Abstract
Neuroinflammation plays a key role in the progression and pathogenesis of postoperative cognitive dysfunction, but it does not always occur in the local response to primary injury. In this study, we revealed that probiotics alleviate cognitive dysfunction associated with neuroinflammation in cardiac surgery. Rats were administered a probiotic or placebo once a day by gavage for 2 weeks until the day of surgery. Cardiac surgery was induced by ischemia/reperfusion of the left coronary artery. Key factors, such as the gut microbiome, the gut barrier and the blood-brain barrier (BBB), were systematically investigated to determine whether changes in the gut microbiome lead to neuroinflammation. We used 16S rDNA sequencing to confirm that cardiac surgery induced intestinal flora dysbiosis by altering the number of organisms rather than the structure in the cecum microbiome, which occurs at the same time as damage to the gut barrier. Cardiac surgery also increased BBB permeability, suggesting that disruption of the microbiome may increase the likelihood of neuroinflammation. Probiotics-induced alterations in the intestinal flora significantly reduced the level of inflammatory cytokines (IL-6 and IL-1β). Importantly, we found that the administration of probiotics significantly improved spatial memory impairment in rats after cardiac surgery, as measured by the Morris water maze. Overall, dysbiosis of the gut flora may aggravate cognitive impairment associated with neuroinflammation after cardiac surgery, and probiotics may attenuate this effect.
Keywords: Probiotics, cardiac surgery, postoperative complications, neurocognitive disorders, microbiome
Introduction
Cognitive dysfunction is a common central nervous system complication after anesthesia and surgery. Postoperative cognitive dysfunction (POCD) is characterized by dementia-like symptoms, such as memory impairment, attention loss, inability to plan, and difficulty in switching between tasks [1,2]. Previous studies have reported an incidence of cognitive dysfunction of 14-50% three months after cardiac surgery and 5-20% after non-cardiac surgery [3-5]. Many factors, such as brain hypoperfusion, abnormal hormone secretion and immune inflammatory response, are currently believed to be involved in the mechanism of POCD [6-8]. However, as far as we know, none of the mechanisms discussed above have been used to prevent or treat POCD in cardiac surgery patients; therefore, the pathogenesis of POCD after cardiac surgery remains to be further studied.
The gut is called the body’s “second brain” because it has many of the same neural functions as the brain that can affect mood and mental health [9,10]. New evidence suggests that intestinal flora disorders are linked to brain immune dysfunction, which is associated with processes of mental health damage [11]. Sampson and his colleagues proposed a new mechanism, in which intestinal flora imbalance is also an important factor in the progression of neuroinflammation [12]. However, whether intestinal flora are involved in postoperative cognitive dysfunction has not been reported. Therefore, we hypothesize that dysbiosis of gut flora may aggravate cognitive impairment associated with neuroinflammation after cardiac surgery and that probiotics may attenuate this effect.
Materials and methods
Animals and experimental groups
This study was carried out in accordance with NIH Guidelines for the Care and Use of Animals and approved by the Animal Care and Use Committees of Harbin Medical University. All efforts were made to minimize animal discomfort and number of animals used. Ten-week-old SD rats weighing 280-300 g were purchased from the Second Affiliated Hospital of Harbin Medical University (Harbin, China). Animals were housed in a temperature-controlled room and received a standard diet and drinking water.
The experiment was divided into two parts. In part 1, to verify that changes in the intestinal flora are involved in the neuroinflammatory response after cardiac surgery, we divided the animals into two groups: control (Con) and cardiac surgery (Cardiac). In part 2, to determine whether probiotics-induced alterations in the intestinal flora improve cognitive functions after cardiac surgery, the animals were divided into four groups: control (Con), cardiac surgery (Cardiac), cardiac surgery + probiotics (CSP), and control + probiotics (CPO).
Induction of cardiac surgery
The rats were intraperitoneally (IP) administered 3% pentobarbital sodium (0.2 ml/100 g) to induce anesthesia, intubated and then mechanically ventilated using an animal respirator. Tail artery catheterization was performed to dynamically monitor blood pressure, and a left thoracotomy was then performed to expose the heart. A 5-0 surgical silk suture was used to ligate the left anterior descending (LAD) coronary artery at 3 mm from its origin for 30 min. A successful occlusion was deemed to have been formed when the color of the left ventricle became pale and ST segment changes were observed on an ECG monitor. After hemodynamics was observed to be maintained at a steady level, the ligature was removed and the thorax was closed. Buprenorphine (0.03 mg/kg bw, IP) was administered to all experimental rats after closing the thorax to manage postoperative pain.
Intestinal histopathologic analysis
The rats were sacrificed on postoperative days 0, 7, 14, and the ileum was dissected 5 cm above the cecum. After cleaning with normal saline, the dissected tissues were fixed in 4% formalin for 48 h and then used to generate paraffin sections. Sections were later obtained and stained using hematoxylin and eosin (H&E). Intestinal morphologic alterations were classified using Chiu pathology scores [13].
Measurement of intestinal flora
To determine the gut microbial community, the flora of the intestinal tract was assessed using 16S rDNA sequencing on postoperative day 7 [14]. Briefly, fresh feces (1 g) were collected from each rat and stored in sterile tubes at -80°C in an anaerobic environment. DNA samples were isolated and extracted using a QIAamp® DNA Kit. Following sample quality inspection, the V3/4 region of the 16S rDNA was amplified using the 341F (CCTACGGGNGGCWGCAG) and 785R (GACTACHVGGGTATCTAATCC) primers to construct a 16S rDNA library. An Illumina® Miseq Sequenator was used to conduct high-throughput sequencing (HTS). Raw data were then analyzed to determine the diversity and abundance of the microbial population in the samples.
