Abstract
β3 integrin (ITGB3), also known as CD61 or GP3A, is one of the most widely studied components in the integrin family. As an adhesion receptor on the cell surface, ITGB3 participates in reprogramming tumor metabolism, shaping the stromal and immune microenvironment, facilitating epithelial to mesenchymal transition (EMT) and endothelial to mesenchymal transition (End-MT) and maintaining tumor stemness, etc. Recent studies proposed various intervention strategies against ITGB3 and have achieved promising outcomes in several types of tumor. Here, we review the adaption response and cellular crosstalk in the tumor microenvironment mediated by ITGB3, as well as its upstream and downstream signaling pathways. Lastly, we focus on the inhibitors of ITGB3, ultimately indicating that ITGB3 is a promising target in the tumor microenvironment.
Keywords: ITGB3, tumor microenvironment, metabolism, immune, stemness
Introduction
Tumors are serious disease risk that threatens human health. The malignancy of a tumor is driven by multiple factors, comprising proliferative signal pathways that promote angiogenesis, stimulate metastasis and invasion, as well as energy metabolism reprogramming and evasion of immune surveillance [1]. Increasing evidence emphasizes on the significance of the tumor microenvironment, and the regulation of this microenvironment is the most promising strategy for tumor therapy. It is necessary to screen for cross-functional targets that are robustly associated with tumor malignancy and microenvironment reprogramming.
As the major cell adhesion receptors for the extracellular matrix (ECM), integrins are widely expressed on the cell membrane [2]. Integrins exert notable biological roles in linking cells to counter-receptors on other cells and ligands in the ECM, which lead to the changes in tumor cell behavior and microenvironment status by activating a variety of signal transduction pathways [3]. Integrin activation can also regulate ECM assembly and the polarity of migrating cells, thereby mediating tumor metastasis and non-tumor cell infiltration [4]. As a matter of fact, microenvironmental influences on cell behavior can be determined by the pattern of integrin expression on the cell surface [5]. Thus, integrins could function as the hub family that connects tumor cells and their surrounding microenvironment.
β3 integrin (ITGB3), also known as CD61 or GP3A, is one of the most widely studied members of the integrin family, which exerts diverse crucial roles in malignant tumor progression and in the reprogramming of the tumor microenvironment. The present study provides an overview of the multi-functional roles that involve with ITGB3, such as metabolic reprogramming, epithelial to mesenchymal transition (EMT), endothelial to mesenchymal transition (End-MT), stemness regulation and drug resistant acquisition, pro-angiogenesis, stromal and immune microenvironment re-education. We also discussed the regulation network of ITGB3. Furtherly we concluded the potential drug or inhibitors that target ITGB3, which might provide new concepts in ITGB3 targeted therapy. Therefore, ITGB3 has a promising future as a novel target for comprehensive advanced cancer therapy strategies by targeting the tumor microenvironment, including anti-angiogenesis therapy, anti-stemness therapy, metabolism regulation therapy, and even immunotherapy.
General description of ITGB3
As a heterodimer, ITGB3 has two main forms. The β3 subunit is mainly accompanied by αIIb and αv, both of which can distinguish ligands containing an RGD tripeptide active site selectively, such as vitronectin and fibronectin [5,6]. This improved the development of cilengitide, a cyclic peptide as well as an αvβ3-targeted antagonist, which has shown encouraging outcomes in some phase I/II trials [7]. αIIb β3 integrin is highly expressed in platelets, where it is associated with the pathogenesis of Glanzmann thrombasthenia [8] and might be involved in platelet tumorigenesis [9,10]. Additionally, αvβ3 integrin overexpression was observed on angiogenetic endothelial cells and tumor cells, thereby promoting invasion and migration in several malignant tumors [11-15]. Furthermore, ITGB3 is regarded as a robust prognostic factor related to poor survival in non-small cell lung cancer, breast cancer, cervical cancers, pancreatic ductal adenocarcinoma, T-cell acute lymphoblastic leukemia and gliomas [16-22].
ITGB3 and metabolic reprogramming
Hypoxia and PH dependent adaption
Hypoxia is a critical biological process in the metabolism atlas of tumors, which also influences glucose metabolism and could even induce neo-angiogenesis [23,24]. αvβ3 integrin is an important mediator of hypoxia-related biological process, and is transcriptionally upregulated under hypoxia in human microvascular endothelial cells and malignant cancer cells with a hypoxia induced factor 1A (HIF1A) dependent manner [25,26]. Furthermore, evidence demonstrated that epidermal growth factor receptor vIII (EGFRvIII) and ITGB3 tented to form complexes in the environment of hypoxia and vitronectin enrichment, which complexes could robustly accelerated the malignant process of glioblastoma [27].
Cancer cells’ survival depends on a favorable acid-base balance, especially prefer acidic microenvironment [28,29]. Evidences suggested that αvβ3 integrins can be stimulated by an extracellular acidic PH. Moreover, the acidic microenvironment of tumors also facilitates cell invasion by promoting the activity of matrix metalloproteases, which can bind with the β3 integrins [30].
Glucose and lipid metabolism
Glucose transporter type 3 (GLUT3) is a critical glucose transporter that has essential functions in the mediation of glucose metabolism. Analyses of multiple datasets reveal that GLUT3 exhibited a robust positive correlation with ITGB3. During glioblastoma progression, knockdown of ITGB3 strongly inhibited GLUT3 expression, glucose uptake, lactate production and even the levels of glycolysis [22]. Binding of the integrin ligand milk fat globule-EGF factor 8 (MFGE8) with αvβ3 integrin assists the uptake of fatty acid by regulating the location of CD36 and fatty acid transport protein 1 (FATP1) from cytoplasmic vesicles to the cell surface [31].
ITGB3 and tumor cell heterogeneity
ITGB3 and epithelial-to-mesenchymal transition (EMT)
Epithelial-to-mesenchymal transition (EMT) has complex effects on carcinoma progression and metastasis [32]. ITGB3 is regarded as an EMT biomarker in colorectal cancer, prostate cancer, and breast cancer etc. [33,34]. Studies have reported that ITGB3 was up-regulated during EMT, while it is expressed at a low level in normal epithelial tissues [32]. Silencing of ITGB3 inhibited metastasis and EMT in malignant breast cancer mammary epithelial cells [35,36]. Notably, ITGB3 is involved in EMT mainly under the induction of transforming growth factor-β (TGF-β), a core regulator of the malignant features of tumors [37]. TGF-β1 mediates the upregulation of αvβ3 integrin expression at the transcription level, and then induces the auto-stimulation of the phosphorylation of the type II TGF-β receptor on its tyrosine sites via SRC and the stimulation of MAPK, thus mediating the progression of EMT [35,38,39]. Additionally, the strengthening effect of fibroblast growth factor 1 (FGF1) in EMT, which is induced by TGF-β in epithelial cells, also requires enhanced expression of integrin αvβ3 [40].
