Abstract
Background and Aims
Herbivory by large mammals imposes a critical recruitment bottleneck on plants in many systems. Spines defend plants against large herbivores, and how early they emerge in saplings may be one of the strongest predictors of sapling survival in herbivore-rich environments. Yet little effort has been directed at understanding the variability in spine emergence across saplings.
Methods
We present a multispecies study examining whether and how sapling size, spine type and species' environmental niche (light and precipitation environment) influence early emergence and biomass investment in spines. A phylogenetically diverse pool of 45 species possessing different spine types (spines, prickles and thorns; that are derived from distinct plant organs: leaf, epidermis or cortex, and branch, respectively), were grown under common-garden conditions, and patterns of spine emergence and biomass allocation to spines at 5 and 15 weeks after transplanting were characterized.
Key Results
Spine type and species' resource niche were the main factors driving early emergence and investment patterns. Spines emerged earliest in leaf spine-bearing species, and latest in thorn-bearing species. The probability of early spine emergence increased with decreasing precipitation, and was greater in species from open than from closed habitats. Sapling investment in spines changed with plant mass but was contingent on spine type and habitat type.
Conclusions
Different spine types have strikingly different timing of expression, suggesting that developmental origins of spines play a critical role in sapling defences. Furthermore, species from different precipitation and light environments (open vs. closed habitats) showed contrasting patterns of early spine expression, suggesting that resource limitation in their native range may have driven divergent evolution of early defence expression.
Keywords: Developmental constraints, early emergence, large herbivore, prickle, resource environment, sapling, spine, thorn, spinescence
Introduction
In systems where large mammalian herbivores (hereafter, large herbivores) are abundant, they have severe impacts, via persistent defoliation, on the survival and growth of woody plants at juvenile stages (Bond, 2008; Staver and Bond, 2014; Churski et al., 2017). Herbivory by smaller browsing and mixed-feeding mammals on seedlings and saplings represents a significant bottleneck in tree recruitment in herbivore-rich systems (Prins and van der Jeugd, 1993; Staver and Bond, 2014). In these systems, being defended early in development is potentially crucial for tree survival. A growing body of literature has revealed spines to be an essential and specific structural defence against small to large herbivores, particularly in adult plants (Cooper and Owen-Smith, 1986; Hanley et al., 2007; Shipley, 2007; Charles-Dominique et al., 2017). Spines (like most structural defences) become more important in plant defence during the sapling stages (Hanley et al., 2007; Barton and Koricheva, 2010; Ochoa-López et al., 2015). Even though deployment of spines in saplings varies substantially across species (Grubb, 1992), little effort has been directed at understanding the sources of this variation. Understanding variability among species in the early expression of spines could provide insights into survivorship of spiny saplings and help to explain their distribution across environmental gradients (Myers and Bazely, 1991; Grubb, 1992; Moles et al., 2011; Schmidt et al., 2013; Tindall et al., 2016).
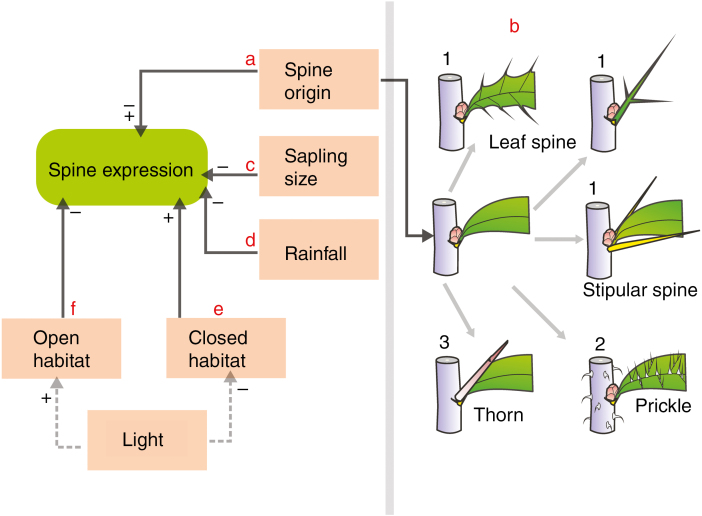
Here, we examined early emergence and investment in spines in saplings across a large number of species. We describe the constitutive onset of spines across species from different habitats. Plant defence theories posit that variation in defence expression across species is driven by differences in plant growth rate (Herms and Mattson, 1992), environmental resource supply (Coley et al., 1985; Tomlinson et al., 2016), plant internal resource pools (Bryant et al., 1983) and risk of herbivory (McKey, 1974; Rhoades, 1979). However, differences in expression of physical defences (e.g. pubescence and spines) may also be partly explained by differences in the origin of the plant tissue/organ modified (Hanley et al., 2007; Barton, 2016), but this source of variation is currently not accounted for by plant defence theories (Barton and Boege, 2017). We formulated an integrative framework (Fig. 1) that incorporates predictions from both plant defence (Stamp, 2003) and developmental constraints theories (Barton and Boege, 2017) to examine early spine expression in saplings.
Fig. 1.
Conceptual diagram showing how different factors are predicted to affect early spine expression in saplings. Symbols at the end of arrows indicate positive (+), negative (–) or positive and negative effects (±). Solid and broken lines represent predictions that were tested and implied but not directly tested in this study, respectively.
Spines can be produced from different plant organs (Bell and Bryan, 2008) that could impose a strong developmental constraint on their expression in saplings (Fig. 1A). Spines, derived from modified leaves and leaf parts, are likely to incur significant cost in terms of lost photosynthesis, but may be the earliest to emerge (Fig. 1B, shown with ‘1') as they can be deployed simultaneously with the growth of first leaves. Prickles, derived from outgrowths of the epidermis or cortex, have lower construction costs (Bazely et al., 1991), but their emergence may be delayed because they undergo longer developmental phases (Kellogg et al., 2011; Gallenmüller et al., 2015). Thorns, derived from stems and auxiliary meristems, should be slowest to emerge as early plant growth involves first the development of the primary stem (and associated leaves) before lateral branching can occur to form thorns (Barthelemy and Caraglio, 2007; Bell and Bryan, 2008). Further, building thorns involves activation and lignification of auxiliary meristems which require substantial biomass investment and are therefore likely to be affected by overall plant size to a greater extent than spines or prickles. In addition to these allocation costS and anatomical constraints, the timing of spine expression may reflect adaptation to resource availability.
Plant defence theory generally predicts trade-off between growth and defence investment (Stamp, 2003) due either to resource allocation constraints (Herms and Mattson, 1992) or to shared plant regulatory pathways (Campos et al., 2016; Zuest and Agrawal, 2017). Spines are thought to incur allocation costs (Craine et al., 2003) by diverting carbon pools from building other plant vegetative parts (e.g. stem and leaves) (Skogsmyr and Fagerstom, 1992). Importantly, unlike many chemical defences, allocation to spines represents fixed investment that cannot be recycled (Grubb, 1992). For young saplings, such fixed investment may be particularly costly due to limited total resource pools (Boege and Marquis, 2005). Given that realized plant size (i.e. total biomass) reflects growth rate (e.g. fast-growing species produce bigger saplings) and absolute internal resource pools (Bazzaz et al., 1987), species producing larger saplings (i.e. those with a fast biomass accumulation rate) are probably selected for lower constitutive investment in spines (Fig. 1C). In contrast, slow-growing species (producing smaller saplings) may invest heavily in spines to reduce loss of limited resources (Coley et al., 1985; Herms and Mattson, 1992).
