Abstract
Tetrodotoxin (TTX) is an extremely potent paralytic toxin responsible for yearly illness and death around the world. A clinical measurement is necessary to confirm exposure because symptoms of TTX intoxication cannot be distinguished from other paralytic toxins. Our group has developed an online solid phase extraction hydrophilic interaction liquid chromatography (HILIC) method for the analysis of TTX in human urine with tandem mass spectrometry. The reportable range for the method was 2.80 – 249 ng/ mL in urine with precision and accuracy within 15% as determined for all quality control samples. No isotopically-labeled internal standard is available for TTX; thus a surrogate internal standard, voglibose, was investigated to compensate for matrix effects and ionization suppression. However, upon evaluation, voglibose was ineffective for this purpose. This new online method rapidly identifies TTX, facilitating the work of public health authorities and providing support to monitoring programs worldwide.
Keywords: Tetrodotoxin, Online SPE, HILIC, Marine toxins, Paralytic
1. Introduction
Tetrodotoxin (TTX) is an extremely potent paralytic toxin. With an oral median lethal dose (MLD, or LD50) of approximately 334 μg/ kg body weight for mice, TTX is one of the most poisonous non-protein substances known. It has been estimated that merely 2 mg of pure toxin could kill a grown man (Cayman Chemical, 2014; N.V., 1999; Noguchi and Ebesu, 2001). Found in many types of marine and land animals, TTX is responsible for yearly illness and death in Eastern and Southeastern Asian countries, as well as Australia and parts of South America (Islam et al., 2011).
Most human poisonings occur following ingestion of toxin-containing animals, as TTX is not destroyed by cooking. Pufferfish, also called fugu, is a well-known TTX-containing fish that is considered a delicacy in many Asian countries and must be prepared by trained chefs to minimize exposure. Small gastropods (snails and slugs) and fish containing this toxin are often collected recreationally in China and prepared and consumed in-home (Arakawa, 2010). Consequently, Taiwan, Japan, and China report high incidences of TTX intoxication, relative to other parts of the world (Yang et al., 1996). To reduce the number of poisonings, import and sale of potentially hazardous fish is highly regulated (United States Food and Drug Adminstration, 1989). Guidelines for sale and preparation of edible pufferfish published by the Japanese Ministry of Health and Welfare reduced the mortality rate of TTX poisoning in Japan to 6.4% (1995–2007) (Noguchi and Ebesu, 2001; Noguchi and Arakawa, 2008). Despite such regulations poisonings continue to occur (United States Food and Drug Adminstration, 1989).
Multiple human exposure incidents have been documented in the United States resulting from ingestion of toxin from both native species (in this case, newts) and imported goods (dried and packaged fish) in Oregon, California, and Minnesota (Bradley and Klika, 1981; Centers for Disease Control and Prevention, 1996; 2015). In the Minnesota poisoning case, leftover fish was determined to be a type of puffer contaminated with up to 72.3 ng/mL TTX. Given the potency of this toxin and the potential for toxic levels in animals, TTX is recognized as a significant threat to worldwide public health (Centers for Disease Control and Prevention, 2015).
Symptoms of TTX poisoning are similar to those resulting from exposure to other toxins and thus may be insufficient for diagnosis. Historically, TTX exposure has been determined by identifying the toxin in the food source; however, if no food remains, the presence of TTX cannot be verified. The best approach to confirm exposure is through the measurement of specific biomarkers in biological fluids, such as urine or blood, of the victim (Leung et al., 2011). Multiple studies have identified TTX in the urine and serum of exposed humans, with higher concentrations consistently detected in urine than in serum (Hwang et al., 2005; Leung et al., 2011). The majority of reported detected concentrations range from <1 to 14 ng/mL in serum/blood, and <1 to 460 ng/mL in urine; in several cases urine tested positive when serum did not. Additionally, TTX has been detected in urine up to five days post exposure, but in serum it has been detected for only a few hours following ingestion (O’Leary et al., 2004; Oda et al., 1989). This data supports the measurement of TTX in urine as the optimal biomarker to diagnostically confirm exposure to this toxin.
TTX has been measured in urine and plasma using gas chromatography with mass spectrometry (GC-MS), liquid chromatography with tandem mass spectrometry (LC-MS/MS), and enzyme-linked immunosorbent assays (ELISA) (Kurono et al., 2001). TTX has been successfully separated under reversed phase (C18) and hydrophilic interaction liquid chromatographic (HILIC) conditions (Akaki and Hatano, 2006; Diener et al., 2007). Sample clean-up has also been utilized to reduce matrix effects, including C18 and WCX off-line or manual solid phase extraction (SPE), as well as multiple ultrafiltration steps (O’Leary et al., 2004; Tsai et al., 2006; Yu et al., 2010). Although these methods achieve limits of detection as low as 0.13 ng/mL in urine, they are complex processes, requiring larger sample volumes (1 to 50 mL), and multiple sample preparation steps such as manual SPE, freeze-drying, reconstitution, and filtration. (Cho et al., 2012; Fukushima, 2005; Jen et al., 2008; O’Leary et al., 2004; Tsai et al., 2006; Wu et al., 2014; Yu et al., 2010). Online SPE, used in the present study, streamlines the sample preparation process, requires minimal sample preparation prior to instrumental analysis, uses reduced sample volumes, decreases waste production, and minimizes matrix effects (Kuklenyik et al., 2004; Schebb et al., 2011; Shaner et al., 2014). Our lab has previously developed an online method to improve the sensitivity of an existing offline preparation method for the detection of saxitoxin and neosaxitoxin (STX and NEO) in human urine (Johnson et al., 2009). Sample preparation time was reduced by 66% from 3 h to 1 h prior to loading on the instrument, eliminating much of the analyst interaction with the samples. Overall analysis time is further reduced by the online extraction system that chromatographically separates one sample while simultaneously extracting the following sample (Bragg et al., 2015).
