Abstract
Accumulating evidence has revealed that human gingiva-derived mesenchymal stem cells (GMSCs) are emerging as a new line of mesenchymal stem cells and may have the potential to control or even treat autoimmune diseases through maintaining the balance between Th and Treg cells. Given that GMSCs have a robust immune regulatory function and regenerative ability, we investigated the effect of GMSCs on preventing T cell-mediated bone marrow failure (BMF) in a mouse model. We observed that GMSCs markedly improved mice survival and attenuated histological bone marrow (BM) damage. Moreover, we found GMSCs significantly reduced cell infiltration of CD8+ cells, Th1 and Th17 cells, whereas increased CD4+Foxp3+ regulatory T cells (Tregs) differentiation in lymph nodes. GMSCs also suppressed the levels of TNF-α, IFN-γ, IL-17A and IL-6, but IL-10 was increased in serum. The live in vivo imaging identified that GMSCs can home into inflammatory location on BM. Our results demonstrate that GMSCs attenuate T cell-mediated BMF through regulating the balance of Th1, Th17 and Tregs, implicating that application of GMSCs may provide a promising approach in prevention and treatment of patients with aplastic anemia.
Keywords: GMSC, bone marrow failure, Th1, Th17, Treg
Introduction
Human aplastic anemia (AA) is a rare autoimmune disease characterized by severe pancytopenia and bone marrow failure (BMF) [1]. A decreased number and function of hematopoietic stem and progenitor cells contributes to anemia, neutropenia, and thrombocytopenia [2]. In patients with AA, massive destruction of hematopoietic stem and progenitor cells by activated T lymphocytes containing Th1, Th17 cells is responsible for the development of pancytopenia, marrow hypoplasia and obliteration of the hematopoietic microenvironment destruction [3-5]. An abnormal quantity and function of regulatory T cells (Tregs) in peripheral blood of patients with AA has been previously reported and has emerged as the major cause of this condition [6].
Mesenchymal stem cells may have a unique advantage in treating BMF since they possess both the reparative, regenerative and immune suppressive properties that could not only suppress dysfunctional responses but also drive the recovery mechanisms that restore hematopoietic function [7]. Recent studies have demonstrated that the ability of MSC to downregulate T-cell priming, proliferation, and cytokine release is deficient in patients with AA, persists indefinitely after immunosuppressive therapy, but seems to be restored after BMF of mesenchymal stem cells (MSCs) from normal donors [8,9]. As a major component of the hematopoietic microenvironment, MSCs were found to be aberrant in acquired AA [10]. Paradoxically, MSCs from patients with AA were more readily induced to differentiate into adipocytes and had poor proliferation potential and a deficient support of hematopoietic colony-forming activity [11]. The pathophysiology of BMF has been best evaluated by animal models that also provide suitable systems for testing new therapeutic strategies. Thus, it seems important to explore whether other tissue-derived MSCs are able to prevent and treat AA. Recently, we and others have reported that human gingival tissue-derived MSCs (GMSCs) shared similar phenotypic and functional characteristics as other MSCs in multiple animal experimental models including colitis, collagen-induced arthritis, wound healing, and contact hypersensitivity [12-17]. In contrast to adipose-derived stem cells, BM-derived MSCs (BMSCs), and umbilical cord-derived MSCs (UC-MSCs), GMSCs have unique advantages, such as a more retrievable source, faster proliferation, and no potential risk of tumorigenesis during cell culture [18-20]. However, the effects of human GMSCs in a BMF animal model system have never been reported previously.
In the present study, we employed the multifaceted effects of human GMSCs on regulating the pathological developments, immunological homeostasis and homing dynamics of aplastic anemia animal model in vivo. We established a murine AA model and found that adoptive transfer of human GMSCs markedly improved the symptoms of AA mice through modification of infiltrated T cells, especially the Th1, Th17 and Treg cell populations. The distribution and homing dynamics of labeled GMSCs in AA mice were also observed in vivo. These findings recapitulated the therapeutic effects of human GMSCs against human aplastic anemia.
Materials and methods
Ethics statements
The research has been carried out in accordance with the World Medical Association Declaration of Helsinki. The ethics has been approved for studying human tissues. Human GMSCs tissues was obtained under informed consents from the healthy subjects who underwent wisdom teeth surgery in the Third Hospital at the Sun Yat-sen University. The animal study was carried out in accordance with the recommendations of the animal use protocol, which was approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University (Approve number: 160520). All institutional and national guidelines for the care and use of laboratory animals were followed.
Animals
Inbred C57BL/6 (B6, H2b/b) and hybrid CB6F1 (H2b/d) mice were purchased from the Charles River Laboratories (Beijing, China). They were maintained at animal facilities of the Sun Yat-sen University. Females were used at 8 to12 weeks of age. Donor and recipient C57BL/6 mice were matched for age and sex in each specific experiment.
Induction of BMF in AA mice
AA mice were established as previously described [21]. Briefly, CB6F1 mice were injected with 5 × 106 B6 LN cells in 0.2 mL PBS through the tail vein 4 to 6 hours after 5.0 Gy total body irradiation (TBI) from Rs2000 (Rad Source, USA), whereas mice received 5 Gy TBI without LN cell infusion as the TBI mice. The control mice were not received any treatment. The day when mice received TBI with or without LN cells was named as the 0th day. Mice received normal care, and were monitored daily for signs of disease until be humanely euthanized for tissue/cell collection at 14th day. For survival studies, mice were considered lethally induced on the day they were no longer able to take food or water, at which time they were humanely euthanized.
Isolation, culture and infusion of GMSCs
Human gingiva samples were collected following routine dental procedures at the Division of Dentistry in the Third Hospital at the Sun Yat-sen University, which were approved by the medical ethics committees of Institutional Review Boards (IRB) in the Third Hospital at the Sun Yat-sen University (IRB 2018-02-195-01). The characteristic information of donors for GMSCs was listed in Table S1. Human GMSCs were prepared from these tissues as previously described [16,22,23]. Briefly, gingival tissues were obtained from discarded tissues of patients who undergone routine dental procedures. Tissues were digested by dispase II (2 mg/mL) at 4°C overnight or 37°C 2 h followed with collagenase IV (4 mg/mL) at 37°C for 0.5 h (shaking the tissue every few minutes) to avoid fragment. Then the collected cell suspension was filtered through a 70-μm cells trainer and was centrifuged to harvest cell deposition. The cell deposition was re-suspended and transferred to 10 cm dish with MEM alpha medium containing 10% fetal bovine serum, 100 U/mL penicillin/100 μg/mL streptomycin at 37°C in an incubator with 5% CO2 and 95% O2. After 72 h, the non-adherent cells were discarded and the plastic-adherent cells were GMSCs. For GMSC characterization markers detection, GMSC were examined by flow cytometry with mAbs for human CD29, CD34, CD39, CD44, CD45, CD73, CD80, CD86, CD90, CD105, HLA-ABC, HLADR and CD11B. Characteristics of donor are shown in Figure S1. Fresh cells or stored GMSCs from the third to the fifth passages were used in the experiments. The primary cultured human dermal fibroblasts were used as a control since them share similar morphologies but lack the functional activities of GMSCs. Primary human dermal fibroblasts were isolated from the foreskin dermis of children aged between 6 and 8 years who underwent surgery in the Third Affiliated Hospital at the Sun Yat-sen University, which was approved by the medical ethics committee of IRB in the institute. Informed consents were obtained from all guardians of the donor participants. The dermal tissue was minced into small pieces and allowed to adhere to tissue culture flasks for 30 min in a CO2 incubator at 37°C. Then Dulbecco’s modification of Eagle’s medium supplemented with 10% fetal bovine serum was added to the flasks for allowing fibroblasts to grow out of tissue pieces and attaching to the culture flask until cells reached 80-90% confluence. Then fresh cells or stored Fibroblasts from the third to the fifth passages were used in the experiments. After AA mice were established, at the 6th day, 2 × 106 GMSCs or fibroblasts in 0.2 ml PBS were injected via the tail veins to per AA mouse. For GMSCs prevention experiments, GMSCs were injected via the tail veins to AA mice on the day 0.
