Abstract
Objective: Post-infectious irritable bowel syndrome (PI-IBS) is a common functional gastrointestinal (GI) disorder that occurs after acute GI infection. Recent studies showed that microRNAs were involved in the occurrence and development of IBS. Here, we elaborated the role of miR-510 in the occurrence of PI-IBS and analyzed its mechanism. Methods: We detected the expressions of miR-510 and PRDX1 in colonic mucosal tissues by qRT-PCR, Western blot and immunohistochemistry. Furthermore, we transfected Caco-2 cells with miR-510 mimic, anti-miR-510, si-PRDX1, and control, then evaluated the cell viability and apoptosis by CCK8 assay and flow cytometry, assessed expression levels of PRDX1 by qRT-PCR and Western blot analysis, and pro-inflammatory cytokines by qRT-PCR and ELISA. Results: MiR-510 expression was downregulated and negatively correlated with TNF-α, whereas PRDX1 expression was upregulated in PI-IBS colonic mucosal tissues. LPS at concentrations of 5 and 10 μg/ml can significantly induce inflammatory injury in Caco-2 cells. MiR-510 overexpression aggravated the injury induced by LPS, as reflected by increased cell viability, decreased apoptosis, and less production of pro-inflammatory cytokines. miR-510 mimic transfection in cells significantly suppressed the mRNA and protein expression levels of PRDX1. Furthermore, the inflammatory injury induced by LPS was exacerbated by upregulating PRDX1 expression when miR-510 was knocked down. Conclusion: MiR-510 downregulation in intestinal tissue might contribute to PI-IBS via targeting PRDX1. The results of this study will not only enrich the pathogenesis of PI-IBS but also make us understand the biological activity of miR-510 and provide important experimental basis for PI-IBS clinical treatment targeting miR-510.
Keywords: Post-infectious irritable bowel syndrome, miR-510, inflammation, peroxiredoxin 1
Introduction
Irritable bowel syndrome (IBS) has been considered as a common functional gastrointestinal (GI) disorder [1,2]. It is characterized by the presence of chronic or recurrent abdominal pain or bloating and discomfort [3,4]. After acute GI infection, 3% to 35% of IBS patients develop post-infectious irritable bowel syndrome (PI-IBS) [5]. PI-IBS patients have more severe intestinal symptoms, such as colonic hypercontractility, than IBS patients [6]. Although PI-IBS is not a life-threatening disease similar to bowel cancer, the occurrence of diarrhea and abdominal pain needs to be treated through stress management and diet control, which result in a serious decline in quality of life, unemployment, and increased medical costs [7]. Although the exact pathogenesis of PI-IBS remains unclear, growing evidence shows that persistent inflammatory response in intestinal tissue is a prominent pathological feature of PI-IBS [8]. The number of T-lymphocytes and enterochromaffin cells in intestinal tissues in a PI-IBS mouse model increased significantly, suggesting a possible persistent inflammatory reaction after the recovery of intestinal infection [8]. In the PI-IBS mouse model, the expression levels of interferon-γ, IL-1β, IL-17, and IL-12 in intestinal tissues were significantly higher than those of control mice, and the expression levels of IL-10 and IL-4 were significantly decreased [9,10].
MicroRNAs (miRNAs) are small non-coding RNAs that regulate the genome expression by binding to the 3’-untranslated region (UTR) of the target mRNA complementarily, causing the target mRNA to be cut or transcriptionally suppressed [11,12]. By regulating the expression of target genes, miRNA can participate in the occurrence and development of various diseases, such as cancer and inflammation. Recently, some miRNAs were found to be associated with GI disorders, including IBS [13-15]. Currently, miRNAs associated with IBS-D include miR-23b, miR-378, hsv2-miR-H11, and miR-29 [16-19].
Our previous study showed that the miR-510 mRNA expression in the colonic mucosal tissues was dramatically decreased in IBS-D [20]. However, the specific mechanism by which miR-510 participates in the development and progression of IBS-Dis not clear. In this work, we investigated the expression and biological function of miR-510 in PI-IBS. We also studied its potential possible mechanism.
Methods
Statement of ethics
Colonic mucosal tissues from PI-IBS and control cases were obtained from the Department of Gastroenterology in the Affiliated Hospital of Yangzhou University. All patients signed informed consent first and then underwent screening colonoscopies. This study was approved by the ethics committee of the College of Medicine of Yangzhou University.
Colonic mucosal tissue collection
Thirty-six patients with PI-IBS and 36 normal controls were included in this study. The diagnosis of PI-IBS was based on the Rome IV Diagnostic Criteria [1]. The colonic mucosal tissues of PI-IBS patients and controls were collected from the Department of Gastroenterology in the Affiliated Hospital of Yangzhou University. All participants signed informed consent and underwent colonoscopies. The study was approved by the ethics committee of the College of Medicine of Yangzhou University. The colonic mucosal tissues were treated with liquid nitrogen and then transferred to a -80°C refrigerator.
Cell culture
Human epithelial colorectal adenocarcinoma Caco-2 cells and human embryonic kidney 293 cells (HEK293) were obtained from the Peking Union Medical College Cell Bank (Beijing, China). Cell lines were cultured in Dulbecco’s modified Eagle’s medium (Gibco, USA) containing 10% heat-inactivated fetal bovine serum (Gibco, USA), penicillin, and streptomycin (Sigma-Aldrich, St. Louis, MO, USA) in a 5% CO2 incubator with saturated humidity at 37°C.
