Abstract
In the present study, the effects of albiflorin (ALB) on the pulmonary inflammation induced by ovalbumin (OVA) in an asthmatic mouse model were investigated. Airway hyperreactivity (AHR) in asthmatic mice was detected using the acetylcholine stimulation test. Eosinophilia cells in the serum of asthmatic mice were counted. Hematoxylin and eosin (H&E) staining was used to observe pathological changes in lung tissue. Inflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α were detected in bronchoalveolar lavage fluid (BALF) and lung tissue using enzyme-linked immunosorbent assay (ELISA). Western blotting was used to detect the mitogen-activated protein kinase/nuclear factor kappa B (MAPK/NF-κB) signaling pathway in the lungs of asthmatic mice. The results from the present study indicated that ALB dramatically suppressed the expression of inflammatory cytokines including IL-1β, IL-6, and TNF-α, and inflammatory cells. In addition, ALB significantly decreased malondialdehyde (MDA) content as well as increased superoxide dismutase (SOD) activity. ALB also alleviated AHR in asthmatic mice and improved pathological changes in the lungs. In addition, ALB inhibited the MAPK/NF-κB signaling pathway in the lungs of the asthmatic mice. Thus, ALB appears to inhibit lung inflammation in asthmatic mice via regulation of the MAPK/NF-κB signaling pathway.
Keywords: Albiflorin, asthma, inflammation, MAPK, NF-κB
Introduction
Bronchial asthma (also referred to as asthma) is a chronic inflammatory disease of the airways that can seriously affect human health [1]. Currently, there are approximately 100 million asthmatic patients in the world; the prevalence rate continues to increase by 50% every decade [2]. Approximately 250,000 die from asthma, typically before the age of 15 years [3]. In western and developing countries, patients with severe asthma only account for 5-10% of all asthma patients; however, all asthma cases require treatment, which results in a significant economic burden worldwide [4]. Due to the industrialization progress and adoption of westernized lifestyles in China, the prevalence rate of asthma continues to rise, especially among children [5]. The prevalence rate of asthma in China has increased by approximately 1.5% in recent years, corresponding to more than 10,000 asthma additional patients every year [6]. Therefore, continuing research on the pathogenesis of asthma, as well as implementation of reasonable and effective measures for prevention and control of the disease, is important. Asthma is a chronic inflammatory disease of the airways involving various cells, including inflammatory and structural cells such as eosinophils, mast cells, lymphocytes, neutrophils, smooth muscle cells, airway epithelial cells, and various cell components [7]. The pathogenesis of asthma involves the interaction of genetic and environmental factors, as well as neuroendocrine and immune mechanisms [8]. Thus, the pathogenesis is complex, and is characterized by airway inflammation and hyperresponsiveness; and airway weight plastic is the main pathophysiological feature [9]. Chronic inflammation of the airway induces airway hyperresponsiveness and a wide variety of reversible airflow restrictions, resulting in decreased lung function [10,11]. Moreover, repeated inflammation eventually leads to structural changes of the airways, including airway remodeling, repair of airway punctum epithelial injury, goblet cell proliferation and metaplasia, bronchial gland hyperplasia, subepithelial basement membrane thickening, collagen deposition, smooth muscle layer hyperplasia and hypertrophy, neovascularization, and infiltration of inflammatory cells around the airways [12-14]. Airway remodeling also leads to airway hyperresponsiveness. According to the pathological characteristics of chronic inflammation due to asthma, an anti-inflammatory strategy is the most important method for asthma treatment [15,16]. Glucocorticoid (GC), which is the most effective anti-inflammatory drug, has become the treatment of choice for airway inflammation in cases of asthma [17,18]. However, due to the many side effects associated with long-term use of GC, the search for new and effective anti-inflammatory drugs, to replace or be used in conjunction with GC for the treatment of asthma, is the current focus of anti-inflammatory drug research.
The monoterpene glycosides, albiflorin (ALB) and paeoniflorin (PF), are major constituents of P. alba Radix [19] and reportedly exert many pharmacological activities, including antioxidant and anti-inflammatory effects [20-22]. However, research on the effects of ALB in asthma remains limited. In the present study, the effects of ALB in ovalbumin (OVA)-induced mouse model of asthma were investigated.
Materials and methods
Reagents
ALB was obtained from the National Institutes for Food and Drug Control (Beijing, China). Dexamethasone (Dex) was provided by Yi Feng Drug Store (Nanjing, China). OVA (Escherichia coli serotype 0111:B4, No. L-2630) was provided by Sigma-Aldrich (St. Louis, MO, USA). Tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were obtained from Nanjing KeyGen Biotech. Co., Ltd. (Nanjing, China). Malondialdehyde (MDA) and superoxide dismutase (SOD) kits were bought from Shanghai Enzyme-linked Biotechnology Co., Ltd (Shanghai, China). All antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
Animals
Female BABL/C mice weighing 18-22 g were purchased from Nanjing Qinglong Mountain Animal Breeding Co., Ltd (animal approval number: SCXK (Su) 2016-0008).
