Abstract
Covalent binding of G protein-coupled receptors by small molecules is a useful approach for better understanding of the structure and function of these proteins. We designed, synthesized and characterized a series of 6 potential covalent ligands for the histamine H3 receptor (H3R). Starting from a 2-amino-pyrimidine scaffold, optimization of anchor moiety and warhead followed by fine-tuning of the required reactivity via scaffold hopping resulted in the isothiocyanate H3R ligand 44. It shows high reactivity toward glutathione combined with appropriate stability in water and reacts selectively with the cysteine sidechain in a model nonapeptide equipped with nucleophilic residues. The covalent interaction of 44 with H3R was validated with washout experiments and leads to inverse agonism on H3R. Irreversible binder 44 (VUF15662) may serve as a useful tool compound to stabilize the inactive H3R conformation and to study the consequences of prolonged inhibition of the H3R.
Keywords: G protein-coupled receptor (GPCR), Histamine H3 receptor, covalent binder, isothiocyanate
1. Introduction
The histamine H3 receptor (H3R) was discovered in 1983 by Arrang et al. [1] as the third representative of the G protein-coupled receptor (GPCR) subfamily of histamine receptors (now known to consist of H1R, H2R, H3R, and H4R). As an autoreceptor, H3R presynaptically modulates the synaptic histamine level, while as a heteroreceptor it regulates the levels of other neurotransmitters (e.g., acetylcholine, noradrenaline, serotonin, dopamine, glutamate). Due to the high expression in brain areas such as the cerebral cortex, hypothalamus tuberomammillary nucleus, hippocampus, and striatum, H3R regulates several physiological processes of the central nervous system including sleep-wake cycle, learning and memory processes, food intake, and susceptibility to seizures [2,3,4]. As such, H3R attracts considerable interest as a therapeutic target from industry and academia alike. The cloning of the human receptor in 1999 by Lovenberg et al. [5] and the discovery of several structurally diverse drug-like ligands in the 2000s [6,7] gave a strong impetus to H3R research. Although this boom resulted in a large amount of novel H3R ligands, several clinical trials ongoing in the early 2010s [8,9] have so far resulted in only one approved H3R ligand (Wakix, approved in 2016 by EMA and in 2019 by FDA) [10,11] suggesting that H3R pharmacology is not yet fully understood and complementary approaches are needed to pave the way forward.
One such approach is the use of covalent small-molecule binders, which can for example help in the stabilization of H3R in the active or inactive conformation. This in turn could aid in crystallization of a ligand-receptor complex (no crystal structure of H3R has been published to date), as well in the exploration of the binding site, conformation, and signaling properties of the protein. Targeted covalent inhibitors (TCIs) are ligands designed to bind irreversibly to a nucleophilic amino acid residue when bound to the targeted protein. A typical strategy of TCI design is linking a pharmacophore moiety (anchor) that leads the ligand into the binding pocket to a reactive moiety (warhead) which reacts with a residue creating a covalent bond [12,13]. In the field of kinases the use of TCIs has resulted in marketed drugs in past years [12], while the progress in covalent GPCR ligands is still limited in comparison [13]. Nonetheless, aided by advances in GPCR crystallography, covalent ligands targeting the orthosteric binding site of GPCRs have been disclosed [14,15,16,17,18,19,20,21,22,23]. The targeted GPCR residue is mostly a cysteine with diverse warheads being used, such as a disulfide [17,19,23], isothiocyanate [18], or Michael acceptor [15]. Other residues have been targeted as well with various warheads, i.e., lysine with activated ester [16] or arylsulfonyl fluoride [20], tyrosine with an arylsulfonyl fluoride [22], or asparagine with an aziridinium ion [17]. Indeed, recent extensions of the diversity in warheads give an opportunity for the fine-tuning of the reactivity [24,25].
While no covalent ligand for H3R has been reported to date, the use of covalent strategies within the histamine receptor family is not without precedent. In the 1940s, covalent histamine-protein complexes were explored for pharmacological investigation using different warheads on histamine [26,27]. More recently, mutated H1R has been targeted by a disulfide-containing ligand [17]. Furthermore, our group has reported the discovery of a H4R covalent ligand VUF14480 (1, Figure 1) which acts as a partial H4R agonist [15]. Its submicromolar affinity (pKi = 6.3) for human H4R is high enough to ensure sufficient recognition by the receptor, whereupon the ethenyl moiety acts as a Michael acceptor forming a covalent interaction with C983.36 [15].
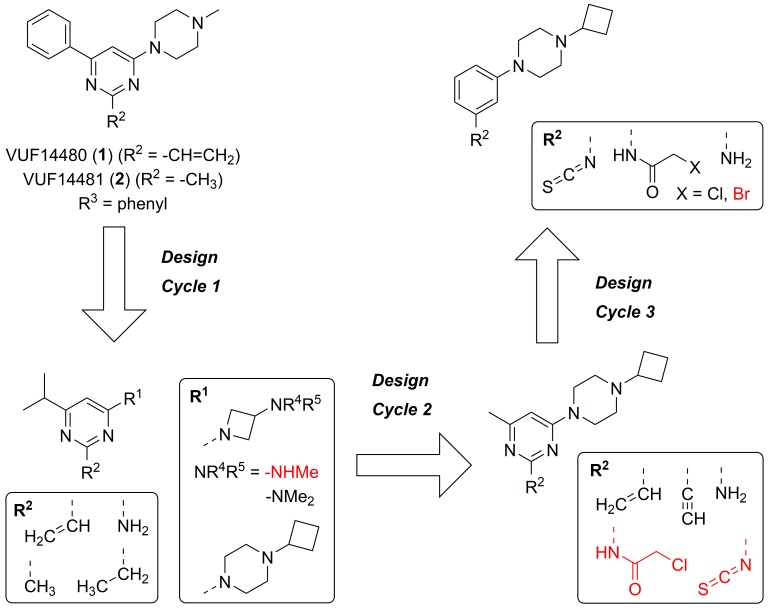
Figure 1.
The three design cycles of the potential H3R covalent ligands and their non-covalent reference compounds. Red compounds proved too unstable to be isolated in pure form.
In this study, we describe the design and synthesis of covalent H3R ligands, ultimately resulting in the discovery of a covalent inverse agonist targeting the H3R.
2. Results
2.1. Design
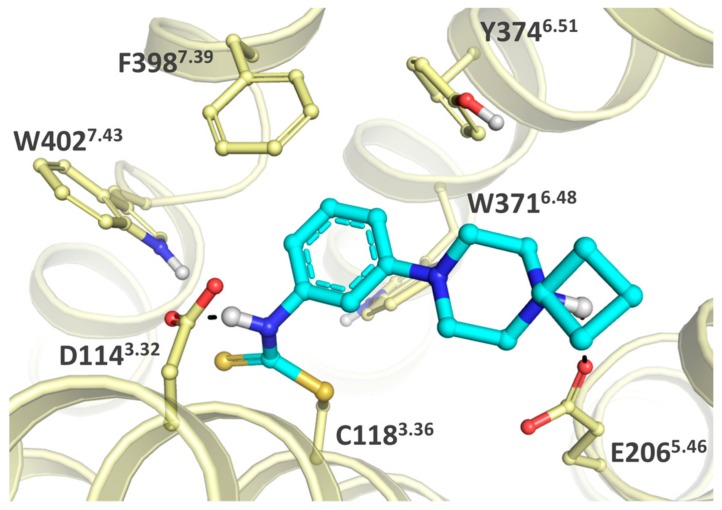
Arguably, a H4R covalent ligand can be a suitable starting point for covalent H3R ligands given the high homology of H4R and H3R (43% full sequence identity, 50% predicted transmembrane bundle identity) [28]. Hence, the covalent interaction of Michael acceptor VUF14480 (1) with H4R [15] was used as a template for the design of potential H3R covalent ligands (Figure 1). Similarly to the H4R binding pocket, C3.36 is also accessible in the H3R binding pocket (C1183.36) to potentially serve as nucleophilic moiety [29] (throughout this manuscript, we use both the UniProt residue numbers and the GPCR-specific Ballesteros–Weinstein residue numbering scheme [30]). Given the relatively high similarity of the H3R and H4R orthosteric binding pockets [29], the C1183.36 residue of H3R might be targeted with a similar combination of anchor, central core and warhead moieties as 1 contains. Several design cycles were needed to tune this scaffold into a H3R covalent ligand suitable for our purposes. In-house results (unpublished data) suggested that H4R/H3R selectivity of this scaffold could be switched from H4R to H3R by suitable modification of the anchor and substituent pattern of pyrimidine core. Thus, the aim of the first design cycle was the identification of a suitable anchor (R1). Three different anchors were chosen based on publicly available and in-house data: (1) replacement of the methyl substituent by a cyclobutyl group [31], (2) replacement of the piperazine moiety by a 3-(methylamino)azetidine moiety, (3) replacement of the piperazine moiety by a 3-(dimethylamino)azetidine moiety to assist in synthetic feasibility (vide infra). Based on in-house results, the phenyl group at Position 6 was changed to an isopropyl group to favor H3R selectivity. Both the pyrimidine ring as central core and the vinyl group as warhead were unchanged. Instead, the warhead (R2) was addressed in the second design cycle whilst the group at Position 6 was changed to a methyl group to prevent the more lipophilic isopropyl moiety from targeting a lipophilic subpocket next to the cysteine, thereby preventing the warhead from achieving its appropriate orientation. The vinyl unit was changed to an ethynyl moiety in order to potentially improve the orientation of the reactive carbon atom. Isothiocyanate and halomethylamido derivatives were also designed. Notably, isothiocyanates are known to have high reactivity toward cysteines and reduced albeit still substantial reactivity toward lysines [13,32,33] depending on the pH, while chloroacetamides show reactivity only to cysteines [13]. Yet in our work, both warhead types caused synthetic obstacles (vide infra) because of too high chemical reactivity that we attribute to the electron-withdrawing pyrimidine nitrogen atoms. This reactivity issue combined with biological inactivity of other compounds in this second cycle (vide infra) necessitated a third design cycle comprising of scaffold hopping to the less electron-withdrawing phenyl ring as central core. This switch in core was supported by a ChEMBL search on H3R ligands containing a phenyl group connected with a 3-carbon linker to a basic amino moiety. Figure 2 illustrates the envisioned strategy key to the third design cycle, with C1183.36 forming a covalent bond with the isothiocyanate moiety. The binding mode of a representative ligand from this design cycle (Figure 1 top right, R2 = -NCS) was evaluated by covalent docking using GOLD (version 5.2.1). The binding site of the H3R protein is characterized by two acidic residues that are known to interact with basic groups of the ligands, i.e., D1143.32 and E2065.46. Residue D1143.32 is considered the anchoring point for the basic amines of the endogenous ligands of aminergic GPCRs [34]. Residues in TM5 are also involved in ligand binding and in the case of H3R it is residue E2065.46 that is anticipated to interact with the imidazole ring of the endogenous agonist histamine [35]. Interestingly, the predicted binding mode of the isothiocyanate analog is flipped to allow covalent bond formation between the isothiocyanate and C1183.36, resulting in a binding mode in which the basic piperazine moiety interacts with E2065.46 through an ionic interaction. The nitrogen atom of the dithiocarbamate that is formed after covalent bond formation is able to interact with D1143.32 (Figure 2) through a hydrogen bond.