Species from resource-limited environments generally grow more slowly and have low post-defoliation regrowth capacity, and are therefore expected to invest heavily in constitutive defences (Coley et al., 1985; Swihart and Bryant, 2001; Stamp, 2003). Thus, we predicted that saplings of spiny species from low precipitation and light environments are probably selected for greater expression of spines relative to those from high precipitation and light environments (Fig. 1D–F. Indeed the general distribution of spiny plants suggests that environments characterized by low precipitation may select for greater incidence of spines (Grubb, 1992; Charles-Dominique et al., 2016). For instance, the proportion of spiny plants generally increases with decreasing rainfall (Grubb, 1992; Schmidt et al., 2013). However, the distribution of spinescence across light gradients is in contrast to the expectation of greater spinescence under low light conditions. Spiny plants tend to be more common in ‘open' than in ‘closed' (e.g. forests) habitats (Myers and Bazely, 1991; Grubb, 1992; Charles-Dominique et al., 2016; Osborne et al., 2018), However, it remains unknown if saplings of spiny species from closed habitats (i.e. low light niche) have been selected for early and greater investment in spines than those from open habitats (i.e. high light niche).
To understand whether spiny plants with different spine types or from distinct environments have evolved different strategies for early spine expression (in order to survive the limitations encountered in their native environments), we sampled 45 spinescent species across the angiosperm phylogeny that naturally occur under diverse environments and grew them under common-garden conditions. We then assessed constitutive expression of spines (i.e. baseline defence expression) by quantifying emergence timing and biomass investment in spines at two temporal periods (at 5 and 15 weeks after transplanting). We specifically tested the following four predictions: (1) Sapling size is negatively related to emergence time and biomass investment in spines, reflecting growth–defence trade-offs due to allocation costs and (2) spines (i.e. leaf and stipular spines) and prickles are the fastest to emerge (because leaves are produced immediately upon germination and prickles generally incur low biomass investment) while thorns are likely to be delayed due to architectural constraints in saplings and their expression is potentially tied to sapling size. Across species, emergence and investment in spines: (3) scales negatively with mean annual precipitation and (4) is greater and independent of sapling size for species from closed compared with open environments, potentially reflecting the cost of herbivory associated with plants growing under more arid and lower light conditions.
MATERIALS AND METHODS
Seed collection and germination
We sampled seeds of spiny species from the living collections of Xishuangbanna Tropical Botanical Garden of the Chinese Academy of Science (XTBG-CAS), located in Menglun, Yunnan, China (21°55'38''N, 101°15'6''E); the XTBG-CAS savanna field station in Yuanjiang Valley, Yunnan (23°28'15''N, 103°10'37''E); the Germplasm Bank of Wild Species, Kunming Institute of Botany (KIB-CAS); and from South Africa by Tomlinson et al. (2012). In total we germinated seeds of 45 species that were mostly natives to tropical China (ten species), South-east Asia (15 species) and Africa (13 species; see Supplementary data Table S1 for details) and distributed across wet to arid and closed to open habitats.
We classified species according to their spine types as: stipular spines, leaf spines, thorns or prickles (Grubb, 1992; Gutschick, 1999; Bell and Bryan, 2008). Here, we placed Berberis species in the ‘leaf spines' group to differentiate them from ‘stipular spines' because spines in Berberris are produced from both leaves and stipules. Our sample included species belonging to 17 plant families (Supplementary data Table S1), of which 13 species (six families) possess prickles, 21 species (12 families) possess thorns, five species (all Berberis) possess leaf spines and six species (two families) possess stipular spines. We initially attempted to germinate additional leaf-spiny species of Ilex (e.g. I. cornuta and I. aquifolium) and Hakea (H. oleifolia, H. erinacea and H. prostrata), but were unsuccessful.
We grouped species into ‘closed' (forest; 16 species) or ‘open' habitats (savanna, thicket and grassland; 29 species) based on descriptions from the online flora covering the geographic distribution of the species (e.g. Flora of China: http://efloras.org/;http://worldwidewattle.com/) as well as published references (Coates-Palgrave, 2002). We complemented this with expert opinion for species with widespread distributions. For thorns and prickles, we have representative species in both open and closed habitats, but all stipular and leaf spine species are from open habitats only (Supplementary data Table S1).
We derived mean annual precipitation (MAP) per species based on the distribution range of each species. We determined the MAP for each species using species distribution data (GBIF.org: http://www.gbif.org/occurrence; accessed 9 August 2017, doi:http://doi.org/10.15468/dl.zrarz4) and combined it with precipitation data produced by Fick et al. (2017; WorldClim: http://worldclim.org/version2) using the zonal statistics tool in ArcMap desktop GIS (version 10.2, Esri Inc. CA, USA).
Greenhouse experiment
Seeds were germinated on either agar or river sand, and after 10 d were transplanted into a greenhouse located in XTBG-CAS for the experiment. The greenhouse was covered with shade netting from March to October to reduce irradiance levels to 40–50 % of full sunlight. This was necessary to reduce desiccation and mortality of the young seedlings, particularly of the forest species. Temperature and relative humidity in the greenhouse ranged over the course of the study from 19.7 to 33.3 °C and from 42 to 100 %, respectively. At the time of transplanting, each seedling was placed in a plastic tube of 10 cm diameter and 80 cm length. We chose deep pots to allow adequate space for taproot growth and reduce pot-binding effects, particularly for species from open habitats (Tomlinson et al., 2012). Tubes were filled with river sand mixed with Osmocote 18-6-12 N-P-K fertilizer (8–9 month mixture) at a concentration of 5 kg of fertilizer m–3 of river sand. Each tube was irrigated for 1 min twice daily using an automated irrigation system (EZ Pro™ Jr, Signature Control Systems Inc., Peoria, IL, USA).