Blood cell counts and peripheral blood smears
At the 6th, 10th and 14th day, 20 μL peripheral blood was collected from the tail vein. Complete blood counts were performed using a Mindray BC-5800 plus blood cell analyzer, and 5 μL peripheral blood was obtained for blood smear, and microscopic observation for lymphoproliferative activity and quantitation of nucleated cells.
Bone marrow mononuclear cell count and histologic examination
On the 14th day, mice were sacrificed by CO2 and cervical dislocation. BM cells were removed from the right femur by elution with PBS and centrifuged to harvest BM cells for count. The left femurs were fixed with 10% formalin, and stained with H&E. Histologic images were obtained by photography of microscopic sections.
RNA extraction and real-time RT-PCR quantitation
On the 14th day, mice were sacrificed as described above. Total RNA was isolated from lymph nodes by Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The first strand cDNAs were synthesized from 2 μg of total RNA in a 20 μL reaction using reverse transcriptase (5 × All-In-One-RT MasterMix, abm, USA). Next, a 2 μL aliquot of reverse transcription product was amplified with SsoFast™ EvaGreen (Bio-Rad, USA). The specific primers were designed from GenBank and synthesized by BGI (Shenzhen, China). The thermal profile reactions were performed in a real-time PCR system (Roche, Germany). The mocycler conditions included a three-step schedule as follows: 95°C for 10 min, 95°C for 15 s, and 60°C for 60 s for 40 cycles. The amplified products were quantified by measuring the calculated cycle thresholds (CT) for individual targets and β-actin mRNA. The 2-ΔΔCT method was used for quantification and statistical analysis. The primer sequences are listed in Table S2.
Enzyme-linked immunosorbent assay
Blood samples were collected from the retro-orbital sinus using EP tubes after the 14th day. Blood specimens (without anticoagulant) were kept at room temperature for 30 min, followed by centrifugation at 12000 g, 10 min. Sera were collected and stored at -80°C. The levels of, TNF-α, INF-γ, IL-6, IL-17A and IL-10 were detected by an ELISA assay (Bioo scientific, USA). To determine the levels of soluble cytokines such as IFN-γ and IL-17A, animal LN cells were harvested and cultured in fresh media on 12-well plates with PMA (50 ng/ml) and ionomycin (500 ng/ml) for 5 hours and then culture media was collected and concentrated by 100 KD ultra filtration device (Millipor, USA), and supernatants were subjected to an ELISA assay (ELISA kit, Bioo scientific, USA). OD values were read in the plates at 450 nm wavelength, using standard concentration/standard curves, and corresponding values were calculated based on the standard curves.
Surface and intracellular staining using a flow cytometry for murine samples
Lymph nodes obtained from mice were surface and intracellularly stained with fluorescent-conjugated antibodies. For Foxp3 staining, cells were fixed and permeabilized using the Foxp3 staining buffer set (eBioscience) according to the manufacturer’s protocol. For IFN-γ and IL-17 intracellular staining, cells were harvested and cultured in fresh media on 12-well plates with PMA (50 ng/ml), ionomycin (500 ng/ml) and Brefeldin A for 5 hours and then fixed with IC fixation buffer using the intracellular staining buffer set (Biolegend).
GMSC in vivo distribution
To track the GMSC distribution in vivo in AA model, a live in vivo imaging method was conducted. GMSC were re-suspended at a concentration of 1 × 106 cells/ml in PBS with 5 µM DiR (Red) (Thermo, MA, USA). After mixing, cells were incubated in the DiR/PBS solution for 15 min at 37°C in the dark, and then washed three times with PBS at a centrifugation of 300 g for 5 min. The final cells were re-suspended in PBS for injection into mice via tail vein immediately. In vivo tracking experiments, TBI or AA mice that had been intravenously injected with DIR-labeled cells 24 hrs later were imaged using the Bruker In Vivo MS FX PRO Imager (Bruker, Billerica, MA, USA) with the IVIS 200 small animal imaging system (PerkinElmer, Waltham, MA, USA) using the Ex filter at 700 nm and the Em filter at 780 nm. Background fluorescence was measured and subtracted by setting up a background measurement (Ex filter, 530 nm). The color image represents the spatial dose distribution of fluorescence, and white photographs of the mice were collected at the same time. Images were acquired and analyzed using the Living Image 4.0 software (PerkinElmer), as previously described [24].
Statistical analyses
All results are expressed as Mean ± SEMs. Multiple and student’s t tests were used for statistical analyses. The data were analyzed using SPSS 20.0 software, and p values below 0.05, 0.01 and 0.001 were assessed as statistically significant.
Results
Established a mouse model of immune-mediated BMF
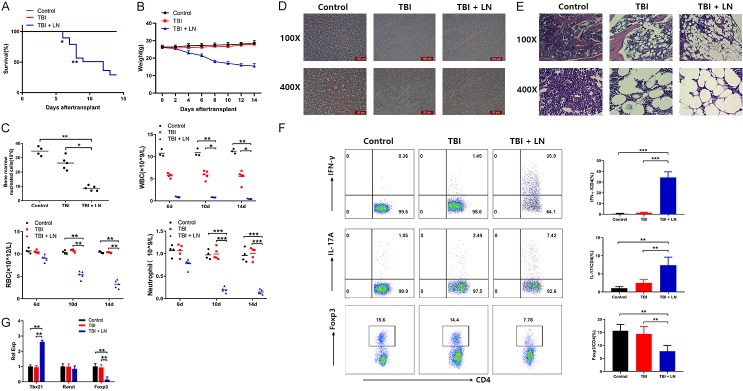
To explore the mechanisms of human AA, we optimized an animal model of immune-mediated BMF by infusion of B6 LN cells into recipient CB6F1 mice after irradiation. We observed the general changes of mice until 14 days. As expected, mice received 5 Gy TBI without LN cell infusion survived without marrow replacement. Whereas mice received LN cells after irradiation showed symptoms of malaise, anorexia, activity decrease, piloerection and arched shape, and began to die at the 6th day (P<0.05), also showed a significantly different death rate compared to control mice or TBI mice at the 8th day (P<0.01) (Figure 1A). The body weight can well reflect the life activities of AA mice, an increasing weight loss was also observed in TBI+LN mice compared to control mice or TBI mice (Figure 1B). Since BMF is the main pathologic feature of AA, we examined the BM cellularity. TBI+LN mice showed a significant reduction in the number of BM nucleated cells (Figure 1C left top). The changes of peripheral blood cells are considered as the main cause of anemia. The complete blood counts showed a significant reduction of TBI+LN mice in the number of WBC (Figure 1C right top), RBC (Figure 1C left bottom) and neutrophil compared to control mice or TBI mice (Figure 1C right bottom) at the 10th and 14th day. Additionally, peripheral blood smear also showed that the nucleated cells of TBI+LN mice were reduced (Figure 1D). We specially examined the damage of BM, by hematoxylin and eosin staining of BM specimens obtained from sternum of mice. Microscope images showed that, myeloproliferation was extremely low, the number of BM specimens obtained with nucleated cells was significantly reduced, a large number of fat cells replaced the hematopoietic cells, and megakaryocytes were almost absent in TBI+LN mice (Figure 1E). To confirm that the infiltrating T cells in TBI+LN mice were responsible for disease pathogenesis, we used flow cytometry to detect the percentages of Th1 (CD4+IFN-γ+), Th17 (CD4+IL-17+) and Treg (CD4+Foxp3+) cells in CD4+ cells isolated from lymph nodes of mice. TBI+LN mice had extremely high levels of Th1 and Th17 cells, as well as a 2.7-fold reduction of Treg cells compared to TBI mice (Figure 1F). In addition, we also used qPCR to examine Tbx21, RoRγt and Foxp3 mRNA expression levels that are main transfects of Th1, Th17 and Treg cells in spleen tissues. TBI+LN mice showed a dramatically increased Tbx21 and decreased Foxp3 level, although no significant differences of RoRγt mRNA were observed among all group mice (Figure 1G).
