Abstract
Background: Human epidermal growth factor receptor 2 (HER2) initiates a variety of signals that lead to the invasion and metastasis of gastric cancer. Though drugs targeting HER2 have been applied in clinical practice, drug resistance remains a big challenge. This study aimed to propose a new therapeutic target by exploring the regulating pathway of HER2. Methods: Reverse transcription polymerase chain reaction (RT-PCR), western blot and immunohistochemistry staining were used respectively to detect the expression of HER2, Twist, E-cadherin and Fascin1 in both HER2 knockdown and overexpressed cell lines. Trans-well chamber assay and wound healing assay were used to detect the invasive ability of gastric cancer cells. The correlation between HER2 and Twist was analyzed based on specimens obtained from 118 patients with gastric cancer. Results: HER2 silencing decreased the expression of Twist (P<0.05) and increased the expression of E-cadherin (P<0.05), while the expression of Fascin1 remained unchanged (P>0.05) and the migration and invasion abilities of cancer cells were weakened (P<0.01). On the contrary overexpression of HER2 increased the expression of Twist (P<0.05) and decreased the expression of E-cadherin (P<0.05), while the expression of Fascin1 still remained unchanged (P>0.05), and the migration and invasion abilities of cancer cells were enhanced (P<0.01). Our data indicated that the HER2 kinase domain was not involved in the regulation of Twist or E-cadherin. In addition, the expression of HER2 was positively correlated with the EMT-related transcription factor Twist in gastric cancer tissues. Conclusion: HER2 could promote the invasion and migration of gastric cancer cells by down-regulating E-cadherin and up-regulating Twist, which indicated that E-cadherin and Twist were both promising therapeutic targets.
Keywords: HER2, twist, fascin1, gastric carcinoma, invasion, migration
Introduction
Gastric cancer (GC), being the fourth most common cancer and the second leading cause of cancer death, has become significant health problem worldwide, especially in China, South Korea and Japan [1]. Based on data from the International Agency for Research on Cancer, approximately 950,000 new cases of gastric cancer were diagnosed in 2012, with 700,000 deaths globally [2]. At present, adequate surgical resection is the only curative remedy for gastric cancer [3]. The patients with advanced gastric cancer who have lost the opportunity of surgery can choose targeted therapies, such as trastuzumab, a monoclonal antibody against HER2 [4,5].
HER2, also known as ERBB2, is a member of the human epidermal growth factor receptor (EGFR) family. It is a transmembrane tyrosine kinase receptor that regulates cell proliferation, differentiation, and survival in a variety of tumors such as breast cancer, gastric cancer, colon cancer, bladder cancer, and biliary cancer [6,7]. Up to date, trastuzumab is the only target approved as the first-line treatment of HER2 positive metastatic gastric cancer [8]. However, the ubiquitous resistance of trastuzumab is a major clinical problem in the treatment of HER2-positive cancer, which limits the application of HER2 inhibitors to a large extent [9]. In view of this, there appears to be an urgent need to explore the downstream HER2 signaling pathway in gastric cancer.
Herein, we investigated the effects of HER2 on the migration and invasion of gastric cancer cells BGC823, MKN45, and SGC7901 and demonstrated that HER2 promoted the invasion and migration of gastric cancer cells by down-regulating the expression of E-cadherin and up-regulating the expression of Twist, whereas the expression of Fascin1 was not affected.
Materials and methods
Cell culture
Human gastric cancer cell lines, BGC823, SGC7901 and MKN45, were maintained in Dulbecco modified Eagle medium (DMEM; Gibco, CA, USA) supplemented with 10% fetal bovine serum (FBS) as described in [10] and were cultured at 37°C in an incubator filled with 5% CO2 atmosphere. When the cells were in the exponential phase of growth and reached 80% confluence, the routine medium would be replaced by serum-free DMEM without antibiotics for 24 h to transfect plasmid into the cells.