Transfection
In this study, the RNA oligoribonucleotides including miR-510 mimics, miR-510 inhibitor (anti-miR-510), mimic control (miRcon), miRNA inhibitor control (anti-miR-con), si-PRDX1, and negative control si-PRDX1 (NC-si-PRDX1) were synthesized by GeneChem (Shanghai, China). Caco-2 cells were independently transfected with 200 nM miR-510 mimics, miRcon, anti-miR-510, anti-miR-con, si-PRDX1, and NC-si-PRDX1 for 48 h according to the manufacturer’s instructions of Lipofectamine 2000 (Invitrogen, San Diego, CA, USA).
Luciferase reporting assay
The 3’-UTR sequences (containing miR-510 binding sites), which include the wild type (WT) and mutant (MUT) of PRDX1, were amplified from human genomic DNA by polymerase chain reaction (PCR) and cloned into the pGL3-luciferase basic vector (Promega, Wisconsin, WI, USA). Next, using Lipofectamine™ 2000 (Invitrogen, San Diego, CA, USA), the HEK293 cells were co-transfected with miRNAs (miR-510 mimics and miRcon), the reporter vectors (WT or MUT), and the Renilla control vector (Promega, Wisconsin, WI, USA). After 24 h post transfection, the luciferase activity was detected using a Dual-Luciferase Assay Kit (Promega, Madison, WI, USA).
Quantitative real-time PCR (qRT-PCR)
MiRNAs and total RNA were isolated from cells and tissues using the mirVana miRNA Isolation Kit (Ambion, Austin, TX, USA) and Trizol reagent (Invitrogen, Carlsbad, CA, USA). Then, cDNA was obtained using the All-in-One™ miRNA First-Strand cDNA Synthesis Kit (GeneCopoeia, Rockville, MD, USA) and the PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara Bio, Inc., Dalian, Japan). qRT-PCR was performed using the All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia, Rockville, MD, USA) and PrimeScript™ RT Master Mix (Takara Bio, Inc., Dalian, Japan) on the 7500 Real-Time PCR System (Applied Biosystems, White Plains, NY, USA). Relative expression levels of the genes were calculated using the 2-ΔΔCt method [21].
Western blot assay
Proteins were extracted from the colonic mucosal tissues and Caco-2 cells using RIPA buffer according to the manufacturer’s instructions. Protein concentration was measured using the BCA Protein Assay Kit (Beyotime, Beijing, China). Equal amounts of total proteins from tissues and cells were separated on sodium dodecyl sulfate-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). The human PRDX1 (1:1000, Abcam, Cambridge, MA, USA) served as the experimental antibodies, whereas beta-actin (Abcam) served as an internal reference.
Immunohistochemistry
Two colonic mucosa samples were taken from each patient during endoscopy. Then samples were fixed by 4% paraformaldehyde, and embedded with paraffin. Expression of PRDX1 was detected by immunohistochemistry. After deparaffinage, sections were incubated with 3% H2O2 for 15 min and 3% BSA for 30 min. Anti-PRDX1 antibody (monoclonal, 1:500; Abcam, Cambridge, MA, USA) was added and stored at 4°C overnight. Then, samples were labeled with HRP, stained with DAB, and re-stained with hematoxylin. Then, sections were dehydrated and mounted. Section was photographed with a microscope (Nikon, Japan).
Cell viability assay
Viability assays of Caco-2 cells were conducted using Cell Counting Kit-8 (CCK-8, Biosharp, China). Approximately 5×103 Caco-2 cells/well were seeded in a 96-well plate and treated with LPS or other interference factors for different hours. Then, 10 μl/well CCK8 solution was added to the cell culture medium. The cells were incubated at 37°C for 1 h in 5% CO2 and humidified 95% air. By using a Microplate Reader, the absorbance was measured at 450 nm. All experiments were repeated three times.
Apoptosis assay
To identify and quantify the apoptotic cells, the flow cytometry analysis experiment was performed using the Annexin V-FITC/PI Apoptosis Detection Kit (Beijing Biosea Biotechnology, Beijing, China). After treatment with LPS, cells (2×105 cells/well) were washed twice with cold PBS, added with the binding buffer including RNase A and FITC-Annexin V (Sigma-Aldrich, St. Louis, MO, USA), and then incubated for 1 h at room temperature in the dark. Subsequently, apoptotic cells were determined by flow cytometry (BD Biosciences).
ELISA
The culture supernatant was collected after treatment with LPS and other interference factors. Then, we measured the concentrations of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) by ELISA (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. All experiments were repeated thrice, and data were expressed as mean ± standard error in the entire manuscript. Independent samples T-test was used in analyzing the difference of means between the two groups. One-way analysis of variance was used in comparing the difference among three groups and more than three groups. P<0.05 indicated a statistically significant result.