Figure 1.
Induction of BMF in AA mice recapitulates human disease. CB6F1 mice received 5 × 106 B6 LN 4 to 6 hours after 5.0 Gy irradiation. All mice were sacrificed at the 14th day and were examined for followed assays. Survival rates (A) and weight loss (B) were estimated by time course after irradiation with or without B6 LN reception; (C) The changes of BM nucleated cells and peripheral blood were estimated at day 6, 10 and 14; (D) Peripheral blood smears were estimated by microscope; (E) Left femurs were stained with hematoxylin and eosin at the 14th day and were imaged by microscope; (F) The expression levels of CD4+IFN-γ, CD4+IL-17A and CD4+Foxp3 in LN cells were performed by flow cytometry; (G) The relative quantity of Tbx21, RoRγt and Foxp3 in LN cells were measured by qPCR. Bar (C) shows the Mean ± SD for 4-6 mice from each other group. All data above are representative of three independent experiments (mean ± SEM). *P<0.05, **P<0.01, ***P<0.001. Authors have no conflict of interest.
Therapeutic administration of GMSCs prolongs the survival of AA mice
To determine whether GMSCs have therapeutic effect on AA mice, we injected GMSCs or control fibroblast cell to AA mice. Phenotypic analysis indicated that the fibroblast and GMSC populations were broadly similar (Figure S1). Moreover, the phenotypic feature of both cell populations has a difference on the expression of immune suppressor molecules. Phenotypic analysis indicated that the GMSCs used in this study were a mixed population. As showed in Figure 2A, at the 8th day, GMSCs treated AA mice showed a significant difference compared to fibroblasts-treated or PBS treated AA mice (P<0.05). At the 11th day, GMSCs treated AA mice notably prolonged survival of AA mice compared to fibroblasts treated or PBS treated AA mice (P<0.01). In the 14th day, fibroblasts-treated or PBS treated AA mice were all died but 50% of GMSCs treated AA mice were still survived. We also used weight change to evaluate the disease degrees of AA mice, we found that GMSCs treatment significantly reversed the body weight loss of AA mice (Figure 2B).
Figure 2.
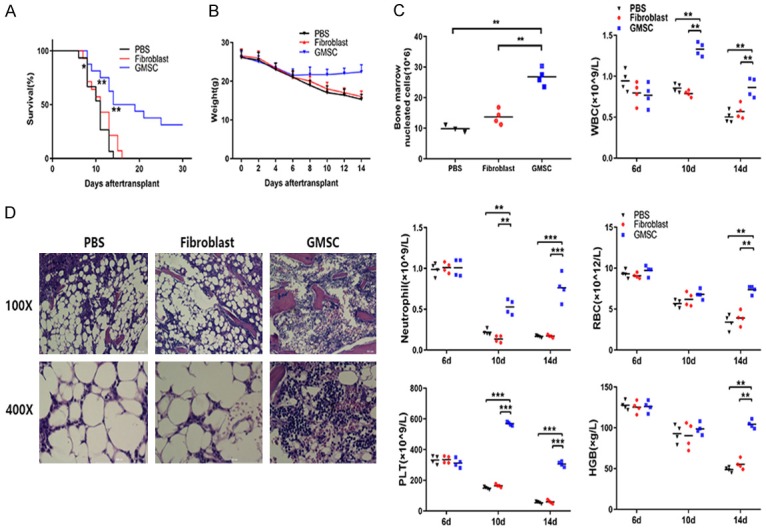
GMSCs attenuate lethal BMF in AA mice. GMSCs were injected via the tail veins to AA mice at 6th day. All mice were sacrificed at the 14th day and were examined for followed measures. Survival rate (A) and body weight changes (B) of AA mice were estimated by time course after GMSCs transplantation; (C) The number of BM nucleated cells and complete blood counts were performed using a blood cell analyzer in AA mice with or without GMSCs treatment; (D) Left femurs of AA mice were stained with hematoxylin and eosin and were imaged by microscope after GMSCs transplantation. Bar (C) show the mean ± SD for 3-5 mice from each other group. All data above are representative of three independent experiments (mean ± SEM). *P<0.05, **P<0.01, ***P<0.001. Authors have no conflict of interest.
To test whether GMSCs treatment affects AA disease severity by protecting BM and blood cells, we evaluated the following indicators including BM nucleated cells, WBC, neutrophil, RBC, PLT and HGB. In the BM, PBS treated AA mice and fibroblast treated AA mice produced severe BM hypoplasia, while GMSCs treated AA mice dramatically reversed this reduction (Figure 2C left top). For peripheral blood cells, at the 6th day, there was no significant differences in the numbers of WBC, neutrophil, RBC, PLT counts and mean HGB between GMSCs and without GMSCs treated AA mice. At the 10th day, the WBC, neutrophil and PLT counts of GMSCs infusion AA mice showed a significant increase compared to without GMSCs treated AA mice (1.7-fold to PBS treated AA mice, 1.5-fold to fibroblast treated AA mice in WBC, 4.7-fold to PBS treated AA mice, 5.2-fold to fibroblast treated AA mice in neutrophil and 3.8-fold to PBS treated AA mice, 3.5-fold to fibroblast treated AA mice in PLT). At the 14th day, the RBC, WBC, PLT counts and mean HGB of GMSCs infusion AA mice all increased significantly compared to without GMSCs treatment AA mice (P<0.01 or P<0.001 for all) (Figure 2C).
A separate set of experiments was also performed to assess the function of the transplanted GMSCs on AA mice. Several histopathological changes were observed in the BM. The myeloid, erythroid, and megakaryocytic cells of the GMSCs infusion AA mice were more visible and fat cells were significantly reduced compared to fibroblast treated or PBS treated AA mice (Figure 2D).