Plasmid transfection
The BGC823, MKN45 and SGC7901 cells were seeded on six-well cell culture plates (Costar, USA) at a density of 1×106 cells/well. When reaching 50-70% confluence, the BGC823 cells would be transfected with 10 μl (1 μg/10 μl) HER2-shRNA Plasmid and 10 μl (1 μg/10 μl) control-shRNA Plasmid (Santa Cruz, USA) respectively, as described in [10] while the MKN45 and SGC7901 cells would be transfected with 4 μg HER2 Plasmids (HER2CA with constitutively active mutation in kinase domain, wild type HER2 (HER2WT) and HER2KD with kinase death mutation; Addgene organization, USA) and PCDNA3 respectively, using the Lipofectamine 2000 transfection reagent.
RNA extraction and reverse transcription PCR
After transfection for 24 h, the total RNA of the cells was extracted by using the Trizol reagent (Invitrogen, USA) following the manufacturer’s protocols; specially, 1~5 mg of total RNA was subjected to reverse transcription using the first-strand cDNA synthesis kit (Fermentas, USA). The primer sequences were as follows: HER2: 5’AGGGAAACCTGGAACTCACC3’ (sense), 5’GCACAATCCGCAGCCTCT3’ (antisense); Fascin1: 5’GCGCATCACACTGAGGGCGT3’ (sense), 5’GTGACCTT GCGGCAGCCGAT3’ (antisense); and β-actin: 5’TGACGTGGACATCCGCAA-AG3’ (sense), 5’CTGGAAGGTGGACAGCGAGG3’ (antisense). The PCR conditions were as follows: denaturation at 94°C for 30 s; annealing at 58°C (HER2)/59°C (Fascin1)/57°C (β-actin) for 30 s and elongation at 72°C for 45 s. The PCR products of these genes were analyzed by electrophoresis in 1.5% agarose gel and visualized using UV fluorescence after staining with ethidium bromide. The ratio of the intensity of HER2 and Fascin1 genes to the intensity of β-actin was analyzed by Image Tool 3.0 (University of Texas Health Science Center, SanAntonio, USA).
Western blotting
After transfection for 48 h, as described in [12,13], the total proteins of cells were extracted by cell lysis buffer and quantified using the BCA protein assay Reagent kit (Merck, Germany) following the manufacturer’s protocols. Specifically, 20-30 μg total proteins were electrophoresed by (8% or 10%) SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, USA), which were then incubated in 5% non-fat skim milk buffer with primary antibodies [HER2 (1:400, Santa Cruz, USA); Twist (1:500, Protein Tech, USA); E-cadherin (1:500, Protein Tech, USA); Fascin1 (1:2000, Santa Cruz, USA); β-actin (1:1000, Santa Cruz, USA)] at 4°C overnight. After incubation with a second antibody (1:2000, Jia Mei China) for 2 h at room temperature, the proteins were visualized using an enhanced chemiluminescence (ECL, Bio-RAD, USA) following the manufacturer’s instructions, followed by quantification of bands.
Migration and invasion assay
The migration and invasion of GC cells were examined in 24-well transwell chambers (Millipore, USA) which contain upper and lower culture compartments separated by polycarbonate membranes with 8 mm sized pores. The chambers were additionally coated with 50 μl (120 μg/ml) Matrigel (Millipore, USA) to allow the invasion assay cultured overnight at 4°C. Then, the cells were resuspended and seeded in the upper chamber at a final concentration of 1.0×105/ml for invasion or 1.0×106/ml for migration in DMEM containing 1% FBS. 200 μl cell suspension was added to the upper chamber, and 900 μl DMEM (with 10% FBS) was added to the lower chamber. After 24 h incubation for invasion or 12 h for migration, the residual cells left over the upper surface of the filters were wiped off using cotton swabs. Then, the filters were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. The cells penetrating through the membrane were photographed and counted under a microscope at the magnification of ×200. Each group was tested in triplicate, and each experiment was repeated for three times.
Wound healing assay
After plasmid transfection for 24 h, the cells were resuspended and seeded into 6-well plates with 500 μl suspension at a final concentration of 8×105/ml. When reaching 100% confluence, the cells were scratched, and then incubated and photographed at 0 h, 24 h, 48 h, 72 h and 96 h respectively. The healing rate of the injured area was calculated as (area at 0 h - area at 96 h)/area at 0 h×100%.