Results
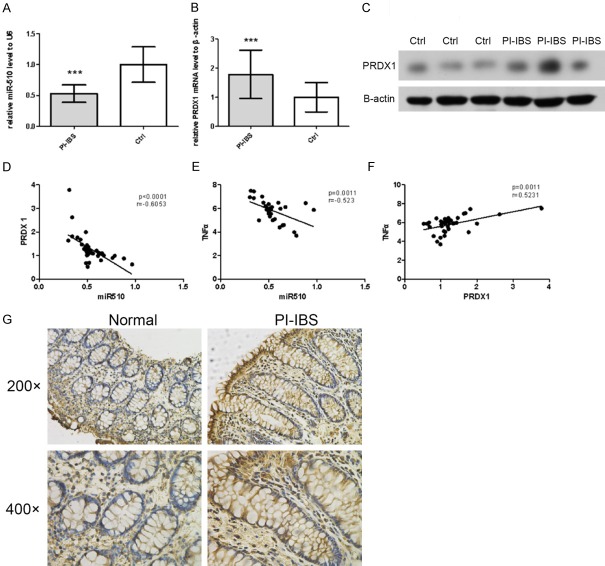
miR-510 was downregulated and PRDX1 was upregulated in colonic mucosal tissues of PI-IBS patients
In order to understand the role of miR-510 in the pathogenesis of PI-IBS, miR-510 expression was detected in the colonic mucosal tissues (from 36 PI-IBS patients and 36 normal controls) by qRT-PCR. Compared with the control group, miR-510 expression was significantly reduced in PI-IBS colonic mucosal tissues (P<0.01; Figure 1A). Furthermore, we detected the expression levels of PRDX1 mRNA in PI-IBS colonic mucosal tissues by qRT-PCR. As shown in Figure 1B, PI-IBS colonic mucosal tissues showed increased PRDX1 expression (P<0.01). Compared with the normal samples, PRDX1 protein expression (by Western blot and immunohistochemistry) in PI-IBS colonic mucosal samples was also significantly increased (Figure 1C, 1G). Given that the above data showed the downregulation of miR-510 and upregulation of PRDX1 in the PI-IBS group, we further carried out a correlation analysis, which showed that the expression of PRDX1 was negatively correlated with miR-510 (Figure 1D). As in our previous study we found that PRDX1 can increase the secretion of pro-inflammatory cytokines and promote inflammatory reactions, we further performed a correlation analysis in the PI-IBS group and found that miR-510 was negatively correlated with serum TNF-α expression, but PRDX1 was positively correlated (Figure 1E and 1F).
Figure 1.
In colonic mucosal tissues of PI-IBS patients, miR-510 was downregulated, and PRDX1 was upregulated. A. Significant downregulation of miR-510 was observed in PI-IBS colonic mucosal tissues by qRT-PCR (left panel). B, C, G. Dramatic upregulation of the PRDX1 mRNA and proteins was observed in PI-IBS patients by qRT-PCR, Western blot (left panel) and Immunohistochemistry. D. Expression levels of PRDX1 were negatively correlated with miR-510 expression in the PI-IBS group. E. ELISA showed that the expression level of miR-510 was negatively correlated with serum TNF-α expression in the PI-IBS group. F. ELISA showed that the expression level of PRDX1 was positively correlated with serum TNF-α expression in the PI-IBS group. *P<0.05.
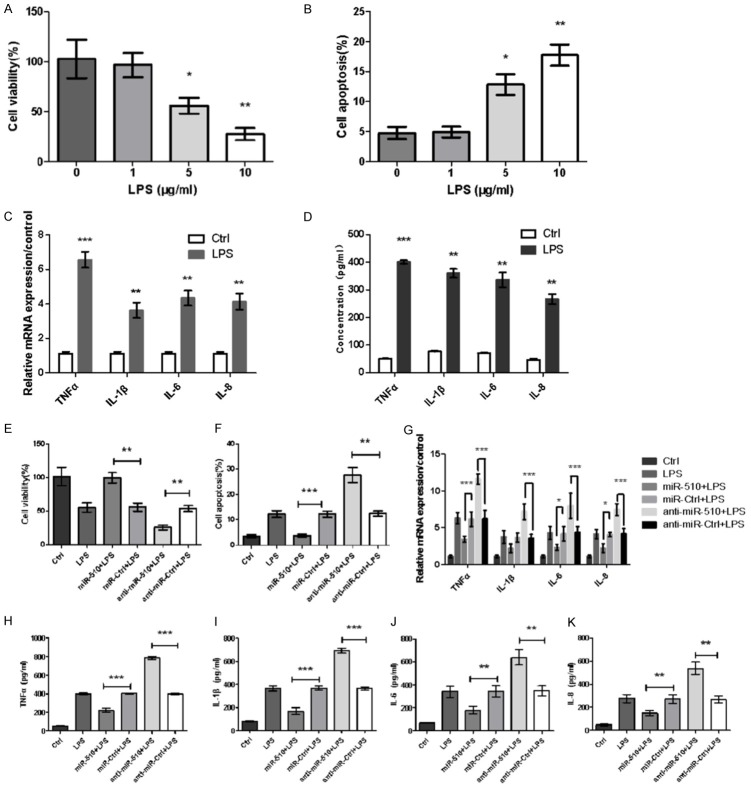
Overexpression of miR-510 alleviated LPS-induced inflammatory injury in Caco-2 cells
First, we found inflammatory injury in Caco-2 cells induced by LPS. When the stimulating concentration of LPS reached 5 and 10 μg/ml, a significant decrease in Caco-2 cell viability was observed (Figure 2A), and a dramatic increase in Caco-2 cell apoptosis was detected (P<0.05 or P<0.01, Figure 2B). Based on the above data, a dose-dependent relationship existed between LPS and Caco-2 cell damage. Thus, 5 μg/ml LPS was selected as the stimulus condition for the following experiments. Given the importance of cytokines in inflammatory diseases, we evaluated the expression of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and IL-8 by qRT-PCR. According to our data, after being injured with LPS, the mRNA levels of pro-inflammatory cytokines significantly increased in Caco-2 cells compared with the control (P<0.01 or P<0.001, Figure 2C). In addition, higher concentrations of the four cytokines were tested in the LPS group than in the control group in cell culture supernatant via ELISA (all P<0.01, Figure 2D).