This study presents a novel method using online solid phase extraction coupled with liquid chromatography and tandem mass spectrometry to rapidly detect tetrodotoxin in human urine. Incorporating online SPE significantly decreases the time between sample receipt and reportable results than methods previously reported. Streamlining the sample preparation process also reduces potential losses associated with concentration and transfer steps while maintaining selectivity, sensitivity, and performance.
2. Experimental section
2.1. Reagents and supplies
Neat tetrodotoxin (≥98%, HPLC grade) and voglibose (≥97%, TLC grade) were purchased from Sigma Aldrich (St. Louis, MO). Deionized water (18Ω) was obtained from a filter system manufactured by AquaSolutions (Jasper, GA). HPLC/spectrophotometer grade acetonitrile and methanol were purchased from Lab Depot (Dawsonville, GA). Ammonium formate (HPLC grade, ≥ 99%), hydrochloric acid (certified ACS Plus), glacial acetic acid (HPLC grade), and formic acid (Optima, LC/MS grade) were purchased from Fisher Scientific (Hampton, NH). Oasis MCX 10 × 1 mm, 30 μm particle size, SPE cartridges were purchased from Waters Corporation (Milford, MA). Pooled urine and a convenience set of individual urine samples was purchased from Tennessee Blood Services (Memphis, TN). All purchased urine was pre-screened by the vendor in accordance with FDA regulations to be free of Hepatitis B, Syphilis, and HIV. This study used de-identified urine acquired from commercial sources and thus the work did not meet the definition of human subjects as specified in 45 CFR 46.102 (f) (Department of Health and Human Services, 2009).
2.2. Preparation of calibration standards and quality controls
TTX solution (22.5 μg/mL) was prepared gravimetrically by adding 0.556 mg of neat material to 24.7 mL of 3 mM HCl. A high concentration working solution (HCWS) was prepared fresh daily by combiningg 18 μL of the 22.5 μg/mL TTX solution with 1012 μL pooled human urine (final concentration: 392.5 ng/mL). Fifty microliters of the HCWS was then added to 650 μL of urine to prepare a low concentration working solution (LCWS, final concentration: 28.0 ng/mL). Using these solutions, ten calibration standards and two quality control samples (QCs) were prepared in a 96-well 2 mL deep well Nunc® plate (Thermo Scientific, Rochester, NY). A solution of neat voglibose (179 ng/mL) was prepared gravimetrically in DI water. From the LCWS, calibration standards 1–4 and a low-end quality control (QCL) sample were prepared, at 2.80, 5.61, 10.3, 25.7, and 7.48 ng/mL, respectively. From the HCWS, calibration standards 5–10 and high-end quality control (QCH) sample were prepared at 52.3, 98.1,124,151,196, 248, and 75.9 ng/mL, respectively. The final volume of each sample was 300 μL (including 25 μL internal standard, and 275 μL of combined working standard and pooled urine). Each of these samples was diluted with 300 μL of 0.1% acetic acid. The entire plate was then heat-sealed, vortexed for approximately 3 min, and centrifuged at 3000 rpm for at least 2 min before placing the plate into the autosampler for analysis.
2.3. Sample preparation and solid phase extraction
Online solid phase extraction (SPE) and HPLC separation was carried out using a Spark Holland Symbiosis (Emmen, The Netherlands) system comprised of a binary pump, a vacuum degasser, an autosampler, a heated column compartment, a high pressure pump for SPE solvent, and an automated cartridge exchanger. The 96-well plate containing all samples to be analyzed was placed into the autosampler of the Symbiosis, maintained at 10 °C. Online SPE was carried out using Waters Oasis Symbiosis/ Prospekt-2 MCX 30 μm 10 × 1 mm mixed-mode (strong cation exchange (SCX)/reversed phase C18) SPE cartridges (Waters Corporation, Milford, MA). The SPE cartridges were loaded into the Symbiosis automated cartridge exchanger prior to analysis. Solid phase extraction, controlled by the software programs Analyst (Sciex, Foster City, CA) and Symbiosis Pro (Spark Holland, Emmen, The Netherlands), began with the conditioning of the cartridge with 1 mL of 100% methanol, followed by equilibration with 1 mL of 0.1% acetic acid in water and loading of 125 μL of sample solution. The cartridge was then washed with 1 mL of 100% water, followed by a wash of 1 mL of 100% acetonitrile, and finally the analytes were eluted with 250 μL of 50% acetonitrile/50% 200 mM ammonium formate (pH 3) directly onto the HPLC column.
2.4. Chromatography conditions
Analytes were eluted directly from the SPE cartridge onto the HPLC column, where the compounds were then separated. Chromatographic separation was achieved using a gradient elution on a HALO Penta-HILIC column (3 × 75 mm, 2.7 μm) (Mac-Mod Analytical, Inc., Chadds Ford PA) maintained at 30 °C. Mobile phase was comprised of (A) 100% acetonitrile and (B) 50 mM ammonium formate, pH 3 (2.5% formic acid). The elution pump program was as follows: initial flow rate of 0.85 mL/min with 5% A/95% B from 0:01 to 2:32 min, mobile phase was adjusted to 20% A/80% B from 2:32 to 4 min, followed by flow rate decrease to 0.5 mL/min from 4:05 to 7:01 min, and finally another change at 7:02 min to 0.85 mL/min flow rate with 5% A/95% B until the end of the run.
