Abstract
Background: The aim of this study was to explore the effects of irisin on human visceral adipose tissue and adipocytes functions. Methods: Fresh human visceral white adipose tissues derived from 11 donors were used to examine the effects of irisin on browning, adipogenesis and osteogenesis gene expression, and anti-inflammatory properties. Preadipocytes were also used to examine the effects of irisin on mitochondrial respiration, adipogenic differentiation, and osteogenic differentiation. Key results: Irisin significantly increased cellular mitochondrial energy metabolism in differentiated visceral adipocytes. Irisin also increased mRNA levels of transcriptional regulators of brite/beige adipocytes (UCP-1, PGC1α, PRDM16, TMEM26, and CD137) in subcutaneous white adipose tissue but not in visceral/brown adipose tissue or their derived mature adipocytes. In parallel, irisin increased the protein levels of UCP-1 in subcutaneous white adipose tissue, but had no effect on the expression of this protein in visceral white adipose tissue and perirenal brown adipose tissue. However, irisin inhibited adipogenic differentiation, promoted osteogenic differentiation in visceral adipocytes, down-regulated adipogenesis, and upregulated osteogenesis genes expression in visceral fat tissue. Moreover, administration of irisin reduced the expression of proinflammatory marker mRNAs in both visceral and subcutaneous white adipose tissue. Conclusions: Our data suggest that (1) irisin may increase mitochondrial respiration and glycolysis in visceral adipocytes by a UCP-1 independent pathway; (2) irisin promotes anti-inflammatory activity on fat tissue.
Keywords: Irisin, thermogenesis, adipogenesis, osteogenesis, human visceral white adipose tissue, anti-inflammation
Introduction
Obesity, a major global epidemic, has become a worldwide health problem due to sedentary lifestyles and the enhanced supply of high calorie food [1]. Many lines of evidence have established that the accumulation of visceral white adipose tissue (vcWAT) is more pathogenic than subcutaneous white adipose tissue (scWAT), as the former carries greater risk of developing obesity, cardiovascular events, atherosclerosis, hypertension, and increases the risk of type 2 diabetes and other metabolic diseases [2-4]. Unfortunately, accumulating evidence shows that different approaches to weight loss (e.g., lifestyle modification, weight loss agents, bariatric surgery, or exercise) have relatively limited effects on the reduction of vcWAT compared with scWAT [2,5].
Over the past years, a number of studies have demonstrated that ‘brite’ fat cells within white adipose depots are able to switch from energy storage to energy dissipation, thereby enhancing whole-body energy expenditure [6-8]. These studies suggested that these ‘brite’ cells have therapeutic potential for fighting metabolic and obesity related diseases. Moreover, several lines of evidence indicate that mature white human adipocytes have the capacity to acquire brown-like features that induces a more oxidative phenotype (transdifferentiation to ‘brite’ fat cells) in vivo [9,10].
Irisin was identified as an exercise-induced myokine in early 2012 [11]. Existing research shows that exercise upregulates the expression of peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), which may trigger a proteolytic cleavage of fibronectin type III domain containing 5 (FNDC5) to produce irisin. Irisin subsequently circulates to fat tissue where it can induce the transition of white fat to brown fat [11]. However, the proteolytic enzyme that cleaves irisin from FNDC5 has yet to be identified [12]. Our previous research shows that irisin can induce the browning of subcutaneous adipocytes by increasing the uncoupling protein 1 (UCP-1), and that this effect is probably mediated by activating the ERK and p38 MAPK signaling pathways [13]. Due to its effect of increasing adaptive thermogenesis, irisin might exhibit antiobesity or antidiabetic effects.
The browning effects of irisin in subcutaneous white tissue from both mice and humans have been clearly documented [11,14]. However, the effects of irisin on visceral fat remain poorly understood. Furthermore, adipocytes derived from obese individuals express the high levels of many inflammatory mediators as obesity causes a chronic low-grade inflammatory state. Compared with scWAT, vcWAT is more infiltrated with inflammatory and immune cells, such as macrophages, which are an important source of inflammatory cytokines (e.g., tumor necrosis factor TNF-α and IL-6) [15,16]. Therefore, the reduction of vcWAT could play a pivotal role in the treatment of metabolic diseases and obesity-induced inflammation.
In this study, we systematically examined the effects of irisin on browning in human primary mature visceral adipocytes and fresh human vcWAT. We observed that irisin increased mitochondrial metabolism of human visceral adipocytes that was independent of the expression of UCP-1 protein. Moreover, the p38 and ERK signaling was not activated by irisin, sharply different with those previously observed in subcutaneous fat. Additionally, we observed that irisin inhibited adipogenesis during differentiation, leading to the suppression of new adipocytes formation, while promoting osteogenic differentiation. Furthermore, our study demonstrated that irisin significantly downregulates the expression of proinflammatory cytokines in fresh human vcWAT and scWAT fragments. Overall, our research provided further insights into the mechanisms of irisin’s action on human visceral adipose tissue and its derived adipocytes.
Methods
Primary human vcWAT and scWAT collection
Visceral adipose tissue samples (omental) were obtained from 9 female and 2 male donors, subcutaneous adipose tissue samples (breast) from 3 female and 2 male donors, and perirenal brown adipose tissue (BAT) from 2 female and 2 male donors (without known infection) through the University of Florida, Department of Pathology Grossing Room. The UF Institutional Review Board approved these studies. Visceral adipose tissue samples were obtained from donors receiving abdominal surgery. Subcutaneous adipose tissue samples were obtained from donors undergoing breast reduction surgery for cosmetic reasons. Perirenal brown fat donors underwent nephrectomy due to renal cell carcinoma. The use of human material conforms to the principles outlined in the Declaration of Helsinki. Fat tissues were processed within 6 hours after collection.