Experimental scheme
Sixty BALB/c mice were randomly divided into five groups: control, OVA, OVA + dexamethasone (Dex, 2 mg/kg), OVA + ALB20 (ALB, 20 mg/kg), and OVA + ALB40 (ALB, 40 mg/kg). Except for the control group, all mice were intraperitoneally and subcutaneously injected with 0.2 mL of sensitizing solution (0.2 mL containing 0.1 mL OVA and 0.1 mL sensitizing solution with AL(OH)3 0.02 mg) on days 1 and 7, respectively [23]. On days 15 and 28, mice were administered 2.5% OVA solution for 20 minutes each day. In the control group, normal saline of equal volume was used instead of sensitizing solution for atomization. Mice in the OVA + Dex, OVA + ALB20, and OVA + ALB40 groups were administered Dex (2 mg/kg), ALB (20 mg/kg) and ALB (40 mg/kg), respectively, 30 minutes before atomization. Mice in the control and OVA groups were administered the same amount of normal saline.
Airway hyperreactivity test (AHR)
Awake mice were placed in a barometric volume recording room 48 h after the last OVA booster immunization, and the average baseline reading was recorded over a 3-min period. Atomization was performed using acetylcholine and the average reading was recorded over a 3-min period. According to the manufacturer’s protocol, the enhanced pause (Penh) was calculated as the airway contraction index, to reflect the extent of the increase in airway reactivity.
Blood cytology
Blood was collected from the eye sockets of mice 24 h after the last excitation. The blood (20 μL) was added to 0.38 mL of counting solution and eosinophils were counted under an optical microscope.
Determination of inflammatory factors in serum and lung tissue
Lung tissue was homogenized in physiological saline at 12,000 rpm and centrifuged at 4°C for 10 min; the supernatant frozen at -80°C for later use. The protein content was determined using the bicinchoninic acid (BCA) method. TNF-α, IL-6, and IL-1β were detected in serum and lung tissue using ELISA kits.
Measurement of SOD and MDA
The oxidative enzyme activities of SOD and MDA in lung tissues were measured by commercialized kits.
Histopathological examination
After the mice were euthanized and the blood collected, the lung tissues were fixed in 10% neutral formalin solution overnight. Then, the tissues were fixed and embedded in paraffin, and cut into 4-mm-thick slices. Paraffin wax was removed and sections were stained with hematoxylin and eosin (H&E). Changes in lung tissue were observed under an optical microscope.
Immunohistochemistry
Immunohistochemistry staining was used to detect the expression of p-P38 and p-NF-κBp65 in the lung tissues. Briefly, the paraffin sections of lungs were deparaffinized, rehydrated and incubated in 3% hydrogen peroxide (H2O2). The sample was incubated with the corresponding primary antibody at 4°C overnight after blocked with 3% BSA. Secondary antibody and three antibodies for were incubated for 20 min at 37°C. Then, samples were stained with DAB and restained with hematoxylin. After dehydrated and dried, the sections were observed under a light microscopy (200×) (Nikon, Tokyo, Japan), and analyzed with Image J software (National Institutes of Health, Bethesda, MD, USA).
Quantitative RT-PCR
RNA was extracted using TRIzol reagent (Takara, Tokyo, Japan) according to the manufacturer’s instructions. cDNA was synthesized via first-strand cDNA synthesis with a PrimeScript RT reagent Kit (Takara, Tokyo, Japan). RT-PCR was performed using the CFX 96 q-PCR system (Bio-Rad, California, USA). A SYBR Green RT-PCR Kit (Takara, Tokyo, Japan) was used for quantitative RT-PCR analyses. All reactions were performed in a 20 μl reaction volume in triplicate. The relative expression levels of target genes were normalized against the level of GAPDH. The following primers were used for RT-PCR: P38 forward: 5’-TGACCCTTATGACCAGTCCTTT-3’; reverse: 5’-GTCAGGCTCTTCCACTCATCTAT-3’; NF-κBp65 forward: 5’-AGCGCGGGGACTATGACTT-3’; reverse: 5’-GCCCGGTTATCAAAAATCGGAT-3’; GAPDH forward: 5’-AGGTCGGTGTGAACGGATTTG-3’; reverse: 5’-TGTAGACCATGTAGTTGAGGTCA-3’.