ITGB3 and the maintenance of stemness
Cancer stem cells (CSCs), a special subpopulation within the tumors, can initiate tumor growth, sustain self-renewal, and retain their differentiative ability, and ITGB3 exert key roles in this process [41,42]. Integrin αvβ3 is essential and adequate to mediate the development of lung, breast, and pancreatic tumor cells towards a stem-like phenotype [43]. Homeobox D3 (HOXD3), an upstream transcription factor linked to ITGB3 expression, could increase stemness traits in breast cancer cells through β3 integrin-mediated Wnt/β-catenin signaling [42]. Mammary stem cells (MaSCs) can undergo oncogenic mutation and develop into cancer stem cells, resulting in the occurrence, metastasis and recurrence of breast cancer. ITGB3 stimulated by TGF-β2 relies on the expansion of pregnancy-related MaSCs and the promotion of stem-like cells in tumors by enhancing Slug expression [44,45]. Moreover, transcription of ITGB3 in the side population (SP), a CSC rich population, is reported to be increased compared with that in the parent cells, demonstrating that ITGB3 expression in CSC-like SP cells is vital for peritoneal metastasis of gastric cancer [41]. In addition, to regulate the differentiative ability of CSCs, ITGB3 can promote trans-differentiation of human umbilical cord mesenchymal stem cells (hUC-MSCs) into primordial germ-like cells (PGCs) [46]. Additionally, HER2/NEU-transformed tumor cells with overexpression of ITGB3 exhibit tumor initiating cell (TIC) characteristics compared with non-transformed mammary epithelial cells [47]. Therefore, we could regard ITGB3 as a promising marker and modulator that maintains the stemness of tumors (Figure 1).
Figure 1.
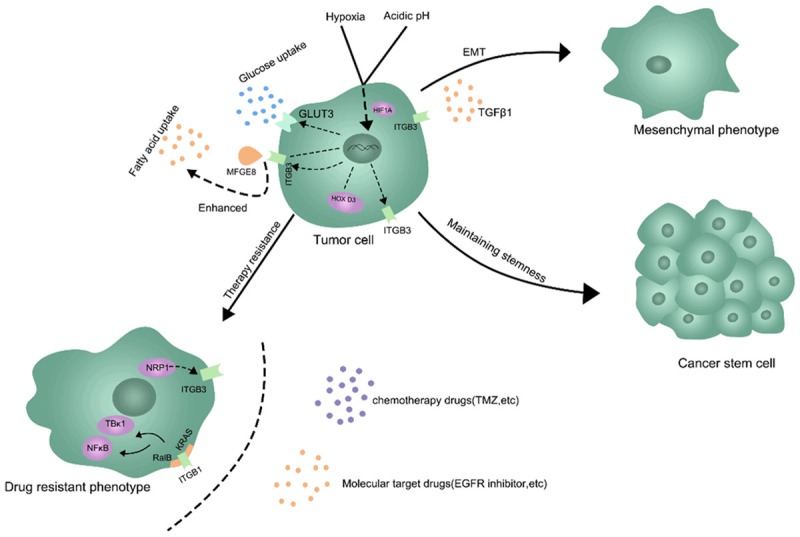
The critical role of ITGB3 in the metabolic reprogramming and tumor cell heterogeneity. ITGB3 can be regulated and adapted in hypoxia and acidic environment. ITGB3 also mediated the glucose and lipid metabolism of tumor cells. Moreover, ITGB3 is involved in the regulation of EMT, stemness maintenance and drug resistance.
ITGB3 and drug resistant tumor cells
Drug resistance is another major feature of malignant tumor cells, which leads to a higher recurrence rate and mortality. In recent years, increasing researches suggested that ITGB3 has a close relationship with drug resistance [48-50]. In glioma cells, the ITGB3 knockdown resulting in an enhanced temozolomide (TMZ) sensitivity by reducing repair of TMZ-induced DNA double-strand breaks [51]. Naik A et al indicated that NRP1-ITGB3 axis also mediated the chemoresistance response of breast cancer cells [52]. Other evidence suggested that ITGB3 inhibition enhances the antitumor activity of ALK inhibitor in ALK-rearranged non-small cell lung cancer (NSCLC) [53]. The overexpression of ITGB3 is also involved in the resistance to EGFR inhibition, Mechanistically due to the complex formed by ITGB3/KRAS/RalB and the activation of TBK1 and NFκB that the complex mediated [43,54].
ITGB3 and the tumor stromal microenvironment
Cross-talking with endothelial cell
Tumor angiogenesis is a complicated process, during which neovasculars are developed from a pre-existing vascular network to satisfy the demand of tumor tissues for oxygen, nutrition and metabolism. ITGB3 is regarded as a marker of angiogenesis, which involves in the key steps of tumor angiogenesis not only by regulating cell-cell, cell-matrix interaction but also involves in several signaling pathways [55]. ITGB3 binds with ECM via its ligand vitronectin and matrix metalloproteinases (MMPs), allowing MMP2 to degrade and remodel the extracellular matrix, which promoted the activation of endothelial cells [56]. Moreover, several new pro-angiogenic regulators such as Angiopoietin-2 and Nogo-B are found to bind with ITGB3, which results is sprouting angiogenesis via focal adhesion kinase (FAK) signaling [57,58]. Meanwhile, the β3 subunit mediates the migration of endothelial cells, by promoting the phosphorylation and activation of VEGFR-2 mechanically [59]. In addition, down regulation of ITGB3 involves the loss of endothelial cell adhesion molecule (ECAM), causing the internalization of VEGFR2 [60]. ITGB3 can also inhibit endothelial cell apoptosis via different mechanisms. For example, α5β3 integrin can bind fibronectin, leading to increased expression of NFκB and the survival ability of endothelial cells, while other researches suggested that αvβ3 inhibits p53 activity and the apoptosis rate of endothelial cells through the MAPK pathway [61,62]. Interestingly, recent study claimed that TGF-β1 improved expression of ITGB3 significantly, inducing the process of End-MT, which enhances endothelial cells’ migration via Notch signaling pathway [63].
Cross-talking with cancer associated fibroblasts (CAFs)
Cancer associated fibroblasts (CAFs) are the most abundant components of the tumor stromal microenvironment [64]. Increasing evidence suggested that CAFs exerting pivotal roles in the tumor microenvironment reprogramming as well as the tumor cells behavior [65,66]. ITGB3 functionally mediated the signal communications between tumor cells and CAFs. For example, CAFs assemble fibronectin and trigger invasion of cancer cells mainly via integrin-αvβ3 [67]. Moreover, Wen S et al found that interaction of interleukin 32 with integrin β3 mediating the cross-talk between CAFs and breast cancer cells plays a crucial role in CAF-induced breast tumor invasiveness [68]. The communications between tumor cells and stromal cells that mediated by ITGB3 were visualized in Figure 2.
Figure 2.
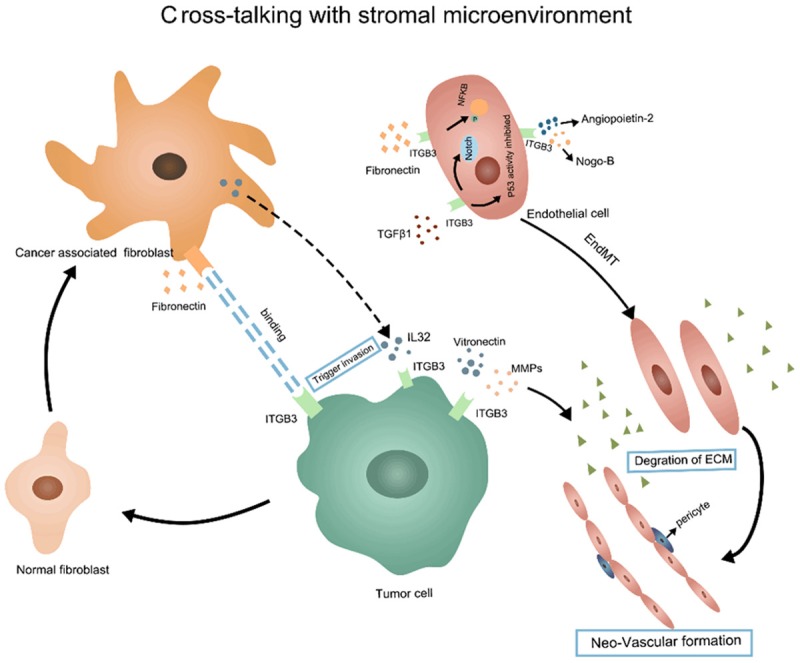
ITGB3 mediated the cross-talking between tumor cells and stromal microenvironment. The ITGB3 mediated signal is critical in the communication between tumor cells and stromal cells like fibroblasts and endothelial cells.