Evaluation of blood-brain barrier (BBB) permeability
To evaluate changes in BBB permeability on postoperative day 7, we developed an approach based on the sequential injection of small-size (Sodium-fluorescein, NaF) and large-size (Rhodamine, RHO70) fluorescent dyes followed by a quantitative assessment of the degree of intravascular and extravasated dyes in brain sections containing the cortex or the hippocampus [15]. The tracers were successively injected into the right femoral vein and circulated for 1 h (NaF) or 20 min (RHO70), and the rats were decapitated to extract the prefrontal cortex and hippocampal tissues, which were quickly dissected, weighed and stored for further use. Following fixation and dehydration, the tissues were embedded in O.C.T. compound medium (Sakura Finetek®, USA) and then quickly frozen at -196°C (liquid nitrogen). The tissues were sliced into 8 µm-thick sections, and confocal imaging was directly performed.
Western blot analysis
The expression levels of the biomarkers (GFAP and Iba-1, respectively) of astrocytes and microglia, matrix metalloproteinase-9 (MMP-9) and cytokines (IL-6 and IL-1β) in both the hippocampus and the prefrontal cortex were determined by Western blotting on postoperative day 7. Samples were homogenized in lysis buffer and then incubated at 0°C for 30 min. After centrifugation, proteins were obtained from the supernatant (16,000 g at 4°C for 20 min). The extracted proteins were separated by electrophoresis at 80 V and then transferred to PVDF membranes with transfer buffer. Then, in 5% skim milk diluted with 1× TBST (120 min at 20°C), the membranes were blocked before probing with the appropriate primary antibodies (goat polyclonal anti-Iba-1 antibody, diluted 1:2000; mouse monoclonal anti-IL 6 antibodies, diluted 1:3000; rabbit polyclonal anti-IL 1β antibodies, diluted 1:5000; rabbit polyclonal anti-GFAP antibodies, diluted 1:10000, all from Abcam®, USA; mouse monoclonal anti-β actin antibodies, diluted 1:2000, ZSGB-BIO®, China; rabbit polyclonal anti-MMP9 antibodies, diluted 1:1000, Affinity Biosciences®, USA) for 12 h at 4°C. Then the membranes were incubated with the appropriate HRP IgG secondary antibodies (peroxidase-conjugated rabbit anti-goat IgG (H + L) diluted 1:2000, ZSGB-BIO®, China; HRP goat anti-mouse IgG (H + L) and HRP goat anti-rabbit IgG (H + L) diluted 1:5000, ABclonal®, USA). The proteins were quantified using ImageJ® densitometric software and detected using a chemiluminescent kit on a Thermo Fisher® FluorChem Imaging System.
Immunofluorescence
The tissues were embedded in O.C.T. complex medium (Sakura Finetek®, USA) after fixation and dehydration, and then frozen rapidly at -196°C (liquid nitrogen). The tissues were sliced into 8 µm-thick sections and stored at -80°C for further use. After heating to room temperature (RT) for 10 min, the sections were refixed in acetone (precooled to 4°C) for 10 min, rinsed in PBS 3 times (5 min each time), and then permeabilized in 0.25% Triton-X100. After the sections were washed with PBS 3 times (5 min each), they were blocked in 5% serum containing 1% glycine and 0.01% Tween 20 for 30 min to retrieve the antigen and then incubated with appropriate primary antibodies (rabbit polyclonal anti-GFAP antibodies, diluted 1:1000; mouse monoclonal anti-IL 6 antibodies, diluted 1:500; goat polyclonal anti-Iba-1 antibody, diluted 1:500; rabbit polyclonal anti-IL 1β antibodies, diluted 1:500, all Abcam®, USA) for 12 h at 4°C. After washing, the sections were incubated with the appropriate conjugated secondary IgG antibodies (donkey anti-goat IgG (H + L) Alexa Flour® 594, Abcam, USA; Cy3 goat anti-rabbit IgG (H + L), diluted 1:200; FITC goat anti-rabbit IgG (H + L), diluted 1:100; Cy3 goat anti-mouse IgG (H + L), diluted 1:200; Abclonal®, USA) in the dark at 37°C for 60 min. The tissues were incubated with DAPI for 3 min and then rinsed in the dark for 3×5 min in PBS. Immunostaining was observed under a fluorescence microscope (LeicaTM DM4000 B, Leica, Germany).
Probiotic administration
A commercial probiotic formulation (Golden bifid, Shuangqi pharmaceutical Co., China) composed of no less than 0.5×107 CFU Bifidobacterium longum, 0.5×106 Lactobacillus bulgaricus and 0.5×106 Streptococcus thermophiles was orally administered to the animals to investigate the therapeutic potential of probiotics. Probiotics (2.0 g/kg) were dissolved in 2 ml (approximately 1% of body weight) of drinking water and administered once a day by gavage for 2 weeks until the day of surgery. The Con groups received only the vehicle.
Open field test
The open field was a square arena, 100×100 cm (length × width) with a 50-cm-high wall. A 60×60 cm square area was defined as the central area. Anxiety-like behavior, such as moving outside of the central area or defecation, and locomotor activity of the rats were measured for 5 min. The arena was cleaned by ethanol after use. All records were acquired by a tracking system (SuperMaze®, China).
Morris water maze
The difference in cognitive function among groups was detected by the Morris water maze (MWM) on postoperative day 21. The spatial acquisition procedure lasted for 5 days. The rats were released at 4 different but fixed points (the W, S, NW, or SE directions of the maze) each day to find the escape platform. If the rats failed to find the platform in 90 s, it was guided to the platform and allowed to remain on the platform for 15 s. The escape platform was removed on day 6 to measure the spatial memory of the rats. The rats were released at the SW direction and allowed to search in the maze for 60 s. All the records were acquired by a tracking system (SuperMaze®, China).