Adipose tissue processing
Preadipocytes from human vcWAT, scWAT, and perirenal BAT were isolated and expanded as described previously [13]. Briefly, adipose tissues were minced into small fragments and placed in DMEM/F12 medium with 10% fetal bovine serum and 100 U/mL penicillin-streptomycin overnight to restore quiescence. Preadipocytes (adipose tissue stromal cells) were obtained from mature adipocytes by ceiling culture or were placed directly in flasks for tissue culture. Preadipocytes were cultured and differentiated following our published protocol [13]. Fully differentiated adipocytes or cultured adipose tissue fragments were treated with or without irisin (50 nM) for the indicated time.
Differentiation of human preadipocytes into mature adipocytes and osteoblasts
Human visceral preadipocytes were cultured in 6-well plates until confluent and then induced with adipogenic or osteogenic differentiation medium for 14 or 21 days as described previously [13], adding fresh medium every third day. To demonstrate the effect of irisin in adipogenesis or osteogenesis, the cells were treated with or without irisin (50 nM) throughout the differentiation period. Adipogenic and osteogenic differentiation was confirmed by oil red O and Alizarin red staining and evaluated by light microscopy [17,18].
Mitochondrial bioenergetics analysis
Preadipocytes derived from visceral, subcutaneous, and brown adipose tissues were fully differentiated into mature adipocytes in adipogenic differentiation medium for 14 days, and then seeded into XF96 Microplates (15,000 cells/well) for 3-4 days in the absence or presence of 50 nM irisin. Mitochondrial respiration analyses were performed using a Seahorse Bioscience XF96 Extracellular Flux Analyzer (Seahorse Bioscience, USA), which enables real-time, simultaneous measurement of oxygen consumption rate (OCR) and extracellular acidification rates (ECAR) [19].
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from cultured cells and adipose tissue using Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription of 2 µg total RNA was performed with the cDNA Reverse Transcription Kit (Promega) according to the manufacturer’s protocols. The 2-ΔΔCt method was used to calculate the relative gene expression normalized to β-actin. All measurements were performed at least three times. Primer sequences are available in Table 1.
Table 1.
Primer sequences for qRT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β-actin | GACCTCTATGCCAACACAGT | AGTACTTGCGCTCAGGAGGA |
| UCP-1 | TAAAAACAGAAGGGCGGATG | GTGGGTTGCCCAATGAATAC |
| PGC-1α | GCTTTCTGGGTGGACTCAAGT | GAGGGCAATCCGTCTTCATCC |
| PRDM16 | AGGACATTGAGCCAGGTGAG | GCTTGGACTGGAAGAGTTCG |
| CD137 | TCTCCGCAGATCATCTCCTT | CTTCTGGAAATCGGCAGCTA |
| TMEM26 | GGCTTCTTGAATTGCACCAT | ATGGAGTCCCAATGTCCAAA |
| ADIPOQ | ATGACCAGGAAACCACGACT | CACCGATGTCTCCCTTAGGA |
| CEBPB | GACAAGCACAGCGACGAGTA | AGCTGCTCCACCTTCTTCTG |
| PPARR | GGCTTCATGACAAGGGAGTTTC | AACTCAAACTTGGGCTCCATAA |
| RUNX | GTGGACGAGGCAAGAGTTTCA | CATCAAGCTTCTGTCTGTGCC |
| OSTERIX | GTCCTATGGCGGGGAGGACTGG | TGGCAGCTGCAAGCTCTCTGTA |
| OPN | GTGGTGATCTAGTGGTGCCAAG | AGGCACCGGCCATGTGGCTAT |
| HSL | CTTCATGTCGCCGCTGCTGG | CGGATGCGCTCCACGCACAG |
| ATGL | CAGCATCTGCCAGTACCTGGTG | AGGCCACGTTGGTGCAGAAGA |
| IL-10 | CATCGATTTCTTCCCTGTGAA | TCTTGGAGCTTATTAAAGGCAT |
| IL-6 | ATGCAATAACCACCCCTGAC | GAGGTGCCCATGCTACATTT |
| TNFα | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT |
| MCP-1 | TCCCAAAGAAGCTGTGATCTTC | AGTGAGTGTTCAAGTCTTCGGA |
| MIP-1α | GAATCATGCAGGTCTCCACTG | CTCTAGGTCGCTGACATATTTC |
Western blot and immunocytochemistry (ICC) analysis
For immunoblotting analysis, 10-40 μg of protein were loaded onto 12% SDS-PAGE gels and transferred onto PVDF membranes. Anti-Phospho-ERK1/2 (#9101, Cell Signaling), anti-Phospho-p38MAPK (#9211, Cell Signaling) and anti-UCP-1 (AB155117, Abcam) antibodies were used for western blotting. Anti-β-actin (A5316, Sigma-Aldrich) was employed as a loading control. For ICC analysis, adipocytes were fixed with 4% paraformaldehyde for 10 min, and blocked in the presence of hydrogen peroxide. Then cells were incubated overnight with anti-UCP-1 antibody (1:500). Horseradish peroxidase-conjugated secondary antibody was used for ICC [13].
