Abstract
The goal of this study was to assess the role of high-mobility group box 1 (HMGB1)-induced endothelial cell (EC) pyroptosis in systemic inflammatory response syndrome (SIRS) following radiofrequency (RF) ablation of hepatic hemangiomas. We enrolled 76 patients with hepatic hemangioma who underwent RF ablation. Serum concentrations of HMGB1, interleukin (IL)-1β, IL-18 and lactate dehydrogenase (LDH) were determined at different time points. Immunohistochemistry staining (IHC) was performed to evaluate the expressions of HMGB1, NLRP3, caspase-1, GSDMD, IL-18 and IL-1β in hepatic hemangioma and sub-ablated hemangioma tissues. In vitro experiments used human umbilical vein endothelial cells (HUVECs) treated with sub-ablative hyperthermia to mimic insufficient RF ablation of hepatic hemangiomas. ELISA and western blotting were performed to quantify HMGB1, NLRP3, caspase-1, GSDMD, IL-18, IL-1β and LDH levels with or without the addition of ethyl pyruvate (EP), a HMGB1 inhibitor, in the medium. Flow cytometry and fluorescent staining were performed to assess pyroptosis of HUVECs. Twenty-nine patients experienced SIRS after RF ablation (29/76, 38.2%). HMGB1, IL-1β and IL-18 levels were significantly correlated with SIRS. IHC staining revealed an obvious increase in HMGB1, NLRP3, caspase-1, GSDMD, IL-18, and IL-1β in the ECs of sub-ablated hemangioma but not in hepatic hemangioma. In vitro experiments showed that subablative hyperthermia led to HMGB1-induced pyroptosis of HUVECs and EP attenuated the pyroptosis of HUVECs. Taken together, these data demonstrate HMGB1-induced ECs pyroptosis may occur during SIRS following RF ablation of hepatic hemangiomas.
Keywords: Endothelial cells, pyroptosis, HMGB1, systemic inflammatory response syndrome, hepatic hemangiomas
Introduction
Hepatic hemangiomas are the most common benign neoplasm of the liver, the majority of which are small and asymptomatic and need no special treatment. However, a minority of hepatic hemangiomas (≥ 5 cm) tend to enlarge and cause symptoms that need active management [1,2]. In recent years, radiofrequency (RF) ablation has been increasingly used for the treatment of hepatic hemangiomas because of its unique advantages, such as minimal invasiveness, definite efficacy, high safety, fast recovery, and wide applicability. However, the relatively long ablation time and large volume of ablated tissues might induce significant systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS) or severe myocardial dysfunction [3-5]. Currently, the underlying pathophysiology of SIRS post hepatic hemangioma ablation is still unknown.
Pyroptosis is a caspase-1-dependent programmed cell death, which features gasdermin D (GSDMD) cleavage and translocation, rapid plasma membrane rupture, and release of inflammatory cytokines, such as IL-1β and IL-18, and intracellular contents [6,7]. The over activation of pyroptosis can result in septic shock, multiple organ dysfunction syndromes, or increase the risk of secondary infection. High-mobility group box 1 (HMGB1) is a ubiquitous nuclear protein that promotes inflammation when released extracellularly after cellular activation, stress, damage, or death. HMGB1 functions in inflammatory disorders via recently elucidated signal and molecular transport mechanisms [8,9]. Moreover, elevated circulating HMGB1 levels significantly contribute to systemic inflammation and multiple organ failure [10,11]. Recent studies have shown that HMGB1 mediates activation of the NLRP3 inflammasome, which in turn, induces pyroptosis of hepatocytes and Hela cells and the subsequent progress of inflammation and injury [12,13]. However, whether HMGB1 is involved in endothelial cell (EC) pyroptosis and subsequent SIRS after RF ablation treatment of hepatic hemangioma has not been investigated.
Hepatic hemangiomas consist of blood-filled cavities fed by the hepatic arterial circulation, with walls lined by a single layer of ECs, and a fibrous tissue between vascular cavities [1]. RF ablation is performed using RF-induced thermal energy to damage the endothelial lining vascular structures to promote thrombosis and induce necrotic coagulation. The target temperature can be set as high as 105°C-115°C during RF ablation; however, only a minority of tissues that surround the electrodes reach that temperature. The actual temperature of the para-tumor tissue is lower than the target temperature because of an abundant blood supply and marked “heat sink” effect of hepatic hemangiomas [4]. Furthermore, a multiple-overlapping ablation technique must be adopted because of the large ablated volume of hepatic hemangiomas [1]. Therefore, there are always insufficient ablation areas around each ablation zone. Based on these findings, we hypothesized that insufficient RF ablation adjacent electrodes will trigger the HMGB1-induced pyroptosis of ECs, release inflammatory factors into the blood, leading to SIRS during RF ablation for hepatic hemangiomas.
The purpose of this study was to investigate the relationship between EC pyroptosis and the occurrence of SIRS, and to assess the role of HMGB1 in the pyroptosis of ECs after RF ablation treatment for hepatic hemangioma.
Materials and methods
Study design
This study was divided into two parts: (1) patient blood and tumor tissue samples were analyzed to assess the relationship between SIRS and HMGB1-induced pyroptosis of ECs; and (2) in vitro experiments to investigate whether insufficient RF ablation induces pyroptosis of ECs and the role of HMGB1 in endothelial pyroptosis. Human umbilical vein endothelial cells (HUVECs) were treated to mimic the scenario of insufficient RF ablation of hepatic hemangiomas. Cells were treated with ethyl pyruvate (EP), an HMGB1 inhibitor.