Figure 2.
Proposed binding mode of a representative compound from the third design cycle (Figure 1 top right, R2 = -NCS) covalently bound to C1183.36. The ligand is shown in cyan sticks and the protein is shown in light yellow sticks and ribbons. Only polar hydrogens are shown in white with protein-ligand hydrogen bonding indicated in black dashed lines. For clarity purposes, parts of the protein are not shown. Residue numbers are shown as UniProt and as GPCR-specific Ballesteros–Weinstein numbers [30].
When studying ligands that form covalent bonds with protein targets, it is essential for pharmacological characterization to have proper non-reactive reference compounds. In our previous work on H4R ligand 1, the 2-methyl-analog 2 had served as non-covalent control [15]. Based on steric and electronic considerations, in the current work several 2-methyl, 2-ethyl and 2-amino-derivatives of the designed compound set were investigated as controls.
2.2. Synthesis
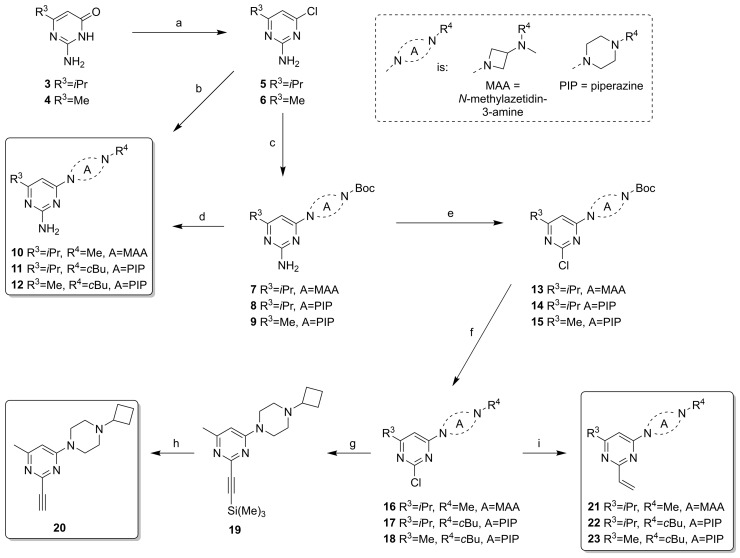
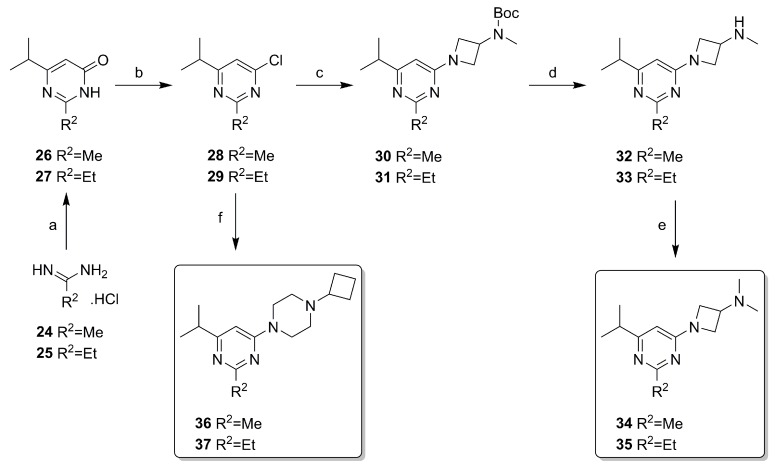
The compounds from Design Cycles 1 and 2 were synthesized as outlined in Scheme 1 and Scheme 2. The core intermediates 5 and 6 were obtained from the appropriate pyrimidin-4(3H)-one derivatives 3 or 4 with POCl3. The Boc-protected intermediates 7–9 were obtained via nucleophilic aromatic substitution on 5 or 6 with aminoazetidine or piperazine moieties under microwave heating at 150 °C. Two 2-amino derivatives (10 and 11) were directly synthesized from N,N-dimethylazetidin-3-amine or 1-cyclobutylpiperazine via an aromatic substitution on 5 or 6, while the other amino derivative 12 was synthesized from the Boc-protected intermediate 9 via deprotection under acidic conditions followed by a reductive amination. Pyrimidines 7–9 were transformed to 2-chloro-pyrimidine intermediates 13–15 by nonaqueous diazotization with tBuONO or iPeONO and SbCl3 [36]. Boc-protection was necessary for this step to prevent by-products. Formation of chlorides 13–15 was followed by deprotection under acidic condition and reductive amination with H2CO or cyclobutanone to afford the intermediates 16–18. A silyl-protected ethynyl group was incorporated via a Sonogashira coupling on intermediate 18 to give 19, followed by removal of the protective group to yield ligand 20. The vinyl derivatives 21–23 were obtained via Suzuki-Miyaura cross-coupling with vinyl boronic acid N-methyliminodiacetic acid (MIDA) ester on the 2-chloro-pyrimidines 16–18 in a microwave at 120 °C. The synthesis of the 3-(methylamino)azetidine analog of 21 (i.e., with R4 = H) was undertaken (not shown), although we anticipated a modest stability because of the simultaneous presence of nucleophilic and electrophilic moieties in the product. Therefore, we designed a protocol in which deprotection on a precursor having R4 = Boc was carried out in a hot D2O/d6-DMSO mixture [37] with the intent to use the solution as is for pharmacological purposes. However, even though product was formed (typically ca. 70% conversion), overall conversion and impurity profiles were not acceptable for further pursuit.
Scheme 1.
Synthesis of pyrimidine-based covalent ligands. (a) POCl3, reflux, 3 h, 26–33%; (b) N,N-dimethylazetidin-3-amine or 1-cyclobutylpiperazine, N,N-Diisopropylethylamine (DIPEA), dioxane, 150 °C, 30 min, μW, 59–65%; (c) t-butyl azetidin-3-yl(methyl)carbamate or t-butyl piperazine-1-carbamate, DIPEA, dioxane, 150 °C, 30 min, μW, 50–85%, 8: used without full purification; (d) 1. HCl, dioxane, rt, 2 h, 2. cyclobutanone, NaBH(OAc)3, dichloromethane (DCM), rt, overnight, 33% (two steps); (e) tBuONO or iPeONO, SbCl3, DCM, rt, 3 h, 23–40%; (f) 1. HCl, dioxane, rt, 2 h-overnight, 2. H2CO or cyclobutanone, NaBH(OAc)3, DCM, rt, overnight, 56–73% (two steps); (g) ethynyltrimethylsilane, CuI, Pd(dppf)Cl2, TEA, DME, 100 °C, 1 h, μW, 54%; (h) K2CO3, MeOH, rt, 1 h, 72%; (i) vinyl boronic acid MIDA ester, Pd(PPh3)4, Na2CO3, DME, H2O, 120 °C, 1 h, μW, 21–62%.