Figure 2.
MiR-510 overexpression alleviated the inflammatory injury induced by LPS in Caco-2 cells. Different concentrations of LPS (0, 1, 5, and 10 μg/ml) were used to stimulate cells for 5 h, and inflammatory injury was observed in Caco-2 cells. At LPS concentrations of 5 and 10 μg/ml, (A) CCK8 assay showed that cell viability was significantly decreased, and (B) flow cytometry showed that cell apoptosis was dramatically increased in Caco-2 cells. (C) mRNA levels and (D) concentrations of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) in the culture supernatant were significantly upregulated in LPS-treated Caco-2 cells compared with the control according to qRT-PCR and ELISA. The inflammatory injury induced by LPS was rescued by miR-510 overexpression. (E) Cell viability was dramatically increased. (F) Cell apoptosis and (G) mRNA expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were significantly decreased, and the concentrations of pro-inflammatory cytokines in the culture supernatants, such as (H) TNFα, (I) IL-1β, (J) IL-6, and (K) IL-8, were significantly decreased. *: P<0.05. **: P<0.01, ***: P<0.001.
Second, our experiment showed that miR-510 overexpression alleviated LPS-induced inflammatory injury in Caco-2 cells. To directly evaluate the effect of miR-510 on the inflammatory injury induced by LPS, a miR-510 mimic or inhibitor was transfected into Caco-2 cells. As shown in Figure 2E and 2F, the transfection of miR-510 mimic resulted in a significant increase in cell viability and markedly reduced cell apoptosis based on LPS stimulation. By contrast, we observed the opposite result with miR-510 inhibitor transfection, showing that cell viability decreased and cell apoptosis increased (P<0.01 or P<0.001, Figure 2E and 2F). Furthermore, the effects of miR-510 on the production of inflammatory cytokines were observed. As shown in Figure 2G-K, a significant reduction in LPS-induced TNF-α, IL-1β, IL-6, and IL-8 production was observed in the miR-510 mimic group (P<0.05, P<0.01, or P<0.001), whereas the opposite effects were noted in the miR-510 inhibitor group (P<0.01 or P<0.001).
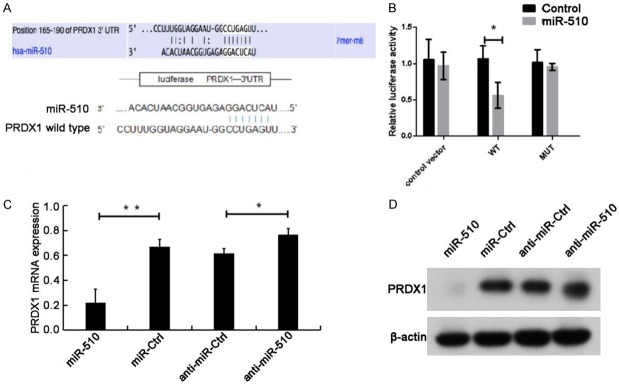
MiR-510 negatively regulated PRDX1 expression in Caco-2 cells by binding to the 3’-UTR of PRDX1 directly
To demonstrate the above conclusions further, we consulted relevant literatures and searched for potential target genes of miR-510 using a bioinformatics software. We found one miR-510 binding site in the 3’-UTR of PRDX1, confirming that PRDX1 is one of the potential targets (Figure 3A). For further verification, the WT 3’-UTR of PRDX1 and its homologous MUT were cloned into the luciferase vector. According to dual-luciferase assays, miR-510 overexpression suppressed the luciferase intensity in WT PRDX1 3’-UTR but not in MUT 3’-UTR (Figure 3B).
Figure 3.
MiR-510 negatively regulated PRDX1 expression in Caco-2 cells by binding to the 3’-UTR of PRDX1 directly. (A) Bioinformatics analyses showed that the PRDX1 3’-UTR is a potential target of miR-510. (B) Dual-luciferase reporter assay exhibited dramatic reduction of luciferase activity in HEK293 cells co-transfected with miR-510 mimics and pGL3-PRDX1-3’-UTR-WT compared with the cells co-transfected with miR-510 mimics and pGL3-PRDX1-3’-UTR-MUT. (C) qRT-PCR and (D) Western blots showed that PRDX1 was downregulated in the miR-510 mimic group but was upregulated in the miR-510 inhibitor group. *: P<0.05. **: P<0.01.