Statistical analysis
Results are presented as mean ± SEM of at least three independent experiments. Each experiment was conducted in triplicate. Statistical significance among multiple groups was analyzed using Prism 7 software by one-way ANOVA followed by post-hoc Dunnett’s test, while Student’s t-test was applied for statistical analysis of two classes of data. P<0.05 was considered significant.
Results
Irisin increases mitochondrial respiration in human visceral adipocytes
To examine the effects of irisin on thermogenesis in mature adipocytes derived from vcWAT, we measured mitochondrial respiration. Figure 1A, 1B show that irisin markedly increased mitochondrial OCR and ECAR in differentiated visceral adipocytes after 4-day treatment. The increased OCR of irisin-treated adipocytes were observed in both basal conditions and after adding the uncoupling agent carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP). Increased mitochondrial respiration was also observed in irisin-treated brown and subcutaneous adipocytes (Figure 1C, 1D). Moreover, the expression of genes (HSL and ATGL) encoding proteins involved in lipid metabolism were also increased in irisin-treated mature visceral adipocytes (Figure 1E). These results demonstrate that irisin can increase cellular thermogenesis and lipolysis in mature visceral adipocytes.
Figure 1.
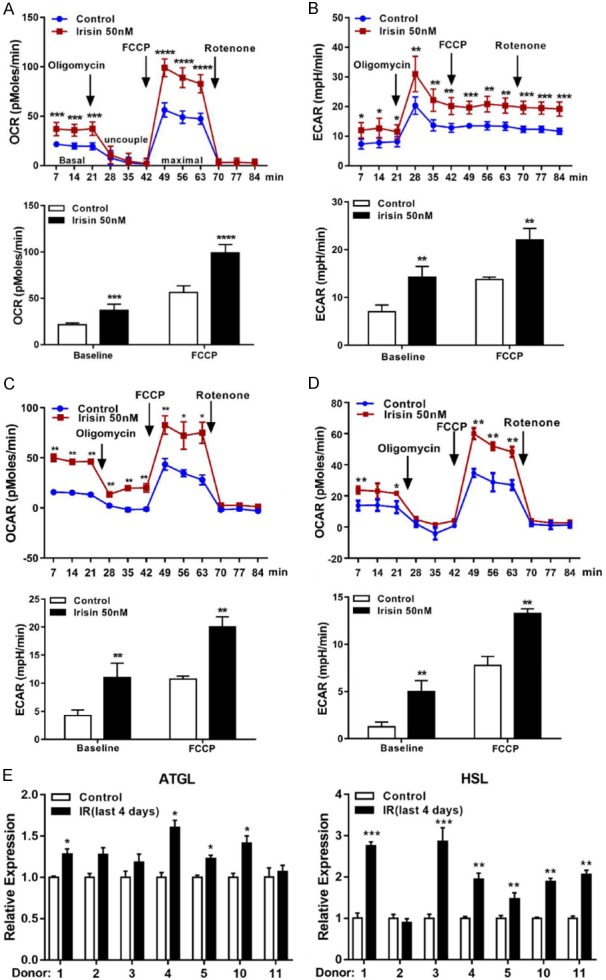
Effects of irisin on adipocytes mitochondrial respiration and lipid metabolism. Fully differentiated mature adipocytes from vcWAT (A, B), perirenal BAT (C), or scWAT (D) were incubated with or without irisin (50 nM) for 4 days. Oligomycin and FCCP were added at the indicated points to determining uncoupled and maximal mitochondrial respiration, respectively. Effects of irisin on cellular OCR and ECAR were measured using an XF96 Extracellular Flux Analyzer. Experimental treatments were performed with 6 technical replicates and 4 biological replicates. (E) The mRNA levels of adipocytes metabolism related genes in human visceral adipocytes were measured by qRT-PCR. Data are expressed as mean ± SEM; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 statistical differenced compared to control.
Irisin has no browning effects on human vcWAT or vcWAT-derived mature adipocytes
Irisin treatment is known to induce the browning of subcutaneous white adipocytes and increase the ability to dissipate energy by UCP-1-mediated thermogenesis [13]. To investigate potential depot differences in responsiveness to irisin, we compared the browning effect of irisin on vcWAT, scWAT, and BAT. Figure 2A shows that irisin insignificantly increased the expression of UCP-1, PRDM16, CD137, and PGC1α genes in vcWAT from most donors (#1, 2, 4, 7, 9, 10, and 11); donors #3, 6, and 8 showed <2-fold upregulation. In contrast, irisin treated scWAT (donors #12 and 13) showed a >10-fold increase. Incubation of irisin with perirenal BAT also had no effect on the expression of browning genes (Figure 2B). Furthermore, the basal expression levels of brite-specific genes (TMEM26 and CD137) in vcWAT were highly variable, and there was no obvious positive correlation between irisin-stimulated UCP-1 and PRDM16 expression and basal expression levels of CD137 and TMEM26 (Figure S1A, S1B). Irisin treatment did not affect the expression of UCP-1 protein in vcWAT and perirenal BAT, although it was significantly up-regulated in scWAT (Figure 2C). Our previous studies show that irisin exerts its browning effects in human subcutaneous fat tissue through ERK and p38 activation. Indeed, as shown in Figure 2D, phosphorylation of p38 (P-p38) and ERK (P-ERK) in human scWAT was enhanced by treating with irisin for 60 min. However, no phosphorylation of ERK and P38 were observed in vcWAT and perirenal BAT.