Patients and blood sample collection
From January 2016 to June 2019, 76 patients with hepatic hemangiomas were treated with RF ablation in our institution. The inclusion criterion for ablation was described in our previously published article [1]. RF ablation was performed using internally cooled cluster electrodes, Cool-tip ACTC 2025 (for laparoscopic procedures) or ACTC 1525 (for CT-guided percutaneous procedures) electrodes, and an RF generator (Covidien Healthcare, Dublin, Ireland).
Blood cell count, CRP, and biochemistry tests to evaluate liver and renal functions were performed before RF ablation and at 1 hour, 1 day, 2 days and 3 days post RF ablation. Blood samples were collected in heparinized tubes before RF ablation and at 1 hour, 1 day, 2 days and 3 days after RF ablation. After sampling, plasma was separated by centrifugation, divided into aliquots, and stored at -70°C until evaluating the serum level of inflammatory cytokines.
All patients gave written informed consent before treatment, which was approved by the investigation and ethics committee of Beijing Chao-yang Hospital, Capital Medical University in accordance with the standards of the Declaration of Helsinki.
Definition of SIRS
SIRS was determined based on the following criteria, including at least two of the parameters: body temperature > 38°C or < 36°C; heart rate > 90 bpm; respiratory rate > 20 breaths/min or PaCO2 < 32 mmHg; and WBC count > 12 × 109/L or < 4 × 109/L [14].
Ablated volume of hemangioma
The ablated volume of hemangioma, considered to be same as the lesion volume of hemangioma before RF ablation, was determined by contrast-enhanced CT or MR before RF ablation to correlate the ablated volume with SIRS. The lesion volumes were calculated using the formula: volume = X × Y × Z × π/6, where X, Y and Z are the maximum diameter in three dimensions (vertical, sagittal and coronal planes when the patients were in a supine position) of the tumor measured by CT or MRI [15].
Immunohistochemistry staining
Hemangioma tissues were excised by laparoscopic resection post RF ablation [16]. Tissues in the region of the sub-ablated hemangioma, located less than 1.0 cm away from the ablation tissues, were collected.
Hepatic hemangioma and subablated hemangioma were fixed with 4% buffered paraformaldehyde, dehydrated, and embedded in paraffin. Five-μm sections were deparaffinized, rehydrated, and rinsed in distilled water. Antigen unmasking was carried out by microwave heating in citrate buffer for 20 minutes. The sections were immunostained with a primary antibody against HMGB1, NLRP3, caspase-1 (Cell Signaling Technology, MA, USA), N-GSDMD, IL-18, and IL-1β (all antibodies from Abcam, Cambridge, UK, except caspase-1) respectively, at 4°C overnight. After incubation with a secondary antibody, the sections were stained with diaminobenzidine. The integrated optical density of positive staining was quantified by Image-Pro Plus software (Media Cybernetics Inc, Bethesda, MD).
In vitro experiments on HUVECs
Cell line and cell culture
HUVECs were collected from fresh cords using standard procedures. The cells were plated on gelatin-coated culture dishes with Endothelial Cell Medium (Sciencell, San Diego, CA, USA), containing 500 ml of basal medium, 25 ml of fetal bovine serum, 5 ml of endothelial cell growth supplement and 5 ml of penicillin and streptomycin solution, then cultured in a humidified incubator at 37°C with an atmosphere of 5% CO2.
Subablative hyperthermia of HUVECs
HUVECs were seeded in 96-well plates at a concentration of 3 × 103/well. After 24-hour incubation, cells were treated by subablative hyperthermia. Subablative treatment was carried out by submerging parafilm-sealed culture plates in a water bath at 45°C for 0, 15, 30, 45, 60 and 75 minutes (37°C was used as a control) to mimic insufficient RF ablation occurring during RF ablation for hepatic hemangiomas [15,17].
Cell viability and LDH release after subablative treatment at different time points were measured. The subablative treatment group was selected for subsequent experiments according to decreased cell viability and elevated LDH release. Another group with subablative treatment was treated with the HMGB1 inhibitor EP (5 mM).
Cell viability assay
Cell viability was evaluated by MTT assays. HUVECs were cultured in 96-well plates at a concentration of 3 × 103/well. MTT solution was added to each well at a final concentration of 0.5 mg/ml and incubated for 4 hours. At the end of incubation, formazan crystals resulting from MTT reduction were dissolved by the addition of 150 ml dimethyl sulfoxide (DMSO) per well. The optical density was read at 570 nm with a plate reader (Model 550, Bio-Rad, California, USA). The mean values were determined from different wells of a representative assay.
ELISA
The levels of HMGB1, LDH and cytokines in serum samples and cell culture supernatants were measured using a human HMGB1 (Elabscience, Wuhan, China), human D-LDH (Elabscience, Wuhan, China), human IL-18 (Elabscience, Wuhan, China) and IL-1β (RayBiotech, Norcross, GA, USA) ELISA Kits. The level of caspase-1 released in cell culture medium was measured using a human caspase-1 ELISA Kit (Jiancheng Bioengineering, Nanjing, China). The absorbance of samples was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, USA).
Western blotting analysis
Western blotting analysis was performed using standard protocols. Primary antibodies were polyclonal anti-caspase-1, polyclonal anti-pro-caspase-1, polyclonal anti-IL-1β, polyclonal anti-NLRP3 and polyclonal anti-HMGB1 (all 1:1000; Abcam, Cambridge, UK); polyclonal anti-GSDMD (1:1000; Proteintech, Rosemont, IL, USA) and anti-β-actin (1:200; Boster, Wuhan, China).