Immunohistochemical staining
The human gastric cancer tissues were obtained from the Department of Pathology of Xiangya Hospital, Central South University. Formalin-fixed, paraffin-embedded tissue sections were used for IHC staining according to the method as described in some of the previous studies [11]. In brief, the sections were first incubated with the primary antibody of anti-HER2 (1:200 dilution; Santa Cruz,) or anti-Twist (1:400 dilution; Abcam), followed by a secondary antibody and diaminobenzidine (DAB, Vector Laboratories, Burlingame, CA) staining. The nuclei were counterstained with Meyer’s hematoxylin. Immunoglobulin G was used as a negative control. The intensity of Twist staining was scored as negative (0), weak (1+), intermediate (2+), and strong (3+), respectively. Two surgical pathologists (Z.L. and X.J.) evaluated the tumor sections independently.
Statistical analysis
The results were presented as mean ± SD for experiments with triplicate measurements. Multiple samples were analyzed by one-way analysis of variance (ANOVA), followed by pairwise comparisons using the L-D-S test. All differences with P-value <0.05 were considered statistically significant.
Results
Depletion of HER2 suppresses the migration and invasion abilities of GC
To select appropriate gastric cancer lines for up- or down-regulating the expression of HER2, the expression of HER2 protein in gastric cancer cell lines MKN45, BGC823 and SGC7901 was detected respectively. As shown in Figure 1A, the expression of HER2 was the highest in BGC823 cells and was the lowest in MKN45 cells. Therefore, the HER2-shRNA plasmids were transfected into the gastric cancer cell line BGC823 to deplete the expression of HER2. The invasive potential of BGC823 was examined using transwell chamber assays, while the control-shRNA plasmids were transfected as a vector control. Compared with the parental and control cells, the migration (Figure 1B) and invasion (Figure 1C) of HER2-shRNA-BGC823 cells were attenuated obviously (P<0.01), and in the wound healing assay, the migratory ability of HER2-shRNA-BGC823 cells were also weakened as expected (P<0.01) (Figure 1D).
Figure 1.
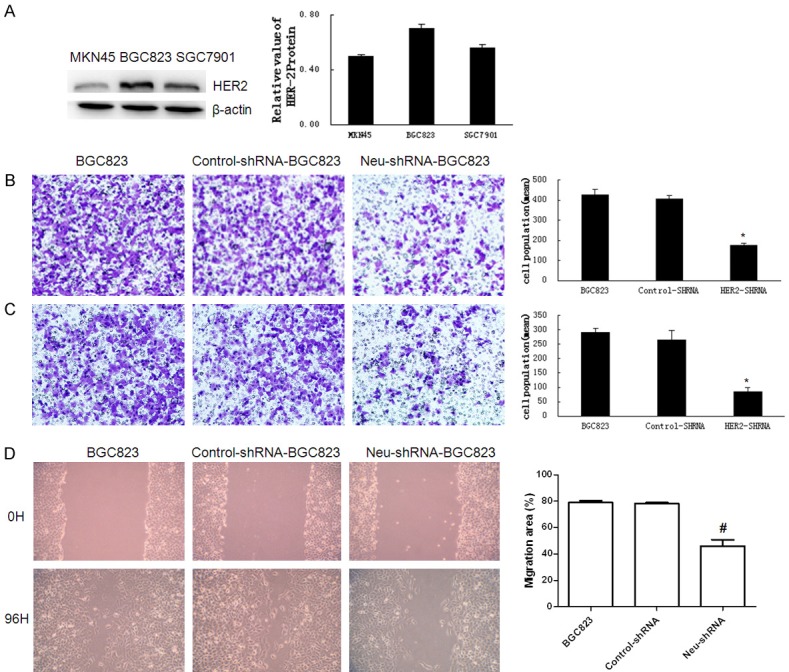
Effect of Neu-shRNA on invasive and migratory ability of BGC823 cells. A. The expression of HER2 in gastric cancer cells BGC823, MKN45, and SGC7901. The expression of HER2 is higher in BCG823 cells compared with the other two cell lines. We chose BCG823 cells for HER2 knock-down test. B, C. Cells migration and invasion ability were evaluated by trans-well chambers. The cells were counted in 5 random fields under a light microscope (magnification, 200). Compared with BGC823 and shRNA-BGC823 cells, the migration and invasion ability were decreased in HER2 knocked-down Neu-shRNA-BGC823 cells. D. Cells migration were evaluated by wound healing (magnification, 200). P<0.01. Cell migration ability was decreased in Neu-shRNA-BGC823 cells compared with control groups.