Scheme 2.
Synthesis of pyrimidine-based reference ligands. (a) methyl 4-methyl-3-oxopentanoate, NaOMe, MeOH, rt, overnight, 91–97%; (b) POCl3, reflux, 3 h, 86–87%; (c) t-butyl azetidin-3-yl(methyl)carbamate, DIPEA, dioxane, 150 °C, 30 min, μW, 34–38%; (d) HCl, dioxane, rt, 3 h-overnight, 77–90%; (e) H2CO, NaBH(OAc)3, DCM, rt, overnight, 82%; (f) 1-cyclobutylpiperazine, DIPEA, dioxane, 150 °C, 30 min, μW, 36–51%.
Non-covalent 2-alkyl-pyrimidines were synthesized with similar synthetic steps, as outlined in Scheme 2. Condensation of the appropriate amidines 24 or 25 and 4-methyl-3-oxopentanoate to 26 and 27 was followed by conversion to chloro-analog 28 and 29 with POCl3. A nucleophilic aromatic substitution on 28 or 29 with t-butyl azetidin-3-yl(methyl)carbamate was performed in a microwave at 150 °C and the obtained Boc-protected intermediate 30 and 31 were deprotected resulting in the monomethyl intermediates 32 and 33. These in turn were subjected to a reductive amination with H2CO to afford 34 and 35. Likewise, cyclobutyl-piperazine products 36 and 37 were obtained from 28 or 29 and 1-cyclobutylpiperazine.
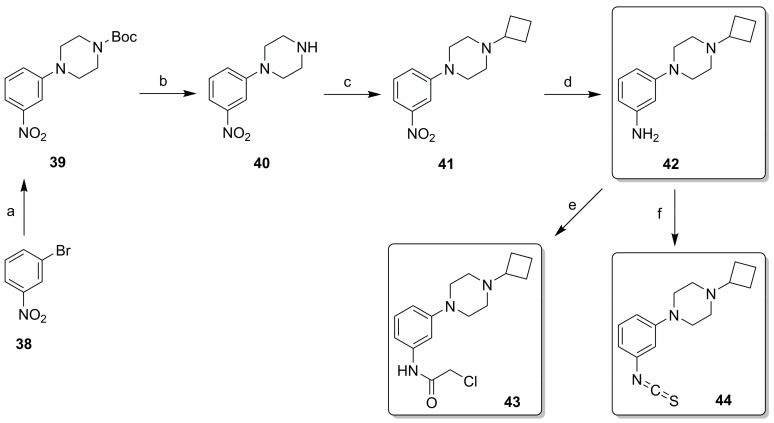
In Design Cycle 2, we also attempted to prepare the designed isothiocyanate and chloroacetamide derivatives from compound 12 via reaction with CSCl2 or 2-chloroacetylchloride, but all attempts were met by complex product profiles. We attribute this to the too high chemical reactivity of the warhead (vide supra), leading to decomposition during workup and purification. All derivatives with phenyl as core ring (i.e., those from the third design cycle) were synthesized as outlined in Scheme 3. Compound 39 was built via a Buchwald–Hartwig amination of bromide 38 with t-butyl piperazine-1-carboxylate. Deprotection to 40 and reductive amination with cyclobutanone yielded 41. The nitro group of 41 was reduced with HCOONH4 and Pd/C to afford key intermediate 42, which was used for incorporating reactive warheads under basic conditions. Chloroacetamide 43 was formed with 2-chloroacetyl chloride, while isothiocyanate 44 was formed with CSCl2. Synthesis of the bromo analog of 43 was also attempted under similar conditions, but the product decomposed during purification. All potential covalent ligands (20–23, 43, 44) were stored as solids in the freezer (−20 °C). Under these conditions, key compound 44 was stable for >2 years (as judged by NMR and LC-MS analysis).
Scheme 3.
Synthetic of phenylpiperazine derivatives. (a) t-butyl piperazine-1-carboxylate, NaOtBu, Pd2(dba)3, Xantphos, dioxane, 80 °C, 1 h, μW, 57%; (b) HCl, dioxane, rt, 1 h, 78%; (c) cyclobutanone, NaBH(OAc)3, DCM, rt, overnight, 76%; (d) HCOONH4, Pd/C, MeOH, H2O, rt, overnight, 25%; (e) 2-chloroacetyl chloride, TEA, DCM, rt, 1 h, 68%; (f) CSCl2, NaHCO3, DCM, H2O, rt, 1 h, 64%.
2.3. Structure-Activity Relationship
Compounds were evaluated for H3R binding in a [3H]N-α-methylhistamine ([3H]NAMH) displacement assay on H3R-expressing HEK293T cell homogenates. The first step of a covalent ligand binding is the reversible binding to the binding site, which is followed by the formation of the covalent bond in case of appropriate orientation of the reactive warhead [13]. Consequently, binding equilibrium cannot be established and therefore an ‘apparent’ affinity (pKi) is reported for potential covalent ligands 1, 20–23, 43 and 44 (Table 1). To identify potential irreversible binders, 100 µM ligand was pre-incubated with human H3R (hH3R)-expressing cell homogenates for 1 h at 25 °C prior to vigorous washout of the compounds. The washed membranes were then subjected to a [3H]NAMH displacement assay to determine the amount of receptors that are still available for [3H]NAMH binding, which is taken as a measure of potential covalent H3R labeling (Table 1).
Table 1.
(Apparent) binding affinity (pKi) of ligands for hH3R expressed on HEK293T cell homogenates, and the amount of unlabeled receptors remaining after 1 h preincubation of hH3R expressing membranes with 100 µM ligand and subsequent washout as determined by radioligand binding. Ligands are considered potential covalent binders when % unlabeled receptors remaining after 1 h pre-incubation with ligand was <50%. Data are mean ± S.E.M of 3 experiments performed in triplicate with the exception of 1 for which mean ± SD is reported for two experiments performed in triplicate.

| ||||||
|---|---|---|---|---|---|---|
| Cmpd. | Central Ring | R1 | R2 | R3 | pKi | % Unlabeled Receptors |
| 2 | Pyrimidine | A | Me | phenyl | 7.1 ± 0.1 | 84 ± 16 |
| 1 | Pyrimidine | A | CH=CH2 | phenyl | < 5 | 41 ± 6 (SD) |
| 10 | Pyrimidine | B | NH2 | iPr | 7.3 ± 0.1 | 100 ± 0.0 |
| 34 | Pyrimidine | B | Me | iPr | < 5 | 87 ± 13 |
| 35 | Pyrimidine | B | Et | iPr | < 5 | 93 ± 7 |
| 21 | Pyrimidine | B | CH=CH2 | iPr | < 5 | 85 ± 15 |
| 11 | Pyrimidine | C | NH2 | iPr | 6.7 ± 0.1 | 96 ± 4 |
| 36 | Pyrimidine | C | Me | iPr | 5.9 ± 0.1 | 96 ± 3 |
| 37 | Pyrimidine | C | Et | iPr | 5.5 ± 0.1 | 89 ± 11 |
| 22 | Pyrimidine | C | CH=CH2 | iPr | 5.5 ± 0.0 | 41 ± 9 |
| 12 | Pyrimidine | C | NH2 | Me | 6.5 ± 0.1 | 91 ± 5 |
| 23 | Pyrimidine | C | CH=CH2 | Me | 5.4 ± 0.1 | 40 ± 8 |
| 20 | Pyrimidine | C | C≡CH | Me | < 5 | 80 ± 2 |
| 42 | Phenyl | C | NH2 | - | 5.9 ± 0.3 | 72 ± 8 |
| 43 | Phenyl | C | NH-CO-CH2-Cl | - | 6.2 ± 0.1 | 86 ± 5 |
| 44 | Phenyl | C | N=C=S | - | 6.5 ± 0.3 | 15 ± 2 |
We expected that H4R covalent binder 1 [15] can also covalently bind H3R due to the high sequence similarity with H4R and the conservation of the anchor residue C3.36 [29]. Although ligands 1 and 2 showed both comparable micromolar affinity for hH4R (pKi 6.3 and 6.4, respectively) [15], ligand 2 showed a remarkable increase in H3R binding affinity (pKi: 7.1), while 1 showed only weak H3R affinity (pKi < 5). Despite its weak apparent affinity, binding of [3H]NAMH to H3R-expressing cell homogenates pre-incubated with 1 after washout resulted in only 41% occupancy of the receptor population by [3H]NAMH, suggesting that 1 irreversibly binds to hH3R since binding of its non-covalent analog 2 is reversible (Table 1).