Statistical analysis
Statistical calculations were performed using SPSS Statistics 18.0 (IBM Corp., Armonk, NY). The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous variables. Normally distributed variables are expressed as the means (SD). Nonparametric variables are expressed as medians (interquartile range). Normally-distributed variables were analyzed using an independent Student’s t-test or one-way analysis of variance (ANOVA). Nonparametric data were compared using the Mann-Whitney rank sum test. The results were considered statistically significant when P<0.05.
Results
Intestinal flora dysbiosis after cardiac surgery in rats
The indices for alpha diversity in the fecal microbiota were similar between the Cardiac and Con groups (observed species: P = 0.808; Chao: P = 0.661, Figure 1A and 1B), indicating that the genus level in the intestinal flora was similar between the Cardiac rats and the Con rats. However, the indices for alpha diversity were significantly different in the cardiac surgery group compared with the control group (Shannon: P = 0033; Simpson: P = 0.042, Figure 1C and 1D), and these results indicated that the quantity of microbiota in the gut significantly differed between the Cardiac rats and the Con rats. In addition, the abundance ratio of genus was calculated according to the genus layers in the species classification unit (Figure 1E).
Figure 1.
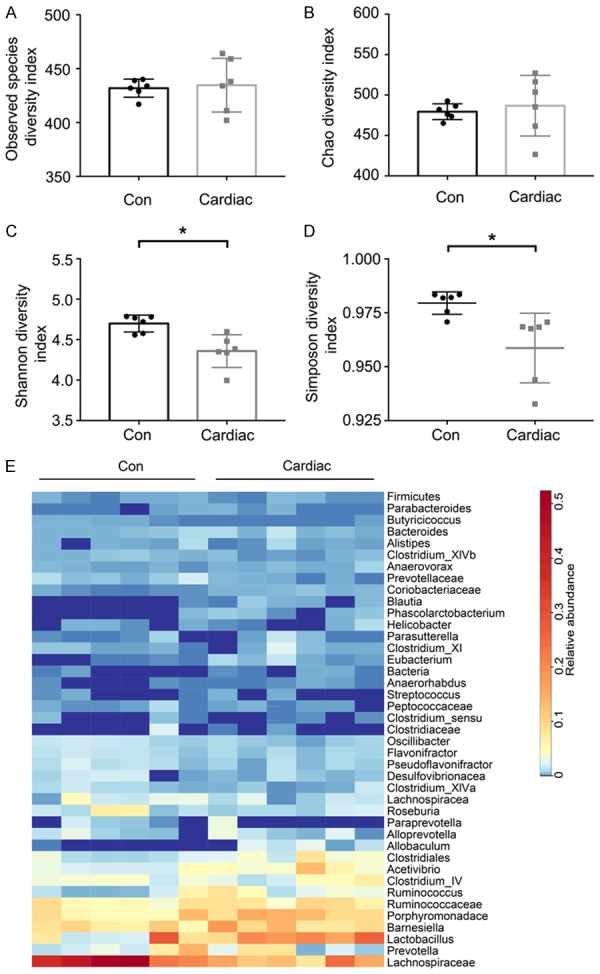
The quantity of microbiome, but not richness, was altered after cardiac surgery in the rats. A and B. Observed species and the Chao index were similar between the Con and Cardiac groups. These results indicated that microbial richness did not differ in the microbiome after cardiac surgery. C and D. The Shannon and Simpson indexes significantly differed between the two groups, indicating that the quantity of microbiome may be altered after cardiac surgery. E. Heat map comparison between the Con group and Cardiac group at the genus level (n = 6). Data for Observed species, the Chao index and the Shannon index were examined using independent Student’s t-tests. The non-normal variables (Kolmogorov-Smirnov test, P<0.05) of Simpson significance were tested by the Mann-Whitney rank sum test. Con = control. *P<0.05.
Taxonomic differences between the Cardiac and Con groups were determined using a logarithmic linear discriminant analysis (LDA) score cutoff value (2.0), and the results suggested that the fecal flora were significantly different between the Cardiac and Con groups. Here, we considered the specific differences observed in taxa at the genus level (Figure 2A). The quantities of Saccharibacteria, Eubacteriaceae, Enterobacteriales, Escherichia/Shigella, Micrococcaceae, etc. were higher in the Cardiac rats than in the Con rats, whereas the quantities of Lachnospiraceae, Paraprevotella and Oscillibacter, etc. were higher in the Con group than in the Cardiac group (Figure 2B).
Figure 2.
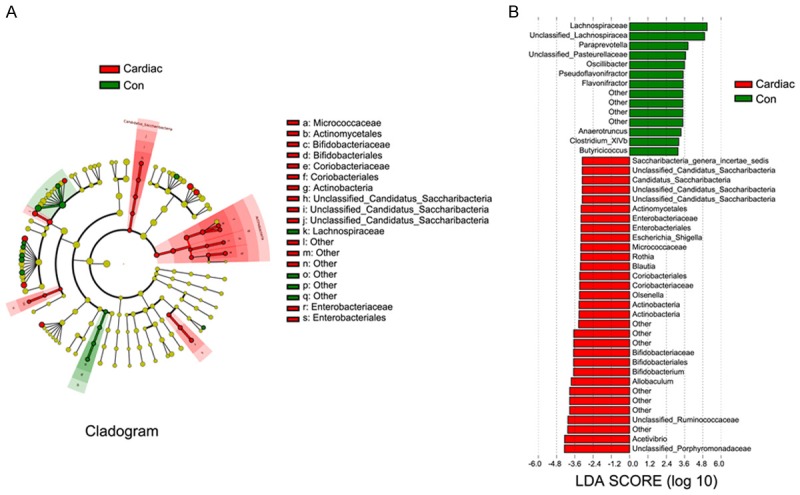
Cardiac surgery induced intestinal flora dysbiosis. A. Phylogenetic distribution of fecal microbiota differences between the Con and Cardiac groups. Red colour is represented with significant differences of taxonomic representation in the Cardiac groups, while green color represents significant differences in taxonomic representation in the Con groups. The fecal microbiota shared between the Con and Cardiac groups is marked by yellow color. B. The taxonomy of the intestinal flora was analyzed by linear discriminant analysis (LDA) with effect size measurements (LEfSe). The length of bars in the chart represents the significance of differences in fecal microbiota between the two groups. For LDA scores >2, P<0.05 (n = 6). Compositions of different species of fecal microbiota associated with cardiac surgery and control rats assessed by LEfSe analysis. Con = control.
