Google Scholar]
- 5.Lerch JK, Puga DA, Bloom O, Popovich PG. Glucocorticoids and macrophage migration inhibitory factor (MIF) are neuroendocrine modulators of inflammation and neuropathic pain after spinal cord injury. Semin Immunol. 2014;26:409–414. doi: 10.1016/j.smim.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammatory cells and cancer: think different! J Exp Med. 2001;193:F23–6. doi: 10.1084/jem.193.6.f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118. [DOI] [PubMed] [Google Scholar]
- 8.Tillmann S, Bernhagen J, Noels H. Arrest functions of the MIF ligand/receptor axes in atherogenesis. Front Immunol. 2013;4:115. doi: 10.3389/fimmu.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, Kooistra T, Fingerle-Rowson G, Ghezzi P, Kleemann R, McColl SR, Bucala R, Hickey MJ, Weber C. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–596. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 10.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosengren E, Bucala R, Aman P, Jacobsson L, Odh G, Metz CN, Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996;2:143–149. [PMC free article] [PubMed] [Google Scholar]
- 13.Shen YC, Thompson DL, Kuah MK, Wong KL, Wu KL, Linn SA, Jewett EM, Shu-Chien AC, Barald KF. The cytokine macrophage migration inhibitory factor (MIF) acts as a neurotrophin in the developing inner ear of the zebrafish, Danio rerio. Dev Biol. 2012;363:84–94. doi: 10.1016/j.ydbio.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Chen T, Leng L, Fan J, Cao K, Duan Z, Zhang X, Shao C, Wu M, Tadmori I, Li T, Liang L, Sun D, Zheng S, Meinhardt A, Young W, Bucala R, Ren Y. MIF produced by bone marrow-derived macrophages contributes to teratoma progression after embryonic stem cell transplantation. Cancer Res. 2012;72:2867–2878. doi: 10.1158/0008-5472.CAN-11-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Chen L, Wang Y, Ding Y, Peng Z, Duan L, Ju G, Ren Y, Wang X. Macrophage migration inhibitory factor promotes proliferation and neuronal differentiation of neural stem/precursor cells through Wnt/beta-catenin signal pathway. Int J Biol Sci. 2013;9:1108–1120. doi: 10.7150/ijbs.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H, Seong HA, Ha H. Critical role of cysteine residue 81 of macrophage migration inhibitory factor (MIF) in MIF-induced inhibition of p53 activity. J Biol Chem. 2008;283:20383–20396. doi: 10.1074/jbc.M800050200. [DOI] [PubMed] [Google Scholar]
- 17.Hanel W, Moll UM. Links between mutant p53 and genomic instability. J Cell Biochem. 2012;113:433–439. doi: 10.1002/jcb.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, Fingerle-Rowson G, Bucala R, Luscher B, Bernhagen J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 19.Oda S, Oda T, Nishi K, Takabuchi S, Wakamatsu T, Tanaka T, Adachi T, Fukuda K, Semenza GL, Hirota K. Macrophage migration inhibitory factor activates hypoxia-inducible factor in a p53-dependent manner. PLoS One. 2008;3:e2215. doi: 10.1371/journal.pone.0002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:E403–12. doi: 10.1210/jc.2010-0417. [DOI] [PubMed] [Google Scholar]
- 21.Amin MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE. Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res. 2003;93:321–329. doi: 10.1161/01.RES.0000087641.56024.DA. [DOI] [PubMed] [Google Scholar]