To further explore the direct effect of miR-510 on PRDX1 expression, we transfected Caco-2 cells with miR-510 mimics or inhibitors and determined their effects on PRDX1. The transfection of miR-510 mimics significantly downregulated the mRNA and protein expression of PRDX1, and miR-510 inhibition dramatically increased the mRNA and protein levels of PRDX1 (Figure 3C and 3D; either P<0.05). The above data suggested that miR-510 can bind to WT PRDX1 3’-UTR and block the expression of PRDX1.
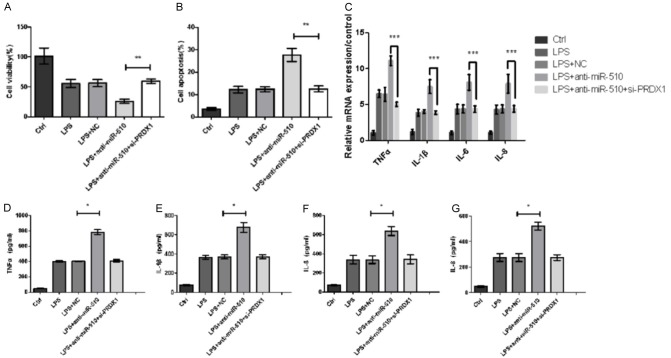
Inflammatory injury induced by LPS in Caco-2 cells depends on the pro-inflammatory factor PRDX1
To understand the effects of PRDX1 on the inflammatory injury induced by LPS, we transfected Caco-2 cells with si-PRDX1 and negative control si-PRDX1 (NC-si-PRDX1). The effects of si-PRDX1 or NC-si-PRDX1 were detected in Caco-2 cells. The remarkable increase of cell viability and decrease of cell apoptosis were measured when silenced with PRDX1 (P<0.01, Figure 4A and 4B). Furthermore, the effects of si-PRDX1 on the production of pro-inflammatory factors were evaluated. As shown in Figure 4C and 4D, the levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were reduced in the si-PRDX1 group (P<0.01 or P<0.001).
Figure 4.
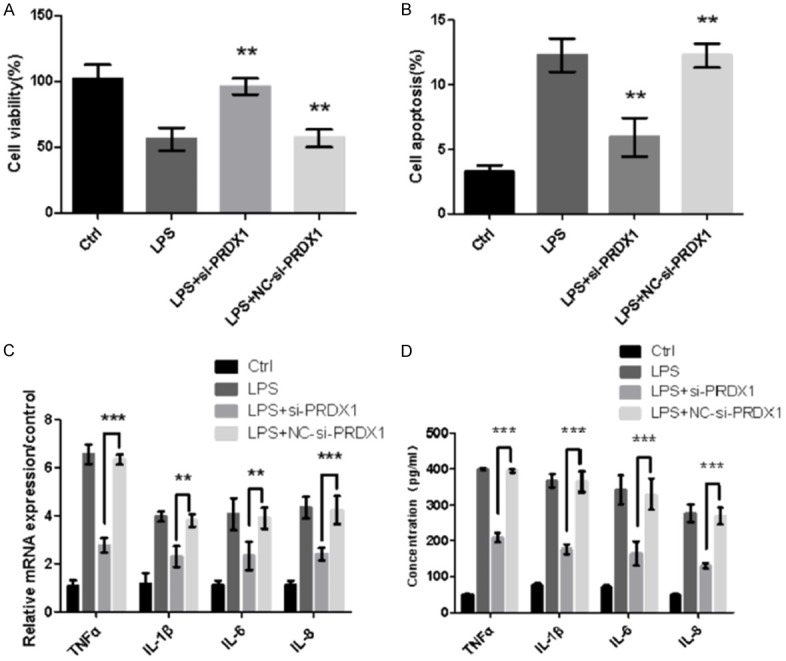
Inflammatory injury induced by LPS in Caco-2 cells depends on the pro-inflammatory factor PRDX1. Silencing of PRDX1 alleviates the inflammatory injury induced by LPS. (A) Cell viability was increased dramatically. (B) Cell apoptosis and (C) mRNA expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were significantly decreased, and (D) the pro-inflammatory cytokine concentrations in the supernatant of cell culture were decreased significantly. **: P<0.01, ***: P<0.001.
Inhibition of miR-510 increased LPS-induced inflammatory injury in Caco-2 cells via PRDX1 overexpression
To verify further whether miR-510 directly targets PRDX1 and then affects LPS-induced inflammatory injury, we transfected Caco-2 cells with si-PRDX1. We found that even when injured with LPS, si-PRDX1 can rescue the damage of cell viability in Caco-2 cells transfected with miR-510 inhibitor (P<0.01) (Figure 5A). Then, silencing of PRDX1 reduced the increase of cell apoptosis induced by LPS stimulation plus miR-510 suppression (P<0.01, Figure 5B). qRT-PCR and ELISA showed that lower levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were detected in the LPS + miR-510 inhibitor + si-PRDX1 group than in the LPS + miR-510 inhibitor group (*: P<0.05, ***: P<0.001, Figure 5C-G).
Figure 5.
MiR-510 inhibition increased LPS-induced inflammatory injury in Caco-2 cells via PRDX1 overexpression. MiR-510 inhibitor or si-PRDX1 was transfected into Caco-2 cells. (A) Cell viability, (B) cell apoptosis, and (C) mRNA expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were measured using qRT-PCR. The concentrations of (D) TNF-α, (E) IL-1β, (F) IL-6, and (G) IL-8 in the supernatant of cell culture medium were examined by ELISA. *: P<0.05, ***: P<0.001.
