Highlights
-
•
Physical exercise maintains or improves cognitive functions, and neurotrophin receptor signaling may play an important role.
-
•
A positive relationship exists between physical exercise and circulating brain-derived neurotrophic factor levels.
-
•
Less clear evidence has been found for a relationship between physical exercise and other neurotrophin levels, such as nerve growth factor, neurotrophin-3, and neurotrophin-4.
-
•
The postexercise variation of brain-derived neurotrophic factor might be associated with improvement of neurocognitive functioning.
-
•
Physical exercise may be an inexpensive and safe strategy for boosting brain-derived neurotrophic factor release, thus preserving or restoring cognitive function.
Keywords: Cognitive function, Neurotrophins, Physical exercise, Sport
Abstract
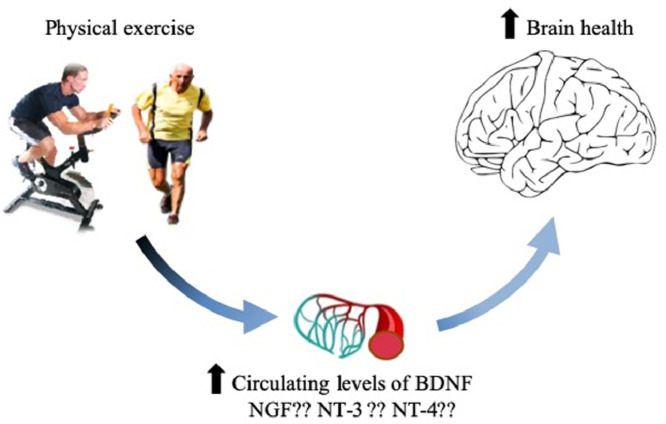
The many important benefits of physical exercise also encompass maintenance or improvement of cognitive functions. Among the various mechanisms underlying the association between physical exercise and brain health, recent evidence attests that neurotrophin receptor signaling may have an important role, because the activation of this pathway leads to growth and differentiation of new neurons and synapses, supports axonal and dendritic growth, fosters synaptic plasticity, and preserves survival of existing neurons. In this review of published evidence, we highlight that a positive relationship exists between physical exercise and circulating brain-derived neurotrophic factor levels and that the postexercise variation of this molecule is associated with improvement of neurocognitive functioning. Less clear evidence has instead been published for other neurotrophins, such as nerve growth factor, neurotrophin-3, and neurotrophin-4. Overall, promotion of adequate volumes and intensities of physical exercise (i.e., approximately 3 months of moderate-intensity aerobic exercise, with 2–3 sessions/week lasting not less than 30 min) may hence be regarded as an inexpensive and safe strategy for boosting brain-derived neurotrophic factor release, thus preserving or restoring cognitive functions.
Graphical abstract
1. Introduction
Neurotrophins are a heterogeneous class of evolutionarily ancient nerve growth factors (NGFs) that exert a kaleidoscope of important biological activities.1 The most important proteins belonging to the neurotrophins family include NGF, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4 (NT-4).2 Each of these molecules can bind to 1 or more cellular receptors and mediate their biological function in vivo (Table 1). Although a thorough discussion of the complexity of the neurotrophin receptor signaling system must be omitted in this article due to space constraints, it is worthwhile mentioning here that the final effect is neurogenesis, thus promoting growth and differentiation of new neurons and synapses, supporting axonal and dendritic growth, fostering synaptic plasticity, and preserving survival of existing neurons.3 All these biological functions finally converge to modulate personality traits, along with executive and cognitive functions (e.g., learning, memory). Accordingly, reliable evidence has been published that an impairment of neurotrophins signaling, especially the BDNF pathway, may be associated with some severe cognitive disorders (e.g., Alzheimer's and Huntington's diseases) or depression.4,5 Hence, it is obvious that strategies aimed at sustaining or even amplifying production of neurotrophins may generate substantial cognitive benefits in humans.6
Table 1.
Neurotrophins and their receptors.
| p75NTR | Trk-A | Trk-B | Trk-C | |
|---|---|---|---|---|
| Nerve growth factor | Yes | Yes | — | — |
| Brain-derived neurotrophic factor | Yes | — | Yes | — |
| Neurotrophin-3 | Yes | Yes | Yes | Yes |
| Neurotrophin-4 | Yes | — | Yes | — |
Abbreviations: p75NTR = 75; Trk = tyrosine kinase.