Western blot analysis
Lungs were homogenized and lysed in extraction buffer for 15 min and centrifuged at 12,000 g at low temperature for 15 min. The supernatant was removed and loaded into a new centrifuge tube. The Enhanced BCA Protein Concentration Assay Kit (P0009; Beyotime, Shanghai, China) was used to detect the protein concentration. Approximately 30 μg of protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and then electrotransferred to poly (vinylidene fluoride) (PVDF) membranes. Next, the membranes were blocked with 5% bovine serum albumin (BSA) for 1.5 h at 37°C, followed by incubation with the primary antibodies overnight at 4°C. Membranes were incubated with peroxidase-conjugated secondary antibody for 1 h at room temperature and then visualized with the Tanon 5200 chemiluminescence imaging system (Tanon Science & Technology Co. Ltd., Shanghai, China). The amounts of the target proteins were analyzed using ImageJ analysis software (NIH, Bethesda, MD, USA) and normalized to the control. Results were obtained from three independent experiments.
Statistical analysis
All experimental results are expressed as means ± standard deviation, and one-way ANOVA followed by Tukey-Kramer multiple comparisons were performed using GraphPad Prism statistical software (GraphPad Software Inc., La Jolla, CA, USA). A P-value < 0.05 was considered statistically significant.
Results
Effects of ALB on AHR in asthmatic mice
As shown in Figure 1, the Penh in all groups was increased compared with the control group. However, the addition of ALB and Dex significantly reduced the Penh in asthmatic mice.
Figure 1.
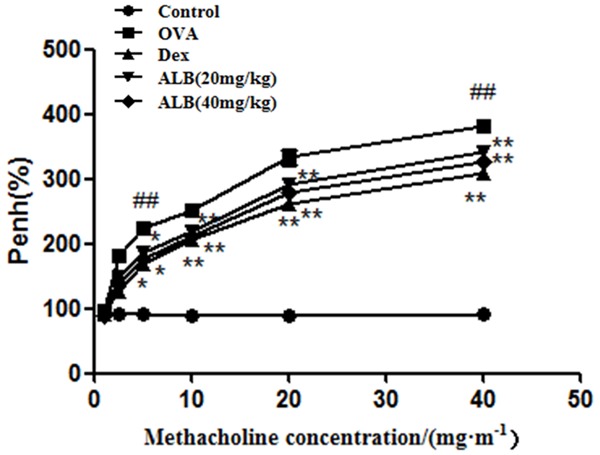
Effects of albiflorin (ALB) on airway hyperreactivity (AHR) in asthmatic mice. Airway hyperreactivity (AHR) in asthmatic mice was detected using the acetylcholine stimulation test after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
Effects of ALB on the number of eosinophils in blood
The eosinophils in the blood of asthmatic mice were significantly increased compared with the control group. The eosinophils in the OVA + Dex, OVA + ALB20, and OVA + ALB40 groups were significantly decreased compared with the OVA group (Figure 2).
Figure 2.
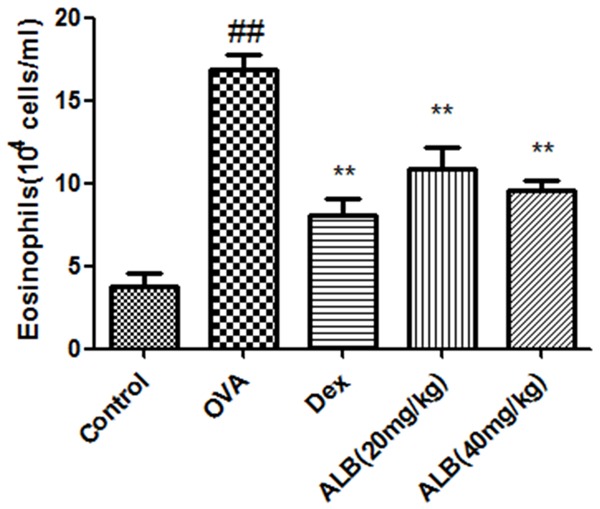
Effects of ALB on the number of eosinophils in the blood of asthmatic mice. The eosinophils were counted under an optical microscope after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
Effects of ALB on inflammatory cytokines in serum of asthmatic mice
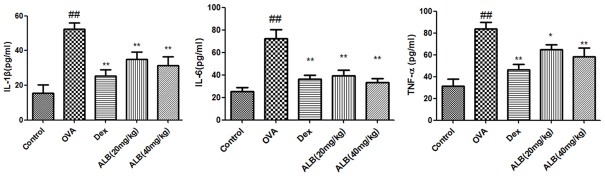
The serum levels of TNF-α, IL-6, and IL-1β in asthmatic mice were significantly increased. The serum levels of TNF-α, IL-6, and IL-1β in OVA + Dex, OVA + ALB20, and OVA + ALB40 groups were significantly decreased compared with the OVA group (Figure 3).
Figure 3.