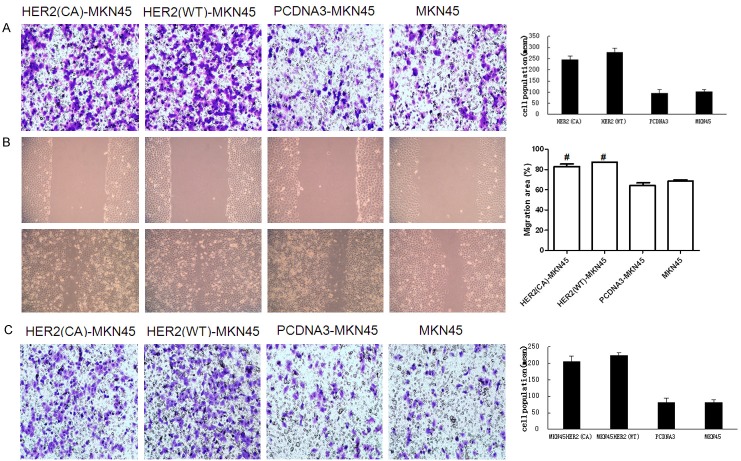
Over-expression of HER2 increases the migratory and invasive abilities of GC
To further confirm the effects of HER2 on the migration and invasion of GC, wild type HER2 plasmids were transfected into the gastric cancer cell line MKN45 which shows the lowest expression of HER2. In transwell chamber assays, the migratory (Figure 2A) ability of HER2WT-MKN45 cells was increased significantly (P<0.01) compared with MKN45 and PCDNA3-MKN45 cells. The wound healing assay showed similar results in MKN45 cells (P<0.01) (Figure 2B). As illustrated in Figure 2C, the invasive potential of HER2WT-MKN45 cells was enhanced significantly (P<0.01). HER2CA, HER2 with constitutively active V659E mutation, was also transfected into the MKN45 cells, and similar results were obtained (Figure 2A-C).
Figure 2.
Effect of HER-2 on invasive and migratory abilities of MKN45 cells. A. Cells migration were evaluated by trans-well chambers. Compared with untreated MKN45 cells and PCDNA3-MKN45 cells, migration and invasion abilities were dramatically increased in HER2 up-regulated MKN45 cells. B. Cells migration were evaluated by scratch assay (magnification, 200). P<0.01. The results were consistent with trans-well chambers. C. Cells invasion were evaluated by Matrigel-coated trans-well chambers. The cells were counted in 5 random fields under a light microscope (magnification, 200). Compared with control groups, cell invasion was increased in HER2(CA)-MKN45 and HER2(WT)-MKN45 cells.
HER2 up-regulates Twist and down-regulates E-cadherin in the protein level
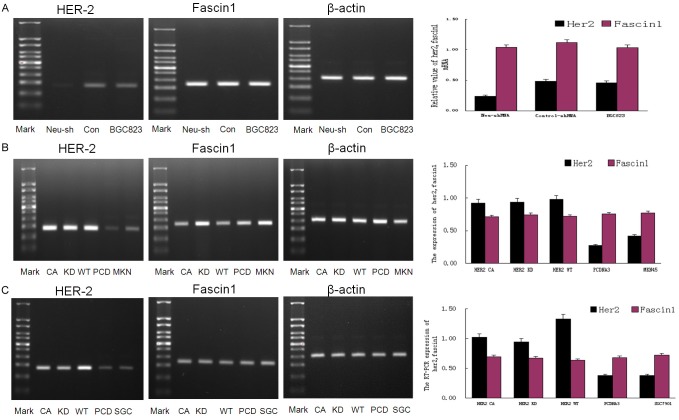
Epithelial-mesenchymal transition (EMT) was thought to be involved in the migratory and infiltrating potential of cancer cells [12], while Twist and E-cadherin play important roles in EMT [12]. On the other hand, Fascin is another important protein that promotes the infiltration of cancer cells [13]. To further elaborate the associations of Twist, E-cadherin and Fascin with HER2-induced EMT, the expressions of Twist, E-cadherin and Fascin1 were detected by western blotting with overexpression or depletion of HER2 in gastric cancer cells. Compared with BGC823 and control-shRNA-BGC823 cells, the expression of HER2 and