GMSCs attenuate T cells-mediated BMF in AA mice
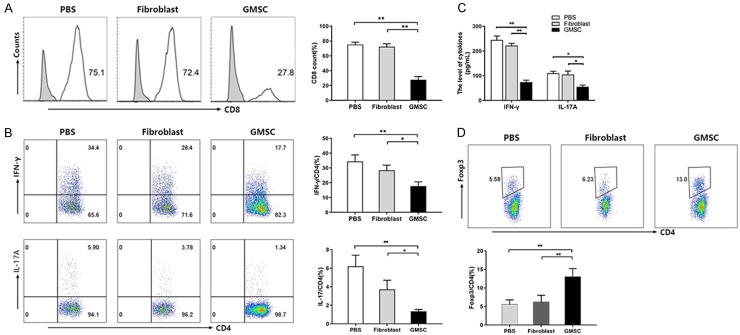
Not only peripheral pancytopenia and BM cell damage, but severe BM aplasia with T-cells infiltration also was observed in AA mice. The disorder of immune function especially cellular immune function is the main pathological mechanism of aplastic anemia. CD4+ cells selectively overexpressed Th1, Th2 and Th17 cells, but hold a low expressed Treg [25]. Th1, Th2 and Th17 can secret a variety of cytokines such as TNF-α, IFN-γ and IL-17, which then act on hematopoietic stem cells. On the other hand, it can promote the proliferation and differentiation of CD8, and mediate the apoptosis and necrosis of hematopoietic stem cells through perforin, granulase, TNF-α and other pathways [26]. To confirm the effects of GMSCs on infiltrated T cells, we first used flow cytometry to examine the frequencies of CD8+ cells in AA mice. As shown in Figure 3A, GMSCs significantly decreased the CD8+ cells percentage in lymph nodes. Among CD4+ cells subsets, GMSCs decreased the absolute ratios of Th1 cells (CD4+IFN-γ+ cells) and Th17 cells (CD4+IL-17+ cells) (Figure 3B). We also used an ELISA to measure the levels of soluble IFN-γ and IL-17A in the supernatants, LN cells obtained from AA mice were cultured in fresh medium on 12-well plates with PMA (50 ng/ml) and ionomycin (500 ng/ml) after 5 hours and then culture medium was collected for an ELISA assay. We found GMSCs also suppressed the protein levels of IFN-γ and IL-17A secreted by infiltrated T cells (Figure 3C). As regulatory T cell plays an important role in regulating immune function, the changes in its quantity and function can further amplify the functions of other immune cells, thus exacerbating aplastic anemia [4,27]. Foxp3, a critical transcriptional factor for Treg differentiation. Interestingly, we found that GMSCs increased frequency of Foxp3+ expression in CD4+ T cells in AA mice (Figure 3D). These data indicate that GMSCs treatment has attenuated infiltrating T pathogenic cells but also increased immune suppressor cells.
Figure 3.
GMSCs inhibit CD8+, Th1, Th17 cells and promote Tregs in AA mice. GMSCs were injected via the tail veins to AA mice at 6th day. All mice were sacrificed at the 14th day and were examined for followed measures. A. CD8+ T cells in LN cells were detected by flow cytometer; B. The expression levels of CD4+IFN-γ+ (Th1) and CD4+IL-17A+ (Th17) cells in LN cells were detected by flow cytometer; C. The protein levels of IFN-γ+ and IL-17A in serum were measured by an ELISA; D. The expression levels of CD4+Foxp3+ (Treg) cells in LN cells were detected by a flow cytometer. All data above are representative of three independent experiments (Mean ± SEM). *P<0.05, **P<0.01. Authors have no conflict of interest.
GMSCs reduce expression of pro-inflammatory cytokines and transcription factors in AA mice
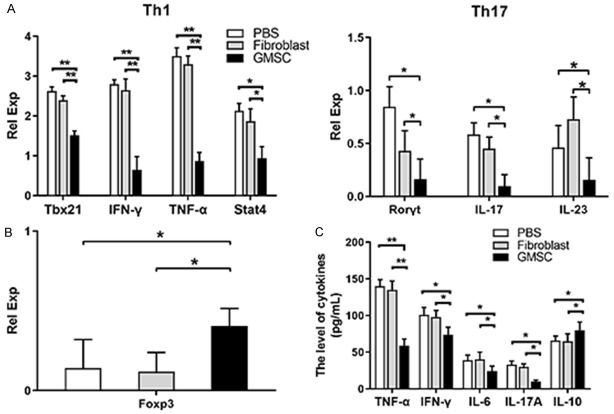
Cytokines are mediated and secreted by activated immune cells and certain stromal cells. CD4+ cells subsets regulate cell physiological function, mediate the inflammatory response, and participate in immune response. To further confirm the critical roles of T-cells mediated BMF and whether GMSCs regulate cytokines or transcription factors levels of Th1, Th17 and Treg cells in AA mice, we further utilized qPCR to detect Tbx21, IFN-γ, TNF-α, stat4, Rorγt, IL-17, IL-23 and Foxp3 in lymph nodes (containing groin, armpit, jaw, elbow and mesenteric lymph nodes) obtained from AA mice at the 14th day. As shown in Figure 4A, GMSCs extremely reduced mRNA expression of Tbx21, IFN-γ, TNF-α, stat4, Rorγt, IL-17, and IL-23. As primary immunosuppressive cells, Tregs also were detected through Foxp3, results showed that Foxp3 mRNA expression levels were significantly increased (Figure 4B). Pro-inflammatory and anti-inflammatory cytokines in serum can well reflect the condition of immunity homeostasis. Accordingly, serum samples were harvested from AA mice at the 14th day, and TNF-α, IFN-γ, IL-6, IL-17A and IL-10 levels were measured by an ELISA assay. Results revealed that GMSCs significantly reduced pro-inflammatory cytokines expression (TNF-α, IFN-γ, IL-6 and IL-17A), but increased anti-inflammatory cytokines IL-10 expression level (Figure 4C).
Figure 4.
GMSCs change inflammatory cytokines or transcription in AA mice. GMSCs were injected via the tail veins to AA mice at 6th day. All mice were sacrificed at the 14th day and were examined for followed measures. A. The relative quantity of cytokines or transcription factors of Th1, Th17 in lymph nodes; B. The relative quantity of Foxp3 in lymph nodes; C. The levels of TNF-α, IFN-γ, IL-6, IL-17A and IL-10 of serum. All data above are representative of three independent experiments (Mean ± SEM). *P<0.05, **P<0.01. Authors have no conflict of interest.
In vivo tracking of GMSCs in AA mice
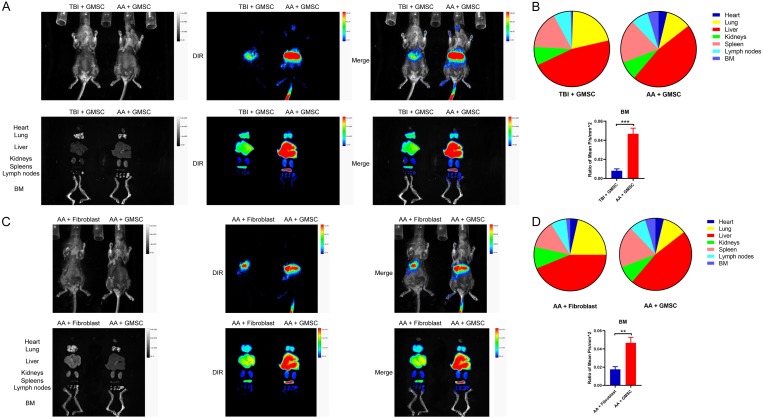
To determine whether transplanted GMSCs migrate to BM to contribute to the therapeutic effect of GMSCs on established AA mice, we also investigated the distribution of GMSC in AA mice using a live in vivo imaging. After transplantation of DIR-labeled cells for 24 h, animals and dissected heart, lung, liver, kidney, spleen, lymph nodes and legs (BM) were subjected to imaging using an in vivo small animal imaging device. First, 2 million DIR-labeled GMSCs were injected to TBI or AA mice for testing whether GMSCs particularly home to inflammatory location. As showed in Figure 5A fluorescent signal in BM was hardly detectable in TBI mouse, conversely, AA mouse image showed the evident fluorescence signal and intensity on BM, although the signal most detained in liver and lung. The fluorescence intensity values are presented in Figure 5B. Next, same numbers of DIR-labeled fibroblasts and GMSCs were injected to AA mice for determining the different distribution between them. Results showed that fluorescent signal in BM was rarely detected in fibroblasts injected mice, but fluorescence was clearly observed in BM in GMSCs injected mice (Figure 5C). The fluorescence intensity values are presented in Figure 5D.
Figure 5.