Alterations in the gut barrier and the BBB after cardiac surgery
Histological measurements of ileal morphology revealed that cardiac surgery significantly affected the intestinal ultrastructure on day 7 (Figure 3A). Compared with the Con group, in the Cardiac group, the epithelial cells of the intestinal villi were partially shed, and microvilli were irregularly arranged, dull, and rough. Additionally, Chiu pathological scores were significantly higher in the Cardiac group than in the Con group on day 7 (P = 0.003, Figure 3B).
Figure 3.

Gut-brain barrier function was damaged after cardiac surgery. A. The intestinal mucosa was obviously damaged after cardiac surgery on postoperative day 7 as measured by H&E staining, indicating that intestinal flora dysbiosis may be most obvious on the seventh day after cardiac surgery. B. Chiu pathology scores revealed obviously higher scores in the Cardiac group on 7 day (n = 8). C. Rhodamine (red) and NaF (green) staining in the hippocampus and the prefrontal cortex. Scale bar = 25 μm. D. The intensity of NaF fluorescence was evidently higher in the Cardiac group than in the Con group, indicating that BBB permeability was significantly higher after cardiac surgery (n = 6). E and F. MMP-9 expression associated with BBB damage was increased after cardiac surgery (n = 6). Significance was tested by independent Student’s t-tests. Con = control, BBB = blood-brain barrier. **P<0.01, ***P<0.001.
In the Con group, both dyes were contained and colocalized within the brain vasculature, and minimal staining was observed extravascularly. Conversely, in the Cardiac group, the large RHO70 dye was contained intravascularly, whereas the smaller NaF dye had partially leaked into the cortex and the hippocampus (Figure 3C). The amount of leaked NaF was higher in the Cardiac group than in the Con group in both the cortex and the hippocampus (cortex: P<0.001; hippocampus: P<0.001, Figure 3D). These results indicated that BBB permeability was significantly higher in the Cardiac group than in the Con group. In addition, MMP-9 synthesis was also significantly higher in the cortex and hippocampus of the Cardiac group than the Con group (P<0.001, Figure 3E and 3F).
Probiotics-induced alterations in the intestinal flora reduced neuroinflammation
The mean optical density of IL-6 (CA1: F (3, 20) = 44.12, P<0.001; DG: F (3, 20) = 44.35, P<0.001) and IL-1β (CA1: F (3, 20) = 28.65, P<0.001; DG: F (3, 20) = 37.39, P<0.001) staining was significantly higher in regions of the hippocampus in rats in the Cardiac group than in rats in the Con group (Figure 4). A significant decrease in the mean optical density of IL-6 and IL-1β staining in regions of the hippocampus and cerebral cortex of the Cardiac group receiving probiotics compared with the Cardiac group receiving the vehicle control (Figure 5).
Figure 4.
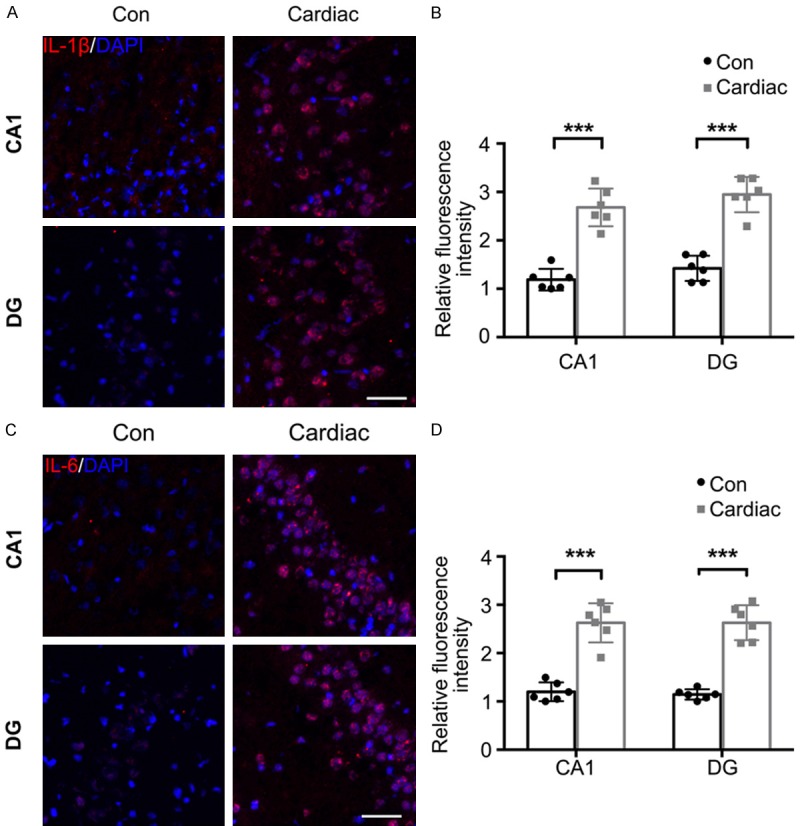
The expression of IL-1β and IL-6 was significantly upregulated following cardiac surgery. The hippocampus was harvested 7 days after cardiac surgery. A. Representative immunostaining images show the distribution and expression of IL-1β in the CA1 and DG regions. Scale bar = 50 μm. B. The mean optical density of IL-1β immunostaining was significantly higher after cardiac surgery. C. Representative images of IL-6 staining. Scale bar = 50 μm. D. IL-6 concentrations in the CA1 and DG regions (n = 6). Significance was tested by independent Student’s t-tests. Con = control; DG = dentate gyrus. ***P<0.001.