Effects of ALB on inflammatory cytokines in the serum of asthmatic mice. Levels of serum of inflammatory cytokines were measured after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
Effects of ALB on inflammatory cytokines in the lungs of asthmatic mice
The levels of TNF-α, IL-6, and IL-1β in the lungs of asthmatic mice were significantly increased. The levels of TNF-α, IL-6, and IL-1β in OVA + Dex, OVA + ALB20, and OVA + ALB40 groups were significantly decreased compared with the OVA group (Figure 4).
Figure 4.
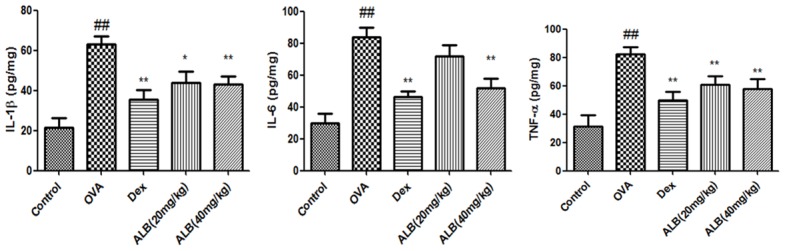
Effects of ALB on inflammatory cytokines in the lungs of asthmatic mice. Levels of serum of inflammatory cytokines were measured after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
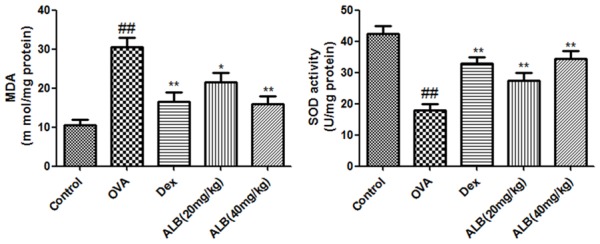
Effects of ALB on MDA and SOD activities in the lungs of asthmatic mice
The lung tissue levels of MDA were significantly increased in the lungs of asthmatic mice accompany with a decrease level of SOD. In comparison with the asthma group, the levels of MDA in ALB and Dex groups were significant decreased while the activity of SOD decreased (Figure 5).
Figure 5.
Effects of ALB on inflammatory cytokines in the lungs of asthmatic mice. Levels of serum of inflammatory cytokines were measured after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
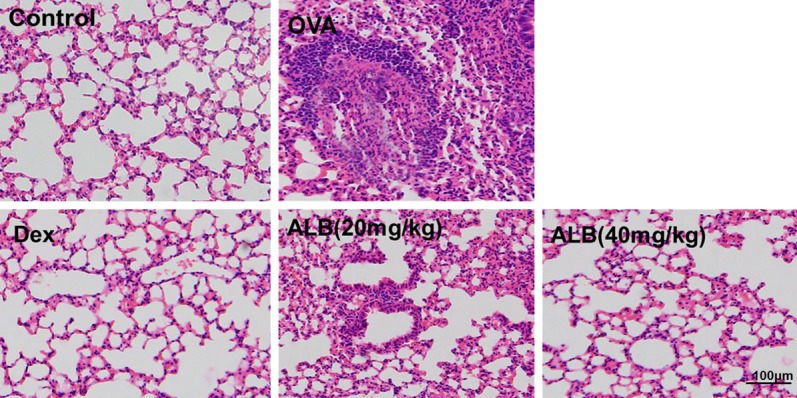
Effects of ALB on pathological changes in the lungs of asthmatic mice
Light microscopy revealed that the lung tissue morphology in the control group was generally normal. In the OVA group, the alveolar capillaries were hyperemic and dilated, and the pulmonary interstitium and alveolar septum were significantly widened; moreover, inflammatory edema and a large number of infiltrated inflammatory cells were observed. In the OVA + ALB20, OVA + ALB40, and OVA + Dex groups, the above changes were significantly alleviated, with only slight hyperemia and dilation of the alveolar capillaries, low-level infiltration of inflammatory cells, and marginal widening of the local focal pulmonary septum (Figure 6).
Figure 6.
Effects of ALB on MDA and SOD in the lungs of asthmatic mice. The activities of MDA and SOD in the lungs were measured after 28 days of the indicated treatment. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
Effects of ALB on the mitogen-activated protein kinase/nuclear factor kappa B (MAPK/NF-κB) signaling pathway in the lungs of asthmatic mice
The expression of MAPK/NF-κB pathway-related mRNA and protein was measured to explore the underlying mechanism of inhibitory effect of ALB on asthma. The mRNA expression of P38 and NF-κB was significantly increased in the asthmic mice compared with the control group, as well as a markedly reduced in the ALB treatment group (Figure 7A). Meanwhile, compared with the control group, the expression levels of p-JNK, p-ERK, p-P38, p-NF-κBP65, and p-IκBα were upregulated in the OVA group. ALB (20 and 40 mg/kg) decreased the expression of p-JNK, p-ERK, p-P38, p-NF-κBP65, and p-IκBα in the lungs of asthmatic mice (Figure 7B). Immunohistochemistry analysis (Figure 7C) suggested the up-regulation of p-P38 and p-NF-κBp65 in the lungs of asthma group mice. ALB or Dex administration decreased the expression of p-P38 and p-NF-κBp65. These results suggested that ALB-medicated protection against asthma involves with the MAPK/NF-κB pathways.