2.5. Mass spectrometry conditions
Tandem mass spectrometry with a Turbospray ionization source operated in positive mode was used to detect all analytes on a Sciex 5500 triple quadrupole mass spectrometer (Foster City, CA). The following MS conditions were constant for all transitions: declustering potential (DP), 55 V; entrance potential (EP), 10 V; collision cell exit potential (CXP), 18.0; curtain gas (CUR), 35 psi; collision gas (CAD), 7 psi; ion spray voltage (IS) 5300 V; source temperature (TEM), 250 °C (interface heater on); ion source gas 1 (heater gas, GS1), 30 psi; ion source gas (nebulizer gas, GS2), 30 psi. Transitions and corresponding collision energies (CE) were as follows: TTX (quantitation ion) 320.1 → 302.1, 35 V; TTX_C (confirmation ion), 320.1 → 162.1, 50 V; Voglibose (surrogate internal standard), 268 → 92, 30 V.
2.6. Data processing and calculations
All data processing, including ion area calculations and linear regression analysis, was carried out using the Analyst software package (Version 1.6.1) from Sciex. Linear regression analysis of the calibration standard concentration versus the ratio of calibration standard ion area to internal standard ion area with 1/x weighting was used for quantitation. All calibration curves with an R2 value of 0.98 or greater were accepted as linear.
The method was validated by the analysis of twenty replicate curves consisting of ten calibration standards and two quality control (QC) levels. Two analysts evaluated QC materials over four months with a maximum of two curves prepared and analyzed per day. Accuracy and precision for the method were determined based on the quantitation ion transition, using all twenty replicates at concentrations of 7.5 and 76 ng/mL.
The method limit of detection (LOD) was determined by using the Taylor method. To calculate the LOD the standard deviation of 20 replicates for each of the three lowest calibration standards was determined (Taylor, 1987). The standard deviations were then plotted versus expected concentrations and the intercept of the least squares regression analysis of this line was used to interpolate the standard deviation of the blank, S0. The LOD was then calculated as 3S0.
Concentrations above the current calibration range were evaluated by preparing two samples at concentrations above the highest calibration standard and diluting them in urine to fall within the calibration range. One high-concentration sample was prepared by adding 20 μL of TTX (22.5 μg/mL) to 730 μL of pooled urine to give a calculated concentration of 599 ng/mL; a second was prepared by adding 30 μL of TTX (22.5 μg/mL) to 720 μL of pooled urine, giving a calculated concentration of 898 ng/mL. These samples were then both diluted to a concentration of 25 ng/mL: 835 μL of the first sample (599 ng/mL) was added to 2 mL of pooled urine, and 55.7 μL of the second sample was added to 2 mL of pooled urine. The diluted samples were then analyzed six times each.
Recovery was evaluated by comparing the peak area response of fortified urine and solvent samples. Samples were prepared by spiking 40 μL of HCWS into 235 μL of pooled urine or water (final concentration: 52.3 ng/mL), then 25 μL of ISTD and 300 μL of 0.1% acetic acid were added. Both samples were extracted, separated, and detected in triplicate as described previously. The method recovery was calculated for the online extraction with the following equation:
Matrix effects and ionization suppression were assessed by post-column infusion. Using an infusion pump and a three-port valve, TTX diluted in mobile phase was delivered at a constant amount (17 μL/min) into the HPLC stream entering the ESI source of the mass spectrometer which was monitoring the infused analyte. Blank urine and water were simultaneously extracted on the online SPE and injected onto the LC column using the method parameters. Suppression or enhancement of the analyte signal is seen when endogenous compounds elute from the column and cause variations in analyte response, providing information on potential interferences present in the method (Bonfiglio et al., 1999).
Twelve individual urine samples from unexposed population were fortified with toxin to represent exposure samples. Individual urines were fortified to two concentration levels within the calibration range. For the higher concentration (52.3 ng/mL), 40 μL of HCWS was added to 235 μL of individual urine with 25 μL of internal standard; for the lower concentration (5.61 ng/mL), 60 μL of LCWS was added to 215 μL of individual urine with 25 μL of internal standard. These samples were treated as unknowns and analyzed in triplicate to check the accuracy of the method. An additional 30 individual urine samples were analyzed with no fortification to evaluate any potential interferences with TTX.
3. Results and discussion
The purpose of this study was to develop a novel method using online SPE-LC-MS/MS to detect TTX in human urine. Online SPE was selected to streamline the sample preparation process, maximizing automation while minimizing potential sample losses and reducing analyst errors. We have developed a sensitive and specific method for the detection of TTX in human urine that successfully reduces waste production, solvent consumption, human error, and analysis time.
3.1. Method optimization
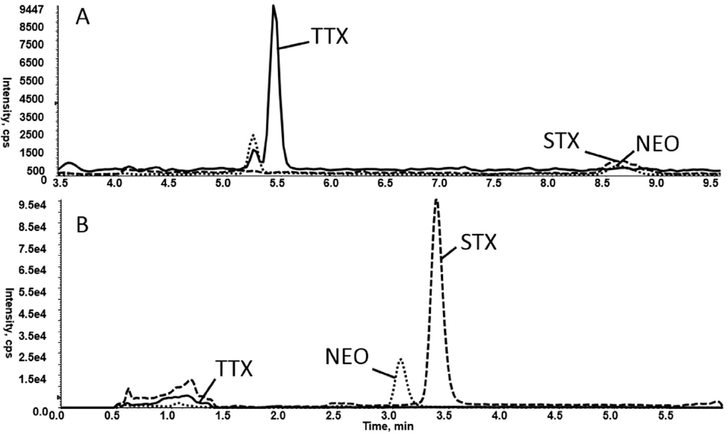
Symptoms of TTX exposure are similar to other paralytic poisons, including saxitoxin (STX) and neosaxitoxin (NEO) (Bragg et al., 2015; Noguchi and Ebesu, 2001); therefore, it is desirable from a clinical perspective to measure these toxins within a single method. Accordingly, TTX was evaluated using the online SPE and LC parameters currently used to isolate STX and NEO from human urine (Bragg et al., 2015). TTX, diluted in 75% acetonitrile/25% methanol, was injected onto an Oasis Symbiosis/Prospekt-2 weak cation exchange (WCX) 10 × 2 mm cartridge. Analytes were eluted onto an Atlantis Silica HILIC (2.1 × 50 mm, 3 pm) column (Waters Corp., Milford MA) and separated isocratically with a mobile phase of 75% acetonitrile/5% methanol/20% ammonium formate buffer at a flow rate of 300 μL/min. This method resulted in a relatively sharp peak for TTX, STX, and NEO from a solvent standard. However, when this experiment was repeated with the analytes prepared in pooled urine, TTX was poorly retained with an unideal peak shape (Fig. 1). Other SPE cartridges, including Hysphere WCX and OASIS MCX (mixed-mode strong cation exchange), were evaluated for sample cleanup. Using the Hysphere WCX SPE cartridge, TTX was recovered but STX and NEO peaks heights were greatly reduced. The analytes were diluted in 0.1% acetic acid in water and analyzed using the Oasis MCX cartridge. TTX response was approximately 5 times greater response compared to the WCX cartridge; STX and NEO, however, were not recovered from the MCX cartridge. At this point it was determined that TTX detection would benefit from a separate detection method from that of STX and NEO.