Discussion
PI-IBS is a common functional GI disorder that occurs after acute GI infection [5]. In the present study, we identified that miR-510 was downregulated and PRDX1 was upregulated in colonic mucosal tissues of PI-IBS patients, and the level of miR-510 was negatively correlated with TNF-α levels. Furthermore, our study showed that miR-510 overexpression decreased the inflammatory injury induced by LPS in Caco-2 cells, indicating the increase in cell viability, decrease in apoptosis, and downregulation of pro-inflammatory cytokines. Our study also found that miR-510 negatively regulated PRDX1 expression in Caco-2 cells by binding to the 3’-UTR of PRDX1 mRNA directly, and miR-510 inhibition aggravated LPS-induced inflammatory reaction in Caco-2 cells via PRDX1 upregulation. In summary, we speculated that decreased miR-510 expression might participate in the inflammatory injury regulation in PI-IBS.
IBS has been traditionally considered as a functional GI disorder. However, recent evidence showed that immune activation, low-grade inflammation [22-26], and distorted mucosal barrier ultrastructure [27-30] have been implicated in IBS. Several studies reported the important function of certain miRNAs in the occurrence and progression of a variety of illnesses. However, few studies have investigated the role of miRNAs in IBS pathogenesis [17,18,31,32]. In our previous studies, we detected the expression level of miR-510 in 53 colonic mucosal tissues with IBS-D and found that miR-510 expression was markedly lower in IBS-D compared with controls [33]. Guo et al. and Zhang et al. found that miR-510 has potential anti-tumor effects [34,35]. Moreover, miR-510 could be used as a new biomarker for diagnosis and as a novel target for treating hypertension [36]. Although we have not explained in the last paper why miR-510 was downregulated in IBS-D, we speculated that it might be associated with local chronic inflammation.
Here, we also found that miR-510 expression was decreased in PI-IBS colonic mucosal tissues, similar to miR-510 downregulation in IBS-D [20]. In the study by Lee et al. and Su et al., PI-IBS had many overlapping features with IBS-D and shared similar pathophysiology and management approaches [37], which can explain the similar downregulation of miR-510 in PI-IBS and IBS-D.
As described in the Results section, miR-510 downregulation in PI-IBS colonic mucosal tissues was negatively correlated with the expression of pro-inflammatory cytokine TNF-α, which prompted us to further evaluate miR-510 and its role in intestinal inflammation. We further investigated the function of miR-510 in Caco-2 cells by transfecting Lipofectamine™ 2000 with miR-510 mimics as described in detail in the Methods section. Here, we found that miR-510 overexpression alleviated LPS-induced inflammatory injury in Caco-2 cells. Subsequent bioinformatics analysis and a previous study [35] showed that miR-510 had a close interaction with PRDX1 (peroxiredoxin 1), which is a class of proteins that play a key role in antioxidation [38] and certain cancer progression [39-41]. PRDX1 promoted the inflammatory response by activating inflammatory signaling pathways, such as NF-κB and MAPK [42,43]. This factor strongly promoted inflammatory response and destroyed innate immunity. Our current research findings have shown that PRDX1 expression is significantly upregulated in colonic mucosal tissues of PI-IBS patients, which aggravated intestinal inflammation. Our study also showed that miR-510 may act as an anti-inflammatory factor by directly binding to the 3’-UTR of PRDX1 mRNA and downregulating PRDX1 expression. In brief, all of the results revealed that miR-510 might serve as an important factor preventing the progression of PI-IBS.
We found that miR-510 expression was downregulated in PI-IBS colonic mucosal tissues and negatively correlated with TNF-α levels. Moreover, miR-510 overexpression alleviated LPS-induced inflammatory injury in Caco-2 cells. Further studies revealed that PRDX1 is a direct target of miR-510. MiR-510 suppression aggravated LPS-induced inflammatory injury in Caco-2 cells by upregulating PRDX1. Overall, our findings will enrich the understanding of PI-IBS pathogenesis and biological activity of miR-510 and provide an important experimental basis for the development of effective PI-IBS drugs based on miR-510.
Acknowledgements
We thank all individuals who participated in this work and the efforts of our team members, namely, Jinfeng Wu, Xiaxin Wu, Sen Han, Zhijie Ling, Shizhen Ding, and Xiaoqin Jia. We also thank Weijuan Gong for her support during the modification process. This study was supported by the National Natural Science Foundation of the Jiangsu Province of China (No. BK20160479), the China Postdoctoral Science Foundation (No. 2017M621845), and the Scientific Research Item of the Chinese Medical Association (No. GJHLQ160014).
Disclosure of conflict of interest
None.