Figure 7.
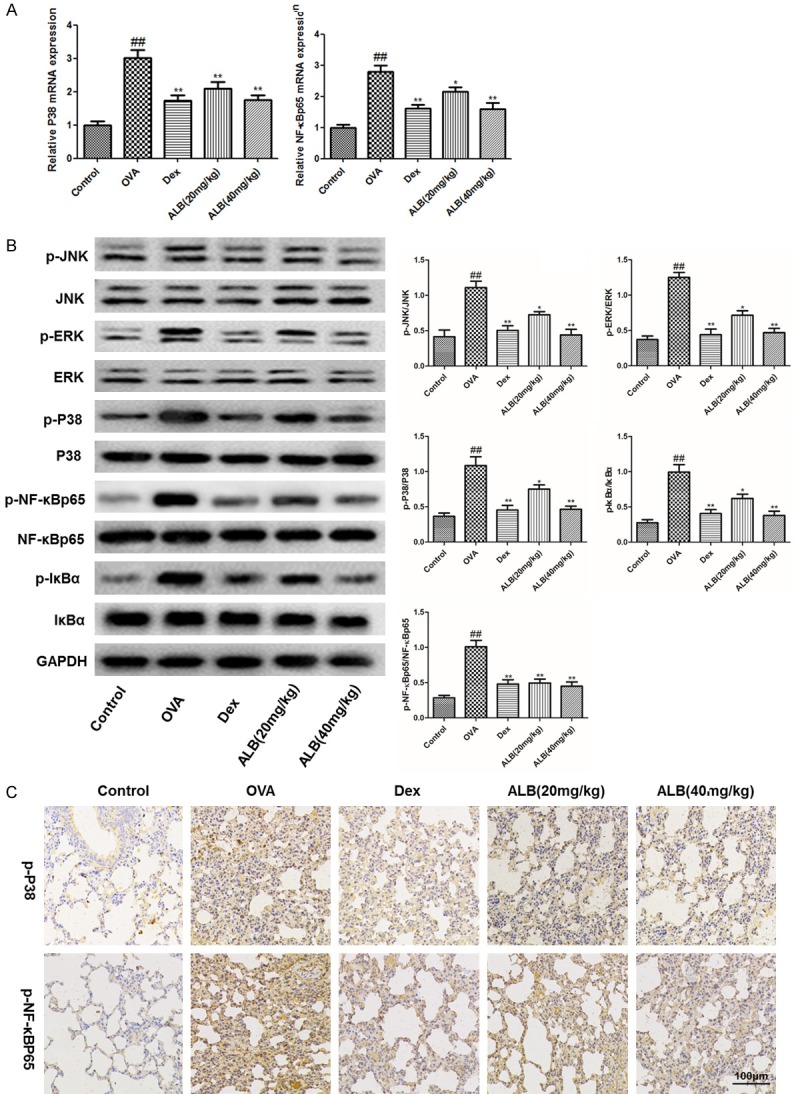
Effects of ALB on the mitogen-activated protein kinase/nuclear factor kappa B (MAPK/NF-κB) signaling pathway in the lungs of asthmatic mice. Expression of indicated mRNA (A) and proteins (B) involved in MAPK/NF-κB signaling pathways. GAPDH was used as an internal control. Comparisons of protein expression among indicated treatment groups are shown. (C) Immunohistochemical staining of lung tissues from indicated treatment groups illustrated presence localization of p-P38 and p-NF-κBP65. The values are presented as means ± standard deviation. ##P < 0.01 and #P < 0.05 versus control group, **P < 0.01 and *P < 0.05 versus OVA group.
Discussion
Bronchial asthma is a chronic inflammatory disease of the airways involving many inflammatory cells (e.g., eosinophils, mast cells, T lymphocytes, and neutrophils) and airway structural cells (airway epithelial cells, smooth muscle cells, and fibroblasts), as well as various cytokines [24,25]. Asthma is a condition characterized by chronic airway inflammation. GC is currently the most effective anti-inflammatory drug for the treatment of asthma [26] and mainly reduces the activation of T lymphocytes by inhibiting the production of cytokines, thus reducing airway inflammation [27,28]. However, the symptoms in asthma patients cannot be completely eliminated.