Fig. 1.
Comparison of methods used to extract marine toxins tetrodotoxin (TTX), saxitoxin (STX), and neosaxitoxin (NEO): A) All analytes extracted from urine matrix using Oasis MCX online SPE cartridge and Halo Penta-HILIC LC column. B) All analytes extracted from urine matrix using Oasis WCX online SPE cartridge and Atlantis Silica HILIC LC column.
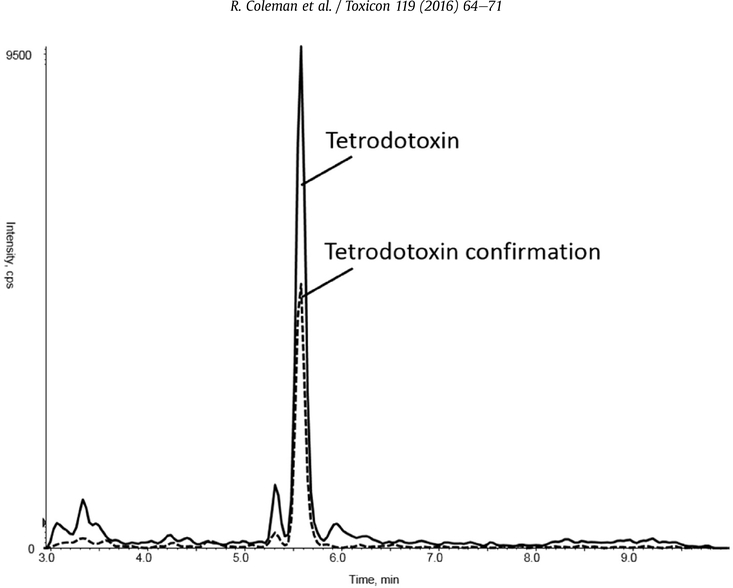
With the updated SPE parameters, the chromatography was then evaluated using the SeQuant ZIC-HILIC (50 × 2.1,3.5 mm PEEK, EMD Millipore, Billerica, MA) and the Halo Penta-HILIC(75 × 3 mm, 2.7 μm, Mac-Mod Analytical, Chadds Ford, PA). For these columns, mobile phase composition was varied step-wise from 90% 50 mM ammonium formate, pH 3, and 10% acetonitrile. Peak shape was poor on the ZIC-HILIC column, but the Halo Penta-HILIC column gave desired retention (4.5 min), resolution from matrix components, and good peak shape (Fig. 2). Using mobile phase conditions identical to those of the MCX SPE elution resulted in sharp, well-resolved peaks.
Fig. 2.
Tetrodotoxin Response: Peak signal intensity of extracted ion chromatograms of tetrodotoxin (TTX, quantitation and confirmation ions, 75 ng/mL) in pooled human urine matrix (first 3 min diverted to waste).
Once the parameters for the online separation and detection of TTX were established, STX and NEO were evaluated using the new method; however, as anticipated, peak shape for these compounds was insufficient for quantitation (Fig. 1).
For detection, both positive and negative ion modes were explored to determine the most abundant fragment ions for TTX. Positive ion transitions were ultimately selected for greatest intensity and specificity from matrix interferences. The transitions selected for quantitation and confirmation ions were 320.1 → 302.1 m/z (corresponding to a loss of water) and 320.1 → 162.1 m/z (corresponding to a loss of C9H8N3O), respectively. The transitions selected were consistent with those reported in previous studies (Cho et al., 2012; Fong et al., 2011; Shoji et al., 2001).
Ideally an isotopically labeled version of the analyte of interest is used to compensate for ionization effects and extraction variabilities from the sample matrix on the analyte. Surrogate internal standards, similar to the analyte of interest, may be used but might not adequately account for those effects, due to retention time and structure differences. However, use of a surrogate internal standard frequently improves quantitative performance by compensating for extraction and recovery of the analyte of interest. Because no isotopically labeled internal standard for TTX was available, voglibose was evaluated as a surrogate. Voglibose is an alpha glucosidase inhibitor prescribed for the treatment of diabetes mellitus (Dabhi et al., 2013), and it was selected for this study because it has a similar structure to TTX, it is not often detected in individual urine samples, and it has been used as an internal standard in other TTX methods (Cho et al., 2012; Tsujimura and Yamanouchi, 2015).