Twist were decreased (P<0.05), the expression of E-cadherin was increased (P<0.05), while the expression of Fascin1 remained unchanged (P>0.05) in the HER2-shRNA-BGC823 transfected cells (Figure 3A). On the contrary, compared with parental and vector control cells, the expression of HER2 and Twist were increased (P<0.05), the expression of E-cadherin was decreased (P<0.05), while the expression of Fascin1 remained unchanged (P>0.05) in MKN45 cells transfected with wild-type HER2 plasmids (Figure 3B); and similar results were obtained in the gastric cancer cell line SGC7901 (Figure 3C). To further investigate whether the HER2 kinase domain is involved in the regulation of Twist and E-cadherin, HER2CA with constitutively active mutation or HER2 KD with kinase death mutation was transfected into the gastric cancer cell line MKN45 and SGC7901 cells. As shown in Figure 3B and 3C, no significant changes were observed among HER2WT, HER2CA and HER2KD transfected cells in both MKN45 and SGC7901 cells, indicating that the HER2 kinase domain is not involved in the regulation of Twist or E-cadherin. To elucidate whether HER2 regulates the expression of Fascin in the mRNA level, Fascin mRNA was detected with RT-PCR after the transfection of HER2-shRNA or HER2 plasmids. As shown in Figure 4, despite of the fluctuations in the expression of HER2, the expression of Fascin showed no distinct changes in BGC823 (Figure 4A), MKN45 (Figure 4B), and SGC7901 cells (Figure 4C). Therefore, HER2 is able to regulate EMT-associated proteins but not Fascin1 in GC cells.
Figure 3.
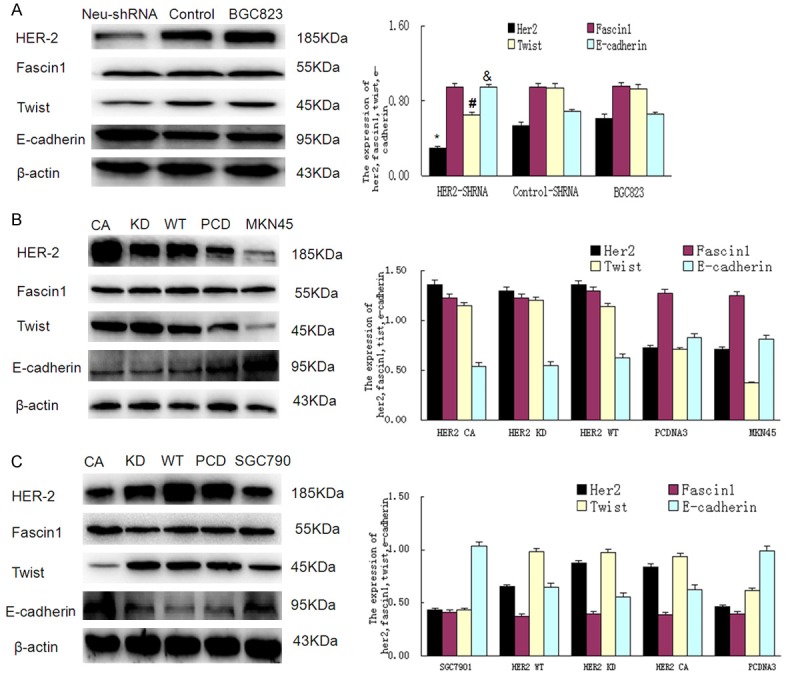
Western blotting analysis of HER-2, Twist, E-cadherin and Fascin1 protein expression in BGC823, MKN45 and SGC7901 cells. A. Compared with control-shRNA-BGC823 and BGC823 cells, the expression of HER2 was decreased in neu-shRNA-BGC823 cells, accompanied with decreased expression of Twist and increased expression of E-cadherin. B. Compared with PCDNA3-MKN45 and MKN45 cells, the expression of HER2 was increased in HER2(CA)-MKN45, HER2(KD)-MKN45 and HER2(WT)-MKN45 cells, accompanied with increased expression of Twist and decreased expression of E-cadherin. C. The western blotting results of SGC7901 reached the same conclusion with MKN45.