Figure 5.
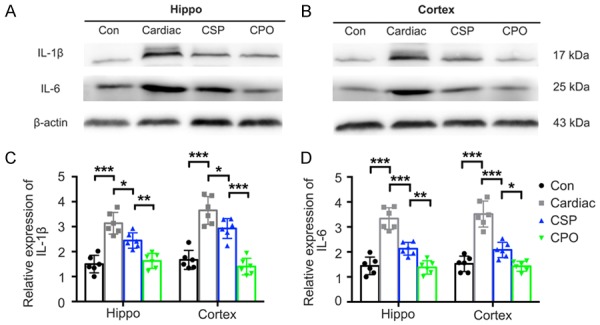
Pretreatment with probiotics alleviated the expression of IL-1β and IL-6 caused by cardiac surgery. A and B. Representative images of IL-1β and IL-6 expression detected by Western blot. C and D. Quantitative results of IL-1β and IL-6 expression in the hippocampus and the prefrontal cortex harvested 7 days after cardiac surgery (n = 6). Significance was tested by one-way analysis of variance. Con = control; CSP = probiotics-treated cardiac surgery; CPO = probiotics-treated control. *P<0.05, **P<0.01, ***P<0.001.
Significantly larger Iba-1-(CA1: P<0.001; DG: P<0.001) and GFAP-(CA1: P<0.001; DG: P = 0.001) positive regions were found in the hippocampus of the Cardiac group than the Con group (Figure 6). In addition, Iba-1 and GFAP expression was significantly higher in the cerebral cortex of rats in the Cardiac group, and these elevations were remarkably inhibited by the probiotic treatment (Figure 7).
Figure 6.
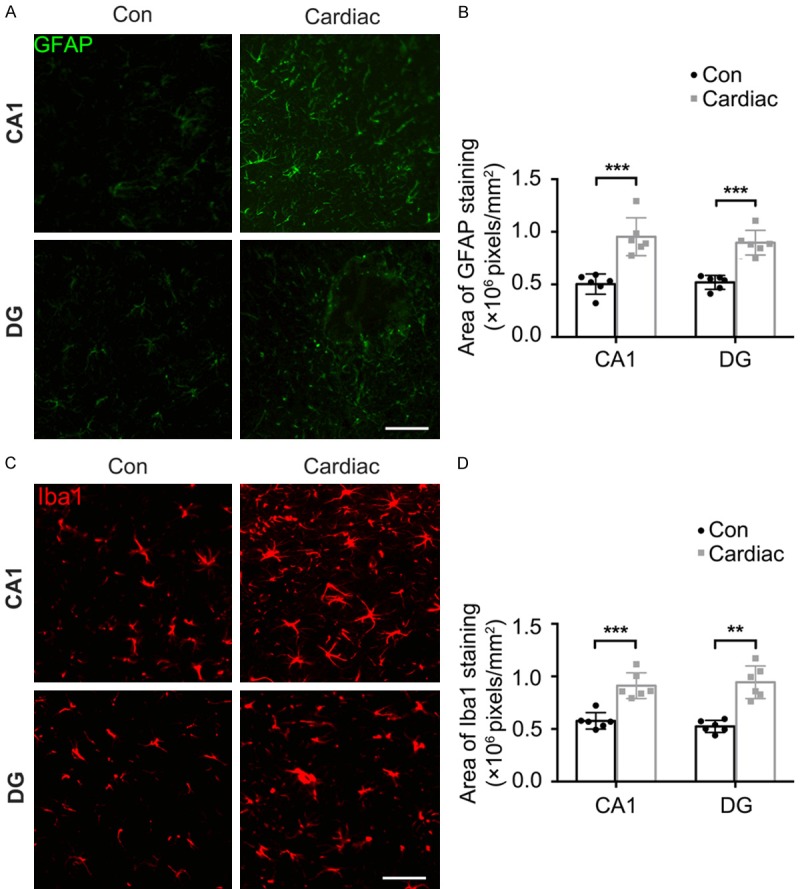
Cardiac surgery induced microgliosis and astrocytosis. The hippocampus was harvested 7 days after cardiac surgery. Immunostaining showed the distribution and expression of biomarkers of microglia and astrocytes (Iba-1 and GFAP). A. Representative images of Iba-1 staining in the CA1 and DG regions. Scale bar = 50 μm. B. The intensity of Iba-1 fluorescence was significantly higher after cardiac surgery. C. Representative images of GFAP staining in the CA1 and DG regions. Scale bar = 50 μm. D. The Intensity of GFAP fluorescence was significantly higher after cardiac surgery (n = 6). Significance was tested by independent Student’s t-tests. Con = control; DG = dentate gyrus. **P<0.01, ***P<0.001.
Figure 7.