In the first design cycle, two H3R aminopyrimidine anchor-moieties (R1 = B or C) were combined with an ethenyl-warhead at Position 2 of pyrimidine core (R2) and an iPr moiety as R3 (Table 1). Good binding affinity was obtained for the amino-derivative 10 while substitution at the R2 position by an alkyl (34, 35), as well as ethenyl moiety (21), significantly reduced the binding affinity (pKi < 5). Washout of 10, 34, 35 and 21 all resulted in high occupancy of the H3R with [3H]NAMH, indicating that receptors are not covalently labeled by 21 nor, as expected, by its non-covalent analogs 10, 34 and 35. To improve H3R binding affinity, the same compound set (R3 = iPr; R2 = NH2, Me, Et, ethenyl) was synthesized with a cyclobutyl-piperazine moiety as anchor (R1 = C). Although the affinity of the amino-analog 11 was reduced compared to 10, the affinities of non-covalent alkyl derivatives 36 and 37 as well as of ethenyl-derivative 22 increased to the micromolar range with respect to their counterparts in series B (Table 1). Washout experiments on non-covalent analogs 11, 36 and 37 show reversibility, while 22 decreased the amount of receptors available for [3H]NAMH binding, indicating that a covalent interaction had been formed between 22 and H3R. To further improve both affinity and covalent labeling, optimization of core substitution (R3) and warhead (R2) were undertaken in the second design cycle. Reducing the iPr moiety to a smaller Me substituent (R3 = Me) resulted in similar binding affinities for both the non-covalent (compare 12 to 11) as well as covalent ethenyl (compare 23 to 22) analog. Similar to 22 and 11, compound 23 led to covalent labeling whereas 12 did not. In contrast, the ethynyl analog 20 displayed poor affinity and is incapable of inhibiting [3H]NAMH binding after washout, suggesting lack of irreversible binding presumably due to inappropriate orientation of the reactive electrophilic carbon. We explored more reactive warheads in order to improve upon the covalent H3R labeling by 22 and 23.
Synthesis of analogs of 22 and 23 with isothiocyanate- and chloromethylketone warheads was attempted, but combining these warheads with the electron-withdrawing pyrimidine core was synthetically challenging. Therefore, in the third and final design cycle, the pyrimidine core was changed to a phenyl group. While use of the phenyl core still resulted in micromolar affinities for non-covalent amino analog 42, the apparent affinity of potential covalent derivatives 43 and 44 was improved with respect to 22 and 23 (Table 1). Non-covalent control 42 and chloroacetamide 43 showed similarly low labeling in washout experiments, for the latter suggesting inappropriate orientation of the electrophilic carbon or low reactivity. Gratifyingly, though, after washout of 44 only 15% of the receptor population was available for [3H]NAMH binding, suggesting that an efficient formation of a covalent interaction with the H3R was formed.
2.4. Covalent Binding to Glutathione and Nonapeptide
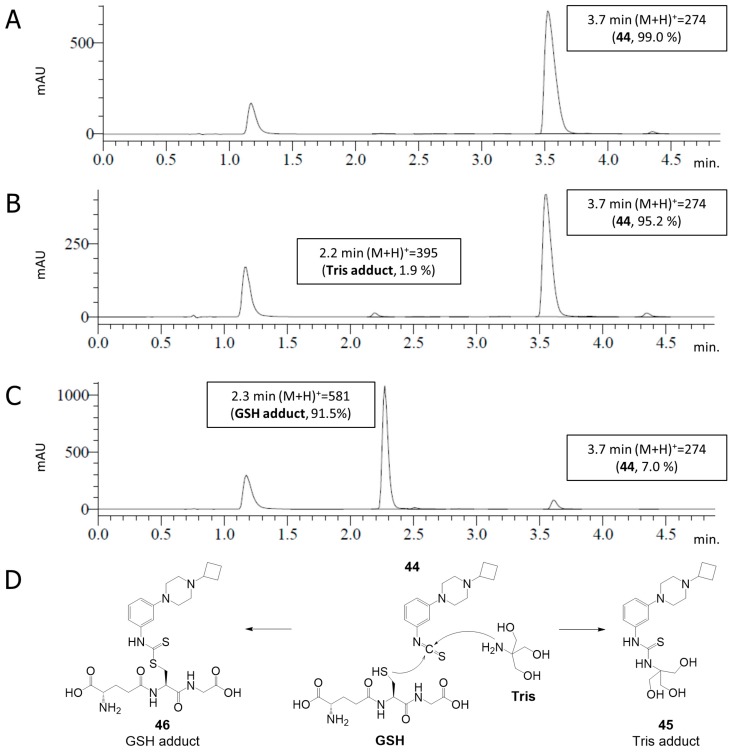
Given the suggested covalent binding to the H3R (Table 1), key isothiocyanate 44 was tested for its chemical stability and reactivity with glutathione (GSH) under the conditions of the pharmacological experiments. Compound 44 (0.40 mM) was dissolved in Tris buffer (50 mM Tris-HCl, pH 7.4) in absence or presence of GSH (1.2 mM). The mixtures were incubated for 10–100 min at room temperature and analysed by LC-MS (Figure 3). Interestingly, the stability experiments showed a very slow reaction with Tris. That is, while 44 remained intact after 10 min incubation (Figure 3A), a very minor adduct of 44 and Tris (45) was visible (1.9%) after 100 min incubation (Figure 3B). Indeed, phenyl isothiocyanates are known to display reactivity towards amines and thiols albeit at different rates (vide supra) [12,32], but exert low reactivity towards alcohols [38]. Therefore, we postulate that the isothiocyanate moiety of 44 binds the amino group of Tris to afford adduct 45 (Figure 3D). Yet, the still high integrity of 44 (95%) at the pharmacologically relevant incubation time (100 min) indicates an appropriate stability of 44 in buffer for our pharmacology assays. Co-incubation with GSH in a 1:3 (44:GSH) molar ratio shows high reactivity, with the GSH adduct 46 visible at high conversion (91.5%) after 10 min incubation (Figure 3C). The reaction of 44 with GSH is postulated to proceed via the thiol group (Figure 3D).
Figure 3.
LC-MS analysis of 44 (0.40 mM) in 50 mM Tris-HCl pH 7.4 without (A,B) and with (C) GSH (1.2 mM). The mixtures were incubated for 10 min (A,C) or 100 min (B) at rt. The incubated mixtures were analysed by LC-MS. A signal from the DMSO used for the stock solution of 44 is visible at 1.2 min. (D) Postulated identified products.
The selectivity of 44 toward nucleophilic residues was further investigated with an oligopeptide KGDYHFPIC. This nonapeptide (NP) is designed to harbor an array of nucleophilic residues, i.e., cysteine, lysine, tyrosine, aspartate and histidine [25]. Analysis by LC-MS/MS can identify the binding to a particular residue from fragmentation patterns. NP and 44 were incubated in phosphate-buffered saline pH 7.4 with 10% MeCN at rt for 16 h. LC-MS analysis indicates a reaction with the NP as evidenced by a NP+44+H+ ion at m/z 1351.5 as well as its doubly-charged ion at m/z 676.5 in the peak at 4.3 min (Figure S1A). The MS/MS spectrum of the two-fold charged NP modified by 44 (m/z 676.5) shows a fragmentation pattern indicative of the cysteine monoadduct of 44 (Figure 4), suggesting selective binding of the isothiocyanate moiety of 44 to the cysteine residue. The non-covalent analog 42 does not show any reaction with NP (Figure S1B).
Figure 4.
MS/MS spectrum of NP modified by 44 (m/z 676.5, see Figure S1A). Two-fold charged species were examined.
2.5. Covalent Labeling of the H3R by 44
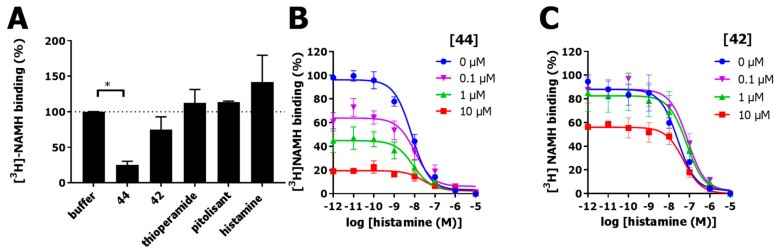
To further validate that 44 binds irreversibly to the H3R, recovery of [3H]NAMH binding after washout of H3R-expressing cell homogenates preincubated with 10 µM 44 or 42 was compared to washout of three reference non-covalent H3R ligands (namely thioperamide, pitolisant, and histamine) with relatively long to short residence times [39]. Indeed, 10 µM 44 could significantly (p < 0.05, one-way ANOVA with Fisher’s LSD test) inhibit recovery of [3H]NAMH binding after washout, while washout of H3R expressing cell homogenates preincubated with 42, histamine, pitolisant, or thioperamide recovered [3H]NAMH occupancy to vehicle levels (Figure 5A).
Figure 5.