- 22.Chesney JA, Mitchell RA. 25 years on: a retrospective on migration inhibitory factor in tumor angiogenesis. Mol Med. 2015;21(Suppl 1):S19–24. doi: 10.2119/molmed.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funamizu N, Hu C, Lacy C, Schetter A, Zhang G, He P, Gaedcke J, Ghadimi MB, Ried T, Yfantis HG, Lee DH, Subleski J, Chan T, Weiss JM, Back TC, Yanaga K, Hanna N, Alexander HR, Maitra A, Hussain SP. Macrophage migration inhibitory factor induces epithelial to mesenchymal transition, enhances tumor aggressiveness and predicts clinical outcome in resected pancreatic ductal adenocarcinoma. Int J Cancer. 2013;132:785–794. doi: 10.1002/ijc.27736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heery R, Finn SP, Cuffe S, Gray SG. Long non-coding RNAs: key regulators of epithelial-mesenchymal transition, tumour drug resistance and cancer stem cells. Cancers (Basel) 2017;9 doi: 10.3390/cancers9040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 27.Bacher M, Schrader J, Thompson N, Kuschela K, Gemsa D, Waeber G, Schlegel J. Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol. 2003;162:11–17. doi: 10.1016/S0002-9440(10)63793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha W, Sevim-Nalkiran H, Zaman AM, Matsuda K, Khasraw M, Nowak AK, Chung L, Baxter RC, McDonald KL. Ibudilast sensitizes glioblastoma to temozolomide by targeting Macrophage Migration Inhibitory Factor (MIF) Sci Rep. 2019;9:2905. doi: 10.1038/s41598-019-39427-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo X, Xu S, Gao X, Wang J, Xue H, Chen Z, Zhang J, Guo X, Qian M, Qiu W, Li G. Macrophage migration inhibitory factor promotes vasculogenic mimicry formation induced by hypoxia via CXCR4/AKT/EMT pathway in human glioblastoma cells. Oncotarget. 2017;8:80358–80372. doi: 10.18632/oncotarget.18673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munaut C, Boniver J, Foidart JM, Deprez M. Macrophage migration inhibitory factor (MIF) expression in human glioblastomas correlates with vascular endothelial growth factor (VEGF) expression. Neuropathol Appl Neurobiol. 2002;28:452–460. doi: 10.1046/j.1365-2990.2002.00416.x. [DOI] [PubMed] [Google Scholar]
- 31.Xu S, Guo X, Gao X, Xue H, Zhang J, Guo X, Qiu W, Zhang P, Li G. Macrophage migration inhibitory factor enhances autophagy by regulating ROCK1 activity and contributes to the escape of dendritic cell surveillance in glioblastoma. Int J Oncol. 2016;49:2105–2115. doi: 10.3892/ijo.2016.3704. [DOI] [PubMed] [Google Scholar]
- 32.Presti M, Mazzon E, Basile MS, Petralia MC, Bramanti A, Colletti G, Bramanti P, Nicoletti F, Fagone P. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol Lett. 2018;16:2881–2886. doi: 10.3892/ol.2018.8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitange GJ, Carlson BL, Schroeder MA, Decker PA, Morlan BW, Wu W, Ballman KV, Giannini C, Sarkaria JN. Expression of CD74 in high grade gliomas: a potential role in temozolomide resistance. J Neurooncol. 2010;100:177–186. doi: 10.1007/s11060-010-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Zhang J, Xie F, Peng J, Wu X. Macrophage migration inhibitory factor promotes Warburg effect via activation of the NFkappaB/HIF1alpha pathway in lung cancer. Int J Mol Med. 2018;41:1062–1068. doi: 10.3892/ijmm.2017.3277. [DOI] [PubMed] [Google Scholar]
- 35.White ES, Flaherty KR, Carskadon S, Brant A, Iannettoni MD, Yee J, Orringer MB, Arenberg DA. Macrophage migration inhibitory factor and CXC chemokine expression in non-small cell lung cancer: role in angiogenesis and prognosis. Clin Cancer Res. 2003;9:853–860. [PubMed] [Google Scholar]
- 36.Huang WC, Kuo KT, Wang CH, Yeh CT, Wang Y. Cisplatin resistant lung cancer cells promoted M2 polarization of tumor-associated macrophages via the Src/CD155/MIF functional pathway. J Exp Clin Cancer Res. 2019;38:180. doi: 10.1186/s13046-019-1166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goto A, Tanaka M, Yoshida M, Umakoshi M, Nanjo H, Shiraishi K, Saito M, Kohno T, Kuriyama S, Konno H, Imai K, Saito H, Minamiya Y, Maeda D. The low expression of miR-451 predicts a worse prognosis in non-small cell lung cancer cases. PLoS One. 2017;12:e0181270. doi: 10.1371/journal.pone.0181270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mawhinney L, Armstrong ME, O’Reilly C, Bucala R, Leng L, Fingerle-Rowson G, Fayne D, Keane MP, Tynan A, Maher L, Cooke G, Lloyd D, Conroy H, Donnelly SC. Macrophage migration inhibitory factor (MIF) enzymatic activity and lung cancer. Mol Med. 2015;20:729–735. doi: 10.2119/molmed.