The distribution of GMSCs in AA mice. (A) 2 million DIR-labeled GMSCs were injected into TBI mice or AA mice. 24 hrs later, digital photo and IVIS images of animals and major organs were presented above; (B) Distribution of fluorescence intensity of organs in (A) and the ratio of BM mean fluorescence intensity values; (C) 2 million DIR-labeled fibroblasts or GMSCs were injected into AA mice. 24 hrs later, digital photo and IVIS images of animals and major organs were presented above; (D) Distribution of fluorescence intensity of organs in (C) and the ratio of BM mean fluorescence intensity values. Representative images from three separated experiments. Authors have no conflict of interest.
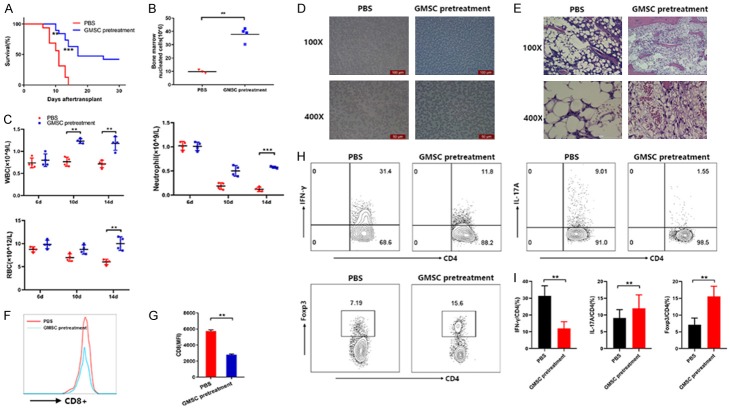
GMSCs pretreatment attenuates lethal BMF in AA mice
All above studies have evaluated the therapeutic potential of GMSCs, which were injected to AA mice at the 6th day. We then also assessed the prevention effects of GMSCs, which GMSC were injected to AA mice at baseline day 0. Survival result showed that GMSCs pretreatment significantly delayed the time point of death at the 10th day (P<0.01). When the 14th day, PBS treated AA mice were all died but 63% of GMSCs pretreated AA mice were still survived. GMSCs pretreatment notably prolonged AA mice survival (Figure 6A). GMSCs pretreated AA mice also showed a significant increased number of BM nucleated cells, WBC, neutrophil and RBC counts (Figure 6B and 6C). The peripheral blood smear showed relatively higher hyperplasia activity and visible nucleated cells (Figure 6D). Importantly, GMSCs pretreatment had significantly less severe disease as measured by hematoxylin and eosin staining of a sternum BM aspirate (Figure 6E). Most notably, GMSCs pretreatment not only reduced CD8+ T cells (Figure 6F and 6G), but also robustly inhibited Th1 (CD4+IFN-γ+), Th17 (CD4+IL-17+) cells and enhanced CD4+ Treg cells subset (Figure 6H and 6I). Thus, in proof-of-concept experiments, GMSCs pretreatment attenuated immune-mediated BMF in AA mice.
Figure 6.
GMSCs significantly prevents disease in AA mice. GMSCs were injected via the tail veins to AA mice at baseline day 0. All mice were sacrificed at the 14th day and were examined for followed measures. (A) Survival estimates of AA mice with GMSCs pretreatment; (B) The numbers of BM nucleated cells; (C) Complete blood counts; (D) Peripheral blood smears; (E) Left femurs were stained with hematoxylin and eosin; (F) The percentage of CD8+ T cells in LN cells was measured by flow cytometer; (G) MFI in (D) was shown; (H) The percentages of CD4+IFN-γ+, CD4+IL-17 and CD4+Foxp3+ cells were measured by a flow cytometer; (I) The statistical analysis of (H) is shown. Bar (B and C) show the mean ± SD for 3-5 mice from each other group. All data above are representative of three independent experiments (mean ± SEM). *P<0.05, **P<0.01.
Discussion
BM transplantation, anti-thymocyte globulin (ATG) and/or corticosteroids, and the requirement of cyclosporine to maintain response, etc, have a clinical therapeutic role in responding to BM-infiltrating T cells dysfunction [28,29]. MSCs transplantation was reported to have an extensive evaluation as a cellular therapy in human AA [30-35], and increasing evidence shows MSCs yield therapeutic effects that were largely mediated by secretion of soluble factors and cytokines [36]. In this study, we found GMSCs attenuated lethal BMF and improved the survival of AA mice, extending the usage of GMSCs in treating autoimmune and inflammatory diseases [16,37-40], and providing evidence that GMSCs represent a convenient cell therapy approach for aplastic anemia.
Ray irradiation severely damages the BM hematopoietic cells and the hematopoietic microenvironment but not causes direct death. When recipient mice were irradiated, mice did not suffer from acute GVHD [41]. Interestingly, the infusion of exogenous lymphocytes causes even more severe bone marrow damage. Thus, a model that CB6F1 mice following adoptive transfer of B6 lymph node cells, shares similar physiological and biological characteristics to patients with AA [21], providing an ideal tool to study the pathogenesis and therapeutic intervention. In this study, CB6F1 mice (the F1 of BALB/c female and C57BL/6 male hybrid) were utilized. Mice were received 5 × 106 B6 LN cells 4 to 6 hours after 5.0 Gy TBI to establish a AA animal model. Our data is consistent with that of Chen et al who developed a model accompanied by severe pancytopenia and marrow hypoplasia and destruction [21].
AA is an immune-mediated disease. The disorder of immune system especially T cells plays an important role in the development of AA. T lymphocytes are the main effector cells in cellular immunity [42,43]. Studies have found that patients with aplastic anemia not only have an abnormal number of T cells, but also have significant changes in the distribution, phenotype and function of T cells. It has been proved that abnormal immune function in aplastic patients is associated with abnormal activity and proliferation of T cells that may kill and inhibit hematopoietic stem/progenitor cells. Abnormal proliferation of CD8+ T cells and macrophages inhibits the proliferation and differentiation of BM hematopoietic stem cells [44]. T lymphocytes can produce hematopoietic stimulating factors and negative hematopoietic regulatory factors after stimulation, but due to the imbalance of lymphocyte cytokines in aplastic anemia patients, excessive negative hematopoietic regulatory factors are produced, showing obvious hematopoietic inhibitory activity [45]. Th1 cells in AA can secrete a variety of immune molecules including IFN-γ, TNF-α directly to promote disease. Immoderate production of IFN-γ, TNF-α, and IL-2 suggests the possibility that the hematopoietic cells are destroyed through Th1 response, as illustrated by the transcriptional up-regulation of inflammatory cytokines, such as TNF-α, stat4, IFN-γ [46-48]. IFN-γ mainly inhibits hematopoiesis through the cell cycle of tissues. TNF-α can up-regulate cell receptor Fas, enhance cell sensitivity to apoptosis through Fas/Fas L, and induce excessive apoptosis of hematopoietic stem/progenitor cells [49]. In addition, Th1 cells can promote the activation of NK, CD8, and macrophage cells which could further secrete various cytokines including IFN-γ, TNF-α, IL-6, IL-2 and then mediate disease progression [2]. Our model is in line with that of Gravano et al who demonstrated a critical role of CD8+ and Th1 cells in immune-mediated BMF [50-52]. We provided evidence that CD8+ T cells, Th1 cells and cytokines of IFN-γ and TNF-α were notably suppressed after consecutive transplantation with GMSCs in AA mice.