References
- 1.Palsson OS, Whitehead WE, van Tilburg MA, Chang L, Chey W, Crowell MD, Keefer L, Lembo AJ, Parkman HP, Rao SS, Sperber A, Spiegel B, Tack J, Vanner S, Walker LS, Whorwell P, Yang Y. Rome IV diagnostic questionnaires and tables for investigators and clinicians. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Oświęcimska J, Szymlak A, Roczniak W, Girczys-Poledniok K, Kwiecień J. New insights into the pathogenesis and treatment of irritable bowel syndrome. Adv Med Sci. 2017;62:17–30. doi: 10.1016/j.advms.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Heitkemper MM, Wiley JW, Camilleri M. 2015 James W. Freston single topic conference: a renaissance in the understanding and management of irritable bowel syndrome. Cell Mol Gastroenterol Hepatol. 2016;2:394–399. e2. doi: 10.1016/j.jcmgh.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mearin F, Ciriza C, Minguez M, Rey E, Mascort JJ, Peña E, Cañones P, Júdez J en nombre de la SEPD, la semFYC, la SEMERGEN y la SEMG. Clinical practice guidelines: irritable bowel syndrome with constipation and functional constipation in adults: concept, diagnosis, and healthcare continuity. Semergen. 2017;43:43–56. doi: 10.1016/j.semerg.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Sundin J, Rangel I, Repsilber D, Brummer RJ. Cytokine response after stimulation with key commensal bacteria differ in post-infectious irritable bowel syndrome (PI-IBS) patients compared to healthy controls. PLoS One. 2015;10:e0134836. doi: 10.1371/journal.pone.0134836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanazawa M, Palsson OS, van Tilburg MA, Gangarosa LM, Fukudo S, Whitehead WE. Motility response to colonic distention is increased in postinfectious irritable bowel syndrome (PI-IBS) Neurogastroenterol Motil. 2014;26:696–704. doi: 10.1111/nmo.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanuri N, Cassell B, Bruce SE, White KS, Gott BM, Gyawali CP, Sayuk GS. The impact of abuse and mood on bowel symptoms and health-related quality of life in irritable bowel syndrome (IBS) Neurogastroenterol Motil. 2016;28:1508–1517. doi: 10.1111/nmo.12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motomura Y, Ghia JE, Wang H, Akiho H, El-Sharkawy RT, Collins M, Wan Y, McLaughlin JT, Khan WI. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–481. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Zhou X, Lan C. Changes of cytokine levels in a mouse model of post-infectious irritable bowel syndrome. BMC Gastroenterol. 2015;15:43. doi: 10.1186/s12876-015-0272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Zhang Y, Deng Z. Imbalanced shift of cytokine expression between T helper 1 and T helper 2 (Th1/Th2) in intestinal mucosa of patients with post-infectious irritable bowel syndrome. BMC Gastroenterol. 2012;12:91. doi: 10.1186/1471-230X-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li N, Long B, Han W, Yuan S, Wang K. microRNAs: important regulators of stem cells. Stem Cell Res Ther. 2017;8:110. doi: 10.1186/s13287-017-0551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Hara SP, Mott JL, Splinter PL, Gores GJ, LaRusso NF. MicroRNAs: key modulators of posttranscriptional gene expression. Gastroenterology. 2009;136:17–25. doi: 10.1053/j.gastro.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thivierge C, Tseng HW, Mayya VK, Lussier C, Gravel SP, Duchaine TF. Alternative polyadenylation confers Pten mRNAs stability and resistance to microRNAs. Nucleic Acids Res. 2018;46:10340–10352. doi: 10.1093/nar/gky666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao L, Wang JY. RNA-binding proteins and microRNAs in gastrointestinal epithelial homeostasis and diseases. Curr Opin Pharmacol. 2014;19:46–53. doi: 10.1016/j.coph.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut. 2010;59:775–784. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Costinean S, Croce CM, Brasier AR, Merwat S, Larson SA, Basra S, Verne GN. MicroRNA 29 targets nuclear factor-kappaB-repressing factor and Claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148:158–169. e8. doi: 10.1053/j.gastro.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Yang L, Larson S, Basra S, Merwat S, Tan A, Croce C, Verne GN. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797–805. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Li Y, Hao Z, Li X, Bo P, Gong W. Association of the serotonin receptor 3E gene as a functional variant in the microRNA-510 target site with diarrhea predominant irritable bowel syndrome in Chinese women. J Neurogastroenterol Motil. 2016;22:272–281. doi: 10.5056/jnm15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z, Jin T, Lu Y. AntimiR-30b inhibits TNF-alpha mediated apoptosis and attenuated cartilage degradation through enhancing autophagy. Cell Physiol Biochem. 2016;40:883–894. doi: 10.1159/000453147. [DOI] [PubMed] [Google Scholar]