(A) Binding of [3H]NAMH to hH3R-expressing HEK293T cell homogenates that were pre-incubated with vehicle, 10 µM 44, 10 µM 42 or 10 µM of non-covalent reference H3R ligands thioperamide, pitolisant and histamine for 1 h at 25 °C, followed by washout. (B,C) Radioligand displacement curves of [3H]NAMH with histamine on cell homogenates preincubated for 1 h at 25 °C with increasing concentrations of (B) 44 or (C) 42. After thorough washing pretreated cell homogenates were subjected to displacement of [3H]NAMH with increasing histamine concentrations. Data are normalized to specific binding of vehicle treated cell homogenates. Data shown are pooled data mean ± S.E.M. of at least three experiments performed in triplicate. * p < 0.05 (one-way ANOVA with Fisher’s LSD test).
Moreover, after washout of cell homogenates pretreated with 0.1–10 µM 44 a concentration-dependent decrease in [3H]NAMH occupancy of the H3R up to 20% is observed, while the affinity of histamine was unaffected (Figure 5B). In contrast, pretreatment with non-covalent analog 42 showed no significant decrease in [3H]NAMH binding up to 1 µM (Figure 5C). However, at 10 µM 42 only 56% [3H]NAMH labeling could be observed despite three vigorous wash steps to remove the ligand.
Due to high homology between H3R and H4R binding of 42 and 44 was evaluated in a NanoBRET-based fluorescent ligand displacement assay on the Nanoluc-H4R. Both 42 and 44 were incapable of displacing the fluorescent tracer clobenpropit-BODIPY from the Nanoluc-H4R up to a concentration of 10 µM (Figure S2), indicating that binding of 44 and 42 is selective to H3R.
2.6. Functional Characterization
Functional characterization of 44 in a [35S]GTPyS assay showed that 44 inhibited basal H3R signaling with a pEC50 value of 7.1 ± 0.3 (Figure 6A). Moreover, 44 could inhibit H3R activity induced by immepip (31.6 nM) to levels below basal signaling with a pIC50 value of 6.1 ± 0.1, suggesting that it might act as inverse agonist (Figure 6B). In comparison, the marketed H3R inverse agonist pitolisant more potently inhibited immepip-induced H3R activity (pIC50 8.1 ± 0.1), however, inhibition of basal H3R signaling was more pronounced for 44.
Figure 6.
Functional characterization of 44 as measured by [35S]GTPγS binding to hH3R expressing HEK293T cell homogenates at 25 °C for 1 h. (A) Inhibition of basal hH3R activity by 44. (B) Inhibition of 31.6 nM immepip-induced hH3R activity by 44 or pitolisant.
3. Discussion
Covalent small-molecule ligands can be useful tools for the better understanding of GPCR structure and function [14,17]. Despite intense investigations on the histamine receptor GPCR family [2], histamine receptors have only been targeted with covalent ligands sporadically [15,17]. The aim of the current study was the identification of a covalent H3R ligand. The high homology of H3R and H4R [28] suggested that the published covalent partial H4R agonist VUF14480 [15] was a suitable starting point. The first design cycle resulted in a cyclobutyl-piperazine anchor moiety, which was combined with a change in warhead in the second design cycle, resulting in Michael acceptor vinyl-ligands 22 and 23 with micromolar apparent affinity for H3R. In a subsequent scaffold hopping, the pyrimidine core was changed to phenyl in the third design cycle, providing the phenyl-isothiocyanate 44 with subnanomolar apparent affinity for H3R and no measurable interaction with H4R up to least 10−5 M.
The alkyl-isothiocyanate moiety as reactive warhead has been regularly used for covalent targeting, e.g., for labeling of cannabinoid receptors [18,40,41], and were found to predominantly interact with cysteine residues. Under physiological conditions alkyl-isothiocyanate rapidly form adducts with cysteines, however, in the presence of lysines, a slow but irreversible reaction could also form lysine adducts [32] as was observed with the some reactive phenyl isothiocyanates [33]. According to our reactivity/stability assays, phenyl-isothiocyanate 44 forms an adduct with cysteine in a rapid reaction without significant aqueous decomposition. A more in-depth nonapeptide assay shows that the reaction is selective for cysteine in the presence of other nucleophilic residues, and formation of lysine adducts did not significantly occur during an incubation period of 16 h.
Determination of washout resistance by radioligand binding is a frequently used method for the validation of a postulated irreversible interaction between a potential covalent ligand and the targeted receptor [15,16,17,18,19,20,21,22,23]. In this experimental design, the bound ligand 44 was washed out after the preincubation with hH3R expressing cell homogenate and showed a concentration-dependent labeling of the receptor, while the non-covalent control 42 and reference ligands recovered radioligand binding to vehicle levels. This result indicates the irreversible nature of 44 binding to the binding pocket.
The unraveling of the crystal structure of H1R [42] has significantly impacted research on the histamine receptor family. However, the moderate similarity between H1R and H3R causes limitations in obtaining H3R models based on the structure of H1R [29]. Covalent ligands have been shown to facilitate GPCR crystallization [14,43]. We therefore believe that the identified covalent inverse agonist 44 could aid in stabilizing the H3R in the inactive conformation and as such function as a tool compound in H3R crystallization. The covalent binder 44 might also serve as a useful extension of the diverse H3R compound set for studying H3R signaling [39,44].
In conclusion, we designed and synthesized potential covalent ligands for H3R. Three successive design cycles led to identification of 44 (VUF15662) as an irreversible H3R binder. Tool compound 44 can be used to stabilize the inactive conformation of hH3R and to evaluate the effects of long-term blockage of H3R signaling.
4. Materials and Methods
4.1. Pharmacology
4.1.1. Materials
[3H]NAMH (specific activity: 79.7 Ci/mmol) and [35S]GTPyS (specific activity 2200 Ci/mmol) were purchased from Perkin Elmer (Boston, MA, USA). All other chemicals were obtained from commercial suppliers and were of analytical grade.
4.1.2. Cell Culture and Transfection
Human embryonic kidney 293T cells (HEK293T) (ATCC, Manassas, VA, USA) were cultured in DMEM (Gicbo, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Bodinco, Alkmaar, the Netherlands) and 1% pen/step (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Two million cells per 10 cm2 dishes were plated 24 h prior to transfection. Cells were transfected using the polyethyleneimine (PEI) method [44] with 2500 ng cDNA encoding the hH3R and 2500 ng empty plasmid pcDEF3.
4.1.3. Preparation of Cell Homogenates
Cell homogenates expressing the hH3R were harvested 48 h after transfection as reported previously [39].
4.1.4. Radioligand Displacement Assays
[3H]NAMH displacement assays were perform in binding buffer [50 mM Tris-HCl pH 7.4, 25 °C] by co-incubation of ~2 nM [3H]NAMH, increasing concentration of unlabeled ligand and cell homogenates expressing the hH3R. Assay mixture was incubated for 2 h at 25 °C before rapid filtration over a PEI-coated GF/C filter with a Perkin Elmer filtermate harvester. Filterplate was dried and 300 min after 25 µL Microsint O was added filterbound radioactivity was measured with a Microbeta scintillation counter (Perkin Elmer).
4.1.5. Receptor Recovery Assay
To assess covalent binding of unlabeled ligands, hH3R expressing cell homogenates were pre-incubated for 1 h with either Tris buffer [50 mM Tris-HCl pH 7.4], 10 µM, 1 µM or 0.1 µM unlabeled ligand, or 100 µM unlabeled ligand for screening, in a thermoshaker (1000 RPM, 25 °C). Subsequently, cell homogenates were vigorously washed according to the procedure described in Nijmeijer et al. (2013) [15] and pelleted cell homogenates were reconstituted in Tris buffer and subjected to radioligand displacement assay (vida supra).
4.1.6. [35S]GTPγs Assay
In [35S]GTPyS assay, hH3R expressing cell homogenates (20 µg/well) were incubated in GTPγs buffer [50 mM HEPES, 150 mM NaCl, 10 mM MgCl2, 4 µM GDP, 0.2 µg saponin, pH 7.4] with ~0.5 nM [35S]GTPγS and increasing ligand concentrations (10−4 M to 10−12 M) for 1 h at 25 °C. Reaction was stopped by rapid filtration of a GF/B filter with a Perkin Elmer filtermate harvester. Filterplate was dried and 25 µL Microsint O was added to measure filterbound radioactivity with a Microbeta scintillation counter (Perkin Elmer) at 500 min.
4.1.7. Chemical Stability and Reactivity of 44
The reactivity of 44 (400 µM) was tested in the mixture of binding buffer (96% 50 mM Tris-HCl pH 7.4 and 4% DMSO) with or without GSH (1.2 mM) at rt. At various timepoints, the mixtures were directly injected and analysed by LC-MS, using the acidic mode elution programme described in the General information of the chemistry experimental section.
4.1.8. Nonapeptide Assay
Analysis of NP (KGDYHFPIC) incubated (16 h, rt) with 44 or 42 was performed according to Ábrányi-Balogh et al. [25].
4.1.9. Data Analysis
All data was analysed and statistical analysis performed using Graphpad prism 7.02 (Graphpad software inc, San Diego, USA). Competition binding curves were fitted using a one-site binding model and affinity (pKi) values were calculated using Cheng–Prusoff equation [45].