Immunofluorescence confocal microscopy
The LIVE/DEAD™ Cell Imaging Kit (488/570) (Invitrogen, Carlsbad, USA) is a sensitive two-color fluorescence cell viability assay optimized for FITC and Texas Red filters. Cells were stained with the LIVE/DEAD Cell Imaging kit in accordance with the manufacturer’s instructions (Molecular Probes). The cells were imaged on a confocal laser scanning microscope (Nikon C2, Japan) to determine whether they were live (green) or dead (red).
Flow cytometry
To assess pyroptosis in HUVECs, active caspase-1 was determined using the FLICA® 660 in vitro Caspase-1 Detection kit (ImmunoChemistry Technologies, Bloomington, USA) as described previously [18]. SYTOX® Green stain (Invitrogen, Carlsbad, USA), a fluorescent nucleic acid dye that only penetrates ruptured cell membranes, was used to mark cells with membrane pore formation. Briefly, after treatment, the cells were harvested and incubated with a caspase-1 detection probe for 60 min at 37°C in the dark. At the end of incubation, unbound FLICA reagent was washed away using a cellular wash buffer. Cells were then stained with 1 μM SYTOX® Green for a further 10 min at 37°C in the dark. The cells were then analyzed using a flow cytometer (Beckman Coulter, CytoFLEX, USA). Pyroptotic cells were defined as double positive for caspase-1 and SYTOX® Green. The double-stained cells were counted as pyroptotic cells, and the rate of pyroptotic cell death was calculated by the formula: (pyroptotic cells/total cells) × 100%.
Statistical analysis
Values were expressed as the mean ± standard deviation (SD). Peak values (PPV) were the highest mean values after therapy. Data were analyzed using the Mann-Whitney U-non-parametric test or one-way ANOVA followed by Dunnett’s Multiple Comparison or Student’s t-test (SPSS, version 17.0). SIRS related risk factors were analyzed using the univariate and multivariate binary logistic regression model method. All descriptive graphs were created using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). Differences with a P-value < 0.05 were considered statistically significant.
Results
Patient characteristics and clinical outcomes
RF ablation was successfully performed for all patients. SIRS appeared in 29 of 76 patients (29/76, 38.2%). Patients were classified according to diagnostic criteria described in the Methods into the SIRS group or Non-SIRS group. Patients’ clinical parameters are summarized in Table 1. There were no significant differences in relevant baseline characteristics between the two treatment groups.
Table 1.
Comparison of clinical parameters between SIRS and Non-SIRS group patients after RF ablation for hepatice hemangiomas
| Non-SIRS group (n = 47) | SIRS group (n = 29) | P value | |
|---|---|---|---|
| Age (y) | 52.04 ± 8.46 | 48.72 ± 13.75 | 0.834 |
| Sex | 0.721 | ||
| Female | 19 (40.43%) | 15 (51.72%) | |
| Male | 28 (59.57%) | 14 (48.28%) | |
| Concomitant illness | |||
| Gallbladder stones | 8 (17.02%) | 5 (17.24%) | 0.674 |
| Type 2 diabetes mellitus | 9 (19.15%) | 4 (13.79%) | 0.579 |
| History of open cholecystectomy | 3 (6.38%) | 2 (6.90%) | 0.756 |
| Chronic hepatitis B | 3 (6.38%) | 2 (6.90%) | 0.632 |
| Hepatic cysts | 6 (12.77%) | 7 (24.13%) | 0.243 |
| History of previous liver surgery | 2 (4.26%) | 1 (3.43%) | 0.768 |
| Volume of hemangiomas (cm3) | 96.75 ± 36.90 | 258.92 ± 167.781 | 0.032 |
| Distribution of lesion, N (%) | 0.598 | ||
| Right lobe | 21 (44.68%) | 14 (48.28%) | |
| Left lobe | 26 (55.32%) | 15 (51.72%) | |
| Approach of RF ablation, N (%) | 0.607 | ||
| Laparoscopic approach | 36 (76.60%) | 24 (82.76%) | |
| Percutaneous approach | 11 (23.40%) | 5 (17.24%) | |
| Time of ablation (min) | 29.59 ± 7.21 | 48.32 ± 13.65 | 0.031 |
| Laboratory findings-pre | |||
| ALT (U/L) | 20.32 ± 11.43 | 23.38 ± 13.61 | 0.847 |
| AST (U/L) | 22.38 ± 6.86 | 21.42 ± 8.47 | 0.649 |
| Total bilirubin (μmol/L) | 13.87 ± 4.23 | 12.55 ± 3.65 | 0.246 |
| BUN (mmol/L) | 4.25 ± 1.43 | 3.64 ± 0.96 | 0.578 |
| Creatinine (μmol/L) | 60.32 ± 12.45 | 59.11 ± 13.25 | 0.467 |
| CRP (mg/L) | 4.24 ± 0.45 | 5.75 ± 6.56 | 0.368 |
| Laboratory findings-post 1 hour | |||
| ALT (U/L) | 74.56 ± 23.34 | 88.56 ± 20.46 | 0.039 |
| AST (U/L) | 118.45 ± 68.46 | 205.67 ± 70.53 | 0.023 |
| Total bilirubin (μmol/L) | 23.54 ± 6.34 | 38.56 ± 13.67 | 0.016 |
| BUN (mmol/L) | 3.34 ± 0.94 | 5.85 ± 1.43 | 0.045 |
| Creatinine (μmol/L) | 60.11 ± 13.43 | 78.44 ± 12.54 | 0.046 |
| CRP (mg/L) | 5.01 ± 0.63 | 7.01 ± 2.01 | 0.032 |
| Pyroptosis-related index-post 1 hour | |||
| HMGB-1 (pg/ml) | 505.08 ± 85.86 | 1027.58 ± 147.55 | 0.003 |
| IL-1β (pg/ml) | 2.95 ± 0.61 | 9.81 ± 1.03 | 0.014 |
| IL-18 (pg/ml) | 50.39 ± 12.58 | 149.44 ± 14.56 | 0.004 |
| LDH (U/L) | 250.78 ± 101.46 | 708.29 ± 113.57 | 0.012 |
SIRS, systemic inflammatory response syndrome; RF, radiofrequency; ALT, alanine aminotransferase; AST, aspartate transaminase, BUN, blood urea nitrogen; CRP, C-reactive protein; IL-1β, interleukin-1β; IL-18, interleukin-18; LDH, lactate dehydrogenase.