Transplanted seedlings were allowed to grow for a minimum of 5 (between 5 and 8 weeks) and 15 (between 15 and 19 weeks) weeks before harvesting (hereafter week 5 and 15, respectively) for trait measurements. Here, we referred to the post-5 weeks plants as ‘saplings' because the fourth to fifth leaves had emerged on most plants and cotyledons had dropped in most species, suggesting that these plants were no longer dependent on stored energy reserves from the cotyledons (Hanley et al., 2004; Barton and Hanley, 2013), and thus capturing a potential shift in resource status for many species. Selection for early or late defence expression should be most apparent at week 5 given that plants have just transitioned from the seedling to sapling stage and have limited resource pools (Boege and Marquis, 2005). At week 15, saplings should generally have more resources to allow for investment in defence. Where possible, we harvested at least five individuals per species at each harvest date. However, for 13 species (including all species with leaf spines), we did not have enough individuals and therefore harvested all individuals of these species at week 15. The greenhouse experiment was conducted in two temporal blocks from July to December in both 2015 and 2016. Thirteen species (three prickles, five stipular spines and five thorns) were grown in 2015, whereas 32 species (five leaf spines, ten prickles, one stipular spine and 16 thorns) were grown in 2016 (Supplementary data Table S1). Greenhouse conditions (minimum, mean and maximum temperature and relative humidity) did not differ between 2015 and 2016 (P > 0.05), and preliminary analyses showed that there was no need to include ‘Year' as a random factor in the models described below.
Trait measurements
We characterized early spine expression using both the presence of spines on saplings and the biomass invested in spines. We inspected and recorded the presence of spines as our measure of spine emergence (yes = 1 and no = 0) on each individual at week 5. At week 15, we again checked the presence of spines on each individual (for those that did not produce spines at week 5).
We carefully removed all spines borne on all plant organs to estimate spine mass fraction (SPMF; our second response variable). The SPMF is commonly reported as a measure of biomass investment in defence (Bazely et al., 1991; Gowda, 1997; Pisani and Distel, 1998; Craine et al., 2003; Gowda and Palo, 2003). Here, we preferred SPMF over other measures of investment in spines (e.g. spine length or density) because it gives an indication of biomass allocation cost that can easily be related to total investment in other plant parts such as leaves (Skogsmyr and Fagerstom, 1992; Craine et al., 2003). The SPMF was estimated only at week 15 as spines were too small to allow for accurate estimation of spine mass at week 5. In all cases, we detached spines from the plant organ using either sharp razor blades or utility knives. For estimation of thorn mass, we included only modified branches with pointed and lignified tips (most species that produced thorns had only thorns emerging and few true branches that were clearly different). We thus removed thorns from the base (attached to the primary stem). The leaf spine species in our data set often produced whole-leaf modified spines or produced clearly visible spines from modified leaf tips. For species with prickles, we removed prickles from both the leaves and the stem.
Biomass of all plants harvested at any of the two sampling dates was divided into leaf, stem and root tissues, dried to constant weight and weighed. We added the leaf, stem, root and spine tissue masses to obtain the total sapling biomass (mass, g, our variable for plant size) and subsequently determined organ mass fractions. We computed the SPMF (g g–1, measured only during week 15 harvesting) as the ratio of spine mass to sapling mass for each individual.
Data analyses
Given the number of species in our data set, and the possibility that phylogeny may influence the examined relationships, we conducted both ordinary and phylogenetically adjusted analyses (Garamszegi, 2014; Lajeunesse and Fox, 2015). Phylogenetically adjusted models were used here to assess whether our conclusions were influenced by the sampled taxa and not to infer any evolutionary pattern. Both phylogenetic and non-phylogenetic models produced qualitatively similar results. We therefore, for simplicity, describe the analyses without phylogenetic corrections here. A detailed description of the phylogenetic methods and results can be found in the supplementary materials (Supplementary data Method S1; Tables S2–S5).
Given that all species with leaf spines and stipular spines were from open habitats and none from closed habitats, we did not have sufficient overlap to conduct multilinear regressions across all predictors. Therefore, we tested sub-set combinations of predictors on spine variables. We analysed early spine expression (presence and biomass investment in spines) in two ways. First, spine emergence was treated as a binary response (yes = 1, no = 0). When examined at week 5 and 15, all individuals of a given species either produced spines or did not. Therefore, we analysed the spine emergence data set at the species level (where a species either produced spines or did not). To test predictions 1 and 3 (i.e. the general relationships between sapling size and a species precipitation niche on spine emergence), we implemented independent binomial regression (generalized linear model) models where the presence of spines is predicted by log-transformed sapling size and MAP (i.e. mean annual precipitation under which the species grows in its natural environment). We tested for the interactive effects of sapling mass and spine type (i.e. mass × spine types, prediction 2) and sapling mass and habitat (i.e. mass × habitat interaction, prediction 4) on spine emergence by implementing similar binomial models. Where the interaction term was not significant, we implemented additional univariate model testing for differences in spine emergences for different spine types and habitat type. All the above models were fitted separately for each week (i.e. week 5 and 15) because we were also interested in exploring whether our results changed across these two temporal periods. Binomial models were performed using the ‘glm' function in R version 3.4.4 (R Core Team, 2018). For some levels of spine type (e.g. leaf spine at 5 weeks and stipular spines at 15 weeks), all species had produced spines (i.e. spine presence = 1 for all observations). Complete separation [i.e. where a linear combination of predictors is perfectly predictive of the outcome – in our case a level of the factor had only ‘success' observations – (Gelman et al. 2008)] can significantly bias model estimates (Bolker, 2015). Thus, for the models testing for differences in spine emergence between species with different spine types, we used the function ‘bayesglm' from the ‘arm' package (Gelman and Su, 2018). The ‘bayesglm' function adopts a Bayesian inference approach and uses minimally informative priors to derive stable regression estimates (Gelman et al., 2008; Gelman and Su, 2018). We used the default settings for all analyses with the ‘bayesglm' function.
Secondly, we analysed differences in SPMF (log-transformed) with linear mixed effect models (here, species was treated as a random effect). Similar to the binomial models above, we first tested for the independent effects of sapling mass (measure of size, prediction 1) and MAP (prediction 3) on SPMF. We further ran models testing for sapling mass × spine type (prediction 2) and sapling mass × habitat (prediction 4) effect on SPMF. We fitted all the linear mixed models using the ‘lmer' function implemented in the ‘lme4' package (Bates et al., 2015). All statistical analyses described here were conducted in R version 3.4.4 (R Core Team, 2018).
RESULTS
Spine emergence in saplings
At 5 and 15 weeks, spines emerged in 47 % (21 of 45) and 69 % (31 of 45) of species, respectively. Spine emergence was remarkably conserved within species at the two sampling dates. When inspected at 5 or 15 weeks, all individuals of a given species either bore spines or did not (except for individuals of Bombax ceiba and Pyracantha fortuneana).