Figure 4.
RT-PCR analysis of HER-2 and Fascin1 expression in BGC823 (A), MKN45(B) and SGC7901 cells (C). (A) Compared with control-shRNA-BGC823 and BGC823 cells, the RNA expression of HER2 was decreased in neu-shRNA-BGC823 cells. There was no significant difference considering the expression of Fascin 1 among groups. (B) Compared with PCDNA3-MKN45 and MKN45 cells, the expression of HER2 was increased in HER2(CA)-MKN45, HER2(KD)-MKN45 and HER2(WT)-MKN45 cells. There was no significant difference considering the expression of Fascin 1 among groups. (C) The RT-PCR results of SGC7901 reached the same conclusion with MKN45.
Twist correlates to the expression of HER2 in clinical gastric cancer specimens
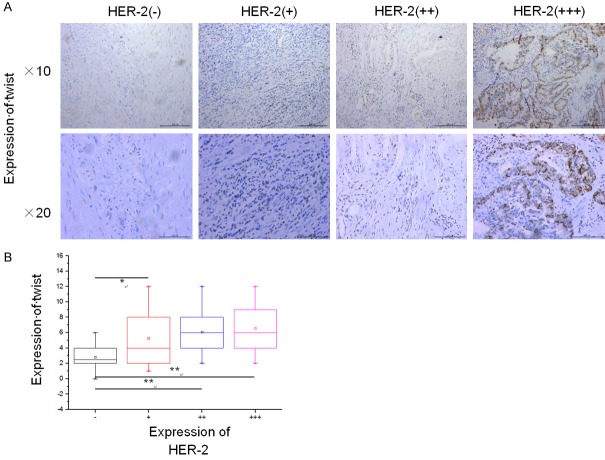
It was reported that HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells [14], while twist was believed to be a key regulator of EMT in carcinoma cells [12]. However, no study has been conducted yet with a particular focus on the correlation between HER2 and Twist. Our data showed that the expression of Twist was higher in GC cells accompanied by a higher intensity of HER2 expression (Figure 5A, 5B and Table 1), and HER2 was positively correlated with Twist in 118 clinical gastric cancer subjects (P<0.05).
Figure 5.
Immunohistochemical staining results and analysis. A. ICH staining of Twist in gastric cancer tissues with different HER2 expression levels. B. Box diagram of Twist expression level in gastric cancer tissues with different HER2 expression levels. Pairwise comparison of twist expression levels in gastric cancer tissues of the four groups. (*P<0.05; **P<0.01).
Table 1.
Relationship between HER2 and Twist expression in clinical gastric cancer tissues
| Twist staining | HER2 level | Total | |||
|---|---|---|---|---|---|
|
| |||||
| - | + | ++ | +++ | ||
| - | 2 | 16 | 10 | 0 | 28 |
| + | 0 | 10 | 10 | 10 | 30 |
| ++ | 0 | 8 | 17 | 5 | 30 |
| +++ | 0 | 3 | 10 | 17 | 30 |
| Total | 2 | 37 | 47 | 32 | 118 |
Discussion
Around 20% of advanced gastric or gastro-esophageal junction cancer patients are HER2-positive. HER2 targeting has been demonstrated to improve the overall survival and progression free survival in HER2 overexpressed cancers [15]. In the clinical practice of gastric cancer, trastuzumab administration is now a standard first-line treatment [16]. Nevertheless, despite the success in some therapeutic cases, the ubiquitous resistance has largely decreased its therapeutic benefit.
The development of cancer metastasis is proven to be related to EMT which can be mediated by the overexpression of HER2 [17,18]. Our data confirmed that the overexpression of HER2 enhanced the migration and invasion of gastric cancer cells, while the depletion of HER2 inhibited the invasion and migration of GC. Therefore, HER2 plays an important role in the migration and invasion mechanism of gastric cancer cells.