The many important benefits of physical exercise on human health have long been recognized and mostly include a decreased risk of cardiovascular disease, diabetes, cancer, osteoporosis, and so forth.7,8 Only recently, however, it has been acknowledged that physical exercise may also generate substantial benefits on executive and cognitive functions through a mechanism that seems to depend on exercise type, intensity, and volume.9 In general, physical exercise of sufficient intensity and duration (i.e., approximately 3 months of moderate-intensity aerobic exercise, with sessions lasting not less than 20 min) seems to be capable of triggering neurogenesis, which potentiates synaptic plasticity and creates new synapses and neural circuits that ultimately can contribute to the optimization of brain plasticity and fitness.10, 11, 12 Notably, the impact of acute exercise cannot be discounted because it is essential for priming some specific cellular pathways that then translate into structural and functional adaptations typically observed in subjects engaged in regular physical exercise.12 Physical exercise can therefore be regarded as an enhancer of environmental factors that may actively promote neuroplasticity by repeated or chronic release of some important biochemical mediators.
The combination of these 2 previous observations, that is, the essential contribution of neurotrophins to brain biology and the impact of physical exercise on cognitive function, leads to the hypothesis that these 2 aspects may somehow be interrelated and that physical exercise may be regarded as a promising option for fostering neurotrophins production, ultimately improving human brain functioning.9,13
Although a large number of investigation studies have been carried out to explore the relationship between physical exercise and neurotrophins in cellular and animal models, the results of these studies cannot be straightforwardly translated to humans, because substantial differences exist between human and animal brains in terms of anatomy, biochemistry, biology, and function.14,15 Moreover, the exercise protocols typically used in animal studies are very different from those used in human studies, so the exercise-induced biological changes in animals must always be interpreted with caution. The current article provides an updated overview of the interplay between physical exercise, neurotrophins, and cognitive function as reported in studies involving humans.
2. Neurotrophins release triggered by physical exercise
The number of studies that have investigated the exercise-related biology of neurotrophins in healthy and diseased humans has grown exponentially during the past decades. The vast majority of studies have focused on BDNF, and data from those studies have been recently reviewed in several comprehensive meta-analyses (Table 2).
Table 2.
Summary of meta-analyses that have explored the effects of physical exercise on BDNF.
| Authors | Study population | n (studies/total participants included) | Conclusions |
|---|---|---|---|
| Szuhany et al. (2015)16 | No exclusion criteria | 29/1111 | Acute increase in BDNF after exercise Chronic increase in BDNF after exercise programs Regular exercise training amplifies the BDNF response to acute exercise Aerobic exercise more effective than resistance training No dependency on BDNF variation based on age |
| Dinoff et al. (2016)17 | Healthy subjects | 29/910 | Chronic increase in BDNF after exercise programs Aerobic exercise more effective than resistance training No association between BDNF variation and exercise duration and intensity No dependency on BDNF variation based on age, sex, and body mass index |
| Dinoff et al. (2017)18 | Healthy subjects | 55/1180 | Acute increase in BDNF after exercise Slightly greater effect on acute BDNF variation from aerobic exercise than from resistance training Association between BDNF variation and aerobic capacity No association between BDNF variation and exercise duration and intensity (but significant variation only observed >30 min) BDNF variation higher in men than in women No dependency on BDNF variation based on age or body mass index |
| Feter et al. (2019)19 | No exclusion criteria | 25/2152 | Chronic increase in BDNF after exercise programs Association between BDNF variation, aerobic capacity, and exercise volume No dependency on BDNF variation based on age, sex, health status, or exercise intensity |
| Dinoff et al. (2018)20 | Patients with major depressive disorders | 6/1176 | No chronic increase in BDNF after exercise programs |
| Kurebayashi and Otaki (2018)21 | Patients with major depressive disorders | 5/199 | No chronic increase in BDNF after exercise programs |
| Mackay et al. (2017)22 | Patients with neurologic disorders | 11/303 | Chronic increase in BDNF after exercise programs |
| Hirsch et al. (2018)23 | Patients with Parkinson's disease | 4/100 | Chronic increase in BDNF after exercise programs |
Abbreviation: BDNF = brain-derived neurotrophic factor.