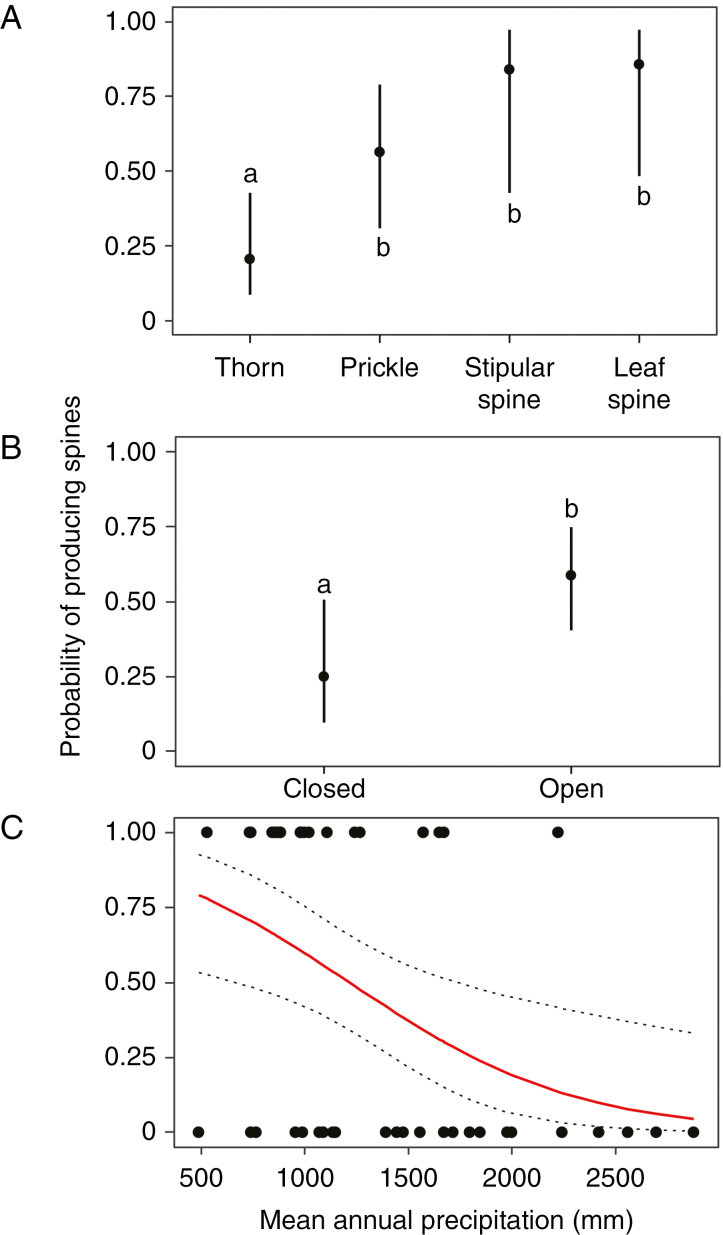
Binomial models indicated that spine emergence was affected by spine type, species' light and precipitation niche (Table 1). As predicted, probability of spine emergence at week 5 (Fig. 2A; Supplementary data Table S6) was significantly lower in species possessing thorns (19 %) compared with species with prickles (54 %), stipular spines (83 %) and leaf spines (100 %, all from the Berberis genus). These patterns persisted to week 15. By 15 weeks, all species with stipular spines, 85 % of species with prickles and 43 % of species with thorns had produced spines.
Table 1.
Results of generalized linear (glm) and mixed effect (LMMs) models testing the effects of plant size, mean annual precipitation, habitat and spine type on probability of emergence and biomass investment in spines in saplings of 45 species grown under greenhouse conditions
| Model | d.f. | Spine emergence (week 5) | Spine emergence (week 15) | Spine investment (SPMF, week 15) | |||
|---|---|---|---|---|---|---|---|
| LRT | P-value | LRT | P-value | LRT | P-value | ||
| log(mass) | 1 | 0.634 | 0.426 | 0.009 | 0.921 | 2.369 | 0.114 |
| MAP | 1 | 9.519 | 0.002 | 9.937 | 0.001 | 1.863 | 0.173 |
| Spine type | 3 | 16.762 | <0.001 | 19.241 | <0.001 | 9.529 | 0.023 |
| Habitat | 1 | 4.852 | 0.028 | 4.746 | 0.029 | 2.197 | 0.138 |
| Log (mass) × spine type | |||||||
| Log(mass) | 1 | 0.188 | 0.980 | 0.000 | 1.000 | 0.00 | – |
| Spine type | 3 | 4.708 | 0.319 | 4.844 | 0.184 | 20.691 | <0.001 |
| Log(mass) × spine type | 3 | 0.727 | 0.948 | 2.181 | 0.536 | 26.793 | <0.001 |
| Log(mass) × habitat | |||||||
| Log(mass) | 1 | 0.113 | 0.74 | 1.591 | 0.207 | 0.00 | – |
| Habitat | 1 | 4.199 | 0.04 | 5.767 | 0.016 | 6.060 | 0.014 |
| Log(mass) × habitat | 1 | 0.308 | 0.58 | 1.622 | 0.203 | 7.575 | 0.006 |
Significant effects (i.e.P < 0.05) were assessed with the likelihood ratio test (LRT) and are highlighted in bold. Spine emergence data were assessed 5 and 15 weeks after transplanting seedlings to the common garden whereas spine mass fraction (SPMF) was determined at week 15 only.
Fig. 2.
Probability of spine emergence after 5 weeks (A) across spine types, (B) between open and closed habitats and (C) along the precipitation preference gradient. For both (A) and (B), circles represent mean probability and error bars are 95 % confidence intervals for the means. For (C), the solid line represents mean predicted probability and dashed grey lines show the 95 % confidence intervals. For (A) and (B), differences in spine emergence were assessed using multiple comparison tests (Tukey).
In contrast to our prediction, the probability of spine emergence was significantly higher in open habitat species relative to closed habitat species at both 5 and 15 weeks (Fig. 2B; Table 1). Fifty-nine per cent and 80 % of species from open habitat, relative to 25 % and 50 % of species from closed habitat, produced spines at 5 and 15 weeks, respectively. Across species (at both 5 and 15 weeks), probability of spine emergence was negatively correlated (Fig. 2C; Table 1) with MAP (i.e. species from higher rainfall environments were less likely to produce spines). We found no associations between spine emergence and sapling size (mass) (Table 1). Further, there was no significant sapling mass × spine types or sapling mass × habitat interaction effect on spine emergence (Table 1). The relationships between spine emergence and the predictor variables were qualitatively similar when analysed at both week 5 and 15 (Table 1).
Biomass allocation to spines in saplings
At 15 weeks, constitutive biomass investment in spines (SPMF) varied from <0.1 % to 15 % of sapling mass across individuals (mean = 2 %; s.d. = 2.6 %). Expressed as the equivalent of leaf mass (i.e. spine mass as a fraction of leaf mass), sapling investment in spines ranged from 1 to 46 % of leaf mass (mean = 5 %; s.d. = 7.4 %) across individuals.