The levels of HMGB1, IL-1β, IL-18 and LDH post RF ablation were higher than at pre-operation. Levels of serum HMGB1, IL-1β, IL-18 and LDH were higher in the SIRS group after RF ablation compared with the Non-SIRS group (P < 0.05). The levels of HMGB1, IL-1β and IL-18 were increased immediately post RF ablation and reduced slowly from 1 hour after RF ablation. The peak occurred at 1-hour post procedure (Figure 1A-C). Serum LDH levels were elevated immediately after RF ablation and reached a peak 1 day after RF ablation and decreased subsequently compared to baseline values (P < 0.05), with the peak occurring 1-day post procedure (Figure 1D).
Figure 1.
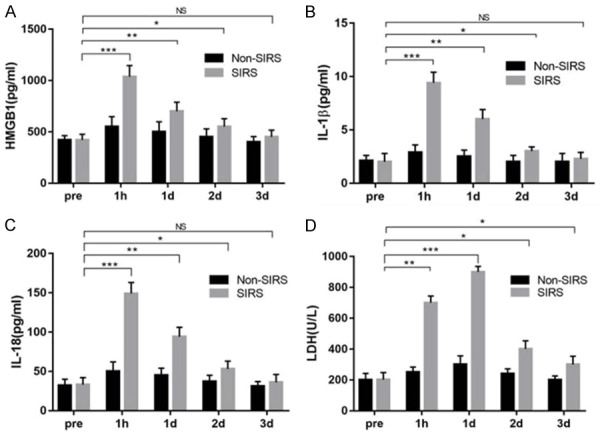
Levels of serum HMGB1, IL-1β, IL-18, and LDH at pre-RF ablation and 1 hour, 1, 2, and 3 days post-RF ablation. A. Serum HMGB1 increased immediately after RF ablation compared with preoperative levels and reduced slowly from 1-hour post-RF ablation. The peak occurred at 1-hour post procedure. B. Serum IL-1β levels were significantly elevated immediately after RF ablation compared with preoperative levels and reduced slowly from 1 hour after RF ablation. The peak of IL-1β occurred 1-hour post procedure. C. Serum IL-18 levels were significantly elevated immediately after RF ablation compared with preoperative levels and reduced slowly from 1 hour after RF ablation. The peak of IL-1β occurred at 1-hour post procedure. D. Serum LDH increased immediately after RF ablation compared with preoperative levels and reduced slowly from day 2 after RF ablation the peak occurred at 1-day post procedure. *P < 0.05, **P < 0.01, ***P < 0.001, NS = not significant.
The mean volume of hemangiomas in the SIRS group was significantly larger than among those in the Non-SIRS group (P < 0.05). Ablation time was significantly longer among patients in the SIRS group than among those in the Non-SIRS group (P < 0.05) (Table 1).
Risk factors of SIRS
By univariate analysis, the volume of hemangiomas (P = 0.032), time of ablation (P = 0.031), AST (P = 0.017), serum total bilirubin (P = 0.032), BUN (P = 0.035), creatinine (P = 0.033), CRP (P = 0.043), HMGB-1 (P = 0.023), IL-1β (P = 0.038), IL-18 (P = 0.017) and LDH (P = 0.019) were significant risk factors that adversely affected the occurrence of SIRS. Multivariate analysis confirmed that volume of hemangiomas (P = 0.042, odds ratio [OR] = 1.457, 95% confidence interval [CI] = 0.690-1.689), time of ablation (P = 0.039, OR = 1.356, 95% CI = 0.968-1.790), HMGB-1 (P = 0.028, OR = 1.765, 95% CI = 0.886-2.422), IL-1β (P = 0.039, OR = 1.798, 95% CI = 0.632-3.765), and IL-18 (P = 0.024, OR = 1.458, 95% CI = 0.788-4.742) were independent risk factors associated with SIRS after RF ablation for hepatic hemangioma (Table 2).
Table 2.