3.2. Method validation
Validation data was collected for a period of four months by two analysts. In order to characterize method accuracy and precision, two quality control samples were prepared and analyzed with each calibration curve, with no more than two runs per day. The expected concentration for QCL (n = 20) was 7.48 ng/mL and the calculated average concentration was 7.60 ng/mL, giving an accuracy of 102% with a CV of 6.33%. The expected concentration for QCH (n = 20) was 75.9 ng/mL and the calculated average concentration was 76.8 ng/mL, giving an accuracy of 101% with a CV of 8.04%. Both quality control levels (n = 6) were also evaluated for accuracy (95.0–106%) and precision (<10%) (Table 1). Three calibrator levels (2.8, 52.3, and 249 ng/mL; n = 20 for each level) also demonstrated high accuracies of 94.8–99.5% with CVs of 15% or less, further confirming the performance characteristics of the method. Accuracy and precision for the method fell within the guidelines recommended by the FDA (U.S. Department of Health and Human Services, 2001).
Table 1.
Accuracy and precision of QC and select calibrator samples quantitated. With and without the voglibose internal standard.
| Sample expected conc (ng/mL) | Calculated with voglibose |
Calculated without voglibose |
||||
|---|---|---|---|---|---|---|
| Calc conc (ng/mL) | Accuracy of mean (%) | Precision (% CV) | Calc conc (ng/mL) | Accuracy of mean (%) | Precision (% CV) | |
| Within-day variation (n = 4) | ||||||
| 7.48 | 7.96 | 106% | 8.40% | 7.84 | 105% | 5.10% |
| 75.9 | 72.3 | 95.0% | 8.03% | 74.3 | 98.0% | 6.91% |
| Between-day variation (n = 20) | ||||||
| 7.48 (QCL) | 7.60 | 102% | 6.33% | 7.89 | 106% | 11.50% |
| 75.9 (QCH) | 76.8 | 101% | 8.04% | 77.0 | 101% | 4.27% |
| Calibration samples (n = 20) | ||||||
| 2.80 | 2.76 | 98.5% | 10.1% | 2.91 | 104.0% | 15.1% |
| 52.3 | 49.6 | 94.8% | 7.33% | 50.2 | 96.0% | 5.90% |
| 249 | 248 | 99.5% | 3.74% | 248 | 99.4% | 2.80% |
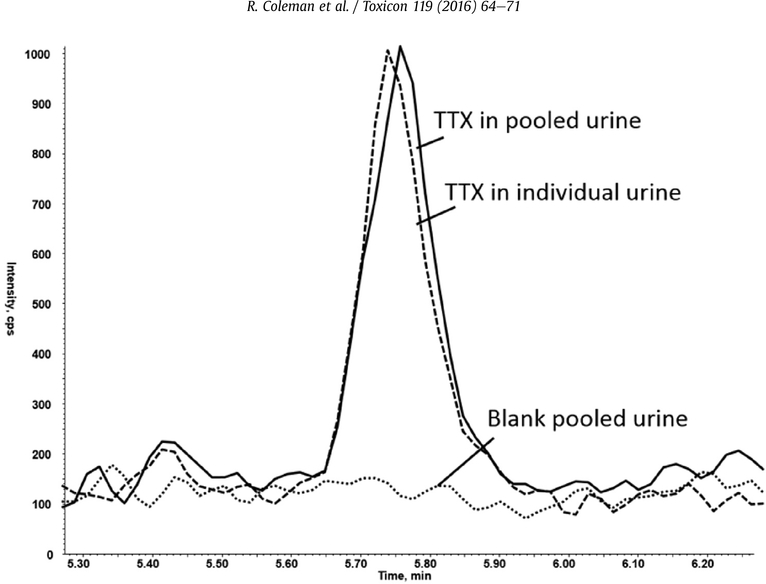
To assess for possible matrix interferences, forty-two individual urine samples from unexposed persons were analyzed as unknown samples (Fig. 3). No peaks were detected at the retention time for TTX, indicating minimal matrix interferences were present.
Fig. 3.
Comparison of Tetrodotoxin Response: Tetrodotoxin (TTX) in spiked pooled urine (5 ng/mL), TTX spiked individual urine (5 ng/mL) and blank pooled urine.