4.2. Modelling
The homology model of H3R was constructed according to Hauwert et al. [46].
The covalent compound docking (Figure 2) was performed using GOLD version 5.2.1 using the Piecewise Linear Potential (PLP) fitness function. A flood fill of the orthosteric binding site was used as reference for the docking site. The sulfur atom of C1183.36 was assigned as covalent anchor point and the pocket residues W1103.28, D1143.32, Y1153.33, E2065.46, W3716.48, and W4027.43 were treated as semi-flexible by sampling different rotamers from the GOLD rotamer library.
4.3. Chemistry
4.3.1. General Information
Chemicals and solvents were obtained from commercial suppliers and were used without further purification. Dry DCM and dioxane were obtained from PureSolv solvent purification system by Inert®. All reactions were carried out under an inert N2 atmosphere. Microwave reactions were performed with Biotage Initiator microwave system. TLC analyses were performed with Merck F254 alumina silica plates using UV visualization or staining. Column purifications were carried out automatically using Biotage Isolera and Silicycle Ultra Pure silica gel. Melting point (Mp) for final compounds was determined using a Büchi M-565 melting point apparatus with a rate of 1 °C/min. NMR spectra were recorded on a Bruker 250 or 500 MHz spectrometer. Chemical shifts are reported in ppm (δ), and the residual solvent was used as internal standard (δ 1H NMR: CDCl3 7.26; D2O 4.79; 13C NMR: CDCl3 77.16). Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, p = pentet, hept = heptet, br = broad signal, m = multiplet, app = apparent), coupling constants (Hz) and integration. HRMS spectra were recorded on Bruker microTOF mass spectrometer using ESI in positive ion mode. Analytical HPLC-MS analyses were conducted using a Shimadzu LC-20AD liquid chromatograph pump system connected to a Shimadzu SPDM20A diode array detector with MS detection using a Shimadzu HPLC-MS 2010EV mass spectrometer. The column used is an Xbridge C18 5 mm column (50mm × 4.6 mm). Acidic mode: Solvent B (MeCN/0.1% formic acid) and solvent A (water/0.1% formic acid), flow rate of 1.0 mL/min with a run time of 8 min. For compounds which retention time (tR) was less than 1.5 min with acidic solvent system, a basic solvent system was used. Basic mode: Solvent B (MeCN/10% buffer), Solvent A (water/10% buffer). The buffer is a 0.4% (w/v) NH4HCO3 solution in water, adjusted to pH 8.0 with NH4OH. The analysis was conducted using a flow rate of 1.0 mL/min with a total run time of 8 min. Gradient settings (basic and acidic system): start 5% B, linear gradient to 90% B in 4.5 min, then isocratic for 1.5 min at 90% B, then linear gradient to 5% B in 0.5 min, then isocratic for 1.5 min at 5% B. Unless specified otherwise, all compounds have a purity of ≥95%, calculated as the percentage peak area of the analysed compound by UV detection at 254 nm. Yields reported are not optimized. The compounds described in Table 1 were checked for the presence of PAINS substructures as described by Baell and Holloway [47], and no PAINS substructures were identified.
4.3.2. Synthesis
The syntheses of final compounds 10–12, 20–23, 34–37 and 43 are described in the Supplementary Materials.
Tert-butyl 4-(3-nitrophenyl)piperazine-1-carboxylate (39). A solution of bromide 38 (808 mg, 4.00 mmol) and NaOtBu (461 mg, 4.80 mmol) in dioxane (15 mL) was degassed with N2 for 10 min. Next, Pd2(dba)3 (183 mg, 0.20 mmol), Xantphos (347 mg, 0.60 mmol) and tert-butyl piperazine-1-carboxylate (1.12 g, 6.00 mmol) were added. The reaction mixture was heated for 1 h at 80 °C under microwave irradiation. The reaction mixture was diluted with water (40 mL) and extracted with DCM (3 × 30 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash chromatography (heptane:EtOAc 5:0 to 4:1) gave the title compound as an orange solid (700 mg, 57%). 1H NMR (500 MHz, CDCl3) δ 7.72 (t, J. = 2.2 Hz, 1H), 7.71–7.67 (m, 1H), 7.39 (t, J. = 8.2 Hz, 1H), 7.20 (dd, J. = 8.3, 2.1 Hz, 1H), 3.61 (t, J. = 5.2 Hz, 4H), 3.24 (t, J. = 5.1 Hz, 4H), 1.49 (s, 9H). HPLC-MS (acidic mode): tR = 5.3 min, purity: 98.6%, [M + H]+: 308.
1-(3-Nitrophenyl)piperazine (40). To a solution of carbamate 39 (1.20 g, 3.90 mmol) in dioxane (20 mL) was added HCl in dioxane (4N, 9.75 mL, 39.0 mmol). The reaction mixture was stirred for 1 h at rt. The solvent was removed under reduced pressure. The residue was mixed with satd. aq. Na2CO3 (40 mL) and extracted with DCM (3 × 30 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. The title compound was obtained as a brown solid (633 mg, 78%). 1H NMR (500 MHz, CDCl3) δ 7.71 (s, 1H), 7.66 (d, J. = 8.0 Hz, 1H), 7.37 (t, J. = 8.2 Hz, 1H), 7.19 (d, J. = 8.3 Hz, 1H), 3.28–3.21 (m, 4H), 3.10–3.02 (m, 4H). HPLC-MS (acidic mode): tR = 2.6 min, purity: 97.3%, [M + H]+: 208.
1-Cyclobutyl-4-(3-nitrophenyl)piperazine (41). To a solution of amine 40 (630 mg, 3.04 mmol) in DCM (20 mL) was added cyclobutanone (273 μL, 3.66 mmol). After 10 min of stirring at rt, NaBH(OAc)3 (966 mg, 4.56 mmol) was added and the resulting mixture was stirred at rt overnight. The reaction mixture was quenched with satd. aq. Na2CO3 (30 mL) and extracted with DCM (3 × 15 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash chromatography (DCM:MeOH 20:0 to 19:1) gave the title compound as a yellow oil (600 mg, 76%). 1H NMR (500 MHz, CDCl3) δ 7.71 (t, J. = 2.3 Hz, 1H), 7.65 (dd, J. = 8.1, 1.5 Hz, 1H), 7.37 (t, J. = 8.2 Hz, 1H), 7.18 (dd, J. = 8.3, 2.1 Hz, 1H), 3.38–3.23 (m, 4H), 2.80 (p, J. = 7.9 Hz, 1H), 2.51 (t, J. = 5.1 Hz, 4H), 2.13–2.04 (m, 2H), 2.00–1.87 (m, 2H), 1.81–1.57 (m, 2H, overlaps with residual water). HPLC-MS (acidic mode): tR = 2.8 min, purity: 97.8%, [M + H]+: 262.
3-(4-Cyclobutylpiperazin-1-yl)aniline (42). To a solution of nitrocompound 41 (59 mg, 0.23 mmol) in MeOH (2 mL) was added HCOONH4 (71 mg, 1.13 mmol) as a solid followed by a suspension of Pd/C (10%, 24 mg) in water (2 mL). The reaction mixture was stirred at rt overnight. The mixture was filtered over Celite and the filtrate was concentrated in vacuo. The residue was diluted with aq. Na2CO3 (1.0 M, 10 mL) and extracted with DCM (3 × 5 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by flash chromatography (DCM:MeOH 20:0 to 19:1). The selected fractions were collected and the solvents were evaporated. The residue was dissolved in aq. HCl (1.0 M, 10 mL), washed with EtOAc (2 × 5 mL) and c-hexane (3 × 10 mL). The pH of the aqueous layer was adjusted to 10 with satd. aq. Na2CO3 and extracted with EtOAc (3 × 10 mL). The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuo. The title compound was obtained as an off-white solid (13 mg, 25%). Mp: 92.5–92.7 °C. 1H NMR (500 MHz, CDCl3) δ 7.04 (t, J. = 8.0 Hz, 1H), 6.36 (dd, J. = 8.2, 2.3 Hz, 1H), 6.25 (t, J. = 2.3 Hz, 1H), 6.21 (dd, J. = 7.7, 2.0 Hz, 1H), 3.59 (br, 2H), 3.26–3.10 (m, 4H), 2.78 (p, J. = 7.9 Hz, 1H), 2.56–2.37 (m, 4H), 2.12–2.02 (m, 2H), 2.00–1.88 (m, 2H), 1.80–1.64 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 152.6, 147.4, 130.0, 107.1, 107.1, 103.0, 60.4, 49.6, 48.8, 27.1, 14.4. HPLC-MS (basic mode): tR = 4.1 min, purity: 98.4%, [M + H]+: 232. HR-MS [M + H]+ calcd for C14H22N3+: 232.1808, found 232.1818.