Univariate and multivariate analysis of clinical parameters related to SRIS after RF ablation for hepatic hemangiomas
| Parameter | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| Age | 0.968 | 0.843-1.146 | 0.845 | |||
| Gender | 2.022 | 0.563-8.438 | 0.413 | |||
| Co-morbidities | 1.854 | 0.732-7.689 | 0.375 | |||
| Volume of hemangiomas | 1.389 | 1.254-1.864 | 0.032 | 1.457 | 0.690-1.689 | 0.042 |
| Distribution of lesion | 1.056 | 0.363-5.245 | 0.845 | |||
| Approach of RF ablation | 2.743 | 0.424-8.695 | 0.453 | |||
| Time of ablation | 1.238 | 1.263-1.905 | 0.031 | 1.356 | 0.968-1.790 | 0.039 |
| ALT-post | 1.075 | 1.378-1.589 | 0.057 | |||
| AST-post | 1.034 | 1.057-1.564 | 0.017 | 1.432 | 0.890-1.267 | 0.732 |
| Total bilirubin-post | 1.256 | 1.497-1.935 | 0.032 | 0.964 | 0.578-1.327 | 0.375 |
| BUN-post | 2.143 | 1.131-5.148 | 0.035 | 4.541 | 0.463-9.974 | 0.431 |
| Creatinine-post | 1.343 | 1.422-1.975 | 0.033 | 1.242 | 0.853-1.246 | 0.785 |
| CRP-post | 2.265 | 1.444-4.660 | 0.043 | 5.052 | 0.590-12.56 | 0.151 |
| HMGB-1-post | 1.685 | 1.246-1.846 | 0.023 | 1.765 | 0.886-2.422 | 0.028 |
| IL-1β-post | 1.424 | 1.057-1.864 | 0.038 | 1.798 | 0.632-3.765 | 0.039 |
| IL-18-post | 1.343 | 1.234-1.569 | 0.017 | 1.458 | 0.788-4.742 | 0.024 |
| LDH-post | 1.339 | 1.034-1.459 | 0.019 | 1.059 | 0.783-1.863 | 0.325 |
SIRS, systemic inflammatory response syndrome; RF, radiofrequency; ALT, alanine aminotransferase; AST, aspartate transaminase; BUN, blood urea nitrogen; CRP, C-reactive protein; IL-1β, interleukin-1β; IL-18, interleukin-18; LDH, lactate dehydrogenase.
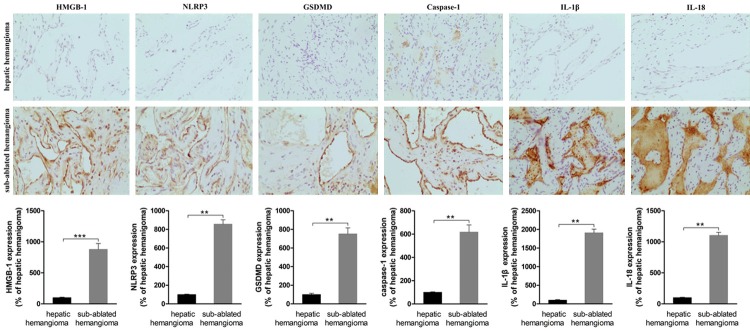
Significant increase in HMGB1-induced pyroptosis of ECs of sub-ablated hemangiomas
The levels of HMGB1, NLRP3, caspase-1, GSDMD, IL-1β and IL-18 in ECs of sub-ablated hemangiomas were significantly higher than in hemangiomas that were not ablated (Figure 2).
Figure 2.
Immunohistochemistry staining of hepatic hemangioma and sub-ablated hemangioma was compared. Representative images are show (200 ×). The expression of HMGB1, NLRP3, caspase-1, GSDMD, IL-1β and IL-18 was low in the endothelial cells of hepatic hemangioma. The expression of HMGB1, NLRP3, caspase-1, GSDMD, IL-1β and IL-18 along the vascular endothelial of sub-ablated hemangioma was significantly increased. *P < 0.05, **P < 0.01, ***P < 0.001.
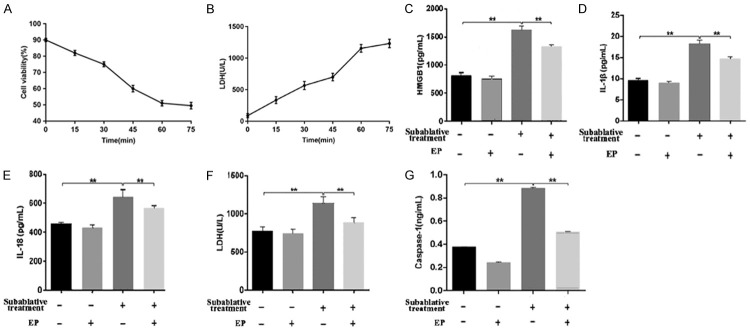
Subablative treatment induces the pyroptosis of HUVECs
The viability of cells was significantly lower after heat treatment for different times. Cell viability was approximately 50% after 60 minutes of heat treatment. LDH release after 60 minutes of heat treatment was significantly higher than those of after 15, 30, 45 minutes of heat treatment respectively (P < 0.05) (Figure 3A, 3B). The cell group treated for 60 minutes was used for subablative treatment.
Figure 3.
The HUVECs group heat treated of 45°C for 60 minutes was used for subablative treatment. (A) The viability of HUVECs was significantly lower after heat treatment of 45°C for different times. Cell viability was approximately 50% after 60 minutes of heat treatment. (B) LDH release after 60 minutes of heat treatment was significantly higher than those of after 15, 30, 45 minutes of heat treatment respectively. (C-G) The supernatant levels of HMGB1, IL-1β, IL-18, LDH and caspase-1 of each group were measured y the method of ELISA. Supernatant evels of (C) HMGB1, (D) L-1β, (E) IL-18 (F) LDH, and (G) caspase-1 increased significantly after subablative treatment and were reduced obviously when EP was added. *P < 0.05. **P < 0.01.
ELISA was used to detect the concentrations of caspase-1, LDH, IL-18 and IL-1β in supernatants. Compared with the control group, the concentrations of caspase-1, LDH, IL-18 and IL-1β in the subablative treatment group were significantly increased (P < 0.01) (Figure 3D-G). Western blotting analysis showed that the expressions of cleaved caspase-1 (caspase-1α), IL-18, IL-1β and cleaved GSDMD (p30) were upregulated in the subablative treatment group compared with controls (Figures 4, 5).
Figure 4.