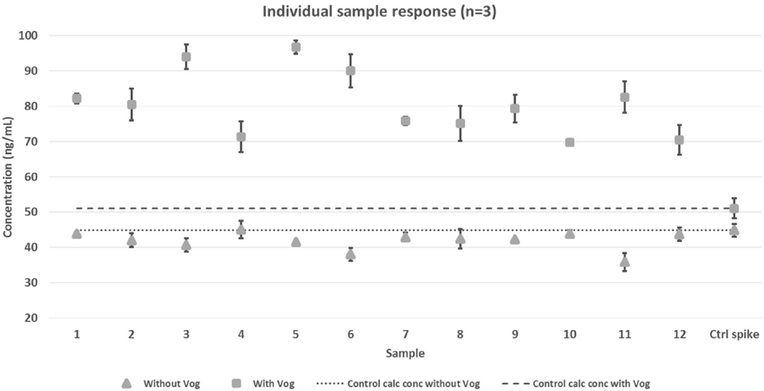
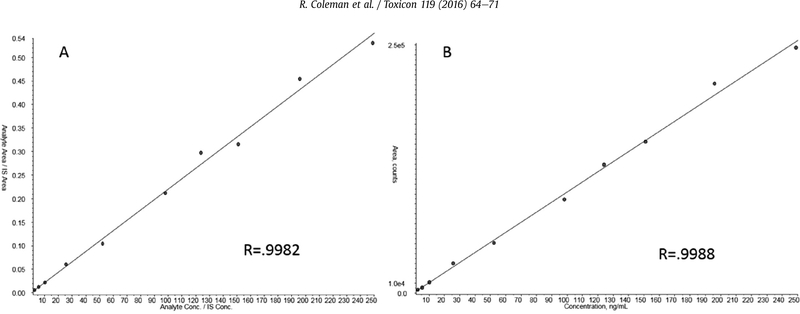
Exposure samples were not available at the time of method validation; therefore fortified urine samples were used to evaluate method accuracy and precision when applied to unknown samples. Twelve individual urines were spiked with a known amount of toxin and analyzed. The mean calculated concentration of spiked TTX in the individual samples was 80.7 ng/mL with a precision of H.2%. Although the variability of TTX between the individual urines was acceptable, the bias (158% accuracy) was extremely high (Fig. 4). Further data analysis showed that the variation of voglibose and TTX response was greatly increased in the individual urines when compared to pooled urine, causing skewed calculations. Voglibose response compared to TTX was consistent throughout the validation of the quality control samples, which were prepared in pooled urine. However, variability of voglibose and TTX was significantly increased in individual urine samples. The individual samples were thus re-quantitated without using voglibose, which resulted in a mean of 42.0 ng/mL, improved accuracy to 93.2%, and decreased variation to 6.27% CV (Fig. 4). A second set of twelve individual urines were spiked at a lower concentration of TTX (5.8 ng/mL) and quantitated with and without voglibose and confirmed this trend (data not shown). Given these results, the QC values used to characterize the method were re-calculated without the internal standard. The variation (4.27–11.5%CV) and accuracy (101–106%) were similar to the results obtained with the internal standard for the 20 replicates (Table 1) and well within the recommended limits (U.S. Department of Health and Human Services, 2001). Calibration curves quantitated with and without voglibose also had similar linearity (Fig. 5). This collective data indicates that the use of voglibose as an internal standard is not essential for the quantitation of TTX from urine and may even cause significantly biased results when individual suspected exposure samples are evaluated. The data presented for the remainder of this study were quantitated without internal standard.
Fig. 4.
Individual Sample Response: Comparison of calculated concentrations quantitated With and Without Voglibose.
Fig. 5.
Tetrodotoxin Calibration Curves: Comparison of curves calculated with Voglibose (A) and without Voglibose (B).
All calibration curves were linear with a coefficient of determination (R2) greater than or equal to 0.98 across a range of 2.8–249 ng/mL for TTX. The LOD was calculated, using the Taylor approach, to be 0.302 ng/mL. The lowest calibrator, at a concentration of 2.8 ng/mL, was reproducibly quantifiable with a signal-to-noise ratio of 5 or greater, supporting a lower reportable limit of 2.8 ng/mL.
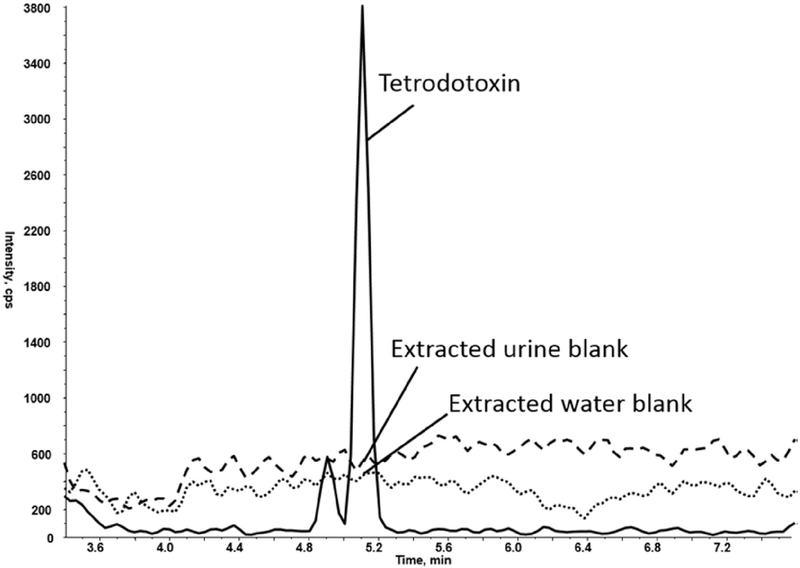
Recovery was determined by comparing the extraction of TTX from water to that of TTX from urine. This recovery, calculated to be 102%, incorporated the extraction efficiency and matrix impacts on ionization efficiency, as the online SPE instrumentation did not permit an independent determination of extraction efficiency. This high recovery indicated that minimal losses resulted from the extraction and matrix effects combined. Ionization suppression was directly assessed by infusion of TTX with extracted urine matrix. The TTX response resulting from a water extraction and a urine extraction were comparable in the retention time for TTX, confirming that minimal matrix effects were present (Fig. 6).
Fig. 6.
Assessment of Matrix Effects: Post-column infusion of TTX with extracted urine and water samples.
Ruggedness was evaluated for three method parameters. Column temperature was varied by ±15% (method setting is 30 °C); formic acid content in mobile phase B was assessed at 1% and 5% (method is 2.5%); and acetic acid content of sample diluent prior to sample extraction was assessed at 0.01% and 1% (method is 0.1%). Samples were run in triplicate at each of these settings, monitoring analyte peak area and retention time of TTX. Altering the column temperature had little effect on the TTX peak area counts, whereas a change in column temperature by ±15 °C resulted in a retention time shift of ±30 s. Altering the formic acid content in the mobile phase resulted in no significant change in analyte peak area (9% CV) nor retention time. Varying the concentration of acetic acid added during the dilution step of sample preparation did not cause significant change in TTX peak area response (6% CV) nor retention time.