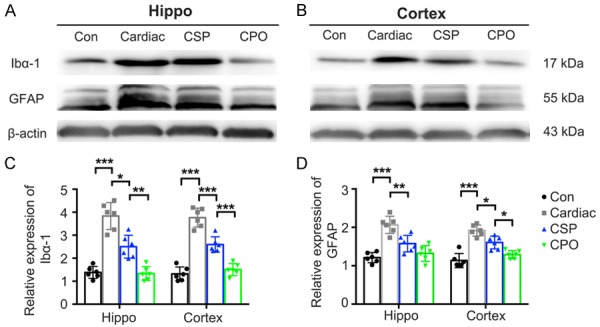
Pretreatment with probiotics inhibits microgliosis and astrocytosis caused by cardiac surgery. A and B. Representative images of Iba-1 and GFAP expression detected by Western blot. C and D. Quantitative data of Iba-1 and GFAP in the hippocampus and the prefrontal cortex harvested 7 days after cardiac surgery (n = 6). Significance was tested by one-way analysis of variance. Con = control; CSP = probiotics-treated cardiac surgery; CPO = probiotics-treated control. *P<0.05, **P<0.01, ***P<0.001.
Modulation of the gut microbiota alleviates cognitive dysfunction
A significant decline in the escape latency in the MWM was observed in all experimental groups during the spatial acquisition process (Figure 8A), which indicated that all groups of rats could learn the platform location. Overall, no significant difference was observed in spatial acquisition among the groups (Figure 8A). During the probe trial, the time spent in the target annulus was significantly shorter (F (3, 44) = 6.99, P = 0.44, Figure 8B), the number of times the animals swam across the site where the platform was located was significantly smaller (F (3, 44) = 9.50, P = 0.14, Figure 8C), and the percentage of the distance swam in the target sector was smaller (F (3, 44) = 11.43, P = 0.34, Figure 8D) in the Cardiac group than in the CSP group. These results indicate that the memory index was significantly better in the probiotic-treated Cardiac group than in the vehicle-treated Cardiac group. Locomotor activity and anxiety-like behavior were measured in the open field test (OFT). No significant difference was observed among the groups in distance moved (Figure 8E), time spent in the central area (Figure 8F) and defecation (fecal boli count) (Figure 8G). These results indicate a lack of group-related differences in locomotor activity or anxiety-like behavior.
Figure 8.
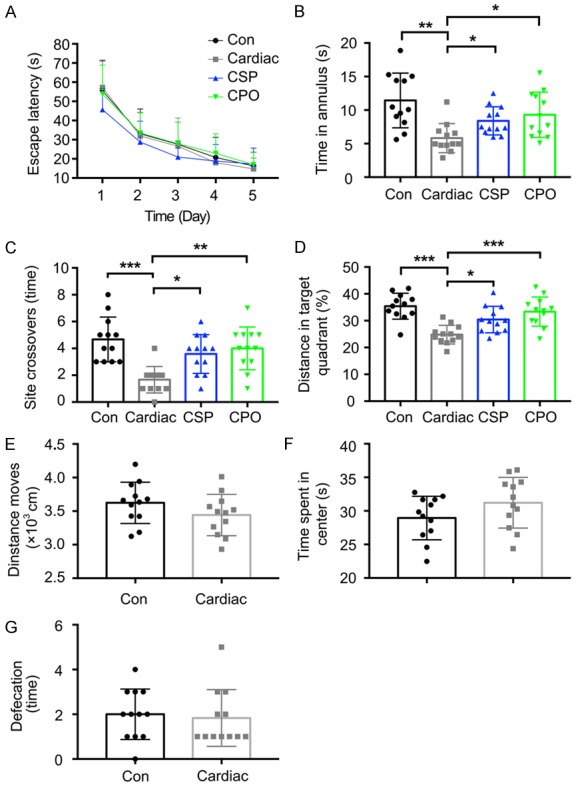
Pretreatment with probiotics attenuated learning and memory impairment caused by cardiac surgery. Rats began MWM and open field testing at 3 weeks after cardiac surgery. A. After a 5-day MWM training session, escape latency was declined in all experimental groups. B and C. The time spent in the target quadrant and the time spent swimming across the site where the platform had been located were both significantly shorter in the Cardiac group than in the Con group, whereas pretreatment with probiotics significantly prolonged these times. D. Pretreatment with probiotics increased the percentage of the distance traveled while swimming in the target sector during the probe trial. E-G. No significant difference in exploratory behaviors or general activity was observed during the test between the Cardiac and Con groups (n = 12). Significance was tested by one-way analysis of variance (ANOVA). Con = control; CSP = probiotics-treated cardiac surgery; CPO = probioticstreated control. *P<0.05, **P<0.01, ***P<0.001.
Discussion
In recent years, the host interactions of intestinal flora have become a focus of attention [16,17]. A growing body of evidence suggests that intestinal flora play an important role in the pathogenesis of obesity, Alzheimer’s disease, atherosclerosis, diabetes and other diseases [18-20]. However, to our knowledge, few studies have examined the effects of gut flora on cognitive function after cardiac surgery. In this study, we found that gut disorders caused by cardiac surgery can damage the gut barrier and increase BBB permeability, thereby possibly exacerbating neuroinflammation and causing POCD. Importantly, we also tested whether probiotic-induced alterations in the intestinal flora could counteract POCD and biological effects.
To our knowledge, we are the first to directly observe changes in intestinal flora in animal models of POCD after cardiac surgery. We found that the gut flora is dysbiotic after cardiac surgery. The potentially high-frequency imbalance of intestinal flora can increase the growth of proteobacteria such as Rothia, Acetivibrio and Shigella (Figure S1), which mainly include LPS-containing Gram-negative bacteria, and the integrity of the intestinal barrier can be destroyed because tight junction proteins are inhibited [21]. This “leaky gut” in cardiac surgery rats allows the intestinal LPS and pathogenic bacteria to escape from the intestinal tract, thereby activating innate immune cells in the intestinal tract and triggering the inflammatory response [13]. These findings suggest that cardiac surgery induces gut inflammation, leading to low levels of systemic and neuroinflammatory development.