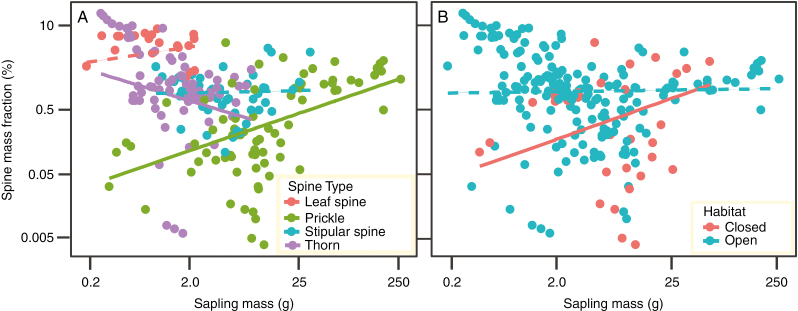
There was no detectable relationship between saplings mass or precipitation niche and investment in spines (SPMF). However, investment in spines (SPMF) differed by spine type (Table 1), with the highest investment in species with leaf spines (mean ± s.d. 5.2 ± 2 %), followed by species with thorns (2.5 ± 3.5 %), stipular spines (1 ± 0.9 %) and the lowest investment in species possessing prickles (0.7 ± 0.9 %). Importantly, there were significant differences in the trend of biomass allocation to spines (SPMF) with sapling size (mass) for the different spine types (i.e. significant mass × spine type interaction) (Fig.3A; Table 1). Allocation to spines was relatively fixed (no significant change in SPMF with sapling mass) for species possessing stipular spines and leaf spines (Fig. 3A). However, SPMF increased for species with prickles but decreased for species possessing thorns (Fig. 3A) with increasing sapling mass.
Fig. 3.
Relationship between sapling mass and biomass investment (as a percentage of total sapling mass) for species (A) possessing different spine types and (B) from different habitats grown under common-garden conditions for 15 weeks.
On average, species from open habitat invested approx. 2.3 times more biomass in spines relative to species from closed habitats. The relationship between sapling mass and SPMF was contingent on a species' light niche (i.e. significant sapling mass × habitat interactions). For open habitat species, SPMF was independent of sapling size. In contrast, SPMF was positively associated with sapling mass for species of closed habitat (Fig. 3B).
Discussion
We have provided the first detailed evaluation of constitutive patterns of early expression of spines among saplings of 45 spinescent species, grown under common conditions. Our approach has allowed us to explore general patterns in spine expression and to evaluate the influence of sapling size, spine type and species' environmental niche (light and precipitation niche) on early expression of spines. Our results suggest that: (1) the inherent constraints imposed by the developmental pathway of the spine (spine type) and (2) the resource environment (light and rainfall) under which species evolved, are two important factors determining constitutive trajectories of early spine expression. These findings were consistent across the two temporal stages examined, namely weeks 5 and 15 after transplanting. Moreover, the timing of spine emergence, when considered at the two sampling dates, was remarkably conserved within species.
Plant defence theory predicts a tight coupling between growth and defence, particularly under limited resource availability (Coley et al., 1985; Herms and Mattson, 1992; Stamp, 2003). Although this prediction has been largely examined with respect to chemical defences (Bergelson and Purrington, 1996; Koricheva, 2002; Zuest and Agrawal, 2017), for physical defences this relationship was less explored. Here, we tested whether sapling size was related to early emergence and investment in spines. In contrast to our prediction, we showed that sapling size was uncoupled from emergence of spines at both week 5 and 15. This suggests that onset of spines, in early saplings, per se may be unrelated to plant size but rather determined by other factors. While continuous sampling (e.g. weekly) may yet have revealed a link between plant size and spine emergence, the scope of our study characterizing 45 species constrained such an approach. Further studies focusing on fewer species could provide more precise developmental trajectories for sapling growth and spinescence. Although spine emergence was decoupled from sapling size (within the time window assessed), we observed that, across species, total biomass allocation to spines does depend on sapling size, but is contingent upon species light niche and spine type. Thus, while sapling size is linked to biomass allocation to spines, the nature of this relationship may be mediated by other factors such that a general assessment of growth–defence trade-off across spiny plants, without adequate consideration of the spine type or resource environment, may fail to detect any pattern (Bergelson and Purrington, 1996; Koricheva, 2002; Moles et al., 2013).
Spine types (leaf spine, stipular spine, thorns and prickles) are derived from separate organs or tissues (leaf blades, stipules, branches and epidermis, respectively; (Bell and Bryan, 2008), and we found that their timing of emergence is strikingly different. Spines derived from leaf parts (leaf spines and stipular spines) and prickles were the earliest to emerge, while thorns, which are modified branches, were the last to emerge. Interestingly, we detected no interaction between spine type and sapling size. This means that the slow pace with which thorns emerge across species is not driven by size limitation (independent from either growth rate or absolute internal resource pools). Instead, our results suggest that the developmental sequence in which organs are produced – which follows a typical order in most vascular plants (i.e. organogenesis, growth and branching) (Barthelemy and Caraglio, 2007) – exerts a strong constraint on the timing of emergence of spines. Thus, the developmental pathway of spines is vital for predicting how quickly species will be protected during their most vulnerable early life stages, and the timing of spine emergence reflects anatomical and architectural constraints perhaps more than any other factor (Villamil et al., 2013).
We observed divergent patterns of investment in spines for the different spine types across sapling sizes. For leaf and stipular spines, the total biomass of spines was uncoupled from sapling size, suggesting that growth and defence may not trade-off in these species. On the contrary, species with prickles invested more in spines and species with thorns invested less as sapling size increased. It seems unlikely that prickles could be resource limited to incur significant allocation cost (Bazely et al., 1991) and therefore respond to sapling size. Kellogg et al. (2011) have shown that prickles in Rubus follow a four-stage developmental progression until the final prickle development is completed. Thus, the link between plant size and investment in prickles might be the result of completing these developmental phases earlier in fast growing individuals (and not due to increased resource pools for allocation to spines). The negative relationship between sapling size and investment in thorns (at week 15) possibly points to a trade-off between growth rate (which determines sapling size) and defence allocation (Herms and Mattson, 1992; Zuest and Agrawal, 2017). Activation and lignification of auxiliary meristems to produce thorns potentially consumes more resources and incurs higher allocation costs in saplings than other types of spines. Here our results imply that regulation and expression of spines differ for the different spine types and therefore lumping these traits generally as ‘spinescence' (see, for example, Grubb, 1992; Hanley et al., 2007; Moles et al., 2013) potentially limits our ability to predict their ecological performance.
Our analysis suggests that early spine expression across species is influenced by a species' native resource environment (precipitation and light niche). We found a significant negative relationship between precipitation and spine emergence (Fig. 2C). This result supports the prediction that species from resource-limited environments (here, low precipitation) are selected for greater constitutive defence expression (Coley et al., 1985; Swihart and Bryant, 2001). In low precipitation environments, both photosynthesis and growth are constrained by water limitation (e.g. during the dry season). Here, plants cannot grow quickly to reach heights that would allow them to rapidly escape ground-based herbivores (Grubb, 1992) and thus must invest more in defences (Coley et al., 1985; Herms and Mattson, 1992). In contrast, emergence and investment in spines was greater in species from open than those from closed habitats (Figs 2B and 3B). These results partially contradict the hypothesis that defence is higher in species from low-resource environments (here species from a closed habitat: Coley et al., 1985). It may be energetically expensive for saplings (e.g. due to low light supply) to allocate limited carbohydrates to spines under closed environments. Consistent with this notion, we observed increased allocation to spines with increasing sapling size for species from closed habitats (Fig. 3B), suggesting that spiny species adapted to closed habitats may allocate more resources in defence when sufficient mass has been reached (Grubb, 1992; Swihart and Bryant, 2001).