Figure 2.
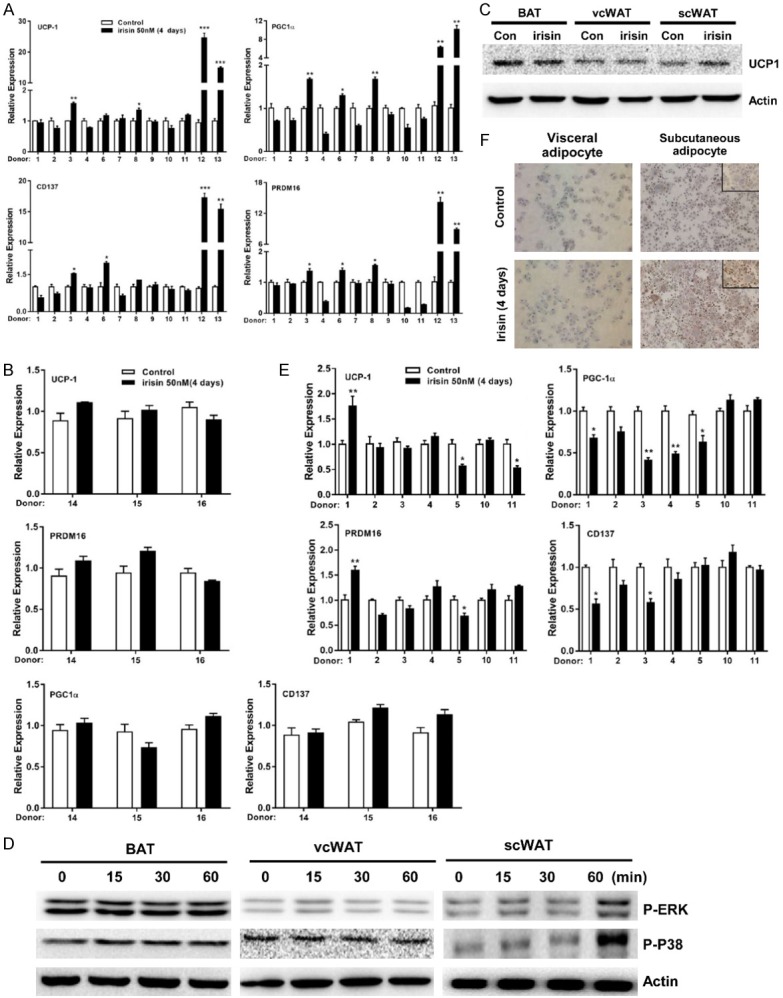
The browning effect of irisin in human adipose tissue and differentiated mature adipocytes from vcWAT. mRNA expression of brown/brite related genes UCP-1, PGC1α, CD137 and PRDM16 in human vcWAT (donors #1-11), scWAT (donors #12-13) (A), and BAT (donors #14-16) (B) after incubate with or without irisin (50 nM) for 4 days. (C) UCP-1 protein expression in response to 4 days irisin stimulation in three different adipose tissue were detected by western blotting. (D) Perirenal BAT, vcWAT, or scWAT were treated with or without irisin (50 nM) for 15, 30, or 60 min, respectively. Levels of P-ERK and P-p38 were assessed by western blotting. (E) mRNA levels of brown/brite related genes in differentiated mature visceral adipocytes after incubate with or without irisin (50 nM) for 4 days. (F) UCP-1 protein expression was detected by immunocytochemistry (ICC) in vcWAT and scWAT derived mature adipocytes. Data are expressed as mean ± SEM; *P<0.05, **P<0.01 and ***P<0.001 statistical differenced compared to control.
We further examined the expression of browning related genes (UCP-1, PGC1α, CD137, and PRDM16) in human visceral adipocytes. Figure 2E shows that except for a slight increase in a few samples, irisin treatment did not affect the transcription of these genes. Moreover, the expression of general adipose genes (PPARR, CEBPB, and ADIPOQ) were upregulated after adipogenic differentiation, but showed no difference in the differentiation groups treated with or without irisin (Figure S2). As shown in Figure 2F, expression of UCP-1 protein increased robustly in irisin-treated subcutaneous adipocytes, but not in irisin-treated visceral adipocytes. Lower or absent beige gene expression in human visceral adipocytes or vcWAT may account for the modest response of these adipocytes to irisin.
Irisin inhibits adipogenic differentiation and promotes osteogenic differentiation in human visceral adipocytes
Our previous work indicates that irisin markedly reduces adipogenic differentiation of human subcutaneous adipocytes. Therefore, we further examined whether irisin has the same effect on human visceral adipocytes. As shown in Figure 3A, mature adipocytes accumulation was reduced by 20-60% in the presence of irisin vs. medium alone after 18 days of differentiation, and expression of adipose genes CEBPB and ADIPOQ were also reduced (Figure 3B). Interestingly, expression of browning related genes (PRDM16 and PGC1α) was also significantly reduced (Figure 3B), despite no browning effects observed on irisin-treated vcWAT fragments (Figure 2). The reduced adipose genes expression was obtained from fresh visceral and subcutaneous fat tissue after irisin treatment for 4 days (Figures 3C and S3A). Collectively, our results demonstrated that irisin exerts an inhibitory effect on human visceral-derived preadipocytes adipogenesis.
Figure 3.