In studies of inflammation, the expression and activity of MMP-9 is recognized as a destructive agent that promotes BBB damage [21-23]. Toft-Hansen et al. showed that metalloproteinases are associated with leukocyte infiltration in neuroinflammation [24]. Agrawal et al. further found that MMP-9 is an important factor in the selective elimination of β-dystroglycan, which anchors astrocyte end feet to the astrocyte basement membrane and is important for white blood cell penetration through the basal membrane of the brain parenchyma and, therefore, consequential to damage in the BBB [25]. In our study, we found that MMP-9 levels were higher in cardiac surgery rats, suggesting that the inflammatory response triggered by gut microbiota dysbiosis was at least partially responsible for the damage induced in the BBB. Therefore, we next assessed BBB permeability by measuring NaF content in brain tissues. The rate of extravasation of NaF is indicative of the rate of entry of small molecules, such as solutes and ions [15]. Hu et al. found that orthopedic surgery induced BBB damage; however, the specific mechanism by which it did so was unclear [26]. In our study, we found that BBB permeability was increased after cardiac surgery and that it aggravated cognitive deficits by increasing the exposure of the brain to various cytokines, including IL-1β and IL-6.
There is growing evidence that brain inflammation after POCD is a risk factor for cognitive dysfunction [5,8,27]. Probiotics are thought to have anti-inflammatory properties for a number of reasons. For example, lactobacillus helveticus reduced inflammatory markers, including prostaglandin E2, nitric oxide synthase, and IL-1β, in the brain [28], while Bifidobacterium longum R0175 reduced the levels of IL-8 and TNF-α [29]. Lactobacillus helveticus R0052 reduced IL-6 and IL-1β levels but did not significantly affect TNF-α levels [30], suggesting that each probiotic exerts a specific effect on the synthesis of proinflammatory cytokines. Consistent with these reports, in this study, the cardiac surgery rats showed neuroinflammation, which was attenuated by the administration of probiotics, possibly by regulating anti-inflammatory effects.
In conclusion, the intestinal disorders caused by cardiac surgery aggravate neuroinflammation, at least to some extent, by damaging the permeability of the intestinal barrier and the BBB. These adverse effects on cognition may be eliminated by probiotics, which can inhibit the imbalance of gut flora. These findings may explain the mechanism of cognitive dysfunction after cardiac surgery, and will facilitate further exploration of the treatment of POCD.
Acknowledgements
This work was supported by a grant for a project funded by the China Postdoctoral Science Foundation (No. 2019M651301), the Fundamental Research Funds for the Provincial Universities (No. 31041180128), the Spark Research Fund from the Fourth Affiliated Hospital of Harbin Medical University (No. HYDSYXH201911) and the Health and Family Planning Commission of Heilongjiang Province (No. 2018310). The authors thank Baixiang Li, M.D., Ph.D., from the Department of Toxicology, the College of Public Health, Harbin Medical University, Harbin, Heilongjiang Province, China, for his assistance in the Morris water maze study.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG Nomenclature Consensus Working Group. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129:872–879. doi: 10.1097/ALN.0000000000002334. [DOI] [PubMed] [Google Scholar]
- 2.Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, Oh ES, Crosby G, Berger M, Eckenhoff RG Nomenclature Consensus Working Group. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–1012. doi: 10.1016/j.bja.2017.11.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontes MT, McDonagh DL, Phillips-Bute B, Welsby IJ, Podgoreanu MV, Fontes ML, Stafford-Smith M, Newman MF, Mathew JP Neurologic Outcome Research Group (NORG) of the Duke Heart Center. Arterial hyperoxia during cardiopulmonary bypass and postoperative cognitive dysfunction. J Cardiothorac Vasc Anesth. 2014;28:462–466. doi: 10.1053/j.jvca.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kok WF, van Harten AE, Koene BM, Mariani MA, Koerts J, Tucha O, Absalom AR, Scheeren TW. A pilot study of cerebral tissue oxygenation and postoperative cognitive dysfunction among patients undergoing coronary artery bypass grafting randomised to surgery with or without cardiopulmonary bypass. Anaesthesia. 2014;69:613–622. doi: 10.1111/anae.12634. [DOI] [PubMed] [Google Scholar]
- 5.Hovens IB, van Leeuwen BL, Mariani MA, Kraneveld AD, Schoemaker RG. Postoperative cognitive dysfunction and neuroinflammation; cardiac surgery and abdominal surgery are not the same. Brain Behav Immun. 2016;54:178–193. doi: 10.1016/j.bbi.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zheng B, Lai R, Li J, Zuo Z. Critical role of P2X7 receptors in the neuroinflammation and cognitive dysfunction after surgery. Brain Behav Immun. 2017;61:365–374. doi: 10.1016/j.bbi.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95:1134–1141. doi: 10.1097/00000539-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Tang N, Jiang R, Wang X, Wen J, Liu L, Wu J, Zhang C. Insulin resistance plays a potential role in postoperative cognitive dysfunction in patients following cardiac valve surgery. Brain Res. 2017;1657:377–382. doi: 10.1016/j.brainres.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Lyte M. Microbial endocrinology: host-microbiota neuroendocrine interactions influencing brain and behavior. Gut Microbes. 