Due to the important role of eosinophils and macrophages in bronchial asthma, histopathological observation of external weak blood and lung tissue [29], whether GC can reduce the increase of eosinophils, and infiltration thereof into bronchial lung tissue seen during asthma, is an important indicator of the inflammatory effect of drugs on bronchial lung tissue in asthma patients [30]. The results of the present study showed that eosinophils in the OVA group were increased compared with the control group. The addition of ALB (20 and 40 mg/kg), and Dex (2 mg/kg) decreased eosinophils in the serum of asthmatic mice.
Clinically, bronchial asthma is characterized by airway hyperresponsiveness, reversible airway obstruction, and allergic airway inflammation [31]. Asthma causes many inflammatory processes and produces several inflammatory cytokines, such as TNF-α, IL-6, and IL-1β [32]. TNF-α is produced by monocytes, and is involved in inflammation and immune regulation [33]. IL-1β is considered an important cytokine involved in tissue destruction and edema [34]. IL-6 has various immunoregulatory functions and can enhance the immune response [35]. Compared with our control group, OVA increased TNF-α, IL-6, and IL-1β levels in bronchoalveolar lavage fluid (BALF) and lung, while ALB and Dex reduced those levels in asthmatic mice. Moreover, in this study, OVA increased the levels of MDA, while ALB and Dex reduced the activities of MDA. Meanwhile, the decreased activity of SOD was detected in the asthma group, and ALB significantly increased the level of SOD.
In several studies, MAPK/NF-κB was shown to be an important signaling pathway in asthma attacks [36]. MAPK/NF-κB participates in airway inflammation, airway remodeling, apoptosis, and the proliferation of asthma [37]. At rest, NF-κB binds to NF-κB inhibitor protein (IκBa), becomes inactive, and resides in the cytoplasm. Many inflammatory stimuli lead to NF-κB activation and induce the expression of various growth factors, cytokines, chemokines, and transcription factors [38], which play an important role in the body’s inflammatory and immune responses, cell growth and development, cell apoptosis, and participate in the onset and development of bronchial inflammation [39]. MAPKs a group of serine-threonine protein kinases activated by different extracellular stimuli, such as cytokines, neurotransmitters, hormones, and cell stress, as well as cell adhesion [40], play an important role in the inflammatory response [41]. In previous studies, MAPK activation was associated with the onset of asthma inflammation, and played an important role in the disease [42]. In the present study, the expression levels of p-JNK, p-ERK, p-P38, p-NF-κBP65, and p-IkBa were upregulated in the OVA group compared with the control group. ALB (20 and 40 mg/kg) decreased the expression of p-JNK, p-ERK, p-P38, p-NF-κBP65, and p-IkBa in the lungs of asthmatic mice.
The above experimental results showed that ALB can inhibit eosinophils in weak blood outside of the context of the mouse model of asthma, as well as inflammatory cytokines in BALF and lung in the mouse asthma model. In addition, ALB can inhibit activation of the MAPK/NF-κB signaling pathway. In summary, ALB improved lung inflammation in the mouse model of asthma and thus is a potential therapeutic reagent for asthma. Whether MAPK/NF-κB is the only signaling pathway that regulates the expression of inflammatory factors should be further investigated.
Disclosure of conflict of interest
None.
References
- 1.Shimoda T, Obase Y, Nagasaka Y, Nakano H, Ishimatsu A, Kishikawa R, Iwanaga T. Lung sound analysis helps localize airway inflammation in patients with bronchial asthma. Sci Rep. 2017;10:99–108. doi: 10.2147/JAA.S125938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alyasin S, Amin R, Neamati S. Evaluation of asthmatic patients referred to jahrom hospital and clinic. Iran J Allergy Asthma Immunol. 2004;3:145–148. [PubMed] [Google Scholar]
- 3.D’Amato G, Vitale C, Molino A, Stanziola A, Sanduzzi A, Vatrella A, Mormile M, Lanza M, Calabrese G, Antonicelli L, D’Amato M. Asthma-related deaths. Multidiscip Respir Med. 2016;11:37. doi: 10.1186/s40248-016-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diasjúnior SA, Reis M, de CarvalhoPinto RM, Stelmach R, Halpern A, Cukier A. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43:1368–1377. doi: 10.1183/09031936.00053413. [DOI] [PubMed] [Google Scholar]