Twist, which was discovered in 1995, is a transcription factor with a helical-loop-helical structure that participates in the regulation of dermal development, bone development, tumor formation, invasion and metastasis through multiple signaling pathways [19]. Recent studies suggest that twist can promote EMT to strengthen the migration ability of cells [14], while EMT is an important mechanism of the invasion and migration of gastric cancer. However, whether HER2 mediates Twist remains unclear. Our data demonstrated that Twist could be regulated by HER2, at least in part, and this positive correlation was reassured by the expression level of HER2 and the staining intensity of Twist in clinical gastric cancer tissues. In addition, HER2 also shown to mediate the expression of E-cadherin, which might be explained by the fact that Twist could inhibit the expression of E-cadherin [20,21]. Moreover, our study also suggested that the HER2 kinase domain was not involved in the regulation of Twist or E-cadherin.
The Fascin1 gene encodes a 55 KD cytoskeletal protein which is able to bind to F-actin proteins, that are localized in the core actin bundles of microspikes, filopodia and actin-based protrusions underneath the plasma membrane and that have been implicated in cell motility, cancer cell invasion and metastasis [13,22-24]. Our previous study, demonstrated that fascin expression induced by TGF-β depended on smad3, at least in part, and on smad3 linker phosphorylation [25]. Some earlier studies have revealed that Twist and Fascin1 were over-expressed in gastric cancer and associated significantly with lymph-node and distant metastases, and poor prognosis [26]. Elevated levels of fascin have been detected in many types of metastatic tumors and have been correlated with clinically aggressive phenotypes, poor prognosis and shorter survival [27]. However, despite of the HER2 knockdown or overexpression, the protein and mRNA levels of Fascin1 remained unchanged in BGC823, MKN45 and SGC7901 cells, indicating that Fascin1 is not mediated by the HER2 regulation pathway.
In conclusion, our results demonstrated that HER2 could down-regulate the expression of E-cadherin and up-regulate the expression of Twist to promote the invasion and migration of gastric cancer. Novel therapies should be targeted at the HER2 downstream signaling pathway molecules for GC patients with trastuzumab resistance.
Acknowledgements
This work was supported by the College Students’ Innovative Training Plan Program (201610533257). We would like to thank all laboratory members for their critical work of this manuscript, and apologize to those not mentioned due to space limitations. This work was supported by the College Students’ Innovative Training Plan Program (201610533257).
Disclosure of conflict of interest
None.
References
- 1.Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet (London, England) 2016;388:2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Freddie B, Siegel RL, Jacques F, Joannie LT, Ahmedin J. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Van CE, Dicato M, Geva R, Arber N, Bang Y, Benson A, Cervantes A, Diaz-Rubio E, Ducreux M, Glynne-Jones R. The diagnosis and management of gastric cancer: expert discussion and recommendations from the 12th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2010. Ann Oncol. 2011;22(Suppl 5):v1–9. doi: 10.1093/annonc/mdr284. [DOI] [PubMed] [Google Scholar]
- 4.Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, Tang L, Xin Y, Jin J, Zhang YJ, Yuan XL, Liu TS, Li GX, Wu Q, Xu HM, Ji JF, Li YF, Wang X, Yu S, Liu H, Guan WL, Xu RH. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019;39:10. doi: 10.1186/s40880-019-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12:676–682. doi: 10.1038/nrclinonc.2015.132. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J. Clin. Oncol. 2018;36:2532–2537. doi: 10.1200/JCO.2018.77.9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meric-Bernstam F, Johnson AM, Dumbrava EEI, Raghav K, Balaji K, Bhatt M, Murthy RK, Rodon J, Piha-Paul SA. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25:2033–2041. doi: 10.1158/1078-0432.CCR-18-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Li Y, Bhattacharya A, Zhang Y. A recombinant human protein targeting HER2 overcomes drug resistance in HER2-positive breast cancer. Sci Transl Med. 2019;11:eaav1620. doi: 10.1126/scitranslmed.aav1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Z, Müller S, Qian G, Xu J, Kim S, Chen Z, Jiang N, Wang D, Zhang H, Saba NF, Shin DM, Chen ZG. Human papillomavirus 16 oncoprotein regulates the translocation of β-catenin via the activation of epidermal growth factor receptor. Cancer. 2015;121:214–25. doi: 10.1002/cncr.29039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou H, Duan Y, Wei D, Zhang Y, Dai J, Li J, Li X, Zhou J, Liu Z, Jin Z, Zhang Z, Yu Y, Hu Z. Molecular features of pleomorphic xanthoastrocytoma. Hum Pathol. 2019;86:38–48. doi: 10.1016/j.humpath.2018.08.038. [DOI] [PubMed] [Google Scholar]
- 12.Ribatti D. Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res. 2017;353:1–5. doi: 10.1016/j.yexcr.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Kureishy N, Sapountzi V, Prag S, Anilkumar N, Adams JC. Fascins, and their roles in cell structure and function. Bioessays. 2002;24:350–361. doi: 10.1002/bies.10070. [DOI] [PubMed] [Google Scholar]
- 14.Singh S, Chakrabarti R. Consequences of EMT-driven changes in the immune microenvironment of breast cancer and therapeutic response of cancer cells. J Clin Med. 2019;8 doi: 10.3390/jcm8050642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shitara K, Iwata H, Takahashi S, Tamura K, Park H, Modi S, Tsurutani J, Kadowaki S, Yamaguchi K, Iwasa S, Saito K, Fujisaki Y, Sugihara M, Shahidi J, Doi T. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive gastric cancer: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20:827–836. doi: 10.1016/S1470-2045(19)30088-9. [DOI] [PubMed] [Google Scholar]
- 16.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 17.Voutsadakis IA. HER2 in stemness and epithelial-mesenchymal plasticity of breast cancer. Clin Transl Oncol. 2019;21:539–555. doi: 10.1007/s12094-018-1961-x. [DOI] [PubMed] [Google Scholar]
- 18.Ingthorsson S, Andersen K, Hilmarsdottir B, Maelandsmo GM, Magnusson MK, Gudjonsson T. HER2 induced EMT and tumorigenicity in breast epithelial progenitor cells is inhibited by coexpression of EGFR. Oncogene. 2016;35:4244–55. doi: 10.1038/onc.2015.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Cserjesi P, Olson EN. Dermo-1: a novel twist-related bHLH protein expressed in the developing dermis. Dev Biol. 1995;172:280–92. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- 20.Feng MY, Wang K, Song HT, Yu HW, Qin Y, Shi QT, Geng JS. Metastasis-induction and apoptosis-protection by TWIST in gastric cancer cells. Clin Exp Metastasis. 2009;26:1013–23. doi: 10.1007/s10585-009-9291-6. [DOI] [PubMed] [Google Scholar]
- 21.Jung HY, Yang J. Unraveling the TWIST between EMT and cancer stemness. Cell Stem Cell. 2015;16:1–2. doi: 10.1016/j.stem.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Fu H, Hu Z, Wen J, Wang K, Liu Y. TGF-beta promotes invasion and metastasis of gastric cancer cells by increasing fascin1 expression via ERK and JNK signal pathways. Acta Biochim Biophys Sin (Shanghai) 2009;41:648–56. doi: 10.1093/abbs/gmp053. [DOI] [PubMed] [Google Scholar]
- 23.Fu H, Wen JF, Hu ZL, Luo GQ, Ren HZ. Knockdown of fascin1 expression suppresses the proliferation and metastasis of gastric cancer cells. Pathology. 2009;41:655–60. doi: 10.3109/00313020903273100. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Gu Y, Lu W, Liu X, Fu H. Fascin1 promotes gastric cancer progression by facilitatingcell migrationand epithelial-mesenchymal transition. Pathol Res Pract. 2018;214:1362–1369. doi: 10.1016/j.prp.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Cao F, Liu B, Luo X, Ma X, Hu Z. TGF-β induces fascin expression in gastric cancer via phosphorylation of smad3 linker area. Am J Cancer Res. 2015;5:1890–6. [PMC free article] [PubMed] [Google Scholar]
- 26.Luo GQ, Li JH, Wen JF, Zhou YH, Hu YB, Zhou JH. Effect and mechanism of the twist gene on invasion and metastasis of gastric carcinoma cells. World J Gastroenterol. 2008;14:2487–93. doi: 10.3748/wjg.14.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang FK, Han S, Xing B, Huang J, Liu B, Bordeleau F, Reinhart-King CA, Zhang JJ, Huang XY. Targeted inhibition of fascin function blocks tumour invasion and metastatic colonization. Nat Commun. 2015;6:7465. doi: 10.1038/ncomms8465. [DOI] [PubMed] [Google Scholar]