Several studies have reported the association of IL-17 with inflammatory disorders such as rheumatoid arthritis, asthma, multiple sclerosis and lupus [53-57], as well as hematological disorders such as myelodysplastic syndrome and acute myeloid leukemia [58,59]. We hypothesized that Th17 cells could contribute to the development of BMF in AA mice, like in some AA patients [60]. While Treg cells play an immunosuppressive role [61-63], and an abnormal quantity and function of Tregs in peripheral blood of patients with AA has been reported and has emerged as the major cause of the condition [44]. The disorder of Th17/Treg cells plays an important role in the aplastic anemia [27,33,64,65]. Li et al addressed that BMSCs regulate the Treg/Th17 balance by affecting the Notch/RBP-J/FOXP3/RORγt pathway [33]. Here we examined the role of GMSCs in Th17 immune responses in AA mice by regulating the immunity homeostasis. In accordance with the present results, we present evidence that GMSCs markedly suppressed Th17 and increased Treg cell frequency, resulting in the hematologic recover and the survival prolong of AA mice. Interestingly, GMSCs suppressed the secretion of IL-17A and IL-6 but increased anti-inflammatory cytokines of IL-10 levels. It is parallel that GMSCs inhibited Th1, Th17 cells, while promoted the expansion of CD4+ Tregs in AA mice, indicating an immunosuppression ability of GMSCs for immune-mediated BMF. It is possible that GMSC exerted their suppression via their secretion of soluble molecules as we recently observed that exosomes derived from GMSC have a similar function to the parent cells (data not shown). It has been extensively established that IL-10 and/or Treg cells markedly suppress inflammatory and autoimmune responses [63,66-68]. Additionally, MSCs also secret various soluble molecules, such as TGF-β1, HGF, PGE2, IL-10, IDO, NO, HO-1, and HLA-G to exert their immunoregulatory action. Moreover, we recently reported that GMSCs improved xeno-GVHD mice survival in a humanized animal model and suppressed osteoclastogenesis and bone erosion, which involved with CD39-CD73-adenosine signal pathway [22,40]. CD39 regulates immune responses balance by dephosphorylating ATP to ADP [71]. As a result, activated MSCs contribute to the restoration of damaged tissues or organs.
Several studies have shown that when tissues are damaged, MSCs from bone marrow or from the exogenous infusion are generally localized to the inflammatory area and damaged sides with low limited efficiency [69,70]. The current experimental results showed that the GMSCs infusion could localize to the bone marrow with 24 h in the AA model, suggesting that GMSCs were homing to the damaged bone marrow after infusion via blood circulation, and performed hematopoietic support, repair function and local immune regulation of the bone marrow microenvironment by homing to the bone marrow through direct contact or secretion of soluble bioactive molecules.
It is well recognized that MSCs have low immunogenicity portability due to the lack of major histocompatibility complex class II molecules or lymphocyte costimulatory molecules [72]. MSC’ immunoregulatory activity is not constitutive and could be classified as pro-inflammatory MSC1 and immunosuppressive MSC2 phenotype for response to different TLR-priming stimulation [73]. In addition, there is a unified standard protocol to identify the phenotype of MSC characteristics. MSCs are acceptable for clinical application because they are easy to be collected and expanded. Most importantly, recent studies have revealed that GMSC have several advantages over other MSCs. GMSC are highly homogenous and proliferate more rapidly, with stable phenotypes and functional characteristics and no potential risk of tumorigenesis [18-20]. In addition, the safety of GMSC has been recently documented [74].
In conclusion, our findings indicate GMSCs transplantation may treat immune-mediated BMF of aplastic anemia via suppression of Th1 and Th17 cells and enhancement of CD4+Foxp3+ regulatory T cells differentiation. In the near future, we will conduct the clinical trial to determine the safety and efficacy of human GMSCs, particularly GMSCs derived from patients with AA, on patients with AA.
Acknowledgements
This study was supported by the National Key R&D Program of China (2017YFA0105800), the National Natural Science Foundation of China (No. 81671611), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2016ZT06S252), the National Natural Science Foundation of Guangdong Province (No. 2014A030313131) (all to F.H), from Guangxi Science and Technology Base and Specialized Talent grant (to G.Q) and S.Z is supported by the NIH R61 AR073409, R01 059103 and STAR Award.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol. 2008;15:162–168. doi: 10.1097/MOH.0b013e3282fa7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509–2519. doi: 10.1182/blood-2006-03-010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomou EE, Keyvanfar K, Young NS. T-bet, a Th1 transcription factor, is up-regulated in T cells from patients with aplastic anemia. Blood. 2006;107:3983–3991. doi: 10.1182/blood-2005-10-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Latour RP, Visconte V, Takaku T, Wu C, Erie AJ, Sarcon AK, Desierto MJ, Scheinberg P, Keyvanfar K, Nunez O, Chen J, Young NS. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood. 2010;116:4175–4184. doi: 10.1182/blood-2010-01-266098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park M, Park CJ, Cho YW, Jang S, Lee JH, Lee JH, Lee KH, Lee YH. Alterations in the bone marrow microenvironment may elicit defective hematopoiesis: a comparison of aplastic anemia, chronic myeloid leukemia, and normal bone marrow. Exp Hematol. 2017;45:56–63. doi: 10.1016/j.exphem.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Fu R, Liu H, Wang H, Liu C, Wang T, Qi W, Guan J, Li L, Shao Z. Abnormal quantity and function of regulatory T cells in peripheral blood of patients with severe aplastic anemia. Cell Immunol. 2015;296:95–105. doi: 10.1016/j.cellimm.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Zachar L, Bacenkova D, Rosocha J. Activation, homing, and role of the mesenchymal stem cells in the inflammatory environment. J Inflamm Res. 2016;9:231–240. doi: 10.2147/JIR.S121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacigalupo A, Valle M, Podesta M, Pitto A, Zocchi E, De Flora A, Pozzi S, Luchetti S, Frassoni F, Van Lint MT, Piaggio G. T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol. 2005;33:819–827. doi: 10.1016/j.exphem.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 2010;5:e9016. doi: 10.1371/journal.pone.0009016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 11.Chao YH, Peng CT, Harn HJ, Chan CK, Wu KH. Poor potential of proliferation and differentiation in bone marrow mesenchymal stem cells derived from children with severe aplastic anemia. Ann Hematol. 2010;89:715–723. doi: 10.1007/s00277-009-0892-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, Le AD. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–383. doi: 10.1016/j.bbrc.2010.01.126. [DOI] [PubMed] [Google Scholar]