2.1. BDNF
In 2015, Szuhany et al.16 carried out a meta-analysis of all studies addressing the potential association between different exercise paradigms and BDNF concentration in humans. The authors included 29 studies in their analysis, representing a total of 1111 participants (no exclusion criteria were used for the study populations), and concluded that a single session of exercise was effective to immediately increase the serum or plasma concentration of BDNF (Hedges’ g = 0.46; 95% confidence interval (CI): 0.29–0.62; p < 0.001). Postexercise increase in BDNF values was then significantly amplified in subjects who had completed a program of regular exercise training comprising 3–24 weeks (Hedges’ g = 0.58; 95%CI: 0.10–1.07; p = 0.02). The elimination of resistance training intervention (i.e., arm and elbow strength) did not significantly attenuate the effect of acute exercise on BDNF (Hedges’ g = 0.49; 95%CI: 0.34–0.64; p < 0.001), whereas strength/resistance training studies did generate a significant acute impact on BDNF concentration (Hedges’ g = 0.57; 95%CI: −0.24 to 1.37; p = 0.17), thus suggesting that aerobic exercise may be more effective globally in boosting the circulating values of BDNF.
In a subsequent meta-analysis, Dinoff et al.17 explored the impact of exercise interventions lasting not less than 2 weeks on the circulating values of BDNF. Overall, 29 studies met the criteria to be included in the analysis, representing a total 910 participants. In agreement with previous evidence, the resting values of BDNF were found to be significantly increased by exercise interventions (standardized mean difference (SMD) = 0.39; 95%CI: 0.17–0.60; p < 0.001), but a significant impact was only observed after aerobic exercise (SMD = 0.66; 95%CI: 0.33–0.99; p < 0.001) and not after resistance training (SMD = 0.07; 95%CI: –0.15 to 0.30; p = 0.52). No significant difference was noted among different age classes (p = 0.25), between women and men (p = 0.95), between training programs of a duration of less than 12 weeks and of 12 weeks or greater (p = 0.39), or between short- and long-duration exercise (p = 0.97). The same group of authors performed a 2nd meta-analysis that investigated the impact of different types (e.g., running, cycling, and resistance training) and intensities of exercise on circulating BDNF values.18 A total of 55 studies with 1180 total participants were included in the final analysis. In accord with previous evidence, circulating BDNF concentration was increased by approximately 60% immediately after acute exercise (SMD = 0.59; 95%CI: 0.46–0.72; p < 0.001). Both aerobic exercise (SMD = 0.61; 95%CI: 0.46–0.75; p < 0.001) and resistance training (SMD = 0.48; 95%CI: 0.15–0.80; p = 0.004) were associated with immediate postexercise increase of circulating BDNF values, albeit the variation was slightly higher after aerobic training than after resistance training (i.e., 61% vs. 48%). Also, in this 2nd meta-analysis, no significant association was noted between BDNF variation and overall exercise duration (β = 1.822; p = 0.075) and intensity (β = 1.766; p = 0.085). Nevertheless, when the exercise interventions were divided between those lasting 30 min or less (SMD = 0.47; 95%CI: 0.31–0.63; p < 0.001) and those lasting more than 30 min (SMD = 0.81; 95%CI: 0.53–1.09; p < 0.001), a marginally significant difference was observed (p = 0.04), thus supporting the hypothesis that 30 min of exercise is the minimal threshold for producing significant BDNF variations. Interestingly, postexercise BDNF variation seemed to be higher in men than in women (SMD = 0.75 vs. 0.13; p < 0.001), but the variation was not influenced by age (β = –0.877; p = 0.38) or body mass index (β = 1.000; p = 0.33). Finally, greater variations in postexercise circulating BDNF values were observed in patients with higher levels of cardiorespiratory fitness (β = 3.548; p = 0.002).