Beyond the relevance of this work, our results should be considered with caution for two main reasons. First, species were grown under a single common garden (i.e. unnatural conditions for most species) and thus patterns of spine expression may be obscured for some species (Poorter et al., 2016). Determining the right conditions under which to examine early spine expression, for multiple species sourced from contrasted environments, is quite difficult, and the advantage of common-garden studies is that they allow for comparisons across phylogenetically diverse plants under highly controlled and similar environmental conditions. Previous studies examining the effect of resource supply on spinescence have so far produced inconsistent results (Hanley et al., 2007) and thus failed to provide clear patterns on how fertilization, light and water affect spine expression. For instance, fertilization has been reported to have positive (Gowda et al., 2003), negative (Bazely et al., 1991) or no (Pisani and Distel, 1998; Cash and Fulbright, 2005) effect on spine expression. In turn, shading has had no (Bazely et al., 1991) or positive (Barton, 2014) effects on spine production, whereas irrigation has no detectable influence on spine production (Pisani and Distel, 1998; Barton, 2014). Hence, deciding what environmental conditions are best for testing this question is still a challenge.
Secondly, given that spines have most probably evolved as defence mechanisms against large herbivores (Hanley et al., 2007; Charles-Dominique et al., 2016), constitutive patterns of early spine expression are likely to reflect historical patterns of large mammalian herbivory (McKey, 1974; Rhoades, 1979). However, attributing patterns of spine expression observed in this study to large herbivory pressure (or using a proxy) is difficult given the existing data on mammalian herbivores. The majority of our species are native to China and South-east Asia, and have ranges that previously contained significant concentrations of large herbivores (wild cattle, deer, rhinoceros and elephant) similar to the fauna of Africa (Harris et al., 1953; Wharton, 1966; Biasatti et al., 2012; Corlett, 2014). However, the composition and distribution of large mammalian herbivores in South-east Asia have changed remarkably in recent times, and, in most habitats, large herbivores have been extirpated (Corlett, 2007), making it difficult to attribute variations in herbivory pressure to the observed animal densities. Further, some spine types (such as prickles and leaf spines) could also have evolved in response to invertebrate herbivores (Kariyat et al., 2017). Thus, attributing constitutive patterns of spine expression to a specific class of herbivore requires an understanding of which class of herbivore is specifically targeted by these different spine types.
In spite of the potential limitations of inferring evolutionary sources of species variation in common-garden studies, our approach has elucidated some of the general trends in the timing and investment in spines during early plant growth. Indeed our results are consistent with patterns observed for other plant defence types. For instance, similar to our results, there is increasing evidence that expression of chemical (e.g. polyphenols and monoterpenoid: Murray and Hackett, 1991; Goralka and Langenheim, 1996; Fernandez-Lorenzo et al., 1999), physical (e.g. toughness: Rafferty and Lamont, 2007; Kitajima et al., 2013; Mason and Donovan, 2015) and indirect defences (Brouat and McKey, 2000; Villamil et al., 2013) are limited during early ontogeny because key features and pathways are not well developed in juveniles (Barton and Hanley, 2013; Barton and Boege, 2017). Further, while the incidence of sclerophylly, tough leaves (i.e. high leaf mass area) and pubescens (trichomes) is higher in resource-limited environments (e.g. low precipitation and fertility: Coley et al., 1985; Hanley et al., 2007), these physical defence traits respond positively to high light intensities (Groom and Lamont, 1997; Roberts and Paul, 2006; Hanley et al., 2007). Similarly, chemical defences are thought to be greater in species from resource-limited environments (Coley et al., 1985; Stamp, 2003), yet there is substantial evidence that species growing under high-light conditions have greater concentrations of both carbon- and non carbon-based secondary metabolites than those growing under shade (Koricheva et al., 1998; Roberts and Paul, 2006).
Conclusion
We conducted the first study examining the pattern of emergence and investment in spines in woody saplings across a diverse species pool. Generally, our results suggest that variation in the onset of spines depends on the spine type possessed by a species and the environment from which species originate (i.e. light and precipitation niche). Further, we found that, across species, investment in spines was influenced by sapling size but this effect was contingent on spine type and light niche. Due to the striking differences in early spine expression observed in this study, we advocate that understanding effectiveness of the different spine types against large herbivores is an important next step in developing predictive frameworks on how herbivory and environmental resources – and potential changes in these – will shape spiny woody communities in the future. Our study, focusing on structural defence in woody juveniles (most of which are tropical species), complements similar work from temperate and boreal forests which mostly focused on chemical defences. Our findings, together with other studies, provide insights into the roles of herbivory, environment and developmental controls on defence investment in juvenile woody plants. Finally, we suggest that understanding how resource limitation and herbivory interact to influence developmental shifts in different spine types could improve predictions for how future changes in resource supply and herbivore abundances will influence spiny plant communities.
Supplementary data
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Method S1: details of phylogenetic methods used in this study. Table S1: summary information on the 45 species used in this study. Table S2: GenBank accession numbers for DNA sequences of the 45 species used in this study. Table S3: summary of phylogenetically adjusted model testing for factors affecting spine emergence. Table S4: summary of pairwise comparison on probability of spine emergence between spine types based on the phylogenetically adjusted model. Table S5: summary of phylogenetically adjusted models testing for effect of factors on biomass investment in spines. Table S6: summary of pairwise comparison of the probability of spine emergence between spine types based on an ordinary binomial model.
Funding
This work was supported by research grants from the National Natural Science Foundation of China (grants nos 31470449 and 2017PB0093). M.A. was supported by a Chinese Government scholarship.
ACKNOWLEDGEMENTS
We thank Professor William Bond for very helpful discussions. We are grateful to the staff of the Germplasm Bank of Wild Species (Kunming Institute of Botany – Chinese Academy of Science) for helping us to acquire some of the seeds for this study. We thank Yang Dong and Yejing Wang for technical support in running the experiment, and the Public Technology Service Centre (Central Laboratory) of Xishuangbanna Tropical Botanical Garden for allowing us to use some of their equipment for this study. We are grateful to three anonymous reviewers for their helpful suggestions. There is no potential conflict of interest to be disclosed by the authors. K.W.T. and M.A. conceived the study; M.A. collected the data, analysed the data and led the writing of the manuscript; K.W.T., T.C.D. and K.E.B. contributed critically to the drafts and gave final approval for publication. The data supporting the results will be deposited in Dryad (https://doi.org/10.5061/dryad.nk98sf7ph).