ITGB3 and the immune microenvironment
Current evidence shows that ITGB3 affects tumor immunity via both the innate and adaptive immune systems. ITGB3 showed transcriptional upregulation and a progressive increase of surface expression after neutrophils infiltration [69]. H2O2 and HOCl are the reactive oxygen species produced by neutrophils. ITGB3 could act as a regulator to augment TGF-β/H2O2/HOCl signaling, transforming non-metastatic tumors to a metastatic phenotype [70]. Additionally, a study of tuberculosis revealed that αvβ3 integrin expression is improved on monocytes, leading to increased monocyte recruitment [71]. The interaction between ITGB3 and MFGE8 inhibits macrophages to produce IL-1β in a necrotic cell-induced and ATP-dependent manner [72]. As we known, classical activated macrophages exert anti-tumor effects by producing inflammatory cytokines such as IL-1β. Furthermore, βig-h3 is a protein secreted mainly from the ECM of tumor associated fibroblasts, which could interact with ITGB3 expressed on the surface of CD8+ T cell and macrophages, thus leading to the inactivation of CD8+ T cells and F4/80 macrophages [73]. Meanwhile, specific phagocytosis of apoptotic bodies by dendritic cells depends on the engagement of β3 integrin [74,75]. Moreover, human plasmacytoid dendritic cells express CD36 and CD61 (ITGB3), both of which are involved in uptake of apoptotic cells and the induction of immune tolerance [76]. In conclusion, ITGB3 is more likely to be an immunosuppressive target in solid tumors.
Inconsistent results exist concerning ITGB3’s involvement in immune regulation of hematological tumor. Soluble ITGB3 is a robust natural killer (NK)-cell activator against acute myelocytic leukemia (AML) cells. This is accompanied by the induction of cytokines to improve the proliferation of NK cells, which specifically increases the cytotoxicity of NK cell against AML blasts and induces increased granzyme B and FAS ligand transcript levels. Moreover, ITGB3 favors the excretion of pro-inflammatory cytokines, directing a cytotoxic effect and amplify the immune response in acute myeloid leukemia cells [77]. Platelet β3 integrin can interact with fibrinogen, inducing the synthesis of P-selectin, which can mediate inflammation and the Th1 immune response [78]. In conclusion, the function of ITGB3 in immune regulation might be different under varies of circumstances (Figure 3).
Figure 3.
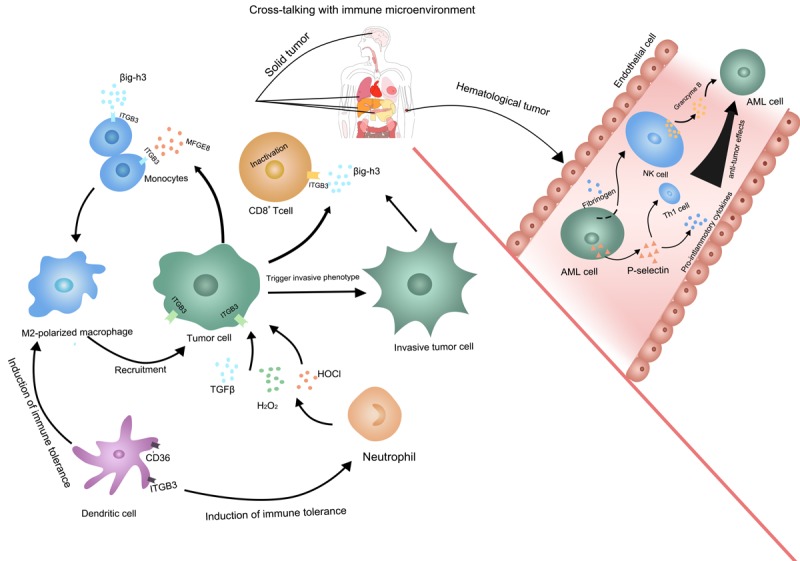
The multiple functions of ITGB3 in tumor immune microenvironment. In solid tumor, the enhanced ITGB3 signaling facilitates an immunosuppressive environment by recruiting M2 type macrophages and neutrophils, and making CD8+ T cells inactivation. While in hematological tumor, ITGB3 promotes NK cell and Th1 cell activation, thus amplifies the anti-tumor effects.
The regulation network of ITGB3
Upstream signaling pathway of ITGB3
TGF-β is the main upstream factor of integrins, which controls the malignant phenotype of tumors, such as invasiveness, stemness, and immune suppression [14]. TGF-β regulates the expression of integrin ligands and stimulates the expression of integrin-associated proteins. Chronic TGF-β exposure leads to increased levels of mesenchymal-like cancer cells with enhanced ITGB3 expression, which is upregulated by constant extracellular signal-regulated kinase (ERK) 1/2 activation [79]. The binding of fibroblast growth factor 1 (FGF1) to β3 integrin is significant for the enhanced TGF-β-stimulated EMT. FGF1 coupled with αvβ3 integrin signaling also increased SMAD2 signaling [40]. In addition to TGF-β and FGF1 signaling, another critical ligand of β3 integrin, MFGE8, is involved in β3 integrin/FAK/PI3K/AKT pathway [80]. HOXD3, an upstream transcription factor linked to β3 expression, activates β3 integrin-mediated WNT/β-catenin signaling, which is critical to maintain cancer stemness [42]. Chronic and continuous production of reactive oxygen species (ROS) can stimulate integrins, and ITGB3 is a core regulator in ROS-mediated activation of the PI3K-AKT-mTOR pathway [34]. Currently, several miRNAs have been proven to target ITGB3 and mediate ITGB3 signaling. For example, miR-483-3p targets ITGB3 directly, thus repressing its downstream FAK/ERK signaling [81]. MiR-98 blocks the proliferation, invasion and metastasis of lung cancer cells by combining with the 3’-UTR of ITGB3 mRNA directly [82]. Moreover, miR-30a-5p and miR-320a can also exert tumor suppressive roles through the inhibition of ITGB3 [83,84].
Downstream signaling pathway of ITGB3
The interaction between ITGB3 and Src is selective and is mediated by the tail of ITGB3. Src combines directly with ITGB3, resulting in the autophosphorylation and activation of SRC [85]. The ITGB3-c-Src signaling axis explains the aggressive behavior of αvβ3 integrin expression in tumors. Blockade of C-SRC kinase activity or decreased expression of endogenous ITGB3 could inhibit anchorage-independent growth and metastatic ability [15]. Focal adhesion kinase (FAK), a non-receptor protein tyrosine kinase, exerting roles in localizing integrins to focal adhesions and assembling integrin signaling molecules. Besides, the autophosphorylation FAK at tyrosine 397 (Y397) produces a binding site for Src [20]. It was revealed that β3 integrin regulated MMP2 expression by activating FAK-PI3K-AKT signaling, contributing to the increased metastatic potential of residual cancer in hepatocellular carcinoma [11]. ITGB3-AKT signaling mediates the proliferation of platelet-induced hemangioendothelioma cells [9]. In addition, ITGB3 can activate the P21 (RAC1) activated kinase 4 (PAK4)-Yes Associated Protein 1 (YAP) axis, which contributes to the enhanced expression of GLUT3, a driver of cancer stem cells and glycolysis ability [22]. Interestingly, YAP-defective cells exhibited displaced expression of β3 integrin [86]. Unliganded αvβ3 integrin can couple to Kirsten rat sarcoma viral proto-oncogene (KRAS), promoting the recruitment and activation of RAS like proto-oncogene B (RALB) to the tumor cell surface, resulting in the activation of NF-κB, a necessary factor for tumor proliferation and self-renewal ability [43]. The regulation network of ITGB3 was summarized in Figure 4.