1-Cyclobutyl-4-(3-isothiocyanatophenyl)piperazine (44). To an ice-cold mixture of aniline 42 (46 mg, 0.20 mmol) in DCM (2 mL) and aq. NaHCO3 (1.0 M, 30 mL) was added dropwise a solution of CSCl2 (18 μL, 0.24 mmol) in DCM (1 mL). The reaction mixture was stirred for 1 h at rt. The reaction mixture was diluted with water (10 mL) and extracted with DCM (3 × 5 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated in vacuo. Purification by flash chromatography (DCM:MeOH 20:0 to 19:1) gave the title compound as a white solid (35 mg, 64%). The compound is stable as a solid in the freezer (−20 °C) over a period of at least 2 years as judged by NMR and LC-MS analysis. Mp: 79.7–80.1°C. 1H NMR (500 MHz, CDCl3) δ 7.18 (t, J. = 8.1 Hz, 1H), 6.81 (dd, J. = 8.5, 2.4 Hz, 1H), 6.72–6.65 (m, 2H), 3.23–3.15 (m, 4H), 2.77 (p, J. = 7.9 Hz, 1H), 2.50–2.42 (m, 4H), 2.11–2.02 (m, 2H), 1.96–1.86 (m, 2H), 1.79–1.64 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 152.2, 134.2, 131.9, 130.0, 116.5, 114.9, 112.6, 60.3, 49.3, 48.3, 27.1, 14.4. HPLC-MS (acidic mode): tR = 3.6 min, purity: 98.9%, [M + H]+: 274. HR-MS [M + H]+ calcd for C15H20N3S+: 274.1372, found 274.1374.
Acknowledgments
We thank Hans Custers for HRMS measurements. Tímea Imre is acknowledged for performing the nonapeptide assay. László Petri is acknowledged for his efforts with the nonapeptide measurements.
Abbreviations
| [3H]NAMH | [3H]N-α-methylhistamine |
| ANOVA | analysis of variance |
| DCM | Dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| DME | 1,2-Dimethoxyethane |
| DMSO | dimethyl sulfoxide |
| GPCR | G protein-coupled receptor |
| GSH | glutathion |
| GTPγS | guanosine 5′-O-[gamma-thio]triphosphate |
| LSD | least significant difference |
| MeCN | Acetonitrile |
| MIDA | N-methyliminodiacetic acid |
| Mp | Melting point |
| NP | nonapeptide (KGDYHFPIC) |
| SAR | structure-activity relationship |
| satd. aq. | saturated aqueous |
| SD | standard deviation |
| SEM | standard error of mean |
| rt | room temperature |
| TCI | targeted covalent inhibitors |
| TEA | Triethylamine |
| Tris | 2-Amino-2-(hydroxymethyl)propane-1,3-diol |
| μW | microwave reaction |
| wt | wild type |
Supplementary Materials
The following are available online. LC-MS analysis of nonapeptide assays with 44 and 42; Nluc-H4R displacement of clobenpropit-BODIPY by 42, 44 and reference ligands; syntheses of final compounds 10–12, 20–23, 34–37 and 43; LC-MS chromatogram and 1H, 13C, HSQC and HMBC NMR spectra for 44 and 42.
Author Contributions
G.W. performed the synthesis and reactivity/stability studies. T.A.M.M. performed the pharmacological experiments. A.J.K., I.S., G.W. and M.W. contributed to the design of the compound set. G.M.K. and P.Á.-B. supervised the nonapeptide assay. G.W. and T.A.M.M. wrote the original draft with input from A.J.K. and P.Á.-B. M.W., H.F.V., I.J.P.d.E. and R.L. conceptualized and supervised the whole research project. G.M.K., P.Á.-B., M.W., H.F.V., I.J.P.d.E. and R.L. reviewed and edited the manuscript.
Funding
This research was funded by The Netherlands Organization for Scientific Research (NWO) TOPPUNT [“7 ways to 7TMR modulation (7-to-7)”] [Grant 718.014.002]. P. Ábrányi-Balogh was supported by the Hungarian Science Foundation OTKA (PD124598) grant.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compound 44 are available from the authors.
References
- 1.Arrang J.M., Garbarg M., Schwartz J.C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 2.Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L.S., Stark H., Thurmond R.L., Haas H.L. International Union of Basic and Clinical Pharmacology. XCVIII. Histamine Receptors. Pharmacol. Rev. 2014;67:601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mocking T.A.M., Bosma R., Rahman S.N., Verweij E.W.E., McNaught-Flores D.A., Vischer H.F., Leurs R. Molecular Aspects of Histamine Receptors. In: Blandina P., Passani M.B., editors. Histamine Receptors: Preclinical and Clinical Aspects. Springer International Publishing; Cham, Switzerland: 2016. pp. 1–49. [Google Scholar]
- 4.Schlicker E., Kathmann M. Role of the Histamine H3 Receptor in the Central Nervous System. Handb. Exp. Pharmacol. 2017;241:277–299. doi: 10.1007/164_2016_12. [DOI] [PubMed] [Google Scholar]
- 5.Lovenberg T.W., Roland B.L., Wilson S.J., Jiang X., Pyati J., Huvar A., Jackson M.R., Erlander M.G. Cloning and Functional Expression of the Human Histamine H3 Receptor. Mol. Pharmacol. 2018;55:1101–1107. doi: 10.1124/mol.55.6.1101. [DOI] [PubMed] [Google Scholar]
- 6.Celanire S., Wijtmans M., Talaga P., Leurs R., de Esch I.J.P. Keynote review: Histamine H3 receptor antagonists reach out for the clinic. Drug Discov. Today. 2005;10:1613–1627. doi: 10.1016/S1359-6446(05)03625-1. [DOI] [PubMed] [Google Scholar]
- 7.Lebois E.P., Jones C.K., Lindsley C.W. The evolution of histamine H3 antagonists/inverse agonists. Curr. Top. Med. Chem. 2011;11:648–660. doi: 10.2174/1568026611109060648. [DOI] [PubMed] [Google Scholar]
- 8.Kuhne S., Wijtmans M., Lim H.D., Leurs R., de Esch I.J.P. Several down, a few to go: Histamine H3 receptor ligands making the final push towards the market? Expert Opin. Investig. Drugs. 2011;20:1629–1648. doi: 10.1517/13543784.2011.625010. [DOI] [PubMed] [Google Scholar]
- 9.Sadek B., Łażewska D., Hagenow S., Kieć-Kononowicz K., Stark H. Histamine H3R Antagonists: From Scaffold Hopping to Clinical Candidates. In: Blandina P., Passani M.B., editors. Histamine Receptors: Preclinical and Clinical Aspects. Springer International Publishing; Cham, Switzerland: 2016. pp. 109–155. [Google Scholar]
- 10.Kollb-Sielecka M., Demolis P., Emmerich J., Markey G., Salmonson T., Haas M. The European Medicines Agency review of pitolisant for treatment of narcolepsy: Summary of the scientific assessment by the Committee for Medicinal Products for Human Use. Sleep Med. 2018;33:125–129. doi: 10.1016/j.sleep.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Harmony Biosciences LLC. [(accessed on 15 August 2019)]; Available online: https://www.harmonybiosciences.com/newsroom/harmony-biosciences-announces-fda-approval-of-wakix-r-pitolisant-a-first-in/
- 12.Zhao Z., Bourne P.E. Progress with covalent small-molecule kinase inhibitors. Drug Discov. Today. 2018;23:727–735. doi: 10.1016/j.drudis.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Weichert D., Gmeiner P. Covalent Molecular Probes for Class A G Protein-Coupled Receptors: Advances and Applications. ACS Chem. Biol. 2015;10:1376–1386. doi: 10.1021/acschembio.5b00070. [DOI] [PubMed] [Google Scholar]
- 14.Kruse A.C., Ring A.M., Manglik A., Hu J., Hu K., Eitel K., Hübner H., Pardon E., Valant C., Sexton P.M., et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504:101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nijmeijer S., Engelhardt H., Schultes S., van de Stolpe A.C., Lusink V., de Graaf C., Wijtmans M., Haaksma E.E.J., de Esch I.J.P., Stachurski K., et al. Design and pharmacological characterization of VUF14480, a covalent partial agonist that interacts with cysteine 983.36 of the human histamine H4 receptor. Br. J. Pharmacol. 2013;170:89–100. doi: 10.1111/bph.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss S.M., Jayasekara P.S., Paoletta S., Gao Z.G., Jacobson K.A. Structure-based design of reactive nucleosides for site-specific modification of the A2A adenosine receptor. ACS Med. Chem. Lett. 2014;5:1043–1048. doi: 10.1021/ml5002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weichert D., Kruse A.C., Manglik A., Hiller C., Zhang C., Hubner H., Kobilka B.K., Gmeiner P. Covalent agonists for studying G protein-coupled receptor activation. Proc. Natl. Acad. Sci. USA. 2014;111:10744–10748. doi: 10.1073/pnas.1410415111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janero D.R., Yaddanapudi S., Zvonok N., Subramanian K.V., Shukla V.G., Stahl E., Zhou L., Hurst D., Wager-Miller J., Bohn L.M., et al. Molecular-Interaction and Signaling Profiles of AM3677, a Novel Covalent Agonist Selective for the Cannabinoid 1 Receptor. ACS Chem. Neurosci. 2015;6:1400–1410. doi: 10.1021/acschemneuro.5b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kling R.C., Plomer M., Lang C., Banerjee A., Hübner H., Gmeiner P. Development of Covalent Ligand-Receptor Pairs to Study the Binding Properties of Nonpeptidic Neurotensin Receptor 1 Antagonists. ACS Chem. Biol. 2016;11:869–875. doi: 10.1021/acschembio.5b00965. [DOI] [PubMed] [Google Scholar]