Literature cited
- Barthelemy D, Caraglio Y. 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99: 375–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE. 2014. Prickles, latex, and tolerance in the endemic Hawaiian prickly poppy (Argemone glauca): variation between populations, across ontogeny, and in response to abiotic factors. Oecologia 174: 1273–1281. [DOI] [PubMed] [Google Scholar]
- Barton KE. 2016. Tougher and thornier: general patterns in the induction of physical defence traits. Functional Ecology 30: 181–187. [Google Scholar]
- Barton KE, Boege K. 2017. Future directions in the ontogeny of plant defence: understanding the evolutionary causes and consequences. Ecology Letters 20: 403–411. [DOI] [PubMed] [Google Scholar]
- Barton KE, Hanley ME. 2013. Seedling–herbivore interactions: insights into plant defence and regeneration patterns. Annals of Botany 112: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton KE, Koricheva J. 2010. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. The American Naturalist 175: 481–493. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48. [Google Scholar]
- Bazely DR, Myers JH, Burke K, Myers H. 1991. The response of numbers of bramble prickles to herbivory and depressed resource availability. Oikos 61: 327–336. [Google Scholar]
- Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF. 1987. Allocating resources to reproduction and defense. BioScience 37: 58–67. [Google Scholar]
- Bell AD, Bryan A. 2008. Plant form: an illustrated guide to flowering plant morphology. Portland, OR and London: Timber Press Inc. [Google Scholar]
- Bergelson J, Purrington CB. 1996. Surveying patterns in the cost of resistance in plants. The American Naturalist 148: 536–558. [Google Scholar]
- Biasatti D, Wang Y, Gao F, Xu Y, Flynn L. 2012. Paleoecologies and paleoclimates of late cenozoic mammals from Southwest China: evidence from stable carbon and oxygen isotopes. Journal of Asian Earth Sciences 44: 48–61. [Google Scholar]
- Boege K, Marquis RJ. 2005. Facing herbivory as you grow up: the ontogeny of resistance in plants. Trends in Ecology and Evolution 20: 441–448. [DOI] [PubMed] [Google Scholar]
- Bolker BM. 2015. Linear and generalized linear mixed models. In: Fox GA, Negrete-Yankelevich S, Sosa VJ, eds. Ecological statistics: contemporary theory and application. Oxford: Oxford University Press, 309–333. [Google Scholar]
- Bond WJ. 2008. What limits trees in C4 grasslands and savannas? Annual Review of Ecology, Evolution, and Systematics 39: 641–659. [Google Scholar]
- Brouat C, McKey D. 2000. Origin of caulinary ant domatia and timing of their onset in plant ontogeny: evolution of a key trait in horizontally transmitted ant–plant symbioses. Biological Journal of the Linnean Society 71: 801–819. [Google Scholar]
- Bryant JP, Chapin FS, Klein DR. 1983. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 40: 357–368. [Google Scholar]
- Campos ML, Yoshida Y, Major IT, et al. 2016. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth–defense tradeoffs. Nature Communications 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash VW, Fulbright TE. 2005. Nutrient enrichment, tannins, and thorns: effects on browsing of shrub seedlings. Journal of Wildlife Management 69: 782–793. [Google Scholar]
- Charles-Dominique T, Davies TJ, Hempson GP, et al. 2016. Spiny plants, mammal browsers, and the origin of African savannas. Proceedings of the National Academy of Sciences, USA 113: E5572–E5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles-Dominique T, Barczi J-F, Le Roux E, Chamaillé-Jammes S. 2017. The architectural design of trees protects them against large herbivores. Functional Ecology 31: 1710–1717. [Google Scholar]
- Churski M, Bubnicki JW, Jędrzejewska B, Kuijper DPJ, Cromsigt JPGM. 2017. Brown world forests: increased ungulate browsing keeps temperate trees in recruitment bottlenecks in resource hotspots. New Phytologist 214: 158–168. [DOI] [PubMed] [Google Scholar]
- Coates-Palgrave M. 2002. Keith Coates-Palgrave's trees of Southern Africa. Cape Town: Struik. [Google Scholar]
- Coley PD, Bryant JP, Stuart CFI. 1985. Resource availability and plant antiherbivore defense. Science 230: 895–899. [DOI] [PubMed] [Google Scholar]
- Cooper SM, Owen-Smith N. 1986. Effects of plant spinescence on large mammalian herbivores. Oecologia 68: 446–455. [DOI] [PubMed] [Google Scholar]
- Corlett RT. 2007. The impact of hunting on the mammalian fauna of tropical Asian forests. Biotropica 39: 292–303. [Google Scholar]
- Corlett RT. 2014. The ecology of tropical East Asia. Oxford: Oxford University Press. [Google Scholar]
- Craine J, Bond W, Lee WG, Reich PB, Ollinger S. 2003. The resource economics of chemical and structural defenses across nitrogen supply gradients. Oecologia 137: 547–556. [DOI] [PubMed] [Google Scholar]
- Fernandez-Lorenzo JL, Rigueiro A, Ballester A. 1999. Polyphenols as potential markers to differentiate juvenile and mature chestnut shoot cultures. Tree Physiology 19: 461–466. [DOI] [PubMed] [Google Scholar]
- Fick SE, Hijmans RJ. 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Gallenmüller F, Feus A, Fiedler K, Speck T. 2015. Rose prickles and asparagus spines – different hook structures as attachment devices in climbing plants. PLoS One 10: e0143850. doi: 10.1371/journal.pone.0143850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi LZ. 2014. Uncertainties due to within-species variation in comparative studies: measurement errors and statistical weight. In: Garamszegi LZ, ed. Modern phyylogenetic comparative methods and their application in evolutionary biology. Berlin Heidelberg: Springer, 157–199. [Google Scholar]
- Gelman A, Jakulin A, Pittau MG, Su YS. 2008. A weakly informative default prior distribution for logistic and other regression models. Annals of Applied Statistics 2: 1360–1383. [Google Scholar]
- Gelman A, Su Y-S.arm: data analysis using regression and multilevel/hierarchical models. R package version 1.10-1. 2018.