Figure 4.
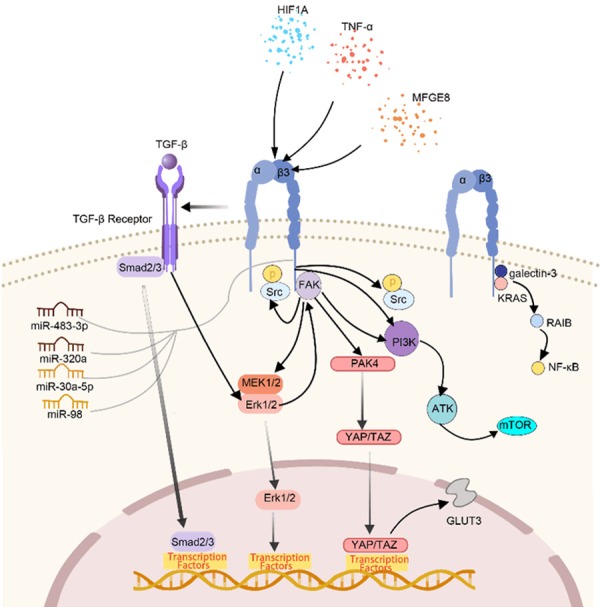
The regulation network of ITGB3 in tumor cells. TGF-β is the main up-stream of ITGB3, other upstream modulators like MEGF8, HOXD3 and several mi-RNAs have been confirmed to regulate ITGB3 expression. The classical downstream signaling of ITGB3 including FAK/PI3K/AKT, MEK/ERK, Akt, YAP/TAZ, KRAS/RalB/NF-κb, etc.
Target therapies for ITGB3
With the development of pharmacological research in ITGB3, several inhibitors targeting ITGB3 have been studied, some of which have been put into clinical trials, including cilengitide, MK0429, and vitaxin. Cilengitide (CLG, EMD 121974), a cyclic RGD peptide, blocks the αv subunit of integrins specifically, and has a high specificity for αvβ3 integrins [7]. CLG inhibits the binding of αvβ3 integrins to the ECM, and has shown anti-angiogenic and anti-tumor effects prospectively in many cancers [87]. CLG can also promote the separation of glioblastoma and mesothelioma cells from the ECM components via exposed RGD sequences, thereby leading to anoikis-dependent apoptosis and inhibition of invasion [7,88]. CLG was validated as a potential strategy to inhibit TGF-β-related malignant features such as invasiveness, stemness, and immunosuppression by targeting αvβ3 integrin in glioblastoma [14]. Phase II clinical trials in non-metastatic castration-resistant prostate cancer have shown that CLG has a good tolerance, although it elicited a negative PSA response [89]. A recent a phase II clinical trial in advanced non-small-cell lung cancer, CLG combined with standard therapy was well tolerated with no unexpected adverse events or dose-limiting toxicities [90]. However, CLG combined with temozolomide chemoradiotherapy exerted little improvement in outcomes in a phase III study of glioblastoma [91].
MK-0429, an orally active and non-peptide αvβ3 integrin antagonist, has high affinity for the purified αvβ3 integrin [92]. This inhibitor blocks the adhesion of HeK293-αvβ3 cells to vitronectin, displayed in the early stages of melanoma metastasis, and the colonization and growth of this murine melanoma in the lungs was achieved by blocking the function of αvβ3 integrin [93]. MK-0429 is undergoing clinical development to treat prostate cancer. Clinical research in men with hormone refractory prostate cancer (HRPC) and metastatic bone disease (MBD) suggested that MK-0429 was generally well tolerated, with evidence of an early reduction in bone turn over, indicating a potential for clinical application [94].
Vitaxin, a synthetic monoclonal antibody that binds an epitope composed of αv and β3 integrin subunits, thus blocking αvβ3 integrins, was implemented in clinical trials for the treatment of stage IV metastatic melanoma and androgen-independent prostate cancer [19]. There are also several kinds of inhibitors targeting ITGB3, the full list of which is presented in Table 1.
Table 1.
The summary of ITGB3 inhibitors
| Inhibitor | Mechanism | Clinical trials | Reference |
|---|---|---|---|
| Cilengitide | Selectively bind to the ligand of integrin αvβ3 | NCT01118676 Phase I NSCLC | PMID: 21269250 |
| NCT00121238 Phase II Prostate cancer | [100] | ||
| NCT01044225 Phase II | PMID: 21049281 | ||
| GBM | [89] | ||
| NCT00689221 Phase III | PMID: 25163906 | ||
| GBM | [91] | ||
| MK-0429 | Selectively inhibit binding of the ligand to integrin β3 | NCT00302471 Phase I | PMID: 20398037 |
| Hormone Refractory Prostate Cancer and Metastatic Bone Disease | [94] | ||
| Vitaxin | Integrin ανβ3-specific monoclonal antibody | NCT00066196 Phase II Metastatic melanoma | PMID: 9600913 |
| NCT00072930 Phase II Metastatic Androgen-Independent Prostate Cancer | [103] | ||
| Luteolin | Inhibit the integrin β3-FAK signal pathway | Null | PMID: 22983392 |
| [104] | |||
| Methylseleninic acid (MSA) | Down-regulate integrin β3 signal pathway | Null | PMID: 28842587 |
| [105] | |||
| Phoyunnanin E | Down-regulate integrins αv and β3 | Null | PMID: 29284478 |
| [106] | |||
| Pinocembrin | Inactivate the integrin β3-FAK-p38α signaling pathway | Null | PMID: 25949790 |
| [107] |
Conclusions and perspective
Currently, increasing numbers of studies are emphasizing the concept of “the tumor microenvironment”. Traditionally, research usually focused on the tumor cells themselves, but ignored the other non-tumor cell components in the tumor microenvironment, as well as the adaption-related changes in the tumor microenvironment, which may be the main reason for the robust resistance by tumors to classical intervention strategies such as chemotherapy and radiotherapy. In recent years, with a more comprehensive understanding of tumor hallmark characteristics, the concept of “the tumor microenvironment”, as well as “microenvironment metabolism reprogramming” and “disorganized microenvironment components” have been emphasized. Moreover, the driving roles of metabolic reprogramming and disordered microenvironment components in tumor malignancy have been validated by large scale cohort studies and biological experiments [95-99]. There is an urgent need to screen for a cross-functional target that is robustly associated with tumor malignancy and microenvironment reprogramming, which has great potential as a promising intervention target in future tumor therapy.