- 22.Guilarte M, Santos J, de Torres I, Alonso C, Vicario M, Ramos L, Martínez C, Casellas F, Saperas E, Malagelada JR. Diarrhoea-predominant IBS patients show mast cell activation and hyperplasia in the jejunum. Gut. 2007;56:203–209. doi: 10.1136/gut.2006.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hod K, Ringel-Kulka T, Martin CF, Maharshak N, Ringel Y. High-sensitive C-reactive protein as a marker for inflammation in irritable bowel syndrome. J Clin Gastroenterol. 2016;50:227–232. doi: 10.1097/MCG.0000000000000327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, Sharkey KA, Ferraz JG, Shaffer E, Vergnolle N. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley S, Garsed K, Singh G, Duroudier NP, Swan C, Hall IP, Zaitoun A, Bennett A, Marsden C, Holmes G, Walls A, Spiller RC. Impaired uptake of serotonin by platelets from patients with irritable bowel syndrome correlates with duodenal immune activation. Gastroenterology. 2011;140:1434–1443. e1. doi: 10.1053/j.gastro.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 26.Chatoo M, Li Y, Ma Z, Coote J, Du J, Chen X. Involvement of corticotropin-releasing factor and receptors in immune cells in irritable bowel syndrome. Front Endocrinol (Lausanne) 2018;9:21. doi: 10.3389/fendo.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez C, Lobo B, Pigrau M, Ramos L, González-Castro AM, Alonso C, Guilarte M, Guilá M, de Torres I, Azpiroz F, Santos J, Vicario M. Diarrhoea-predominant irritable bowel syndrome: an organic disorder with structural abnormalities in the jejunal epithelial barrier. Gut. 2013;62:1160–1168. doi: 10.1136/gutjnl-2012-302093. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Xiao S, Gong Z, Zhu X, Yang Q, Li Y, Gao S, Dong Y, Shi Z, Wang Y, Weng X, Li Q, Cai W, Qiang W. Wuji Wan formula ameliorates diarrhea and disordered colonic motility in post-inflammation irritable bowel syndrome rats by modulating the gut microbiota. Front Microbiol. 2017;8:2307. doi: 10.3389/fmicb.2017.02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters SA, Edogawa S, Sundt WJ, Dyer RB, Dalenberg DA, Mazzone A, Singh RJ, Moses N, Smyrk TC, Weber C, Linden DR, MacNaughton WK, Turner JR, Camilleri M, Katzka DA, Farrugia G, Grover M. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol. 2017;112:913–923. doi: 10.1038/ajg.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martínez C, González-Castro A, Vicario M, Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 2012;6:305–315. doi: 10.5009/gnl.2012.6.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–1664. 1664.e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biton M, Levin A, Slyper M, Alkalay I, Horwitz E, Mor H, Kredo-Russo S, Avnit-Sagi T, Cojocaru G, Zreik F, Bentwich Z, Poy MN, Artis D, Walker MD, Hornstein E, Pikarsky E, Ben-Neriah Y. Epithelial microRNAs regulate gut mucosal immunity via epithelium-T cell crosstalk. Nat Immunol. 2011;12:239–246. doi: 10.1038/ni.1994. [DOI] [PubMed] [Google Scholar]
- 33.Fourie NH, Peace RM, Abey SK, Sherwin LB, Rahim-Williams B, Smyser PA, Wiley JW, Henderson WA. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Exp Mol Pathol. 2014;96:422–425. doi: 10.1016/j.yexmp.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Guo G, Wang G, Zhao J, Wang B, Yu X, Ding Y. Profile of differentially expressed miRNAs in high-grade serous carcinoma and clear cell ovarian carcinoma, and the expression of miR-510 in ovarian carcinoma. Mol Med Rep. 2015;12:8021–8031. doi: 10.3892/mmr.2015.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo QJ, Mills JN, Bandurraga SG, Nogueira LM, Mason NJ, Camp ER, Larue AC, Turner DP, Findlay VJ. MicroRNA-510 promotes cell and tumor growth by targeting peroxiredoxin1 in breast cancer. Breast Cancer Res. 2013;15:R70. doi: 10.1186/bcr3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan R, Mani P, Sivakumar P, Gopinath V, Sekar D. Expression and methylation of circulating microRNA-510 in essential hypertension. Hypertens Res. 2017;40:361–363. doi: 10.1038/hr.2016.147. [DOI] [PubMed] [Google Scholar]
- 37.Lee YY, Annamalai C, Rao SSC. Post-infectious irritable bowel syndrome. Curr Gastroenterol Rep. 2017;19:56. doi: 10.1007/s11894-017-0595-4. [DOI] [PubMed] [Google Scholar]
- 38.Ding C, Fan X, Wu G. Peroxiredoxin 1 - an antioxidant enzyme in cancer. J Cell Mol Med. 2017;21:193–202. doi: 10.1111/jcmm.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu G, Li J, Zhao Y, Liu N, Zhu X, Liu Q, Wei D, Gao C. Identification and verification of PRDX1 as an inflammation marker for colorectal cancer progression. Am J Transl Res. 2016;8:842–859. [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Wu L, Mishra M, Chawsheen HA, Wei Q. Expression of peroxiredoxin 1 and 4 promotes human lung cancer malignancy. Am J Cancer Res. 2014;4:445–460. [PMC free article] [PubMed] [Google Scholar]
- 41.Fiskus W, Coothankandaswamy V, Chen J, Ma H, Ha K, Saenz DT, Krieger SS, Mill CP, Sun B, Huang P, Mumm JS, Melnick AM, Bhalla KN. SIRT2 deacetylates and inhibits the peroxidase activity of peroxiredoxin-1 to sensitize breast cancer cells to oxidant stress-inducing agents. Cancer Res. 2016;76:5467–5478. doi: 10.1158/0008-5472.CAN-16-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ishii T, Warabi E, Yanagawa T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr. 2012;50:91–105. doi: 10.3164/jcbn.11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Z, Xia N, Yuan X, Zhu X, Xu G, Cui S, Zhang T, Zhang W, Zhao Y, Wang S, Shi B. PRDX1 is involved in palmitate induced insulin resistance via regulating the activity of p38MAPK in HepG2 cells. Biochem Biophys Res Commun. 2015;465:670–677. doi: 10.1016/j.bbrc.2015.08.008. [DOI] [PubMed] [Google Scholar]