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin S, Wang M, Liu X, Zhu W, Guo Y, Dai Z, Yang P, Tian T, Dai C, Zheng Y, Hu C, Wei L, Dai Z. Association of genetic polymorphisms in MIF with breast cancer risk in Chinese women. Clin Exp Med. 2017;17:395–401. doi: 10.1007/s10238-016-0439-9. [DOI] [PubMed] [Google Scholar]
- 40.Verjans E, Noetzel E, Bektas N, Schutz AK, Lue H, Lennartz B, Hartmann A, Dahl E, Bernhagen J. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer. 2009;9:230. doi: 10.1186/1471-2407-9-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang P, Liu J, Song Y, Liu Q, Wang C, Qian C, Zhang S, Zhu W, Yang X, Wan F, Liu Z, Luo D. Screening of immunosuppressive factors for biomarkers of breast cancer malignancy phenotypes and subtype-specific targeted therapy. PeerJ. 2019;7:e7197. doi: 10.7717/peerj.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Wang B, Ye C, Yao C, Lin Y, Huang X, Zhang Y, Wang S. Overexpression of macrophage migration inhibitory factor induces angiogenesis in human breast cancer. Cancer Lett. 2008;261:147–157. doi: 10.1016/j.canlet.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 43.Richard V, Kindt N, Decaestecker C, Gabius HJ, Laurent G, Noel JC, Saussez S. Involvement of macrophage migration inhibitory factor and its receptor (CD74) in human breast cancer. Oncol Rep. 2014;32:523–529. doi: 10.3892/or.2014.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richard V, Kindt N, Saussez S. Macrophage migration inhibitory factor involvement in breast cancer (Review) Int J Oncol. 2015;47:1627–1633. doi: 10.3892/ijo.2015.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797–802. doi: 10.1136/gut.2005.078113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He LJ, Xie D, Hu PJ, Liao YJ, Deng HX, Kung HF, Zhu SL. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2015;21:9916–9926. doi: 10.3748/wjg.v21.i34.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon K, Kim N, Park Y, Kim BK, Park JH, Shin CM, Lee DH, Surh YJ. Correlation between macrophage migration inhibitory factor and autophagy in Helicobacter pylori-associated gastric carcinogenesis. PLoS One. 2019;14:e0211736. doi: 10.1371/journal.pone.0211736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng YX, Yang M, Rong TT, Yuan XL, Ma YH, Wang ZH, Shen LS, Cui L. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J Gastroenterol. 2012;18:2253–2261. doi: 10.3748/wjg.v18.i18.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kong F, Deng X, Kong X, Du Y, Li L, Zhu H, Wang Y, Xie D, Guha S, Li Z, Guan M, Xie K. ZFPM2-AS1, a novel lncRNA, attenuates the p53 pathway and promotes gastric carcinogenesis by stabilizing MIF. Oncogene. 2018;37:5982–5996. doi: 10.1038/s41388-018-0387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oliveira CS, de Bock CE, Molloy TJ, Sadeqzadeh E, Geng XY, Hersey P, Zhang XD, Thorne RF. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC Cancer. 2014;14:630. doi: 10.1186/1471-2407-14-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekmekcioglu S, Davies MA, Tanese K, Roszik J, Shin-Sim M, Bassett RL Jr, Milton DR, Woodman SE, Prieto VG, Gershenwald JE, Morton DL, Hoon DS, Grimm EA. Inflammatory marker testing identifies CD74 expression in melanoma tumor cells, and its expression associates with favorable survival for stage III melanoma. Clin Cancer Res. 2016;22:3016–3024. doi: 10.1158/1078-0432.CCR-15-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yaddanapudi K, Rendon BE, Lamont G, Kim EJ, Al Rayyan N, Richie J, Albeituni S, Waigel S, Wise A, Mitchell RA. MIF is necessary for late-stage melanoma patient MDSC immune suppression and differentiation. Cancer Immunol Res. 2016;4:101–112. doi: 10.1158/2326-6066.CIR-15-0070-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751–758. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- 54.Culp WD, Tsagozis P, Burgio M, Russell P, Pisa P, Garland D. Interference of macrophage migration inhibitory factor expression in a mouse melanoma inhibits tumor establishment by up-regulating thrombospondin-1. Mol Cancer Res. 2007;5:1225–1231. doi: 10.1158/1541-7786.MCR-07-0229. [DOI] [PubMed] [Google Scholar]
- 55.Soumoy L, Kindt N, Ghanem G, Saussez S, Journe F. Role of macrophage migration inhibitory factor (MIF) in melanoma. Cancers (Basel) 2019;11 doi: 10.3390/cancers11040529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, Lasnik A, Eaton JW, Mitchell RA. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J Immunol. 2013;190:2984–2993. doi: 10.4049/jimmunol.1201650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, Shariat SF, Zlotta AR, Boorjian SA. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69:300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 58.Meyer-Siegler KL, Leifheit EC, Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC Cancer. 2004;4:34. doi: 10.1186/1471-2407-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gai JW, Wahafu W, Song L, Ping H, Wang M, Yang F, Niu Y, Qing W, Xing N. Expression of CD74 in bladder cancer and its suppression in association with cancer proliferation, invasion and angiogenesis in HT-1376 cells. Oncol Lett. 2018;15:7631–7638. doi: 10.3892/ol.2018.8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taylor JA 3rd, Kuchel GA, Hegde P, Voznesensky OS, Claffey K, Tsimikas J, Leng L, Bucala R, Pilbeam C. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC Cancer. 2007;7:135. doi: 10.1186/1471-2407-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, He J, Peng JY, Duan TH, Cui J, Zhang XS, Zhou FJ, Wang RF, Li J. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36:2095–2104. doi: 10.1038/onc.2016.367. [DOI] [PubMed] [Google Scholar]
- 62.Meyer-Siegler KL, Cox J, Leng L, Bucala R, Vera PL. Macrophage migration inhibitory factor anti-thrombin III complexes are decreased in bladder cancer patient serum: complex formation as a mechanism of inactivation. Cancer Lett. 2010;290:49–57. doi: 10.1016/j.canlet.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang SS, Cen X, Liang XH, Tang YL. Macrophage migration inhibitory factor: a potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget. 2017;8:10650–10661. doi: 10.18632/oncotarget.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kindt N, Descamps G, Lechien JR, Remmelink M, Colet JM, Wattiez R, Berchem G, Journe F, Saussez S. Involvement of HPV infection in the release of macrophage migration inhibitory factor in head and neck squamous cell carcinoma. J Clin Med. 2019;8 doi: 10.3390/jcm8010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kindt N, Preillon J, Kaltner H, Gabius HJ, Chevalier D, Rodriguez A, Johnson BD, Megalizzi V, Decaestecker C, Laurent G, Saussez S. Macrophage migration inhibitory factor in head and neck squamous cell carcinoma: clinical and experimental studies. J Cancer Res Clin Oncol. 2013;139:727–737. doi: 10.1007/s00432-013-1375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lechien JR, Nassri A, Kindt N, Brown DN, Journe F, Saussez S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: a systematic review. Head Neck. 2017;39:2573–2584. doi: 10.1002/hed.24939. [DOI] [PubMed] [Google Scholar]