As a membrane receptor, ITGB3 exhibits a cancer-promoting function through its interactions with the tumor microenvironment. ITGB3 is one of the biomarkers of tumor angiogenesis and has multiple roles in the key steps of angiogenesis. ITGB3 enhances the glycolysis rate and lipid uptake, indicating that ITGB3 is involved in metabolic reprogramming, which is related to poor survival in many malignant tumors (Figure 1). A hypoxic and acidic environment in tumors can also facilitate the expression and activation of ITGB3, which suggested the existence of a positive feedback loop between ITGB3 and microenvironment metabolic reprogramming. In TGF-β-induced EMT and tumor initiating cells, ITGB3 is upregulated, enhancing the migration, invasion, maintaining stemness and thus exhibiting resistance to target therapy (Figure 1). Notably, ITGB3 is highly expressed in non-tumor cells and is associated with the cross-talking between tumor cells and stromal cells as well as immune cells. Furthermore, the signaling crosstalk of ITGB3 includes TGF-β, the main upstream regulator of ITGB3, and a series of downstream regulators, such as FAK, YAP1/TAZ, extracellular signal-regulated kinase (ERK), and AKT, which then leads to cellular behavior changes, including angiogenesis, EMT, apoptosis, maintenance of stemness, and immune suppression.
The multiple functions of β3 integrin in tumor progression and metastasis mean that many inhibitors such as cilengitide, MK-0429, and Vitaxin have already been put into both pre-clinical and clinical trials. Notably, as an αvβ3 integrin-specific inhibitor, cilengitide was the first candidate antiangiogenic drug and has been put into phase I and II clinical trials, which showed encouraging outcomes in pancreatic cells, glioma, and some metastatic solid tumors [100]. However, phase III trials of cilengitide did not show significantly improved outcomes in newly diagnosed glioblastoma, prostate cancer, lung cancer, or malignant melanoma, suggesting that further research is needed [89,90,101]. Recently, some findings lead us to consider the complex roles of β3 integrin, such as its influence on the polarization of M2 macrophages in solid tumor, which have immunosuppressive functions. ITGB3 also could cause CD8+ T cell inactivation. Although the effects of β3 integrin on the tumor immune microenvironment are complicated and confusing, they might explain the unexpected results in phase III trials [102], which indicated that the combination of ITGB3 targeted therapy with classical immunotherapy might be a promising strategy to treat patients resistant to ITGB3 targeting.
Collectively, ITGB3 might regulate cell behavior differently in a variety cell types. Understanding the multiple roles of ITGB3 in the tumor microenvironment is necessary to help direct progress in the design of specific targeting strategies to maximize their clinical effects.
Acknowledgements
We would like to acknowledge all the members in Dr. Meng’s laboratory for help with this study. This work was supported by the National Natural Science Foundation of China [No. 81572831] & [No. 81872054] & [No. 81902546].
Disclosure of conflict of interest
None.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Andriu A, Crockett J, Dall’Angelo S, Piras M, Zanda M, Fleming IN. Binding of alphavbeta3 integrin-specific radiotracers is modulated by both integrin expression level and activation status. Mol Imaging Biol. 2018;20:27–36. doi: 10.1007/s11307-017-1100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15:173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533–548. doi: 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng NC, van Zandwijk N, Reid G. Cilengitide inhibits attachment and invasion of malignant pleural mesothelioma cells through antagonism of integrins alphavbeta3 and alphavbeta5. PLoS One. 2014;9:e90374. doi: 10.1371/journal.pone.0090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118:5996–6005. doi: 10.1182/blood-2011-07-365635. [DOI] [PubMed] [Google Scholar]
- 9.Gu R, Sun X, Chi Y, Zhou Q, Xiang H, Bosco DB, Lai X, Qin C, So KF, Ren Y, Chen XM. Integrin beta3/Akt signaling contributes to platelet-induced hemangioendothelioma growth. Sci Rep. 2017;7:6455. doi: 10.1038/s41598-017-06927-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu XR, Wang Y, Adili R, Ju L, Spring CM, Jin JW, Yang H, Neves MAD, Chen P, Yang Y, Lei X, Chen Y, Gallant RC, Xu M, Zhang H, Song J, Ke P, Zhang D, Carrim N, Yu SY, Zhu G, She YM, Cyr T, Fu W, Liu G, Connelly PW, Rand ML, Adeli K, Freedman J, Lee JE, Tso P, Marchese P, Davidson WS, Jackson SP, Zhu C, Ruggeri ZM, Ni H. Apolipoprotein A-IV binds alphaIIbbeta3 integrin and inhibits thrombosis. Nat Commun. 2018;9:3608. doi: 10.1038/s41467-018-05806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang N, Ma D, Wang L, Zhu X, Pan Q, Zhao Y, Zhu W, Zhou J, Wang L, Chai Z, Ao J, Sun H, Tang Z. Insufficient radiofrequency ablation treated hepatocellular carcinoma cells promote metastasis by up-regulation ITGB3. J Cancer. 2017;8:3742–3754. doi: 10.7150/jca.20816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyazaki YJ, Hamada J, Tada M, Furuuchi K, Takahashi Y, Kondo S, Katoh H, Moriuchi T. HOXD3 enhances motility and invasiveness through the TGF-beta-dependent and -independent pathways in A549 cells. Oncogene. 2002;21:798–808. doi: 10.1038/sj.onc.1205126. [DOI] [PubMed] [Google Scholar]
- 13.Cordes N, Hansmeier B, Beinke C, Meineke V, van Beuningen D. Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines. Br J Cancer. 2003;89:2122–2132. doi: 10.1038/sj.bjc.6601429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth P, Silginer M, Goodman SL, Hasenbach K, Thies S, Maurer G, Schraml P, Tabatabai G, Moch H, Tritschler I, Weller M. Integrin control of the transforming growth factor-beta pathway in glioblastoma. Brain. 2013;136:564–576. doi: 10.1093/brain/aws351. [DOI] [PubMed] [Google Scholar]
- 15.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prevost N, Tarin D, Shattil SJ, Cheresh DA. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–1169. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong QL, An XZ, Guan XM, Ma YM, Li PF, Liang SY, Hu YN, Cui YH, Yu J. Expression of beta-integrin family members in children with T-cell acute lymphoblastic leukemia. Zhongguo Dang Dai Er Ke Za Zhi. 2017;19:620–626. doi: 10.7499/j.issn.1008-8830.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30–5. doi: 10.1097/00006676-200208000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Gruber G, Hess J, Stiefel C, Aebersold DM, Zimmer Y, Greiner RH, Studer U, Altermatt HJ, Hlushchuk R, Djonov V. Correlation between the tumoral expression of beta3-integrin and outcome in cervical cancer patients who had undergone radiotherapy. Br J Cancer. 2005;92:41–46. doi: 10.1038/sj.bjc.6602278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulas P, Willnecker V, Dlugaiczyk J, Laschke MW, Schick B. Mesenchymal-endothelial transition in juvenile angiofibroma? Acta Otolaryngol. 2015;135:955–961. doi: 10.3109/00016489.2015.1042042. [DOI] [PubMed] [Google Scholar]