- 14.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–1868. doi: 10.1002/stem.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29:1849–1860. doi: 10.1002/stem.738. [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Su W, Lin X, Guo Z, Wang J, Zhang Q, Brand D, Ryffel B, Huang J, Liu Z, He X, Le AD, Zheng SG. Adoptive transfer of human gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis via suppression of Th1 and Th17 cells and enhancement of regulatory T cell differentiation. Arthritis Rheum. 2013;65:1181–1193. doi: 10.1002/art.37894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Deng Y, Liang J, Huang F, Qiu W, Zhang M, Long Y, Hu X, Lu Z, Liu W, Zheng SG. Mesenchymal stromal cells attenuate multiple sclerosis via IDO-dependent increasing the suppressive proportion of CD5+ IL-10+ B cells. Am J Transl Res. 2019;11:5673–5688. [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaoka E, Hiyama E, Sotomaru Y, Onitake Y, Fukuba I, Sudo T, Sueda T, Hiyama K. Neoplastic transformation by TERT in FGF-2-expanded human mesenchymal stem cells. Int J Oncol. 2011;39:5–11. doi: 10.3892/ijo.2011.1029. [DOI] [PubMed] [Google Scholar]
- 19.Redaelli S, Bentivegna A, Foudah D, Miloso M, Redondo J, Riva G, Baronchelli S, Dalpra L, Tredici G. From cytogenomic to epigenomic profiles: monitoring the biologic behavior of in vitro cultured human bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2012;3:47. doi: 10.1186/scrt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Lipovsky K, Ellison FM, Calado RT, Young NS. Bystander destruction of hematopoietic progenitor and stem cells in a mouse model of infusion-induced bone marrow failure. Blood. 2004;104:1671–1678. doi: 10.1182/blood-2004-03-1115. [DOI] [PubMed] [Google Scholar]
- 22.Huang F, Chen M, Chen W, Gu J, Yuan J, Xue Y, Dang J, Su W, Wang J, Zadeh HH, He X, Rong L, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells inhibit xeno-graft-versus-host disease via CD39-CD73-adenosine and IDO signals. Front Immunol. 2017;8:68. doi: 10.3389/fimmu.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zeng D, Huang F, Wang J. A protocol for isolation and culture of mesenchymal stem cells from human gingival tissue. Am J Clin Exp Immunol. 2019;8:21–26. [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton EJ, Boddington SE, Nedopil AJ, Henning TD, Demos SG, Baehner R, Sennino B, Lu Y, Daldrup-Link HE. An optical imaging method to monitor stem cell migration in a model of immune-mediated arthritis. Opt Express. 2009;17:24403–24413. doi: 10.1364/OE.17.024403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kordasti S, Marsh J, Al-Khan S, Jiang J, Smith A, Mohamedali A, Abellan PP, Veen C, Costantini B, Kulasekararaj AG, Benson-Quarm N, Seidl T, Mian SA, Farzaneh F, Mufti GJ. Functional characterization of CD4+ T cells in aplastic anemia. Blood. 2012;119:2033–2043. doi: 10.1182/blood-2011-08-368308. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Basu A, Melenhorst JJ, Young NS, Brown KE. Analysis of T-cell repertoire in hepatitis-associated aplastic anemia. Blood. 2004;103:4588–4593. doi: 10.1182/blood-2003-11-3959. [DOI] [PubMed] [Google Scholar]
- 27.Solomou EE, Rezvani K, Mielke S, Malide D, Keyvanfar K, Visconte V, Kajigaya S, Barrett AJ, Young NS. Deficient CD4+ CD25+ FOXP3+ T regulatory cells in acquired aplastic anemia. Blood. 2007;110:1603–1606. doi: 10.1182/blood-2007-01-066258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young NS. Pathophysiologic mechanisms in acquired aplastic anemia. Hematology Am Soc Hematol Educ Program. 2006:72–77. doi: 10.1182/asheducation-2006.1.72. [DOI] [PubMed] [Google Scholar]
- 29.Yang N, Chen J, Zhang H, Dai Z, Yao H, Ma X, Bai J, Zhang Y, Zhang W. Horse versus rabbit antithymocyte globulin in immunosuppressive therapy of treatment-naive aplastic anemia: a systematic review and meta-analysis. Ann Hematol. 2017;96:2031–2043. doi: 10.1007/s00277-017-3136-1. [DOI] [PubMed] [Google Scholar]
- 30.Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J, Ljungman P, Lonnies H, Nava S, Ringden O. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia. 2007;21:1733–1738. doi: 10.1038/sj.leu.2404777. [DOI] [PubMed] [Google Scholar]
- 31.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 32.Broglie L, Margolis D, Medin JA. Yin and Yang of mesenchymal stem cells and aplastic anemia. World J Stem Cells. 2017;9:219–226. doi: 10.4252/wjsc.v9.i12.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Wang L, Pang Y, Jiang Z, Liu Z, Xiao H, Chen H, Ge X, Lan H, Xiao Y. In patients with chronic aplastic anemia, bone marrow-derived MSCs regulate the Treg/Th17 balance by influencing the Notch/RBP-J/FOXP3/RORgammat pathway. Sci Rep. 2017;7:42488. doi: 10.1038/srep42488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao YH, Tsai C, Peng CT, Wu HP, Chan CK, Weng T, Wu KH. Cotransplantation of umbilical cord MSCs to enhance engraftment of hematopoietic stem cells in patients with severe aplastic anemia. Bone Marrow Transplant. 2011;46:1391–1392. doi: 10.1038/bmt.2010.305. [DOI] [PubMed] [Google Scholar]
- 35.Jaganathan BG, Tisato V, Vulliamy T, Dokal I, Marsh J, Dazzi F, Bonnet D. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia. 2010;24:1791–1795. doi: 10.1038/leu.2010.164. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Lu S, Yang S, Xing W, Feng J, Li W, Zhao Q, Wu H, Ge M, Ma F, Zhao H, Liu B, Zhang L, Zheng Y, Han ZC. Impaired immunomodulatory ability of bone marrow mesenchymal stem cells on CD4(+) T cells in aplastic anemia. Results Immunol. 2012;2:142–147. doi: 10.1016/j.rinim.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Zhou L, Dang J, Zhang X, Wang J, Chen Y, Liang J, Li D, Ma J, Yuan J, Chen W, Zadeh HH, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells ameliorate streptozoticin-induced T1DM in mice via suppression of T effector cells and up-regulating treg subsets. Sci Rep. 2017;7:15249. doi: 10.1038/s41598-017-14979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang F, Liu ZM, Zheng SG. Updates on GMSCs treatment for autoimmune diseases. Curr Stem Cell Res Ther. 2018;13:345–349. doi: 10.2174/1574888X13666180220141114. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Huang F, Li W, Dang JL, Yuan J, Wang J, Zeng DL, Sun CX, Liu YY, Ao Q, Tan H, Su W, Qian X, Olsen N, Zheng SG. Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front Immunol. 2018;9:878. doi: 10.3389/fimmu.2018.00878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luo Y, Wu W, Gu J, Zhang X, Dang J, Wang J, Zheng Y, Huang F, Yuan J, Xue Y, Fu Q, Kandalam U, Colello J, Zheng SG. Human gingival tissue-derived MSC suppress osteoclastogenesis and bone erosion via CD39-adenosine signal pathway in autoimmune arthritis. EBioMedicine. 2019;43:620–631. doi: 10.1016/j.ebiom.2019.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu J, Lu L, Chen M, Xu L, Lan Q, Li Q, Liu Z, Chen G, Wang P, Wang X, Brand D, Olsen N, Zheng SG. TGF-beta-induced CD4+Foxp3+ T cells attenuate acute graft-versus-host disease by suppressing expansion and killing of effector CD8+ cells. J Immunol. 2014;193:3388–3397. doi: 10.4049/jimmunol.1400207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan HF, Leng RX, Li XP, Zheng SG, Ye DQ. Targeting T-helper 9 cells and interleukin-9 in autoimmune diseases. Cytokine Growth Factor Rev. 2013;24:515–522. doi: 10.1016/j.cytogfr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol. 2018;9:784. doi: 10.3389/fimmu.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng W, Kajigaya S, Chen G, Risitano AM, Nunez O, Young NS. Transcript profile of CD4+ and CD8+ T cells from the bone marrow of acquired aplastic anemia patients. Exp Hematol. 2004;32:806–814. doi: 10.1016/j.exphem.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F, Gabbas A, Dufour C, Arcese W, Testi G, Broccia G, Carotenuto M, Coser P, Barbui T, Leoni P, Ferster A. Antilymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midolio Osseo (GITMO) Blood. 2000;95:1931–1934. [PubMed] [Google Scholar]