Post exposure urinary concentrations of TTX have been documented above the reportable range for this method (Fong et al., 2011; Hwang et al., 2005); therefore, a dilution protocol was developed and evaluated. Two samples were prepared at concentrations above the reportable range, 598 and 898 ng/mL. Both samples were diluted volumetrically to 25 ng/mL and analyzed as unknowns (n = 6). The average calculated concentration of the diluted samples were 27.1 ng/mL (108% accuracy, 3.11% CV), and 29.9 ng/mL (120% accuracy, 9.72%CV). The accuracy and precision for dilution of a sample at concentration 598 ng/mL was well within recommended guidelines for accuracy and precision, indicating that the dilution protocol is effective and may be used to quantitate unknown samples at concentrations higher than the upper limit of quantitation. Dilution from a higher concentration may be less accurate with more variability and should be carried out with caution.
4. Conclusions
A novel method for the detection of tetrodotoxin in human urine using online SPE coupled with liquid chromatography and mass spectrometry was developed and validated over a clinically-relevant concentration range of 2.80–249 ng/mL. This highly automated, streamlined sample clean-up reduced sample preparation time, as well as eliminated errors and potential samples losses during preparation. The use of voglibose as a surrogate internal standard for quantitation resulted in poor accuracy when fortified individual urine samples were analyzed. The combination of online SPE with an orthogonal chromatographic separation resulted in minimal matrix effects. Inclusion of voglibose in all sample preparation is still recommended to monitor retention time shifts, relative retention times between voglibose and TTX, and variations between sample extractions. Precise and accurate results from both pooled and individual samples without internal standard correction confirm that this method is suitable for identification of human exposures to TTX.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the US Department of Health and Human Services.
Ethical statement
This paper represents experiments carried out under the standard procedures of scientific ethics at the Centers for Disease Control and Prevention. All authors have read the manuscript, agree to its publication in Toxicon, and agree that it has followed the rules of ethics presented by Elsevier’s Ethical Guidelines for Journal Publication. Namely that the material has not, in whole or in part, been published elsewhere, nor is it being considered concurrently by any other publishers. In addition, all authors have been personally and actively involved in a substantive role leading to the publishing of this article.
Transparency document
Transparency document related to this article can be found online at http://dx.doi.org/10.1016/j.toxicon.2016.05.009.
References
- Akaki K, Hatano K, 2006. [Determination of tetrodotoxin in puffer-fish tissues, and in serum and urine of intoxicated humans by liquid chromatography with tandem mass spectrometry]. Shokuhin eiseigaku zasshi. J. Food Hyg. Soc. Jpn 47, 46–50. [DOI] [PubMed] [Google Scholar]
- Arakawa O, 2010. Toxins of pufferfish that cause human intoxications In: Lie A.I.a.H.-J. (Ed.), Coastal Environmental and Ecosystem Issues of the East China Sea. Terrapub and Nagasaki University, pp. 227–244. [Google Scholar]
- Bonfiglio R, King RC, Olah TV, Merkle K, 1999. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 13,1175–1185. [DOI] [PubMed] [Google Scholar]
- Bradley SG, Klika LJ, 1981. A fatal poisoning from the Oregon rough-skinned newt (Taricha granulosa). Jama 246, 247. [PubMed] [Google Scholar]
- Bragg WA, Lemire SW, Coleman RM, Hamelin EI, Johnson RC, 2015. Detection of human exposure to saxitoxin and neosaxitoxin in urine by online-solid phase extraction-liquid chromatography-tandem mass spectrometry. Toxicon 99, 118–124. [DOI] [PubMed] [Google Scholar]
- Cayman Chemical, 2014. Safety Data Sheet, Tetrodotoxin, pp. 1–6. https://www.caymanchem.com/msdss/14963m.pdf.
- Centers for Disease Control and Prevention, 1996. Tetrodotoxin poisoning associated with eating puffer fish transported from Japan - California, 1996. Centers Dis. Control Prev 389–391. www.cdc.gov/mmwr. [PubMed]
- Centers for Disease Control and Prevention, 2015. Tetrodotoxin Poisoning Outbreak from Imported Dried Puffer Fish - Minneapolis, Minnesota, 2014, MMWR. Mobidity and Mortality Weekly Report. [PubMed] [Google Scholar]
- Cho HE, Ahn SY, Son IS, In S, Hong RS, Kim DW, Woo SH, Moon DC, Kim S, 2012. Determination and validation of tetrodotoxin in human whole blood using hydrophilic interaction liquid chromatography-tandem mass spectroscopy and its application. Forensic Sci. Int 217, 76–80. [DOI] [PubMed] [Google Scholar]
- Dabhi AS, Bhatt NR, Shah MJ, 2013. Voglibose: an alpha glucosidase inhibitor. J. Clin. Diagn. Res. JCDR 7, 3023–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services, D., 2009. Part 46: protection of human subjects. In: Services, D.o.H.a.H. (Ed.), DHHS, pp. 1–14. www.hhs.gov. [Google Scholar]
- Diener M, Christian B, Ahmed MS, Luckas B, 2007. Determination of tetrodotoxin and its analogs in the puffer fish Takifugu oblongus from Bangladesh by hydrophilic interaction chromatography and mass-spectrometric detection. Anal. Bioanal. Chem. 389, 1997–2002. [DOI] [PubMed] [Google Scholar]