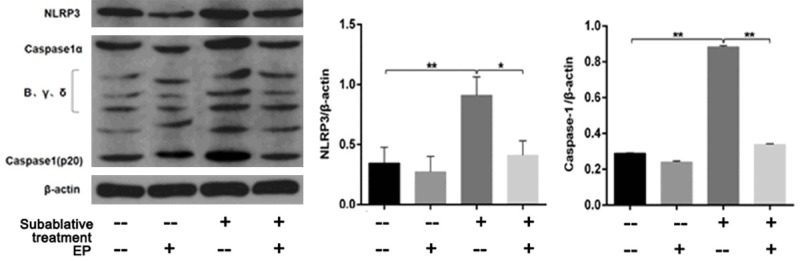
Western blotting analysis was used to detect the protein expression of NLRP3 and cleaved caspase-1 in HUVECs. Subablative treatment significantly increased the expressions of NLRP3 and cleaved caspase-1, while EP efficiently decreased NLRP3 and cleaved caspase-1 expressions. *P < 0.05, **P < 0.01.
Figure 5.
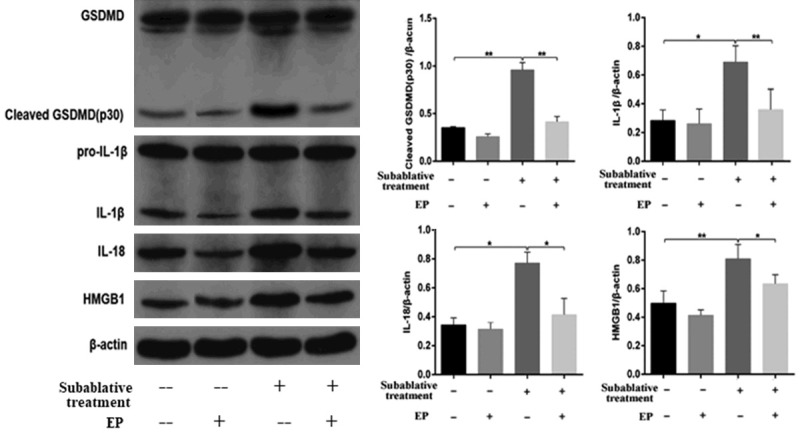
Western blotting analysis was used to detect the protein expression of HMGB1, GSDMD, IL-1β and IL-18 in HUVECs. Subablative treatment significantly increased the expressions of HMGB1, cleaved GSDMD, IL-1β and IL-18 protein, and EP efficiently decreased HMGB1, cleaved GSDMD, IL-1, and IL-18 expressions. *P < 0.05, **P < 0.01.
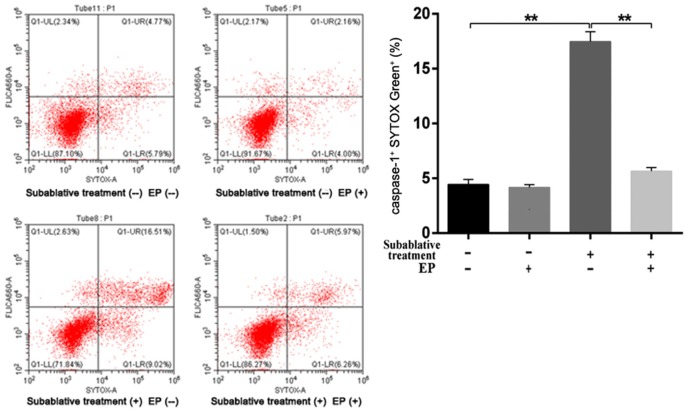
The membrane integrity of each group was assessed by LIVE/DEAD cell staining. As shown in Figure 6, subablative treatment significantly increased the number of dead (red) cells than that of the control group (P < 0.01). Cell pyroptosis quantified by flow cytometry (Figure 7) demonstrated that subablative treatment significantly increased the number of cells with caspase-1 and SYTOX double positive staining, which indicated pyroptosis (P < 0.01).
Figure 6.
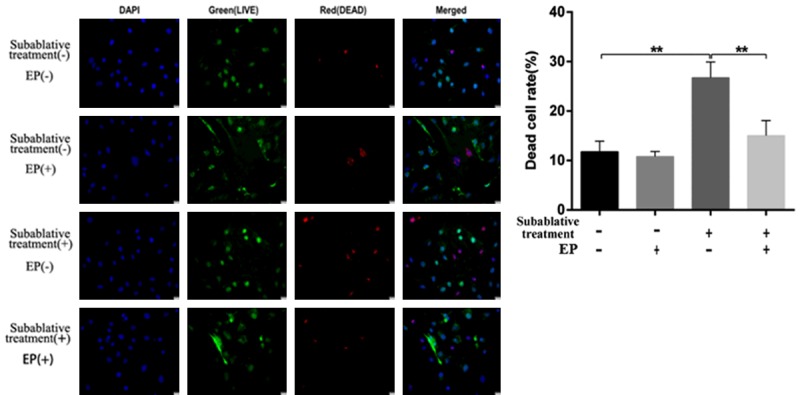
Photomicrographs of LIVE/DEAD cell staining of HUVECs from each group. Subablative treatment significantly increased the number of dead (red) cells, and EP efficiently suppressed the death of HUVECs post-subablative treatment. *P < 0.05, **P < 0.01.
Figure 7.
Pyroptosis was detected using caspase-1/SYTOX Green double staining flow cytometry. Subablative treatment significantly increased the number of cells with caspase-1 and SYTOX double positive staining, which indicated pyroptosis. Furthermore, EP efficiently decreased the number of cells with caspase-1 and SYTOX double positive staining. *P < 0.05, **P < 0.01.
Subablative treatment induces HMGB1 secretion from HUVECs and NLRP3 inflammasome activation
We measured the HMGB1 level in supernatants by ELISA. Increased levels of extracellular HMGB1 were observed for the subablative treatment group compared with the control group (Figure 3C). We examined HMGB1 secretion from HUVECs following subablative treatment. HMGB1 expression detected by western blotting was markedly increased in the subablative treatment group compared with the control group (Figure 4). These results suggest that subablative treatment induces the release of HMGB1.