- 46.Jin J, Chen F, Wang Q, Qiu Y, Zhao L, Guo Z. Inhibition of TNF-alpha by cyclophosphamide reduces myocardial injury after ischemia-reperfusion. Ann Thorac Cardiovasc Surg. 2013;19:24–29. doi: 10.5761/atcs.oa.11.01877. [DOI] [PubMed] [Google Scholar]
- 47.Mao YF, Zheng XF, Cai JM, You XM, Deng XM, Zhang JH, Jiang L, Sun XJ. Hydrogen-rich saline reduces lung injury induced by intestinal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2009;381:602–605. doi: 10.1016/j.bbrc.2009.02.105. [DOI] [PubMed] [Google Scholar]
- 48.Xu Z, Prathapasinghe G, Wu N, Hwang SY, Siow YL, O K. Ischemia-reperfusion reduces cystathionine-beta-synthase-mediated hydrogen sulfide generation in the kidney. Am J Physiol Renal Physiol. 2009;297:F27–35. doi: 10.1152/ajprenal.00096.2009. [DOI] [PubMed] [Google Scholar]
- 49.Hinterberger W, Rowlings PA, Hinterberger-Fischer M, Gibson J, Jacobsen N, Klein JP, Kolb HJ, Stevens DA, Horowitz MM, Gale RP. Results of transplanting bone marrow from genetically identical twins into patients with aplastic anemia. Ann Intern Med. 1997;126:116–122. doi: 10.7326/0003-4819-126-2-199701150-00004. [DOI] [PubMed] [Google Scholar]
- 50.Gravano DM, Al-Kuhlani M, Davini D, Sanders PD, Manilay JO, Hoyer KK. CD8(+) T cells drive autoimmune hematopoietic stem cell dysfunction and bone marrow failure. J Autoimmun. 2016;75:58–67. doi: 10.1016/j.jaut.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Y, Desierto MJ, Chen J, Young NS. The role of the Th1 transcription factor T-bet in a mouse model of immune-mediated bone-marrow failure. Blood. 2010;115:541–548. doi: 10.1182/blood-2009-03-211383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chewning JH, Zhang W, Randolph DA, Swindle CS, Schoeb TR, Weaver CT. Allogeneic Th1 cells home to host bone marrow and spleen and mediate IFNgamma-dependent aplasia. Biol Blood Marrow Transplant. 2013;19:876–887. doi: 10.1016/j.bbmt.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen X, Chang L, Li X, Huang J, Yang L, Lai X, Huang Z, Wang Z, Wu X, Zhao J, Bellanti JA, Zheng SG, Zhang G. Tc17/IL-17A up-regulated the expression of MMP-9 via NF-kappaB pathway in nasal epithelial cells of patients with chronic rhinosinusitis. Front Immunol. 2018;9:2121. doi: 10.3389/fimmu.2018.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li N, Wang JC, Liang TH, Zhu MH, Wang JY, Fu XL, Zhou JR, Zheng SG, Chan P, Han J. Pathologic finding of increased expression of interleukin-17 in the synovial tissue of rheumatoid arthritis patients. Int J Clin Exp Pathol. 2013;6:1375–1379. [PMC free article] [PubMed] [Google Scholar]
- 56.Luo Y, Zheng SG. Hall of fame among pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front Immunol. 2016;7:604. doi: 10.3389/fimmu.2016.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma J, Yu J, Tao X, Cai L, Wang J, Zheng SG. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2010;29:1251–1258. doi: 10.1007/s10067-010-1510-7. [DOI] [PubMed] [Google Scholar]
- 58.Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, Matthews K, Chelliah R, Guinn B, Lombardi G, Farzaneh F, Mufti GJ. IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol. 2009;145:64–72. doi: 10.1111/j.1365-2141.2009.07593.x. [DOI] [PubMed] [Google Scholar]
- 59.Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, Qian J, Jin J, Xu H. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu Y, Hu X, Liu C, Qv X, Xu C. Interleukin (IL)-17 promotes macrophages to produce IL-8, IL-6 and tumour necrosis factor-alpha in aplastic anaemia. Br J Haematol. 2008;142:109–114. doi: 10.1111/j.1365-2141.2008.07161.x. [DOI] [PubMed] [Google Scholar]
- 61.Gu J, Ni X, Pan X, Lu H, Lu Y, Zhao J, Guo Zheng S, Hippen KL, Wang X, Lu L. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol. 2017;14:521–528. doi: 10.1038/cmi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu A, Liu Y, Chen W, Wang J, Xue Y, Huang F, Rong L, Lin J, Liu D, Yan M, Li QZ, Li B, Song J, Olsen N, Zheng SG. TGF-beta-induced regulatory T cells directly suppress B cell responses through a noncytotoxic mechanism. J Immunol. 2016;196:3631–3641. doi: 10.4049/jimmunol.1501740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gao Y, Tang J, Chen W, Li Q, Nie J, Lin F, Wu Q, Chen Z, Gao Z, Fan H, Tsun A, Shen J, Chen G, Liu Z, Lou Z, Olsen NJ, Zheng SG, Li B. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc Natl Acad Sci U S A. 2015;112:E3246–3254. doi: 10.1073/pnas.1421463112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi J, Ge M, Lu S, Li X, Shao Y, Huang J, Huang Z, Zhang J, Nie N, Zheng Y. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood. 2012;120:1624–1632. doi: 10.1182/blood-2011-11-390708. [DOI] [PubMed] [Google Scholar]
- 65.Li H, Lin W, Yan P, Jiang Z, Liu Z, Xiao H, Chen H, Ge X, Hai L, Yang X. In patients with chronic aplastic anemia, bone marrow-derived MSCs regulate the Treg/Th17 balance by influencing the Notch/RBP-J/FOXP3/RORγt pathway. Sci Rep. 2017;7:42488. doi: 10.1038/srep42488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front Immunol. 2018;9:455. doi: 10.3389/fimmu.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25- cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y, Lan Q, Lu L, Chen M, Xia Z, Ma J, Wang J, Fan H, Shen Y, Ryffel B, Brand D, Quismorio F, Liu Z, Horwitz DA, Xu A, Zheng SG. Phenotypic and functional characteristic of a newly identified CD8+ Foxp3- CD103+ regulatory T cells. J Mol Cell Biol. 2014;6:81–92. doi: 10.1093/jmcb/mjt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park BN, Shim W, Lee G, Bang OY, An YS, Yoon JK, Ahn YH. Early distribution of intravenously injected mesenchymal stem cells in rats with acute brain trauma evaluated by (99m)Tc-HMPAO labeling. Nucl Med Biol. 2011;38:1175–1182. doi: 10.1016/j.nucmedbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 70.Cruz M, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser M. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int. 2012;2012:612946. doi: 10.1155/2012/612946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su W, Chen X, Zhu W, Yu J, Li W, Li Y, Li Z, Olsen N, Liang D, Zheng SG. The cAMP-adenosine feedback loop maintains the suppressive function of regulatory T cells. J Immunol. 2019;203:1436–1446. doi: 10.4049/jimmunol.1801306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.You L, Mao L, Wei J, Jin S, Yang C, Liu H, Zhu L, Qian W. The crosstalk between autophagic and endo-/exosomal pathways in antigen processing for MHC presentation in anticancer T cell immune responses. J Hematol Oncol. 2017;10:165. doi: 10.1186/s13045-017-0534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cassano JM, Schnabel LV, Goodale MB, Fortier LA. The immunomodulatory function of equine MSCs is enhanced by priming through an inflammatory microenvironment or TLR3 ligand. Vet Immunol Immunopathol. 2018;195:33–39. doi: 10.1016/j.vetimm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 74.Zhao J, Wang J, Dang J, Zhu W, Chen Y, Zhang X, Xie J, Hu B, Huang F, Sun B, Bellanti JA, Zheng SG. A preclinical study-systemic evaluation of safety on mesenchymal stem cells derived from human gingiva tissue. Stem Cell Res Ther. 2019;10:165. doi: 10.1186/s13287-019-1262-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.