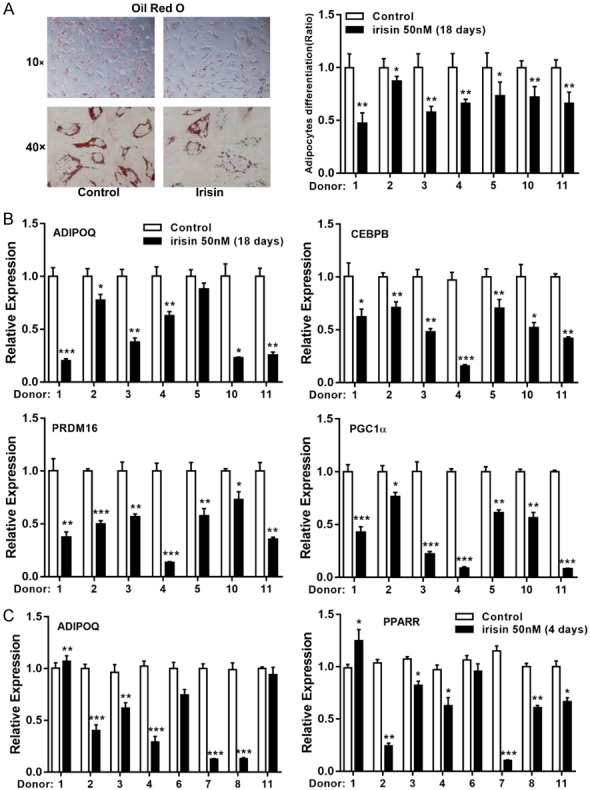
The effect of irisin on adipogenic differentiation. A. The human visceral preadipocytes were induced to adipogenic differentiation with or without irisin (50 nM) for 18 days. Cells were stained with Oil Red O to visualize lipid droplets (left); mature adipocytes were calculated as percentage of control (right). B. Expression of adipogenic genes (CEBPB and ADIPOQ) and browning genes (PGC1α and PRDM16) in differentiated adipocytes were determined by qRT-PCR. C. mRNA expression levels of adipogenic genes (PPARR and ADIPOQ) were measured in vcWAT after 4 days of irisin (50 nM) treatment. Data are expressed as mean ± SEM; *P<0.05, **P<0.01 and ***P<0.001 statistical differenced compared to control.
We next evaluated the effects of irisin on osteoblast differentiation in vitro. Exposure to irisin during the entire differentiation procedure accelerated osteoblast differentiation by increasing mineralization and upregulating the expression of several osteogenic marker genes, including RUNX2, OSTERIX, and OSTEOPONTIN (OPN) (Figure 4A, 4B). Similar results were obtained in fresh visceral and subcutaneous fat tissue under the same treatment (Figures 4C and S3B).
Figure 4.
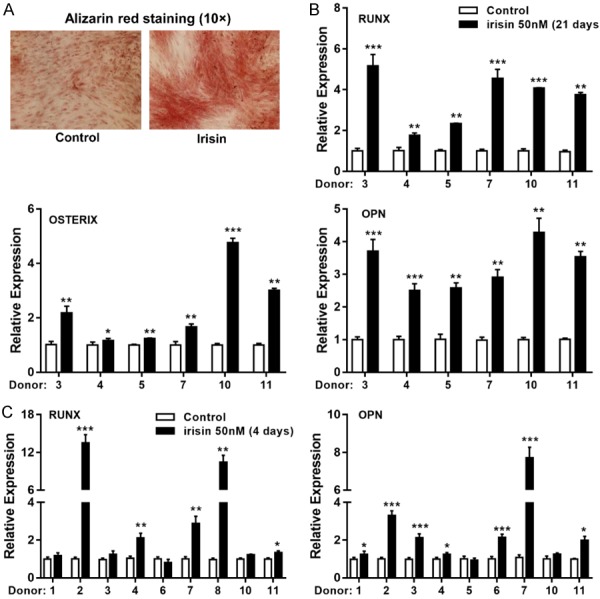
The effect of irisin on osteogenic differentiation. (A) The human visceral preadipocytes were induced to osteogenic differentiation with or without irisin (50 nM) for 21 days, as evidenced by mineral deposition highlighted by Alizarin Red staining. (B) mRNA expression levels of RUNX, OSTERIX and OPN genes were measured in fully differentiated visceral adipocytes (21 days) and (C) in vcWAT after 4 days treatment with irisin directly. Data are expressed as mean ± SEM; * P<0.05, **P<0.01 and ***P<0.001 statistical differenced compared to control.
Irisin reduces the expression of pro-inflammatory cytokines and upregulates an anti-inflammatory cytokine
Previous studies revealed that irisin decreases the expression and secretion of pro-inflammatory markers in LPS-activated macrophages or LPS-activated undifferentiated or differentiated 3T3 cells [20,21]. Therefore, we further explored the anti-inflammatory effect of irisin on human visceral adipose tissue. Compared with primary white adipocytes, scWAT and vcWAT fragments closely represent native fat tissue. As shown in Figure 5A and 5B, irisin reduced the expression of TNF-α, IL-6, MCP-1α, and MIP-1α while enhancing the expression of IL-10 in fresh visceral and subcutaneous fat tissue. These results suggested that irisin has anti-inflammatory properties in human white adipose tissue.
Figure 5.
