Abstract
We demonstrate presence of senescent (p16+, p21+) epithelial cells in the stem and transient amplifying cell niches of the intestine in Crohn’s disease. This may explain why Crohn’s lesions recur in the same places.
To the Editors,
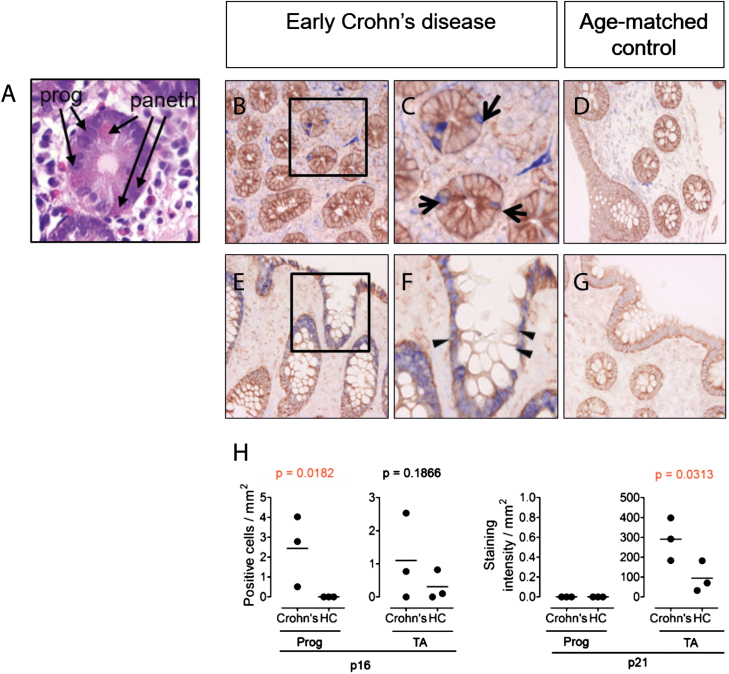
The inflammatory lesions of Crohn’s disease occur most prevalently in the terminal ileum and colon. Recurrent lesions in these areas suggest a defect in the ability of the local intestinal stem cell population to maintain homeostasis of the gut. We have previously demonstrated a reduction in functionality of salivary gland (SG) progenitor cells (SGPCs) isolated from patients with the autoimmune disease primary Sjögren syndrome.1, 2 When we examined tissue from patients with early Crohn’s disease, we observed p16+ cells resident in the stem cell niches of the intestine (Figs. 1A–C). Expression of a second senescence marker, p21, was observed in the transient amplifying (TA) cell niches and the columnar cells of the intestine; Fig. 1E, F). These observations suggest that persistence of lesions in the same intestinal area may be attributable to senescent stem cells, which are no longer capable of proliferating to generate new enterocytes. The reason for the predilection of Crohn’s lesions for the terminal ileum and colon areas remains unknown. Studies have suggested that infection with Mycobacterium avium paratuberculosis (MAP) may be a causal factor in Crohn’s disease.3Mycobacterium avium paratuberculosis is capable of initiating and maintaining chronic inflammation of the intestine. Mycobacterium avium paratuberculosis congregation in potential low traffic areas of the intestine, perhaps where Crohn’s lesions are most often found, may provide an explanation for this. Once inflammation is initiated, pro-inflammatory cytokines such as INF-α, TNF-α, and interleukin (IL)-6 are likely to induce local epithelial cell death and/or stem cell over proliferation.4 Both facets increase pressure on resident stem cells to generate replacement cells and may promote senescence of the stem cell pool. In addition to reduced functionality, senescent cells also express the senescence-associated secretory proteome (SASP) composed of pro-inflammatory cytokines (IL-6, IL-1, CXCL5, CXCL10, CCL1, CCL2, and TNF-α).5 Senescence-associated secretory proteome expression promotes senescence in neighboring cells and therefore represents a potentially pathological phenotype.5 When pro-inflammatory cytokines were applied to healthy SG progenitor cell cultures, proliferation was induced. Presuming the same effect takes place in the intestine, p16+ and p21+ cells in Crohn’s disease may also promote development of replicative senescence in neighboring progenitor cells. Appreciation of the effects of an inflamed environment on epithelial stem cells—and particularly epithelial progenitor cells—is growing and will be crucial to molding our therapeutic options toward not only resolution of inflammation but also recovery of epithelial integrity.
FIGURE 1.
The stem and transient amplifying cell niches of the intestine contain potentially senescent cells in Crohn’s disease. In all immunostains, epithelial growth factor receptor (EGFR, brown) is used as a counterstain to provide tissue architecture. (A) H&E staining of intestinal tissue showing characteristic eosin rich cytoplasmic staining (pink) morphology of paneth cells, confirming localization at level of progenitor cell niche. (B-G) Immunostaining for p16 and p21 (blue) of intestinal tissue from a patient with early Crohn’s disease (B,C,E,F) and age/sex-matched control (D,G). (H) Quantification of p16 and p21 positive cells in progenitor (Prog) and transient amplifying (TA) cell niches in intestinal tissue from early Crohn’s patients (n = 3), compared to age-matched healthy controls (HC). One-tailed Mann Whitney U testing was used for statistical analysis. Exact P-values are given. p16+ or p21+ cells in the progenitor cell niche in the gut were identified based on localization in close proximity to the paneth cells. Methods: Antigen retrieval was performed for 20 minutes with a solution of 1 mM EDTA (pH 8.0) on paraffin sections. Mouse anti-human p16 (Roche clone E6H4, ready to use; blue), mouse anti human p21 (DAKO, clone SX118, 1:20; blue) and rabbit anti-human epithelial growth factor receptor (Abcam clone EP38Y, 1:500; brown) were used with the Lab Vision MultiVision Polymer Detection System anti Mouse-AP anti-rabbit-HRP staining kit (Thermofisher) to detect p16+ and p21+ cells. Arrows denote the progenitor cell niche in the intestine. Triangles denote the transient amplifying cell niche. Intensity of p21 immunostaining in intestine was quantified as pixel intensity.
Author Contribution: XW contributed to the experimental work and data analysis. GD contributed to the patient recruitment and manuscript writing. SP contributed to the study design, data analysis and collection, and first-draft writing.
Supported by: This research was funded by a China Scholarship Council grant (201606220074), Dutch Arthritis Foundation Translational Research grant (T015-052) and a Dutch Arthritis Foundation Long Term Project grant (LLP-29).
REFERENCES
- 1. Pringle S, Wang X, Verstappen GMPJ, et al. Salivary gland stem cells age prematurely in primary Sjögren’s syndrome. Arthritis Rheumatol. 2019;71:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X, Bootsma H, Terpstra J, et al. Progenitor cell niche senescence reflects pathology of the parotid salivary gland in primary Sjögren’s syndrome. Rheumatology. (revised manuscript submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNees AL, Markesich D, Zayyani NR, et al. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015;9:1523–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Onyiah JC, Colgan SP. Cytokine responses and epithelial function in the intestinal mucosa. Cell Mol Life Sci. 2016;73:4203–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gorgoulis V, Adams PD, Alimonti A, et al. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. [DOI] [PubMed] [Google Scholar]