- 20.Yang X., Dong G., Michiels T.J.M., Lenselink E.B., Heitman L., Louvel J., Ijzerman A.P. A covalent antagonist for the human adenosine A2A receptor. Purinergic Signal. 2017;13:191–201. doi: 10.1007/s11302-016-9549-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soethoudt M., Stolze S.C., Westphal M.V., van Stralen L., Martella A., van Rooden E.J., Guba W., Varga Z.V., Deng H., van Kasteren S.I., et al. Selective Photoaffinity Probe That Enables Assessment of Cannabinoid CB 2 Receptor Expression and Ligand Engagement in Human Cells. J. Am. Chem. Soc. 2018;140:6067–6075. doi: 10.1021/jacs.7b11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang X., van Veldhoven J.P.D., Offringa J., Kuiper B.J., Lenselink E.B., Heitman L.H., van der Es D., Ijzerman A.P. Development of Covalent Ligands for G Protein-Coupled Receptors: A Case for the Human Adenosine A3 Receptor. J. Med. Chem. 2019;62:3539–3552. doi: 10.1021/acs.jmedchem.8b02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwalbe T., Huebner H., Gmeiner P. Development of covalent antagonists for β1- and β2-adrenergic receptors. Bioorg. Med. Chem. 2019;27:2959–2971. doi: 10.1016/j.bmc.2019.05.034. [DOI] [PubMed] [Google Scholar]
- 24.Lonsdale R., Burgess J., Colclough N., Davies N.L., Lenz E.M., Orton A.L., Ward R.A. Expanding the Armory: Predicting and Tuning Covalent Warhead Reactivity. J. Chem. Inf. Model. 2017;57:3124–3137. doi: 10.1021/acs.jcim.7b00553. [DOI] [PubMed] [Google Scholar]
- 25.Ábrányi-Balogh P., Petri L., Imre T., Szijj P., Scarpino A., Hrast M., Mitrović A., Fonovič U.P., Németh K., Barreteau H., et al. A road map for prioritizing warheads for cysteine targeting covalent inhibitors. Eur. J. Med. Chem. 2018;160:94–107. doi: 10.1016/j.ejmech.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Fell N., Rodney G., Marshall D.E. Histamine Protein Complexes: Synthesis and Immunologic Investigation: I. Histamine-Azo-Protein. J. Immunol. 1943;47:237–249. [Google Scholar]
- 27.Rodney G., Fell N. Histamine-protein complexes: Synthesis and immunological investigation: II. ß-(5-Imidazoyl) ethyl carbamido protein. J. Immunol. 1943;47:251–259. [Google Scholar]
- 28.Morse K.L., Behan J., Laz T.M., West R.E.J., Greenfeder S.A., Anthes J.C., Umland S., Wan Y., Hipkin R.W., Gonsiorek W., et al. Cloning and characterization of a novel human histamine receptor. J. Pharmacol. Exp. Ther. 2001;269:1058–1066. [PubMed] [Google Scholar]
- 29.Kooistra A.J., Kuhne S., de Esch I.J.P., Leurs R., de Graaf C. A structural chemogenomics analysis of aminergic GPCRs: Lessons for histamine receptor ligand design. Br. J. Pharmacol. 2013;170:101–126. doi: 10.1111/bph.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballesteros J.A., Weinstein H. [19] Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 31.Łażewska D., Kieć-Kononowicz K. New developments around histamine H3 receptor antagonists/inverse agonists: A patent review (2010–present) Expert Opin. Ther. Pat. 2013;24:89–111. doi: 10.1517/13543776.2014.848197. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T., Kawai Y., Kitamoto N., Osawa T., Kato Y. Covalent modification of lysine residues by allyl isothiocyanate in physiological conditions: Plausible transformation of isothiocyanate from thiol to amine. Chem. Res. Toxicol. 2009;22:536–542. doi: 10.1021/tx8003906. [DOI] [PubMed] [Google Scholar]
- 33.Karlsson I., Samuelsson K., Ponting D.J., Törnqvist M., Ilag L.L., Nilsson U. Peptide Reactivity of Isothiocyanates-Implications for Skin Allergy. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep21203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi L., Javitch J.A. The Binding Site of Aminergic G Protein-Coupled Receptors: The Transmembrane Segments and Second Extracellular Loop. Annu. Rev. Pharmacol. Toxicol. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- 35.Uveges A.J., Kowal D., Zhang Y., Spangler T.B., Dunlop J., Semus S., Philip G.J. The Role of Transmembrane Helix 5 in Agonist Binding to the Human H3 Receptor. J. Pharmacol. Exp. Ther. 2002;301:451–458. doi: 10.1124/jpet.301.2.451. [DOI] [PubMed] [Google Scholar]
- 36.Francom P., Janeba Z., Shibuya S., Robins M.J. Nucleic acid related compounds. 116. Nonaqueous diazotization of aminopurine nucleosides. Mechanistic considerations and efficient procedures with tert-butyl nitrite or sodium nitrite. J. Org. Chem. 2002;67:6788–6796. doi: 10.1021/jo0204101. [DOI] [PubMed] [Google Scholar]
- 37.Wang J., Liang Y.L., Qu J. Boiling water-catalyzed neutral and selective N-Boc deprotection. Chem. Commun. 2009:5144–5146. doi: 10.1039/b910239f. [DOI] [PubMed] [Google Scholar]
- 38.Burke T.R., Bajwa B.S., Jacobson A.E., Rice K.C., Streaty R.A., Klee W.A. Probes for Narcotic Receptor Mediated Phenomena. 7. Synthesis and Pharmacological Properties of Irreversible Ligands Specific for μ or δ Opiate Receptors. J. Med. Chem. 1984;27:1570–1574. doi: 10.1021/jm00378a008. [DOI] [PubMed] [Google Scholar]
- 39.Mocking T.A.M., Verweij E.W.E., Vischer H.F., Leurs R. Homogeneous, Real-Time NanoBRET Binding Assays for the Histamine H3 and H4 Receptors on Living Cells. Mol. Pharmacol. 2018;94:1371–1381. doi: 10.1124/mol.118.113373. [DOI] [PubMed] [Google Scholar]
- 40.Mercier R.W., Pei Y., Pandainathan L., Janero D.R., Zhang J., Makriyannis A. hCB2 ligand-interaction landscape: Cysteine residues critical to biarylpyrazole antagonist binding motif and receptor modulation. Chem. Biol. 2011;17:1132–1142. doi: 10.1016/j.chembiol.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pei Y., Mercier R.W., Anday J.K., Thakur G.A., Zvonok A.M., Hurst D., Reggio P.H., Janero D.R., Makriyannis A. Ligand-Binding Architecture of Human CB2 Cannabinoid Receptor: Evidence for Receptor Subtype-Specific Binding Motif and Modeling GPCR Activation. Chem. Biol. 2008;15:1207–1219. doi: 10.1016/j.chembiol.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimamura T., Shiroishi M., Weyand S., Tsujimoto H., Winter G., Katritch V., Abagyan R., Cherezov V., Liu W., Han G.W., et al. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475:65–72. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenbaum D.M., Zhang C., Lyons J.A., Holl R., Aragao D., Arlow D.H., Rasmussen S.G.F., Choi H.J., Devree B.T., Sunahara R.K., et al. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature. 2011;469:236–242. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mocking T.A.M., Buzink M.C.M.L., Leurs R., Vischer H.F. Bioluminescence Resonance Energy Transfer Based G Protein-Activation Assay to Probe Duration of Antagonism at the Histamine H3 Receptor. Int. J. Mol. Sci. 2019;20:3724. doi: 10.3390/ijms20153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng Y.-C., Prusoff W.H. Relationship Between the Inhibition Constant (Ki) and the Concentration of Inhibitor which Causes 50 Per Cent Inhibition (I50) of an Enzymatic Reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 46.Hauwert N.J., Mocking T.A.M., Da Costa Pereira D., Kooistra A.J., Wijnen L.M., Vreeker G.C.M., Verweij E.W.E., de Boer A.H., Smit M.J., de Graaf C., et al. Synthesis and Characterization of a Bidirectional Photoswitchable Antagonist Toolbox for Real-Time GPCR Photopharmacology. J. Am. Chem. Soc. 2018;140:4232–4243. doi: 10.1021/jacs.7b11422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baell J.B., Holloway G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.