More recently, Feter et al.19 published another meta-analysis of 25 studies with 2152 total participants aimed at exploring the chronic effects of exercise on circulating BDNF. No exclusion criteria were applied for selecting the study populations. In the final meta-analysis, exercise was associated with a significant increase in BDNF concentration (mean increase = 596 pg/mL; 95%CI: 472–721 pg/mL; p < 0.001), and this effect was found to be independent of age, sex, and health status. The major contributors to postexercise BDNF variation in the meta-regression analysis were aerobic capacity (p = 0.02) and duration of exercise (p = 0.02).
A 3rd meta-analysis was then published by Dinoff et al.20 It aimed at exploring the effects of long-term exercise interventions on circulating BDNF values in patients with major depressive disorders, diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders. Overall, 6 studies totaling 1176 participants were included in the final analysis, which revealed that exercise interventions were not effective in modifying the baseline concentration of BDNF in these patients (SMD = 0.43; 95%CI: –0.06 to 0.92; p = 0.09). Interestingly, this evidence was then confirmed in another recent meta-analysis published by Kurebayashi and Otaki.21 The authors identified 5 studies with 199 total participants who suffered from major depressive disorders. The authors investigated the effects of physical exercise on circulating BDNF concentration and concluded that the no significant postexercise variation could be noted in these patients (SMD = 0.05; 95%CI: –0.23 to 0.32; p = 0.75).
Unlike the evidence garnered in studies on patients with major depressive disorders, different results were recently published by Mackay et al.22 in patients with neurologic disorders. Overall, 11 studies with 303 total participants were included in the final analysis, which revealed that programs of aerobic exercise (median 12 h/week, lasting 4–24 weeks) were effective in significantly increasing resting BDNF values (SMD = 0.84; 95%CI: 0.47–1.20; p < 0.001). In line with these findings, Hirsch et al.23 recently carried out another meta-analysis that included 4 pre-experimental studies and 2 randomized controlled trials with a total of 100 patients who had idiopathic Parkinson's disease. Overall, physical exercise interventions lasting 28–90 days were effective in significantly increasing circulating BDNF concentration in these patients (SMD = 2.06; 95%CI: 1.36–2.76; p < 0.001).
Another systematic review was recently published by Enette et al.,24 who analyzed the impact of interval and continuous aerobic training on BDNF concentration in subjects aged 60 years or older. Overall, 14 randomized or nonrandomized intervention studies with a total of 988 participants were included in the final analysis. The resting BDNF concentration was found to be significantly increased among participants in 4 of the 5 (80%) studies that applied aerobic interval training protocols and among participants in 6 of the 9 (67%) studies that applied continuous aerobic training.
2.2. Other neurotrophins
Unlike the large volume of data available for BDNF (Table 2), a rather limited number of studies have evaluated the influence of physical exercise on other neurotrophins. An electronic search conducted in Scopus and PubMed using “nerve growth factor” AND “exercise” OR “sport” as search parameters identified a total number of 371 documents, only 12 of which involved humans and investigated the effect of physical exercise on this neurotrophin. The findings from these studies are briefly presented and discussed in this section.
The 1st study that investigated the effect of exercise on circulating NGF values was published by Gold et al.25 in 2003. The authors studied 25 patients with multiple sclerosis and 20 healthy matched controls who were asked to perform a cycle ergometry test for 30 min at 60% of maximal aerobic capacity. Although the immediate postexercise values of BDNF were significantly increased by approximately 30% over the baseline in both patients and controls (cumulative effect, p = 0.03), the concentration of NGF remained virtually unchanged in both groups (cumulative effect, p = 0.09).
Schulz et al.26 studied 15 patients with multiple sclerosis who were engaged in an 8-week aerobic bicycle training program (30 min of cycling at 75% of maximal watts, twice a week). The resting concentration of BDNF was found to be increased after the exercise training period (+17%), whereas the concentration of NGF did not substantially change. Similar results were seen after a 30-min endurance test, with BNDF concentration increasing by approximately 36% and NGF values remaining unvaried.