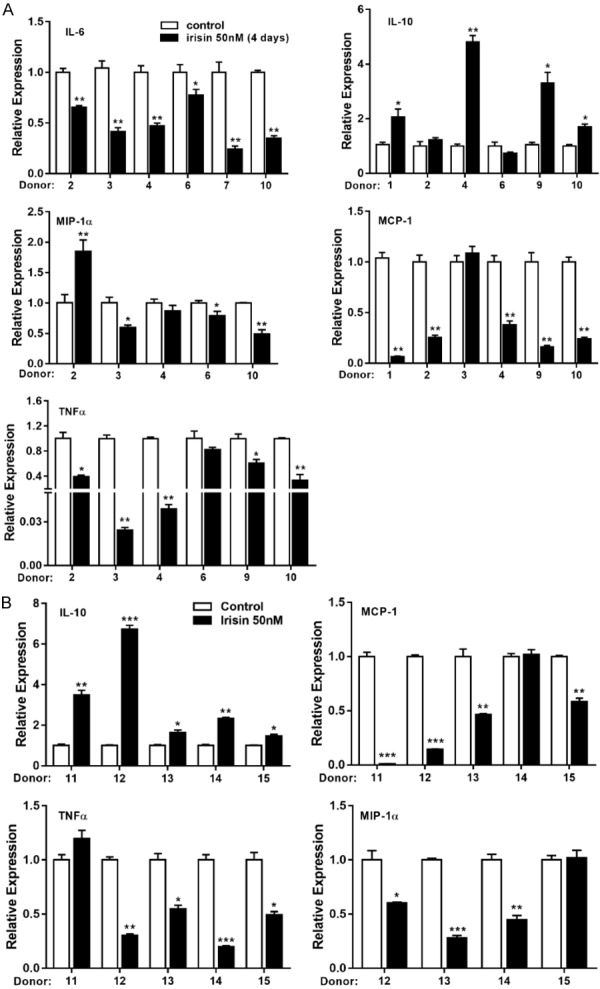
The effect of irisin on inflammation. (A) Human vcWAT and (B) scWAT were treated with or without irisin (50 nM) for 4 days, and the mRNA expression of pro-inflammatory (IL-6, TNFα, MCP-1, and MIP-1α) and anti-inflammatory (IL-10) genes were determined by qRT-PCR. Data are expressed as mean ± SEM; *P<0.05, **P<0.01 and ***P<0.001 statistical differenced compared to control.
Discussion
Irisin, an exercise induced 112 amino acid peptide cleaved from FNDC5, is thought to induce the “browning” of white adipose tissue, increasing total body energy expenditure by enhancing UCP-1-mediated thermogenesis. Irisin is emerging as a promising therapeutic target for the treatment of metabolic diseases. However, its browning effects in human visceral adipose tissue are controversial [11,22,23], and the function of irisin in human cells remains incompletely validated. To this end, we examined the effect of irisin on human visceral fat and summarized all our data on the effects of irisin in three different types of human fat in Table 2.
Table 2.
Effect of irisin on different types of adipocytes
| Subcutaneous | Visceral | Brown | |
|---|---|---|---|
| Expression of browning gene | Increase | No effect | No effect |
| Thermogenesis | Increase | Increase | Increase |
| Adipogenic differentiation | Inhibit | Inhibit | ND |
| Osteogenic differentiation | Promote | Promote | ND |
| Activation of p38/ERK signaling pathway | Yes | No | No |
| Inflammatory cytokines | Decrease pro-inflammation cytokines expression; Increase anti-inflammatory cytokines expression | Decrease pro-inflammation cytokines expression; Increase anti-inflammatory cytokines expression | ND |
Abbreviations: ND, not done.
We found that the expression of UCP-1 and other brown-related genes were enhanced in human scWAT fragments, while there was no obvious change in vcWAT and perirenal BAT after irisin treatment. These effects also were observed in irisin treated mature human adipocytes. Moreover, we examined the level of UCP-1 protein expression in seven human vcWAT samples with or without irisin stimulation, and only very faint protein bands were detected. Some samples had no detectable UCP-1 protein even under extreme exposure. These results suggested that the expression of UCP-1 is insensitive to irisin in human vcWAT. BAT from perirenal fat has the high basal levels of UCP-1 protein, so its regulation appears irisin-independent. Notably, irisin increased mitochondrial metabolism in all three types of adipose tissue-derived primary cultured adipocytes. This is surprising as the thermogenic capability of brown fat is mediated mainly by mitochondrial protein UCP-1, which uncouples the electron transport chain from energy production, resulting in the release of energy as heat. The reasons that the fat depot specificity action of irisin on UCP-1 did not translate into its differences in mitochondrial respiration are unknown, but might involve interactions with other factors modulating the complex mitochondrial biogenesis program.
Wu et al. reported that CD137-high-expressing cellular populations have preferential sensitivity to the browning effects of irisin [6]. However, our studies indicated that the CD137 and TMEM26 basal expression levels had no correlation with the irisin-stimulated UCP-1 or PRDM16 expression in vcWAT. In addition, we previously demonstrated that the browning effect of irisin is mediated by the p38/ERK MAPK pathways [13]. In this study, we did not observe the irisin-induced activation of ERK and p38 MAPK in vcWAT.