- 20.Taherian A, Li X, Liu Y, Haas TA. Differences in integrin expression and signaling within human breast cancer cells. BMC Cancer. 2011;11:293. doi: 10.1186/1471-2407-11-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noh KW, Sohn I, Song JY, Shin HT, Kim YJ, Jung K, Sung M, Kim M, An S, Han J, Lee SH, Lee MS, Choi YL. Integrin beta3 inhibition enhances the antitumor activity of ALK inhibitor in ALK-rearranged NSCLC. Clin Cancer Res. 2018;24:4162–4174. doi: 10.1158/1078-0432.CCR-17-3492. [DOI] [PubMed] [Google Scholar]
- 22.Cosset E, Ilmjarv S, Dutoit V, Elliott K, von Schalscha T, Camargo MF, Reiss A, Moroishi T, Seguin L, Gomez G, Moo JS, Preynat-Seauve O, Krause KH, Chneiweiss H, Sarkaria JN, Guan KL, Dietrich PY, Weis SM, Mischel PS, Cheresh DA. Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell. 2017;32:856–868. e5. doi: 10.1016/j.ccell.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 24.Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430–439. doi: 10.1038/nrc3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Befani C, Liakos P. Hypoxia upregulates integrin gene expression in microvascular endothelial cells and promotes their migration and capillary-like tube formation. Cell Biol Int. 2017;41:769–778. doi: 10.1002/cbin.10777. [DOI] [PubMed] [Google Scholar]
- 26.Sesé M, Fuentes P, Esteve-Codina A, Béjar E, McGrail K, Thomas G, Aasen T, Ramón Y Cajal S. Hypoxia-mediated translational activation of ITGB3 in breast cancer cells enhances TGF-β signaling and malignant features in vitro and in vivo. Oncotarget. 2017;8:114856–114876. doi: 10.18632/oncotarget.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Han L, Dong Y, Tan Y, Li Y, Zhao M, Xie H, Ju H, Wang H, Zhao Y, Zheng Q, Wang Q, Su J, Fang C, Fu S, Jiang T, Liu J, Li X, Kang C, Ren H. EGFRvIII/integrin β3 interaction in hypoxic and vitronectinenriching microenvironment promote GBM progression and metastasis. Oncotarget. 2016;7:4680–94. doi: 10.18632/oncotarget.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swietach P, Vaughan-Jones RD, Harris AL, Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130099. doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ata R, Antonescu CN. Integrins and cell metabolism: an intimate relationship impacting cancer. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paradise RK, Lauffenburger DA, Van Vliet KJ. Acidic extracellular pH promotes activation of integrin alpha(v)beta(3) PLoS One. 2011;6:e15746. doi: 10.1371/journal.pone.0015746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalifeh-Soltani A, McKleroy W, Sakuma S, Cheung YY, Tharp K, Qiu Y, Turner SM, Chawla A, Stahl A, Atabai K. Mfge8 promotes obesity by mediating the uptake of dietary fats and serum fatty acids. Nat Med. 2014;20:175–183. doi: 10.1038/nm.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamuya FA, Duncan MK. aV integrins and TGF-beta-induced EMT: a circle of regulation. J Cell Mol Med. 2012;16:445–455. doi: 10.1111/j.1582-4934.2011.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deep G, Jain AK, Ramteke A, Ting H, Vijendra KC, Gangar SC, Agarwal C, Agarwal R. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer. 2014;13:37. doi: 10.1186/1476-4598-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei Y, Huang K, Gao C, Lau QC, Pan H, Xie K, Li J, Liu R, Zhang T, Xie N, Nai HS, Wu H, Dong Q, Zhao X, Nice EC, Huang C, Wei Y. Proteomics identification of ITGB3 as a key regulator in reactive oxygen species-induced migration and invasion of colorectal cancer cells. Mol Cell Proteomics. 2011;10:M110.005397. doi: 10.1074/mcp.M110.005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galliher AJ, Schiemann WP. Beta3 integrin and src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parvani JG, Gujrati MD, Mack MA, Schiemann WP, Lu ZR. Silencing beta3 integrin by targeted ECO/siRNA nanoparticles inhibits EMT and metastasis of triple-Negative breast cancer. Cancer Res. 2015;75:2316–2325. doi: 10.1158/0008-5472.CAN-14-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silginer M, Burghardt I, Gramatzki D, Bunse L, Leske H, Rushing EJ, Hao N, Platten M, Weller M, Roth P. The aryl hydrocarbon receptor links integrin signaling to the TGF-beta pathway. Oncogene. 2016;35:3260–71. doi: 10.1038/onc.2015.387. [DOI] [PubMed] [Google Scholar]
- 38.Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. alpha(v)beta(3) Integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J Biol Chem. 2004;279:37726–37733. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- 39.Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, Thannickal VJ, Knight DA. Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J Biol Chem. 2008;283:12898–12908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- 40.Mori S, Kodaira M, Ito A, Okazaki M, Kawaguchi N, Hamada Y, Takada Y, Matsuura N. Enhanced expression of integrin alphavbeta3 induced by TGF-beta is required for the enhancing effect of fibroblast growth factor 1 (FGF1) in TGF-beta-induced epithelial-mesenchymal transition (EMT) in mammary epithelial cells. PLoS One. 2015;10:e0137486. doi: 10.1371/journal.pone.0137486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishii T, Yashiro M, Shinto O, Sawada T, Ohira M, Hirakawa K. Cancer stem cell-like SP cells have a high adhesion ability to the peritoneum in gastric carcinoma. Cancer Sci. 2009;100:1397–1402. doi: 10.1111/j.1349-7006.2009.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Zhang Q, Cao Z, Huang Y, Cheng S, Pang D. HOXD3 plays a critical role in breast cancer stemness and drug resistance. Cell Physiol Biochem. 2018;46:1737–1747. doi: 10.1159/000489249. [DOI] [PubMed] [Google Scholar]
- 43.Seguin L, Kato S, Franovic A, Camargo MF, Lesperance J, Elliott KC, Yebra M, Mielgo A, Lowy AM, Husain H, Cascone T, Diao L, Wang J, Wistuba II, Heymach JV, Lippman SM, Desgrosellier JS, Anand S, Weis SM, Cheresh DA. An integrin beta(3)-KRAS-RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat Cell Biol. 2014;16:457–468. doi: 10.1038/ncb2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desgrosellier JS, Lesperance J, Seguin L, Gozo M, Kato S, Franovic A, Yebra M, Shattil SJ, Cheresh DA. Integrin alphavbeta3 drives slug activation and stemness in the pregnant and neoplastic mammary gland. Dev Cell. 2014;30:295–308. doi: 10.1016/j.devcel.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Q, Parris AB, Howard EW, Zhao M, Ma Z, Guo Z, Xing Y, Yang X. FGFR inhibitor, AZD4547, impedes the stemness of mammary epithelial cells in the premalignant tissues of MMTV-ErbB2 transgenic mice. Sci Rep. 2017;7:11306. doi: 10.1038/s41598-017-11751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li B, Liu W, Zhuang M, Li N, Wu S, Pan S, Hua J. Overexpression of CD61 promotes hUC-MSC differentiation into male germ-like cells. Cell Prolif. 2016;49:36–47. doi: 10.1111/cpr.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo PK, Kanojia D, Liu X, Singh UP, Berger FG, Wang Q, Chen H. CD49f and CD61 identify Her2/neu-induced mammary tumor-initiating cells that are potentially derived from luminal progenitors and maintained by the integrin-TGFbeta signaling. Oncogene. 2012;31:2614–2626. doi: 10.1038/onc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong SK, Lee H, Kwon OS, Song NY, Lee HJ, Kang S, Kim JH, Kim M, Kim W, Cha HJ. Large-scale pharmacogenomics based drug discovery for ITGB3 dependent chemoresistance in mesenchymal lung cancer. Mol Cancer. 2018;17:175. doi: 10.1186/s12943-018-0924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johansen S, Brenner AK, Bartaula-Brevik S, Reikvam H, Bruserud Ø. The possible importance of beta3 integrins for leukemogenesis and chemoresistance in acute myeloid leukemia. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kannan N, Nguyen LV, Eaves CJ. Integrin beta3 links therapy resistance and cancer stem cell properties. Nat Cell Biol. 2014;16:397–399. doi: 10.1038/ncb2960. [DOI] [PubMed] [Google Scholar]