- Goralka RJL, Langenheim JH. 1996. Implications of foliar monoterpenoid variation among ontogenetic stages of the California Bay Tree (Umbellularia californica) for deer herbivory. Biochemical Systematics and Ecology 24: 13–23. [Google Scholar]
- Gowda JH. 1997. Physical and chemical response of juvenile Acacia tortilis trees to browsing: experimental evidence. Functional Ecology 11: 106–111. [Google Scholar]
- Gowda JH, Palo RT. 2003. Age-related changes in defensive traits of Acacia tortilis Hayne. African Journal of Ecology 41: 218–223. [Google Scholar]
- Gowda JH, Albrectsen BR, Ball JP, Sjöberg M, Palo RT. 2003. Spines as a mechanical defence: the effects of fertiliser treatment on juvenile Acacia tortilis plants. Acta Oecologica 24: 1–4. [Google Scholar]
- Groom PK, Lamont BB. 1997. Xerophytic implications of increased sclerophylly: interactions with water and light in Hakea psilorrhyncha seedlings. New Phytologist 136: 231–237. [Google Scholar]
- Grubb J. 1992. A positive distrust in simplicity lessons from plant defences and from competition among plants and among animals. Journal of Ecology 80: 585–610. [Google Scholar]
- Gutschick VP. 1999. Biotic and abiotic consequences of differences in leaf structure. New Phytologist 143: 3–18. [Google Scholar]
- Hanley ME, Fenner M, Whibley H, Darvill B. 2004. Early plant growth: identifying the end point of the seedling phase. New Phytologist 163: 61–66. [DOI] [PubMed] [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. 2007. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8: 157–178. [Google Scholar]
- Harris E, And C, Hooijer DA. 1953. Pleistocene mammals from the limestone fissures of Szechwan, China. Bulletin of the American Museum of Natural History 102: 1–134. [Google Scholar]
- Herms DA, Mattson WJ. 1992. The dilemma of plants: to grow or defend. The Quarterly Review of Biology 67: 283–335. [Google Scholar]
- Kariyat RR, Hardison SB, De Moraes CM, Mescher MC. 2017. Plant spines deter herbivory by restricting caterpillar movement. Biology Letters 13: 20170176. doi: 10.1098/rsbl.2017.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg AA, Branaman TJ, Jones NM, Little CZ, Swanson J-D. 2011. Morphological studies of developing Rubus prickles suggest that they are modified glandular trichomes. Botany 89: 217–226. [Google Scholar]
- Kitajima K, Cordero RA, Wright SJ. 2013. Leaf life span spectrum of tropical woody seedlings: effects of light and ontogeny and consequences for survival. Annals of Botany 112: 685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koricheva J. 2002. Meta-analysis of sources of variation in fitness costs of plant antiherbivore defenses. Ecology 83: 176–190. [Google Scholar]
- Koricheva J, Larsson S, Haukioja E, Keinänen M. 1998. Regulation of woody plant secondary metabolism by resource availability: hypothesis testing by means of meta-analysis. Oikos 83: 212–226. [Google Scholar]
- Lajeunesse MJ, Fox GA. 2015. Statistical approaches to the problem of phylogenetically correlated data. In: Fox GA, Negrete-Yankelevich S, Sosa VJ, eds. Ecological statistics: contemporary theory and application. Oxford: Oxford University Press, 261–283. [Google Scholar]
- Mason CM, Donovan LA. 2015. Does investment in leaf defenses drive changes in leaf economic strategy? A focus on whole-plant ontogeny. Oecologia 177: 1053–1066. [DOI] [PubMed] [Google Scholar]
- McKey D. 1974. Adaptive patterns in alkaloid physiology. The American Naturalist 108: 305–320. [Google Scholar]
- Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ. 2011. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology 25: 380–388. [Google Scholar]
- Moles AT, Wallis IR, Foley WJ, et al. 2013. Correlations between physical and chemical defences in plants : tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytologist 198: 252–263. [DOI] [PubMed] [Google Scholar]
- Murray JR, Hackett WP. 1991. Dihydroflavonol reductase activity in relation to differential anthocyanin accumulation in juvenile and mature phase Hedera helix L. Plant physiology 97: 343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers JH, Bazely DR. 1991. Thorns, spines, prickles, and hairs: are they stimulated by herbivory and do they deter herbivores? In: Tallamy DW, Raupp MJ, eds. Phytochemical induction by herbivores. New York: Wiley-Blackwell Publishing, 325–344. [Google Scholar]
- Ochoa-López S, Villamil N, Zedillo-Avelleyra P, Boege K. 2015. Plant defence as a complex and changing phenotype throughout ontogeny. Annals of Botany 116: 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CP, Charles-Dominique T, Stevens N, Bond WJ, Midgley G, Lehmann CER. 2018. Human impacts in African savannas are mediated by plant functional traits. New Phytologist 220: 10–24. [DOI] [PubMed] [Google Scholar]
- Pisani JM, Distel RA. 1998. Inter- and Intraspecific variations in production of spines and phenol in Prosopis caldenia and Prosopis flexuosa. Journal of Chemical Ecology 24: 23–36. [Google Scholar]
- Poorter H, Fiorani F, Pieruschka R, et al. 2016. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytologist 212: 838–855. [DOI] [PubMed] [Google Scholar]
- Prins HHT, van der Jeugd HP. 1993. Herbivore population crashes and woodland structure in East Africa. Journal of Ecology 81: 305–314. [Google Scholar]
- Rafferty C, Lamont BB. 2007. Selective herbivory by mammals on 19 species planted at two densities. Acta Oecologica 32: 1–13. [Google Scholar]
- R Core Team 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rhoades DF. 1979. Evolution of plant chemical defenses against herbivores. In: Rosenthal GA, DH J, eds. Herbivores: their interaction with secondary plant metabolites. New York: Academic Press, 1–55. [Google Scholar]
- Roberts MR, Paul ND. 2006. Seduced by the dark side: integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytologist 170: 677–699. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Traore S, Ouedraogo A, et al. 2013. Geographical patterns of woody plants' functional traits in Burkina Faso. Candollea 68: 197–207. [Google Scholar]
- Shipley LA. 2007. The influence of bite size on foraging at larger spatial and temporal scales by mammalian herbivores. Oikos 116: 1964–1974. [Google Scholar]
- Skogsmyr L, Fagerstom T. 1992. The cost of anti-herbivory and physiological factors ecological. Oikos 64: 451–457. [Google Scholar]
- Stamp N. 2003. Out of the quagmire of plant defense hypotheses. The Quarterly Review of Biology 78: 23–55. [DOI] [PubMed] [Google Scholar]
- Staver AC, Bond WJ. 2014. Is there a ‘browse trap'? Dynamics of herbivore impacts on trees and grasses in an African savanna. Journal of Ecology 102: 595–602. [Google Scholar]
- Swihart RK, Bryant JP. 2001. Importance of biogeography and ontogeny of woody plants in winter herbivory by mammals. Journal of Mammalogy 82: 1–21. [Google Scholar]
- Tindall ML, Thomson FJ, Laffan SW, Moles AT. 2016. Is there a latitudinal gradient in the proportion of species with spinescence? Plant Ecology 10: 1–7. [Google Scholar]
- Tomlinson KW, Sterck FJ, Bongers F, et al. 2012. Biomass partitioning and root morphology of savanna trees across a water gradient. Journal of Ecology 100: 1113–1121. [Google Scholar]
- Tomlinson KW, van Langevelde F, Ward D, et al. 2016. Defence against vertebrate herbivores trades off into architectural and low nutrient strategies amongst savanna Fabaceae species. Oikos 125: 126–136. [Google Scholar]
- Villamil N, Márquez-Guzmán J, Boege K. 2013. Understanding ontogenetic trajectories of indirect defence: ecological and anatomical constraints in the production of extrafloral nectaries. Annals of Botany 112: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton CH. 1966. Man, fire and wild cattle in northern Cambodia. Proceedings of the Annual Tall Timber Fire Ecology Conferrence 5: 23–65. [Google Scholar]
- Zuest T, Agrawal AA. 2017. Trade-offs between plant growth and defense against insect herbivory: an emerging mechanistic synthesis. Annual Review of Plant Biology 68: 513–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.