The current data lead us to propose the following model: (i) Irisin may exert differential effects depending on the location/type of the adipose tissue; (ii) Subcutaneous adipocytes are more likely to undergo browning than visceral adipocytes because subcutaneous adipocytes are smaller and have a greater potential to differentiate (Table 3) [24]; (iii) Adipocytes derived from different progenitor cells exhibit different gene expression patterns and respond differently to irisin [25]; (iv) An irisin-induced thermogenic gene program is mediated by signaling through αV/β5 integrin [26], and the expression of this receptor may differ between various types of adipocytes; (v) The effect of irisin on cellular oxidative metabolism in visceral and brown adipocytes may impact a UCP-1-independent pathway. Other mitochondrial inner membrane anion carrier proteins may also mediate uncoupling, such as adenine nucleotide translocator, ANT1 [27].
Table 3.
Histological characteristics of subcutaneous and visceral fat
| Subcutaneous | Visceral | |
|---|---|---|
| Location | Back and anterior abdominal wall | Mesentery and omentum |
| Ratio of all body fat | 80% | 10-20% |
| Fat cell size | Small | Large |
| Insulin resistant | More sensitive to insulin | More resistant to insulin |
| Glucose uptake | Lower compared with Visceral fat | Higher rate of insulin-stimulated Glucose uptake |
| FFA and TG uptake | Higher | Lower |
| Lipolysis | Lower | Higher |
| Metabolism risks | Lower | Higher |
| Inflammatory cytokines production | Little | More infiltrated with inflammatory cells and is more capable of generating pro-inflammatory factors |
Abbreviations: FFA, free fat acid; TG, triglycerides.
We found that irisin inhibited lipid accumulation by reducing the expression of adipogenic genes (CEBPB and ADIPOQ) during vcWAT derived preadipocytes differentiation. Moreover, the expression levels of browning-related genes (PGC1α and PRDM16) were also lowered, when irisin was present during the entire differentiation period (18 days, Figure 3B), in contrast to the effects of irisin on fresh vcWAT fragments (Figure 2). The possible explanation for the differential actions of irisin on vc-preadipocytes and mature vc-adipose tissue could be due to the epigenetic reprogramming of preadipocytes during the de-differentiation/re-differentiation process, and the loss of adipose tissue native 3D structure. Adipose tissue expansion is characterized initially by adipocytes hypertrophy, followed by an increased number of adipocytes [28]. To evaluate the effect of irisin on adipose tissue, human white adipose tissue derived from subcutaneous and visceral depots were exposed to irisin. We found that the expression levels of several adipogenic genes (PPARR and ADIPOQ) were reduced in both types of fat tissues. Furthermore, irisin can increase gene expression of HSL and ATGL, which encode proteins involved in lipid metabolism. These studies demonstrate the potential effect of irisin to counter obesity by suppressing new adipocytes formation and increasing the metabolic rate in mature adipocytes.
Exercise has widely been recognized to benefit metabolic and skeletal health [29]. Irisin is a myokine produced by skeletal muscle in response to exercise that enhances bone formation and helps prevent fractures [30,31]. It also promotes osteogenesis during subcutaneous fat derived preadipocytes differentiation [13]. We found a similar promotes osteogenesis effect of irisin on visceral fat derived preadipocytes, although preadipocytes derived from vcWAT have less differentiating capacity than those derived from scWAT. In addition, expression of mRNAs encoding osteoblast transcriptional regulators (OPN and RUNX2) were also induced in irisin treated fresh visceral and subcutaneous fat tissue.
Obesity causes a chronic low-grade inflammatory state accompanied by pro-inflammatory macrophage infiltration into white adipose tissue, that is associated with the development of insulin resistance, and increased the risk of cardiovascular disease [32]. Accumulation of subcutaneous and visceral adipose tissue is thought to promote the development of obesity-related mild inflammation [33]. Recently, Agnieszka Irena Mazur-Bialy et al. showed that irisin can decrease the expression of inflammatory markers in LPS-activated macrophages, and that this effect is mediated by inhibition of the TLR4/MyD88 signaling pathway [20]. Another study found that irisin can alleviate the inflammation in adipocytes by inhibiting phosphorylation and activation of nuclear factor kappa B (NFκB) [21]. Irisin also induced the phenotypic switching of adipose tissue macrophages from an M1-like (pro-inflammatory) to an M2-like (anti-inflammatory) phenotype [21,34]. We show here that irisin reduced the expression of a number of proinflammatory cytokine genes (TNF-α, IL-6, MCP-1, and MIP-1α) and increased the expression of the anti-inflammatory cytokine gene (IL-10) in both vcWAT and scWAT. Further mechanistic studies of the effects of irisin on inflammation and macrophage function are needed to provide additional insights, potentially leading to future therapeutic use of irisin as an anti-inflammatory.