Next, we examined NLRP3 inflammasome activation in vitro by western blotting. As shown in Figure 4, NLRP3 protein expression in the subablative treatment group was significantly increased compared with the control group.
HMGB1 inhibitor attenuates HMGB1-induced pyroptosis of HUVECs
ELISA showed that an HMGB1 inhibitor (EP) significantly decreased the concentrations of HMGB1, IL-18, IL-1β, and caspase-1 as well as LDH activity induced by subablative treatment (P < 0.01, respectively) (Figure 3C-G). Western blotting analysis showed that EP decreased the expressions of NLRP3, cleaved caspase-1, IL-1β, IL-18 and cleaved GSDMD in cells with subablative treatment (Figures 4, 5). EP significantly decreased the number of dead (red) cells by LIVE/DEAD cell staining after subablative treatment (Figure 6). Moreover, flow cytometry results revealed the number of double positive cells for caspase-1 and SYTOX Green was reduced in the subablative treatment group (Figure 7). These results indicate the important role of HMGB1 in NLRP3 inflammasome activation and subsequent pyroptosis after the subablative treatment of HUVECs.
Discussion
RF ablation is increasingly accepted as an effective and safe alternative treatment to surgical resection for managing huge hepatic hemangioma. However, SIRS often occurs in patients post RF ablation treatment for hepatic hemangiomas, especially for large hemangiomas [1]. Thus, it is of great importance to explore the pathophysiologic mechanism of SIRS induced by RF ablation for hepatic hemangiomas. In this study, we uncovered an important role for HMGB1-mediated pyroptosis of ECs that contribute to SIRS following RF ablation for the treatment of hepatic hemangiomas.
The initiation of caspase-1 dependent pyroptosis occurs after recognition between inflammatory sensors and molecular patterns. Inflammasomes are composed of different subunits, and the best-characterized is the NLRP3 inflammasome. Activation of the NLRP3 inflammasome can be initiated by numerous stimuli including PAMPs and DAMPs [19,20]. HMGB1 is a widely-expressed and highly-abundant protein that has multiple roles in physiological and pathological processes. Under stress conditions, HMGB1 translocates from the nucleus to the cytosol and then is released into the extracellular space where it acts as a prototypical DAMP [21]. Recent studies reported that high serum HMGB1 levels were associated with inflammation in SIRS [10,11]. Dong et al. [12] confirmed polychlorinated biphenyls 29-pQ (PCB29-pQ) activated the NLRP3 inflammasome, which mediated caspase-1 activation (cleaved caspase-1) that subsequently promoted GSDMD cleavage and translocation to facilitate the release of intracellular inflammatory substances by forming a membrane hole, ultimately leading to pyroptosis. Furthermore, PCB29-pQ-induced HMGB1 release was essential for activation of the NLRP3 inflammasome. Geng et al. [13] demonstrated that HMGB1 mediated heatstroke-induced NLRP3 inflammasome activation and hepatocyte pyroptosis, which in turn promoted liver injury.
In our study, an exploratory multivariate analysis identified baseline patient characteristics as influence indicators for SIRS, including gender, age, concomitant illness, volume of hemangiomas, distribution of lesion, approach of ablation, time of ablation and the levels of ALT, AST, total bilirubin, BUN, creatinine, CRP, HMGB-1, IL-1β, IL-18, and LDH at 1 hour post ablation. Multivariate analysis showed that volume of hemangiomas, duration of ablation, HMGB-1, IL-1β and IL-18 were independent risk factors for SIRS occurrence. Of note, HMGB1 and pyroptosis-related inflammatory cytokines (IL-1β and IL-18) was highly correlated with SIRS occurrence post ablation. Immunohistochemical analysis showed the increased expressions of HMGB-1, NLRP3, caspase-1, GSDMD, IL-18 and IL-1β in endothelial cells of sub-ablated hemangiomas but not in hepatic hemangiomas. Furthermore, in vitro experiments showed that insufficient RF ablation led to HMGB-1-indued endothelial pyroptosis, which was attenuated by an HMGB-1 inhibitor, EP. These findings suggest that HMGB-1 induced endothelial pyroptosis may play a major role in the pathogenesis of SIRS following RF ablation for hepatic hemangiomas.
Our study had some limitations. We did not find an appropriate animal model of hepatic hemangioma that mimics clinical RF ablation therapy. Therefore, we could only indirectly prove that endothelial pyroptosis is related to SIRS following RF ablation for hepatic hemangiomas. Additional experiments to investigate the role of endothelial pyroptosis after RF ablation are required in animal models with orthotopic hepatic hemangiomas.
Conclusions
We demonstrated a novel mechanism by which HMGB1 is involved in the pyroptosis of ECs following insufficient RF ablation. Furthermore, HMGB1-induced EC pyroptosis may be involved in the occurrence of SIRS following RF ablation of hepatic hemangiomas.