- Fong BM, Tam S, Tsui SH, Leung KS, 2011. Development and validation of a high-throughput double solid phase extraction-liquid chromatography-tandem mass spectrometry method for the determination of tetrodotoxin in human urine and plasma. Talanta 83, 1030–1036. [DOI] [PubMed] [Google Scholar]
- Fukushima SOY, 2005. Tetrodotoxin, Drugs and Poisons in Humans: a Handbook of Practical Analysis. Springer, pp. 481–490. [Google Scholar]
- Hwang PA, Tsai YH, Deng JF, Cheng CA, Ho PH, Hwang DF, 2005. Identification of tetrodotoxin in a marine gastropod (Nassarius glans) responsible for human morbidity and mortality in Taiwan. J. Food Prot. 68, 1696–1701. [DOI] [PubMed] [Google Scholar]
- Islam QT, Razzak MA, Islam MA, Bari MI, Basher A, Chowdhury FR, Sayeduzzaman AB, Ahasan HA, Faiz MA, Arakawa O, Yotsu-Yamashita M, Kuch U, Mebs D, 2011. Puffer fish poisoning in Bangladesh: clinical and toxicological results from large outbreaks in 2008. Trans. R. Soc. Trop. Med. Hyg 105, 74–80. [DOI] [PubMed] [Google Scholar]
- Jen HC, Lin SJ, Tsai YH, Chen CH, Lin ZC, Hwang DF, 2008. Tetrodotoxin poisoning evidenced by solid-phase extraction combining with liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 871, 95–100. [DOI] [PubMed] [Google Scholar]
- Johnson RC, Zhou Y, Statler K, Thomas J, Cox F, Hall S, Barr JR, 2009. Quantification of saxitoxin and neosaxitoxin in human urine utilizing isotope dilution tandem mass spectrometry. J. Anal. Toxicol 33, 8–14. [DOI] [PubMed] [Google Scholar]
- Kuklenyik Z, Ye X, Reich JA, Needham LL, Calafat AM, 2004. Automated online and off-line solid-phase extraction methods for measuring isoflavones and lignans in urine. J. Chromatogr. Sci 42, 495–500. [DOI] [PubMed] [Google Scholar]
- Kurono S, Hattori H, Suzuki O, 2001. Sensitive analysis of tetrodotoxin in human plasma by solid-phase extractions and gas chromatography/mass spectrometry. Anal. Lett 34, 2439–2446. [Google Scholar]
- Leung KSY, Fong BMW, Tsoi YK, 2011. Analytical challenges: determination of tetrodotoxin in human urine and plasma by LC-MS/MS. Mar. Drugs 9, 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N.V A.O, 1999. Material Safety Data Sheet, Tetrodotoxin. https://fscimage.fishersci.com/msds/01139.htm.
- Noguchi T, Arakawa O, 2008. Tetrodotoxin-distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. drugs 6, 220–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Ebesu J, 2001. Puffer poisoning: epidemiology and treatment. J. Toxicol 20, 1–10. [Google Scholar]
- O’Leary MA, Schneider JJ, Isbister GK, 2004. Use of high performance liquid chromatography to measure tetrodotoxin in serum and urine of poisoned patients. Toxicon 44, 549–553. [DOI] [PubMed] [Google Scholar]
- Oda K, Araki K, Totoki T, Shibasaki H, 1989. Nerve conduction study of human tetrodotoxication. Neurology 39, 743–745. [DOI] [PubMed] [Google Scholar]
- Schebb NH, Inceoglu B, Rose T, Wagner K, Hammock BD, 2011. Development of an ultra fast online-solid phase extraction (SPE) liquid chromatography electrospray tandem mass spectrometry (LC-ESI-MS/MS) based approach for the determination of drugs in pharmacokinetic studies. Anal. Methods Adv. Methods Appl 3, 420–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner RL, Kaplan P, Hamelin EI, Bragg WA, Johnson RC, 2014. Comparison of two automated solid phase extractions for the detection of ten fentanyl analogs and metabolites in human urine using liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 962, 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji Y, Yotsu-Yamashita M, Miyazawa T, Yasumoto T, 2001. Electrospray ionization mass spectrometry of tetrodotoxin and its analogs: liquid chromatography/mass spectrometry, tandem mass spectrometry, and liquid chromatography/tandem mass spectrometry. Anal. Biochem. 290, 10–17. [DOI] [PubMed] [Google Scholar]
- Taylor JK, 1987. Quality Assurance of Chemical Measurements. Lewis Publishers, Chelsea, Mich. [Google Scholar]
- Tsai YH, Hwang DF, Cheng CA, Hwang CC, Deng JF, 2006. Determination of tetrodotoxin in human urine and blood using C18 cartridge column, ultrafiltration and LC-MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 832, 75–80. [DOI] [PubMed] [Google Scholar]
- Tsujimura K, Yamanouchi K, 2015. A rapid method for tetrodotoxin (TTX) determination by LC-MS/MS from small volumes of human serum, and confirmation of pufferfish poisoning by TTX monitoring. Food Addit. Contam. Part A Chem. Anal. Control, Expo. Risk Assess 32, 977–983. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, 2001. Guidance for industry: bioanalytical method validation. In: Administration, F.a.D.. www.fda.gov/cder/guidance/index.htm.
- United States Food and Drug Adminstration, 1989. Exchange of letters between Japan and the U.S. Food and drug administration regarding puffer fish import conditions for Japanese puffer fish. In: Programs, I. (Ed.), U.S. Department of Health and Human Services; www.fda.gov. [Google Scholar]
- Wu YJ, Lin CL, Chen CH, Hsieh CH, Jen HC, Jian SJ, Hwang DF, 2014. Toxin and species identification of toxic octopus implicated into food poisoning in Taiwan. Toxicon 91, 96–102. [DOI] [PubMed] [Google Scholar]
- Yang CC, Liao SC, Deng JF, 1996. Tetrodotoxin poisoning in Taiwan: an analysis of poison center data. Veterinary Hum. Toxicol 38, 282–286. [PubMed] [Google Scholar]
- Yu CH, Yu CF, Tam S, Yu PH, 2010. Rapid screening of tetrodotoxin in urine and plasma of patients with puffer fish poisoning by HPLC with creatinine correction. Food Addit. Contam. Part A, Chem. Anal. Control Expo. Risk Assess 27, 89–96. [DOI] [PubMed] [Google Scholar]