Acknowledgements
This work supported by research grant R01-AR44731 (WR and LY) and US Army Medical Research research grant W81XWH-15-1-0196 (YD and LY).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- 2.Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes. 2011;6(Suppl 1):13–20. doi: 10.3109/17477166.2011.604326. [DOI] [PubMed] [Google Scholar]
- 3.Ayonrinde OT, Olynyk JK, Beilin LJ, Mori TA, Pennell CE, de Klerk N, Oddy WH, Shipman P, Adams LA. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809. doi: 10.1002/hep.24097. [DOI] [PubMed] [Google Scholar]
- 4.Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1. [DOI] [PubMed] [Google Scholar]
- 5.Chaston TB, Dixon JB. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes (Lond) 2008;32:619–628. doi: 10.1038/sj.ijo.0803761. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn E, Binart N, Lombes M. [Brown, white, beige: the color of fat and new therapeutic perspectives for obesity...] . Ann Endocrinol (Paris) 2012;73(Suppl 1):S2–8. doi: 10.1016/S0003-4266(12)70009-4. [DOI] [PubMed] [Google Scholar]
- 9.Frontini A, Vitali A, Perugini J, Murano I, Romiti C, Ricquier D, Guerrieri M, Cinti S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim Biophys Acta. 2013;1831:950–959. doi: 10.1016/j.bbalip.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Sidossis LS, Porter C, Saraf MK, Borsheim E, Radhakrishnan RS, Chao T, Ali A, Chondronikola M, Mlcak R, Finnerty CC, Hawkins HK, Toliver-Kinsky T, Herndon DN. Browning of subcutaneous white adipose tissue in humans after severe adrenergic stress. Cell Metab. 2015;22:219–227. doi: 10.1016/j.cmet.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke AB, Gomez YH, Daskalopoulou SS. 5 years later: irisin detection still an issue. Eur J Endocrinol. 2017;177:C1–C4. doi: 10.1530/EJE-17-0572. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Xie C, Wang H, Foss RM, Clare M, George EV, Li S, Katz A, Cheng H, Ding Y, Tang D, Reeves WH, Yang LJ. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530–541. doi: 10.1152/ajpendo.00094.2016. [DOI] [PubMed] [Google Scholar]
- 14.Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 15.Bruun JM, Lihn AS, Pedersen SB, Richelsen B. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–2289. doi: 10.1210/jc.2004-1696. [DOI] [PubMed] [Google Scholar]
- 16.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- 18.Paul H, Reginato AJ, Schumacher HR. Alizarin red S staining as a screening test to detect calcium compounds in synovial fluid. Arthritis Rheum. 1983;26:191–200. doi: 10.1002/art.1780260211. [DOI] [PubMed] [Google Scholar]
- 19.Xie C, Zhang Y, Tran TD, Wang H, Li S, George EV, Zhuang H, Zhang P, Kandel A, Lai Y, Tang D, Reeves WH, Cheng H, Ding Y, Yang LJ. Irisin controls growth, intracellular Ca2+ signals, and mitochondrial thermogenesis in cardiomyoblasts. PLoS One. 2015;10:e0136816. doi: 10.1371/journal.pone.0136816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazur-Bialy AI, Pochec E, Zarawski M. Anti-Inflammatory properties of Irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mazur-Bialy AI, Bilski J, Pochec E, Brzozowski T. New insight into the direct anti-inflammatory activity of a myokine irisin against proinflammatory activation of adipocytes. Implication for exercise in obesity. J Physiol Pharmacol. 2017;68:243–251. [PubMed] [Google Scholar]
- 22.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen Crujeiras A, Seoane LM, Casanueva FF, Pardo M. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 25.Gerhard GS, Styer AM, Strodel WE, Roesch SL, Yavorek A, Carey DJ, Wood GC, Petrick AT, Gabrielsen J, Ibele A, Benotti P, Rolston DD, Still CD, Argyropoulos G. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int J Obes (Lond) 2014;38:371–378. doi: 10.1038/ijo.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H, Wrann CD, Jedrychowski M, Vidoni S, Kitase Y, Nagano K, Zhou C, Chou J, Parkman VA, Novick SJ, Strutzenberg TS, Pascal BD, Le PT, Brooks DJ, Roche AM, Gerber KK, Mattheis L, Chen W, Tu H, Bouxsein ML, Griffin PR, Baron R, Rosen CJ, Bonewald LF, Spiegelman BM. Irisin mediates effects on bone and fat via alphaV integrin receptors. Cell. 2018;175:1756–1768. e1717. doi: 10.1016/j.cell.2018.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shabalina IG, Kramarova TV, Nedergaard J, Cannon B. Carboxyatractyloside effects on brown-fat mitochondria imply that the adenine nucleotide translocator isoforms ANT1 and ANT2 may be responsible for basal and fatty-acid-induced uncoupling respectively. Biochem J. 2006;399:405–414. doi: 10.1042/BJ20060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, Cushman SW, Periwal V. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84:S15–S21. doi: 10.3949/ccjm.84.s1.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao X, Nie Y, Ma Y, Chen Y, Cheng R, Yin W, Hu Y, Xu W, Xu L. Irisin promotes osteoblast proliferation and differentiation via activating the MAP kinase signaling pathways. Sci Rep. 2016;6:18732. doi: 10.1038/srep18732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colaianni G, Cuscito C, Mongelli T, Pignataro P, Buccoliero C, Liu P, Lu P, Sartini L, Di Comite M, Mori G, Di Benedetto A, Brunetti G, Yuen T, Sun L, Reseland JE, Colucci S, New MI, Zaidi M, Cinti S, Grano M. The myokine irisin increases cortical bone mass. Proc Natl Acad Sci U S A. 2015;112:12157–12162. doi: 10.1073/pnas.1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smitka K, Maresova D. Adipose tissue as an endocrine organ: an update on pro-inflammatory and anti-inflammatory microenvironment. Prague Med Rep. 2015;116:87–111. doi: 10.14712/23362936.2015.49. [DOI] [PubMed] [Google Scholar]
- 33.Izaola O, de Luis D, Sajoux I, Domingo JC, Vidal M. [Inflammation and obesity (lipoinflammation)] . Nutr Hosp. 2015;31:2352–2358. doi: 10.3305/nh.2015.31.6.8829. [DOI] [PubMed] [Google Scholar]
- 34.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (Lond) 2016;40:434–442. doi: 10.1038/ijo.2015.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