- 5.Ripabelli G, Tamburro M, Sammarco ML, de Laurentiis G, Bianco A. Asthma prevalence and risk factors among children and adolescents living around an industrial area: a cross-sectional study. BMC Public Health. 2013;13:1038. doi: 10.1186/1471-2458-13-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ning LH, Zhang YJ, Wang X, Ma CY, Jia CM, Zhang AP, Wang DM, Song YE, Qu YJ, Song DM. Epidemiological survey of childhood asthma in 2010 in urban Baotou, China. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16:165–9. [PubMed] [Google Scholar]
- 7.Liu G, Cooley MA, Nair PM, Donovan C, Hsu AC, Jarnicki AG, Haw TJ, Hansbro NG, Ge Q, Brown AC, Tay H, Foster PS, Wark PA, Horvat JC, Bourke JE, Grainge CL, Argraves WS, Oliver BG, Knight DA, Burgess JK, Hansbro PM. Airway remodelling and inflammation in asthma are dependent on the extracellular matrix protein fibulin-1c. J Pathol. 2017;243:510–523. doi: 10.1002/path.4979. [DOI] [PubMed] [Google Scholar]
- 8.Mukherjee AB, Zhang Z. Allergic asthma: influence of genetic and environmental factors. J Biol Chem. 2011;286:32883–32889. doi: 10.1074/jbc.R110.197046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z, Zhuang J, Zhao L, Gao X, Luo Z, Liu E, Xu F, Fu Z. Roles of Bronchopulmonary C-fibers in airway Hyperresponsiveness and airway remodeling induced by house dust mite. Respir Res. 2017;18:199. doi: 10.1186/s12931-017-0677-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janssenheininger YM, Irvin CG, Scheller EV, Brown AL, Kolls JK, Alcorn JF. Airway hyperresponsiveness and inflammation: causation, correlation, or no relation? J Allergy Ther. 2012;2012(Suppl 1) doi: 10.4172/2155-6121.S1-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkura N, Fujimura M, Tokuda A, Furusho S, Abo M, Katayama N. Evaluation of airway hyperresponsiveness and exhaled nitric oxide as risk factors for airway remodeling in patients with stable asthma. Allergy Asthma Proc. 2009;30:419–423. doi: 10.2500/aap.2009.30.3253. [DOI] [PubMed] [Google Scholar]
- 12.Filosso PL, Turello D, Magnoni MS. Chronic inflammation and airway remodeling in asthma. Importance of an early treatment. Minerva Pediatr. 2003;55:323. [PubMed] [Google Scholar]
- 13.Tagaya E, Tamaoki J. Mechanisms of airway remodeling in asthma. Allergol Int. 2007;56:331–40. doi: 10.2332/allergolint.R-07-152. [DOI] [PubMed] [Google Scholar]
- 14.Li K, Chen W, Yu B, Duan Z, Zhang W, Qi Q, Zhou R, Zhou W. Effects of fastigial nucleus electrostimulation on airway inflammation and remodeling in an experimental rat model of asthma. Asian Pac J Allergy Immunol. 2016;34:223–228. doi: 10.12932/AP0705. [DOI] [PubMed] [Google Scholar]
- 15.Dong M, Ma C, Wang WQ, Chen J, Wei Y. Regulation of the IL-33/ST2 pathway contributes to the anti-inflammatory effect of acupuncture in the ovalbumin-induced murine asthma model. Acupunct Med. 2018;36:319–326. doi: 10.1136/acupmed-2017-011377. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ. The role of inflammation and anti-inflammatory medication in asthma. Respir Med. 2002;96(Suppl A):S9–15. [PubMed] [Google Scholar]
- 17.Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics. 1993;92:64–77. [PubMed] [Google Scholar]
- 18.Hamid QA, Wenzel SE, Hauk PJ, Tsicopoulos A, Wallaert B, Lafitte JJ, Chrousos GP, Szefler SJ, Leung DY. Increased glucocorticoid receptor beta in airway cells of glucocorticoid-insensitive asthma. Am J Respir Crit Care Med. 1999;159:1600–1604. doi: 10.1164/ajrccm.159.5.9804131. [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZX, Fu J, Ma SR, Peng R, Yu JB, Cong L, Pan LB, Zhang ZG, Tian H, Che CT, Wang Y, Jiang JD. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics. 2018;8:5945–5959. doi: 10.7150/thno.28068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J, Li X, Jiao K, Zhai Z, Sun D. Albiflorin inhibits the formation of THP-1-derived foam cells through the LOX-1/NF-κB pathway. Minerva Med. 2019;110:107–114. doi: 10.23736/S0026-4806.18.05711-7. [DOI] [PubMed] [Google Scholar]
- 21.Wang QS, Gao T, Cui YL, Gao LN, Jiang HL. Comparative studies of paeoniflorin and albiflorin from Paeonia lactiflora on anti-inflammatory activities. Pharm Biol. 2014;52:1189–1195. doi: 10.3109/13880209.2014.880490. [DOI] [PubMed] [Google Scholar]
- 22.Zhu YL, Wang LY, Wang CL, Zhao DP, Wang S, Fei WT, Zhang JJ. Investigation of effects of albiflorin and paeoniflorin on hippocampal BDNF and NO in chronic restraint stress rats. Zhongguo Zhong Yao Za Zhi. 2016;41:4240–4246. doi: 10.4268/cjcmm20162225. [DOI] [PubMed] [Google Scholar]