2014;5:381–389. doi: 10.4161/gmic.28682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 11.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167:1469–1480. e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56:305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Furet JP, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, Doré J, Corthier G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol Ecol. 2009;68:351–362. doi: 10.1111/j.1574-6941.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- 15.Miner JJ, Daniels BP, Shrestha B, Proenca-Modena JL, Lew ED, Lazear HM, Gorman MJ, Lemke G, Klein RS, Diamond MS. The TAM receptor mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med. 2015;21:1464–1472. doi: 10.1038/nm.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandeputte D, Kathagen G, D’hoe K, Vieira-Silva S, Valles-Colomer M, Sabino J, Wang J, Tito RY, De Commer L, Darzi Y, Vermeire S, Falony G, Raes J. Quantitative microbiome profiling links gut community variation to microbial load. Nature. 2017;551:507–511. doi: 10.1038/nature24460. [DOI] [PubMed] [Google Scholar]
- 17.Goodrich JK, Davenport ER, Clark AG, Ley RE. The relationship between the human genome and microbiome comes into view. Annu Rev Genet. 2017;51:413–433. doi: 10.1146/annurev-genet-110711-155532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cattaneo A, Cattane N, Galluzzi S, Provasi S, Lopizzo N, Festari C, Ferrari C, Guerra UP, Paghera B, Muscio C, Bianchetti A, Volta GD, Turla M, Cotelli MS, Gennuso M, Prelle A, Zanetti O, Lussignoli G, Mirabile D, Bellandi D, Gentile S, Belotti G, Villani D, Harach T, Bolmont T, Padovani A, Boccardi M, Frisoni GB INDIA-FBP Group. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Jie Z, Xia H, Zhong SL, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, Zhang D, Su Z, Fang Z, Lan Z, Li J, Xiao L, Li J, Li R, Li X, Li F, Ren H, Huang Y, Peng Y, Li G, Wen B, Dong B, Chen JY, Geng QS, Zhang ZW, Yang H, Wang J, Wang J, Zhang X, Madsen L, Brix S, Ning G, Xu X, Liu X, Hou Y, Jia H, He K, Kristiansen K. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:845. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Hong J, Xu X, Feng Q, Zhang D, Gu Y, Shi J, Zhao S, Liu W, Wang X, Xia H, Liu Z, Cui B, Liang P, Xi L, Jin J, Ying X, Wang X, Zhao X, Li W, Jia H, Lan Z, Li F, Wang R, Sun Y, Yang M, Shen Y, Jie Z, Li J, Chen X, Zhong H, Xie H, Zhang Y, Gu W, Deng X, Shen B, Xu X, Yang H, Xu G, Bi Y, Lai S, Wang J, Qi L, Madsen L, Wang J, Ning G, Kristiansen K, Wang W. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017;23:859–868. doi: 10.1038/nm.4358. [DOI] [PubMed] [Google Scholar]
- 21.Pérez-Hernández M, Fernández-Valle ME, Rubio-Araiz A, Vidal R, Gutiérrez-López MD, O’Shea E, Colado MI. 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) produces edema due to BBB disruption induced by MMP-9 activation in rat hippocampus. Neuropharmacology. 2017;118:157–166. doi: 10.1016/j.neuropharm.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Egashira Y, Zhao H, Hua Y, Keep RF, Xi G. White matter injury after subarachnoid hemorrhage: role of blood-brain barrier disruption and matrix metalloproteinase-9. Stroke. 2015;46:2909–2915. doi: 10.1161/STROKEAHA.115.010351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YJ, Wang ZH, Zhang B, Zhe X, Wang MJ, Shi ST, Bai J, Lin T, Guo CJ, Zhang SJ, Kong XL, Zuo X, Zhao H. Disruption of the blood-brain barrier after generalized tonic-clonic seizures correlates with cerebrospinal fluid MMP-9 levels. J Neuroinflammation. 2013;10:80. doi: 10.1186/1742-2094-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toft-Hansen H, Buist R, Sun XJ, Schellenberg A, Peeling J, Owens T. Metalloproteinases control brain inflammation induced by pertussis toxin in mice overexpressing the chemokine CCL2 in the central nervous system. J Immunol. 2006;177:7242–7249. doi: 10.4049/jimmunol.177.10.7242. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM. Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med. 2006;203:1007–1019. doi: 10.1084/jem.20051342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu N, Guo D, Wang H, Xie K, Wang C, Li Y, Wang C, Wang C, Yu Y, Wang G. Involvement of the blood-brain barrier opening in cognitive decline in aged rats following orthopedic surgery and high concentration of sevoflurane inhalation. Brain Res. 2014;1551:13–24. doi: 10.1016/j.brainres.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen K, Sun Y, Dong W, Zhang T, Zhou N, Yu W, Diao Y, Guo S, Tian Y. Activated A7nachr improves postoperative cognitive dysfunction and intestinal injury induced by cardiopulmonary bypass in rats: inhibition of the proinflammatory response through the Th17 immune response. Cell Physiol Biochem. 2018;46:1175–1188. doi: 10.1159/000489068. [DOI] [PubMed] [Google Scholar]
- 28.Luo J, Wang T, Liang S, Hu X, Li W, Jin F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci China Life Sci. 2014;57:327–335. doi: 10.1007/s11427-014-4615-4. [DOI] [PubMed] [Google Scholar]
- 29.Wagar LE, Champagne CP, Buckley ND, Raymond Y, Green-Johnson JM. Immunomodulatory properties of fermented soy and dairy milks prepared with lactic acid bacteria. J Food Sci. 2009;74:M423–430. doi: 10.1111/j.1750-3841.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 30.Cazzola M, Tompkins TA, Matera MG. Immunomodulatory impact of a synbiotic in T(h)1 and T(h)2 models of infection. Ther Adv Respir Dis. 2010;4:259–270. doi: 10.1177/1753465810379009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