- 51.Christmann M, Diesler K, Majhen D, Steigerwald C, Berte N, Freund H, Stojanovic N, Kaina B, Osmak M, Ambriovic-Ristov A, Tomicic MT. Integrin alphaVbeta3 silencing sensitizes malignant glioma cells to temozolomide by suppression of homologous recombination repair. Oncotarget. 2017;8:27754–27771. doi: 10.18632/oncotarget.10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naik A, Al-Yahyaee A, Abdullah N, Sam JE, Al-Zeheimi N, Yaish MW, Adham SA. Neuropilin-1 promotes the oncogenic Tenascin-C/integrin beta3 pathway and modulates chemoresistance in breast cancer cells. BMC Cancer. 2018;18:533. doi: 10.1186/s12885-018-4446-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noh KW, Sohn I, Song JY, Shin HT, Kim YJ, Jung K, Sung M, Kim M, An S, Han J, Lee SH, Lee MS, Choi YL. Integrin beta3 inhibition enhances the antitumor activity of ALK inhibitor in ALK-rearranged NSCLC. Clin Cancer Res. 2018;24:4162–4174. doi: 10.1158/1078-0432.CCR-17-3492. [DOI] [PubMed] [Google Scholar]
- 54.EGFR inhibitors may induce tumor stemness. Cancer Discov. 2014;4:753. doi: 10.1158/2159-8290.CD-NB2014-073. [DOI] [PubMed] [Google Scholar]
- 55.Demircioglu F, Hodivala-Dilke K. alphavbeta3 Integrin and tumour blood vessels-learning from the past to shape the future. Curr Opin Cell Biol. 2016;42:121–127. doi: 10.1016/j.ceb.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98:119–124. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, Kutschera S, Bartels S, Appak S, Besemfelder E, Terhardt D, Chavakis E, Wieland T, Klein C, Thomas M, Uemura A, Goerdt S, Augustin HG. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cai H, Saiyin H, Liu X, Han D, Ji G, Qin B, Zuo J, Shen S, Yu W, Wu J, Wu Y, Yu L. Nogo-B promotes tumor angiogenesis and provides a potential therapeutic target in hepatocellular carcinoma. Mol Oncol. 2018;12:2042–2054. doi: 10.1002/1878-0261.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang R, Rofstad EK. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. J Exp Clin Cancer Res. 2018;37:92. doi: 10.1186/s13046-018-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Imamaki R, Ogawa K, Kizuka Y, Komi Y, Kojima S, Kotani N, Honke K, Honda T, Taniguchi N, Kitazume S. Glycosylation controls cooperative PECAM-VEGFR2-β3 integrin functions at the endothelial surface for tumor angiogenesis. Oncogene. 2018;37:4287–4299. doi: 10.1038/s41388-018-0271-7. [DOI] [PubMed] [Google Scholar]
- 61.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–93. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stromblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Wang Z, Tian D, Zeng X, Liu Y, Fu Q, Liang A, Zhang Y, Gao Q, Cheng J, Wang Y. Integrin beta3 mediates the endothelial-to-mesenchymal transition via the notch pathway. Cell Physiol Biochem. 2018;49:985. doi: 10.1159/000493229. [DOI] [PubMed] [Google Scholar]
- 64.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, Thoma OM, Kramer V, Waldner MJ, Büttner C, Farin HF, Pešić M, Knieling F, Merkel S, Grüneboom A, Gunzer M, Grützmann R, Rose-John S, Koralov SB, Kollias G, Vieth M, Hartmann A, Greten FR, Neurath MF, Neufert C. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut. 2019 doi: 10.1136/gutjnl-2019-319200. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nature reviews. Nat Rev Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 67.Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, Elkhatib N, Betz T, Gurchenkov B, Vignjevic DM. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly. J Cell Biol. 2017;216:3509–3520. doi: 10.1083/jcb.201702033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, Qin Y, Sun K, Teng Y, Liu M. Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3-p38 MAPK signalling. Cancer Lett. 2019;442:320–332. doi: 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 69.Luo D, McGettrick HM, Stone PC, Rainger GE, Nash GB. The roles of integrins in function of human neutrophils after their migration through endothelium into interstitial matrix. PLoS One. 2015;10:e0118593. doi: 10.1371/journal.pone.0118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng XX, Liu M, Yan W, Zhou ZZ, Xia YJ, Tu W, Li PY, Tian DA. beta3 integrin promotes TGF-beta1/H2O2/HOCl-mediated induction of metastatic phenotype of hepatocellular carcinoma cells by enhancing TGF-beta1 signaling. PLoS One. 2013;8:e79857. doi: 10.1371/journal.pone.0079857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brilha S, Wysoczanski R, Whittington AM, Friedland JS, Porter JC. Monocyte adhesion, migration, and extracellular matrix breakdown are regulated by integrin alphaVbeta3 in mycobacterium tuberculosis infection. J Immunol. 2017;199:982–991. doi: 10.4049/jimmunol.1700128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deroide N, Li X, Lerouet D, Van Vre E, Baker L, Harrison J, Poittevin M, Masters L, Nih L, Margaill I, Iwakura Y, Ryffel B, Pocard M, Tedgui A, Kubis N, Mallat Z. MFGE8 inhibits inflammasome-induced IL-1beta production and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176–1181. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goehrig D, Nigri J, Samain R, Wu Z, Cappello P, Gabiane G, Zhang X, Zhao Y, Kim IS, Chanal M, Curto R, Hervieu V, de La Fouchardiere C, Novelli F, Milani P, Tomasini R, Bousquet C, Bertolino P. Stromal protein betaig-h3 reprogrammes tumour microenvironment in pancreatic cancer. Gut. 2019;68:693–707. doi: 10.1136/gutjnl-2018-317570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rubartelli A, Poggi A, Zocchi MR. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–1900. doi: 10.1002/eji.1830270812. [DOI] [PubMed] [Google Scholar]
- 75.Toth B, Sarang Z, Vereb G, Zhang A, Tanaka S, Melino G, Fesus L, Szondy Z. Over-expression of integrin beta3 can partially overcome the defect of integrin beta3 signaling in transglutaminase 2 null macrophages. Immunol Lett. 2009;126:22–28. doi: 10.1016/j.imlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 76.Parcina M, Schiller M, Gierschke A, Heeg K, Bekeredjian-Ding I. PDC expressing CD36, CD61 and IL-10 may contribute to propagation of immune tolerance. Autoimmunity. 2009;42:353–5. doi: 10.1080/08916930902831969. [DOI] [PubMed] [Google Scholar]
- 77.Skaik Y, Vahlsing S, Goudeva L, Eiz-Vesper B, Battermann A, Blasczyk R, Figueiredo C. Secreted beta3-integrin enhances natural killer cell activity against acute myeloid leukemia cells. PLoS One. 2014;9:e98936. doi: 10.1371/journal.pone.0098936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang H, Lang S, Zhai Z, Li L, Kahr WH, Chen P, Brkic J, Spring CM, Flick MJ, Degen JL, Freedman J, Ni H. Fibrinogen is required for maintenance of platelet intracellular and cell-surface P-selectin expression. Blood. 2009;114:425–36. doi: 10.1182/blood-2008-03-145821. [DOI] [PubMed] [Google Scholar]