Acknowledgements
This study was supported by the program for high-level technical talents in the Beijing health system (2015-03-025) and Beijing Municipal Administration of Hospitals Incubating Program (PX2020011). We thank Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Gao J, Fan RF, Yang JY, Cui Y, Ji JS, Ma KS, Li XL, Zhang L, Xu CL, Kong XL, Ke S, Ding XM, Wang SH, Yang MM, Song JJ, Zhai B, Nin CM, Guo SG, Xin ZH, Lu J, Dong YH, Zhu HQ, Sun WB. Radiofrequency ablation for hepatic hemangiomas: a consensus from a Chinese panel of experts. World J Gastroenterol. 2017;23:7077–7086. doi: 10.3748/wjg.v23.i39.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fodor M, Primavesi F, Braunwarth E, Cardini B, Resch T, Bale R, Putzer D, Henninger B, Oberhuber R, Maglione M, Margreiter C, Schneeberger S, Öfner D, Stattner S. Indications for liver surgery in benign tumours. Eur Surg. 2018;50:125–131. doi: 10.1007/s10353-018-0536-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao J, Ke S, Ding XM, Zhou YM, Qian XJ, Sun WB. Radiofrequency ablation for large hepatic hemangiomas: initial experience and lessons. Surgery. 2013;153:78–85. doi: 10.1016/j.surg.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Gao J, Ji JS, Ding XM, Ke S, Xin ZH, Ning CM, Guo SG, Li XL, Dong YH, Sun WB. Laparoscopic radiofrequency ablation for large subcapsular hepatic hemangiomas: technical and clinical outcomes. PLoS One. 2016;11:e0149755. doi: 10.1371/journal.pone.0149755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Xu L, Yang MM, Ke S, Ding XM, Wang SH, Sun WB. A severe complication of myocardial dysfunction post radiofrequency ablation treatment of huge hepatic hemangioma: a case report and literature review. Open Med (Wars) 2019;14:398–402. doi: 10.1515/med-2019-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao YL, Zhai JH, Chai YF. Recent advances in the molecular mechanisms underlying pyroptosis in sepsis. Mediators Inflamm. 2018;2018:5823823. doi: 10.1155/2018/5823823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma Y, Jiang J, Gao Y, Shi T, Zhu X, Zhang K, Lu K, Xue B. Research progress of the relationship between pyroptosis and disease. Am J Transl Res. 2018;10:2213–2219. [PMC free article] [PubMed] [Google Scholar]
- 8.Morioka N, Miyauchi K, Miyashita K, Kochi T, Zhang FF, Nakamura Y, Liu K, Wake H, Hisaoka-Nakashima K, Nishibori M, Nakata Y. Spinal high-mobility group box-1 induces long-lasting mechanical hypersensitivity through the toll-like receptor 4 and upregulation of interleukin-1beta in activated astrocytes. J Neurochem. 2019;150:738–758. doi: 10.1111/jnc.14812. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi K, Iwamoto H, Sakamoto S, Horimasu Y, Masuda T, Miyamoto S, Nakashima T, Ohshimo S, Fujitaka K, Hamada H, Hattori N. Serum high-mobility group box 1 is associated with the onset and severity of acute exacerbation of idiopathic pulmonary fibrosis. Respirology. 2019 doi: 10.1111/resp.13634. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Yang R, Zou X, Tenhunen J, Tonnessen TI. HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm. 2017;2017:5928078. doi: 10.1155/2017/5928078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang R, Zhu S, Pischke SE, Haugaa H, Zou X, Tonnessen TI. Bile and circulating HMGB1 contributes to systemic inflammation in obstructive jaundice. J Surg Res. 2018;228:14–19. doi: 10.1016/j.jss.2018.02.049. [DOI] [PubMed] [Google Scholar]
- 12.Dong W, Zhu Q, Yang B, Qin Q, Wang Y, Xia X, Zhu X, Liu Z, Song E, Song Y. Polychlorinated biphenyl quinone induces caspase 1-mediated pyroptosis through induction of pro-inflammatory HMGB1-TLR4-NLRP3-GSDMD signal axis. Chem Res Toxicol. 2019;32:1051–1057. doi: 10.1021/acs.chemrestox.8b00376. [DOI] [PubMed] [Google Scholar]
- 13.Geng Y, Ma Q, Liu YN, Peng N, Yuan FF, Li XG, Li M, Wu YS, Li BL, Song WB, Zhu W, Xu WW, Fan J, Su L. Heatstroke induces liver injury via IL-1beta and HMGB1-induced pyroptosis. J Hepatol. 2015;63:622–33. doi: 10.1016/j.jhep.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Wang J, Jin Y, Zhang F, Yang X. Intratumoral radiofrequency hyperthermia-enhanced chemotherapy of liposomal doxorubicin on hepatocellular carcinoma. Am J Transl Res. 2018;10:3619–3627. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Gao J, Yang M, Ke S, Ding X, Kong J, Xu L, Sun W. Intratumoral coagulation by radiofrequency ablation facilitated the laparoscopic resection of giant hepatic hemangioma: a surgical technique report of two cases. Oncotarget. 2017;8:52006–52011. doi: 10.18632/oncotarget.18994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong S, Kong J, Kong F, Kong J, Gao J, Ke S, Wang S, Ding X, Sun W, Zheng L. Insufficient radiofrequency ablation promotes epithelial-mesenchymal transition of hepatocellular carcinoma cells through Akt and ERK signaling pathways. J Transl Med. 2013;11:273. doi: 10.1186/1479-5876-11-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carty M, Kearney J, Shanahan KA, Hams E, Sugisawa R, Connolly D, Doran CG, Muñoz-Wolf N, Gurtler C, Fitzgerald KA, Lavelle EC, Fallon PG, Bowie AG. Cell survival and cytokine release after inflammasome activation is regulated by the Toll-IL-1R protein SARM. Immunity. 2019;50:1412–1424. e6. doi: 10.1016/j.immuni.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y, Wu H. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570:338–343. doi: 10.1038/s41586-019-1295-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandolfi F, Altamura S, Frosali S, Conti P. Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther. 2016;38:1017–28. doi: 10.1016/j.clinthera.2016.02.028. [DOI] [PubMed] [Google Scholar]