- 23.Ma CH, Wang SS, Ma SP, Fan XS. Effects of sanao decoction on allergic airway inflammation in asthma mouse and its component analysis. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18:149–153. [Google Scholar]
- 24.Mastalerz L, Kasperkiewicz H. Effect of inhaled corticosteroids on small airway inflammation in patients with bronchial asthma. Pol Arch Med Wewn. 2011;121:264–9. [PubMed] [Google Scholar]
- 25.Koshino T. Diagnosis and management of asthma. Nihon Rinsho. 1999;57:2107–2112. [PubMed] [Google Scholar]
- 26.Corrigan CJ, Hawrylowicz CM, Lavender P, Lee TH. Glucocorticoid-resistant asthma. Curr Allergy Asthma Rep. 2002;2:144. doi: 10.1007/s11882-002-0009-y. [DOI] [PubMed] [Google Scholar]
- 27.Zhang HP, Wang L, Fu JJ, Fan T, Wang ZL, Wang G. Association between histone hyperacetylation status in memory T lymphocytes and allergen-induced eosinophilic airway inflammation. Respirology. 2016;21:850–857. doi: 10.1111/resp.12774. [DOI] [PubMed] [Google Scholar]
- 28.Dürk T, Duerschmied D, Müller T, Grimm M, Reuter S, Vieira RP, Ayata K, Cicko S, Sorichter S, Walther DJ. Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am J Respir Crit Care Med. 2013;187:476–485. doi: 10.1164/rccm.201208-1440OC. [DOI] [PubMed] [Google Scholar]
- 29.Matucci A, Vultaggio A, Maggi E, Kasujee I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir Res. 2018;19:113. doi: 10.1186/s12931-018-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vural H, Uzun K. Serum and red blood cell antioxidant status in patients with bronchial asthma. Can Respir J. 2000;7:476–80. doi: 10.1155/2000/907478. [DOI] [PubMed] [Google Scholar]
- 31.Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx. 2011;38:555–563. doi: 10.1016/j.anl.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Charrad R, Berraïes A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Anti-inflammatory activity of IL-37 in asthmatic children: correlation with inflammatory cytokines TNF-α, IL-β, IL-6 and IL-17A. Immunobiology. 2016;221:182–7. doi: 10.1016/j.imbio.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Crisafulli C, Galuppo M, Cuzzocrea S. Effects of genetic and pharmacological inhibition of TNF-alpha in the regulation of inflammation in macrophages. Pharmacol Res. 2009;60:332–340. doi: 10.1016/j.phrs.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Bosmann M, Ward PA. Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets. 2014;18:703–714. doi: 10.1517/14728222.2014.902938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jevnikar Z, Östling J, Ax E, Calvén J, Thörn K, Israelsson E, Öberg L, Singhania A, Lau LCK, Wilson SJ, Ward JA, Chauhan A, Sousa AR, De Meulder B, Loza MJ, Baribaud F, Sterk PJ, Chung KF, Sun K, Guo Y, Adcock IM, Payne D, Dahlen B, Chanez P, Shaw DE, Krug N, Hohlfeld JM, Sandström T, Djukanovic R, James A, Hinks TSC, Howarth PH, Vaarala O, van Geest M, Olsson H Unbiased Biomarkers in Prediction of Respiratory Disease Outcomes study group. Epithelial IL-6 trans-signaling defines a new asthma phenotype with increased airway inflammation. J Allergy Clin Immunol. 2019;143:577–590. doi: 10.1016/j.jaci.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Huang W, Song Q, Zheng X, He R, Jie L. Paeonol ameliorates ovalbumin-induced asthma through the inhibition of TLR4/NF-κB and MAPK signaling. Evid Based Complement Alternat Med. 2018;2018:1–8. doi: 10.1155/2018/3063145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chauhan PS, Singh DK, Dash D, Singh R. Intranasal curcumin regulates chronic asthma in mice by modulating NF-κB activation and MAPK signaling. Phytomedicine. 2018;51:29–38. doi: 10.1016/j.phymed.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 38.Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasar A, Marienfeld R, Wirth T, Baumann B. NF-κB: critical regulator of inflammation and the immune response. Transcription Factors. 2004;166:325–376. [Google Scholar]
- 40.Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 41.Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 42.Yuan F, Liu R, Hu M, Rong X, Bai L, Xu L, Mao Y, Hasimu H, Sun Y, He J. JAX2, an ethanol extract of Hyssopus cuspidatus Boriss, can prevent bronchial asthma by inhibiting MAPK/NF-κB inflammatory signaling. Phytomedicine. 2019;57:305–314. doi: 10.1016/j.phymed.2018.12.043. [DOI] [PubMed] [Google Scholar]