- 79.Hong SK, Park JR, Kwon OS, Kim KT, Bae GY, Cha HJ. Induction of integrin β3 by sustained ERK activity promotes the invasiveness of TGFβ-induced mesenchymal tumor cells. Cancer Lett. 2016;376:339–46. doi: 10.1016/j.canlet.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Gao YY, Zhang ZH, Zhuang Z, Lu Y, Wu LY, Ye ZN, Zhang XS, Chen CL, Li W, Hang CH. Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis. 2018;9:845. doi: 10.1038/s41419-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yue J, Lv D, Wang C, Li L, Zhao Q, Chen H, Xu L. Epigenetic silencing of miR-483-3p promotes acquired gefitinib resistance and EMT in EGFR-mutant NSCLC by targeting integrin beta3. Oncogene. 2018;37:4300–4312. doi: 10.1038/s41388-018-0276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ni R, Huang Y, Wang J. miR-98 targets ITGB3 to inhibit proliferation, migration, and invasion of non-small-cell lung cancer. Onco Targets Ther. 2015;8:2689–2697. doi: 10.2147/OTT.S90998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei W, Yang Y, Cai J, Cui K, Li RX, Wang H, Shang X, Wei D. MiR-30a-5p suppresses tumor metastasis of human colorectal cancer by targeting ITGB3. Cell Physiol Biochem. 2016;39:1165–1176. doi: 10.1159/000447823. [DOI] [PubMed] [Google Scholar]
- 84.Sun L, Liu B, Lin Z, Yao Y, Chen Y, Li Y, Chen J, Yu D, Tang Z, Wang B, Zeng S, Fan S, Wang Y, Li Y, Song E, Li J. MiR-320a acts as a prognostic factor and Inhibits metastasis of salivary adenoid cystic carcinoma by targeting ITGB3. Mol Cancer. 2015;14:96. doi: 10.1186/s12943-015-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arias-Salgado EG, Lizano S, Sarkar S, Brugge JS, Ginsberg MH, Shattil SJ. Src kinase activation by direct interaction with the integrin beta cytoplasmic domain. Proc Natl Acad Sci U S A. 2003;100:13298–13302. doi: 10.1073/pnas.2336149100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Horie M, Saito A, Ohshima M, Suzuki HI, Nagase T. YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Nat Commun. 2016;107:1755–1766. doi: 10.1111/cas.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotani N, Honke K, Honda T, Taniguchi N, Kitazume S, Huang R. Integrins as therapeutic targets in the organ-specific metastasis of human malignant melanoma. Oncogene. 2018;37:92. doi: 10.1186/s13046-018-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tucci M, Stucci S, Felici C, Cafforio P, Resta L, Rossi R, Silvestris F. Cilengitide restrains the osteoclast-like bone resorbing activity of myeloma plasma cells. Br J Haematol. 2016;173:59–69. doi: 10.1111/bjh.13922. [DOI] [PubMed] [Google Scholar]
- 89.Alva A, Slovin S, Daignault S, Carducci M, Dipaola R, Pienta K, Agus D, Cooney K, Chen A, Smith DC, Hussain M. Phase II study of cilengitide (EMD 121974, NSC 707544) in patients with non-metastatic castration resistant prostate cancer, NCI-6735. A study by the DOD/PCF prostate cancer clinical trials consortium. Invest New Drugs. 2012;30:749–757. doi: 10.1007/s10637-010-9573-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manegold C, Vansteenkiste J, Cardenal F, Schuette W, Woll PJ, Ulsperger E, Kerber A, Eckmayr J, von Pawel J. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs. 2013;31:175–182. doi: 10.1007/s10637-012-9842-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108. doi: 10.1016/S1470-2045(14)70379-1. [DOI] [PubMed] [Google Scholar]
- 92.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–20. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 93.Pickarski M, Gleason A, Bednar B, Duong LT. Orally active alphavbeta3 integrin inhibitor MK-0429 reduces melanoma metastasis. Oncol Rep. 2015;33:2737–2745. doi: 10.3892/or.2015.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenthal MA, Davidson P, Rolland F, Campone M, Xue L, Han TH, Mehta A, Berd Y, He W, Lombardi A. Evaluation of the safety, pharmacokinetics and treatment effects of an alpha(nu)beta(3) integrin inhibitor on bone turnover and disease activity in men with hormone-refractory prostate cancer and bone metastases. Asia Pac J Clin Oncol. 2010;6:42–8. doi: 10.1111/j.1743-7563.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 95.Cheng W, Ren X, Zhang C, Cai J, Liu Y, Han S, Wu A. Bioinformatic profiling identifies an immune-related risk signature for glioblastoma. Neurology. 2016;86:2226–2234. doi: 10.1212/WNL.0000000000002770. [DOI] [PubMed] [Google Scholar]
- 96.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, Waziri A. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res. 2017;17:6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 99.Zhang CB, Cheng W, Ren X, Wang Z, Liu X, Li G, Han S, Jiang T, Wu A. Tumor purity as an underlying key factor in glioma. Clin Cancer Res. 2017;23:6279–6291. doi: 10.1158/1078-0432.CCR-16-2598. [DOI] [PubMed] [Google Scholar]
- 100.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem. 2010;10:753–768. doi: 10.2174/187152010794728639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim KB, Prieto V, Joseph RW, Diwan AH, Gallick GE, Papadopoulos NE, Bedikian AY, Camacho LH, Hwu P, Ng CS, Wei W, Johnson MM, Wittemer SM, Vardeleon A, Reckeweg A, Colevas AD. A randomized phase II study of cilengitide (EMD 121974) in patients with metastatic melanoma. Melanoma Res. 2012;22:294–301. doi: 10.1097/CMR.0b013e32835312e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Su X, Esser AK, Amend SR, Xiang J, Xu Y, Ross MH, Fox GC, Kobayashi T, Steri V, Roomp K, Fontana F, Hurchla MA, Knolhoff BL, Meyer MA, Morgan EA, Tomasson JC, Novack JS, Zou W, Faccio R, Novack DV, Robinson SD, Teitelbaum SL, DeNardo DG, Schneider JG, Weilbaecher KN. Antagonizing integrin beta3 increases immunosuppression in cancer. Cancer Res. 2016;76:3484–3495. doi: 10.1158/0008-5472.CAN-15-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu H, Beuerlein G, Nie Y, Smith H, Lee BA, Hensler M, Huse WD, Watkins JD. Stepwise in vitro affinity maturation of Vitaxin, an alphav beta3-specific humanized mAb. Proc Natl Acad Sci U S A. 1998;95:6037–6042. doi: 10.1073/pnas.95.11.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruan JS, Liu YP, Zhang L, Yan LG, Fan FT, Shen CS, Wang AY, Zheng SZ, Wang SM, Lu Y. Luteolin reduces the invasive potential of malignant melanoma cells by targeting beta3 integrin and the epithelial-mesenchymal transition. Acta Pharmacol Sin. 2012;33:1325–1331. doi: 10.1038/aps.2012.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai Z, Dong L, Song C, Zhang Y, Zhu C, Zhang Y, Ling Q, Hoffmann PR, Li J, Huang Z, Li W. Methylseleninic acid provided at nutritional selenium levels inhibits angiogenesis by down-regulating integrin beta3 signaling. Sci Rep. 2017;7:9445. doi: 10.1038/s41598-017-09568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petpiroon N, Sritularak B, Chanvorachote P. Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin alphav and integrin beta3. BMC Complement Altern Med. 2017;17:553. doi: 10.1186/s12906-017-2059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen KS, Shi MD, Chien CS, Shih YW. Pinocembrin suppresses TGF-beta1-induced epithelial-mesenchymal transition and metastasis of human Y-79 retinoblastoma cells through inactivating alphavbeta3 integrin/FAK/p38alpha signaling pathway. Cell Biosci. 2014;4:41. doi: 10.1186/2045-3701-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]