In an ensuing investigation, Rokling-Andersen et al.27 conducted a randomized, controlled, 2 × 2 trial including 188 participants in the Oslo Diet and Exercise Study. These participants were randomized to 4 different 1-year protocols: a no intervention protocol (n = 37), a diet protocol (n = 45), an exercise protocol (n = 48), and a diet plus exercise protocol (n = 58). The exercise protocol consisted of 60 min of aerobic training (e.g., fast walking or jogging), 3 times weekly, whereas the dietary intervention increased participants’ intake of fish, vegetables, and fiber-rich products and reduced intake of cholesterol and saturated fats. Neither form of the 2 interventions involving physical exercise was effective in significantly modifying the circulating values of NGF (p = 0.74).
Bonini et al.28 carried out a cross-sectional study including 96 pre-Olympic athletes and 49 matched healthy sedentary controls. The authors reported that the concentration of circulating NGF in athletes was nearly twice that of the controls (368 ± 776 pg/mL vs. 174 ± 484 pg/mL; p < 0.001).
Bansi et al.29 carried out a randomized controlled clinical trial involving 60 patients with multiple sclerosis who engaged in 30 min of controlled cycling daily at 60% of maximal aerobic capacity for 3 weeks performed on a cycle ergometer or an aquatic bike (pool depth, 130 cm). Interestingly, the baseline BDNF concentration increased by 23% (p = 0.046) after water cycling but not after land cycling (p = 0.699), whereas the baseline NGF values did not change after either form of exercise (p = 0.087 after water cycling and p = 0.721 after land cycling).
Kim30 carried out a cross-sectional study that included 22 male adolescents aged between 14 and 18 years. These subjects were divided in 2 groups: those who did not perform any regular exercise program for more than 1 year (control group, n = 9) and those with more than 3 years of training who regularly exercised 18 h/week (trained group, n = 13). Although the concentration of NGF in the trained group was slightly higher than in the control group (6.7 ± 2.8 ng/mL vs. 5.0 ± 2.2 ng/mL), the difference did not reach statistical significance (p > 0.05).
Azali Alamadari and Choobineh31 carried out a study involving 30 subjects (mean age, 58 ± 5 years) with metabolic syndrome, who were randomized to no exercise (n = 14) or an 8-week aerobic training program encompassing 3 sessions/week of exercise at 50%–60% of maximal aerobic capacity (n = 16). Interestingly, a significant increase in resting circulating values of both BDNF (+4%; p < 0.05) and NGF (+48%; p < 0.05) was noted in the exercise group.
Cho et al.32 studied 36 obese middle-aged Korean women (mean age, 55 ± 3 years) who were randomized to no intervention, an 8-week aerobic exercise program (3 sessions/week of 40-min treadmill running at 70% of heart rate reserve), and an 8-week aerobic exercise program (same as before) plus cranial electrotherapy stimulation. The resting concentration of BDNF was found to be significantly increased after the 2 exercise intervention modalities (+17% after exercise alone and +15% after exercise plus cranial electrotherapy stimulation), as were the resting values of NGF (+10% after exercise alone and +9% after exercise plus cranial electrotherapy stimulation). The same authors carried out another intervention study based on a 40-min treadmill exercise at 70% heart rate reserve 3 times/week for 8 weeks in 10 obese (mean age, 23 ± 2 years) and nonobese (mean age, 23 ± 2 years) young men.33 This exercise intervention was effective in increasing the resting concentration of BDNF in both obese and nonobese subjects (1%–20%; p = 0.013), although the circulating levels of NGF remained mostly unchanged in both categories of subjects (p = 0.196). These authors also performed another study including 15 healthy men who were asked to engage in treadmill running under low-, moderate-, and high-intensity conditions.34 In accordance with previous findings, the concentration of BDNF displayed an exercise-volume-dependent immediate increase (+6%, +23%, and +27% after low-, moderate-, and high-intensity exercise, respectively), with values returning to the baseline after 60 min. A similar exercise volume-dependent kinetics was noted for NGF values (+12%, +19%, and +23% immediately after low-, moderate-, and high-intensity exercise, respectively), with values returning to the baseline after 60 min.
In a subsequent study, Mokhtarzade et al.35 randomly assigned 66 patients with multiple sclerosis to no exercise or an 8-week exercise intervention based on cycling at 60%–70% of peak power 3 days/week. Although the exercise intervention was effective in significantly increasing BDNF values in normal weight (n = 33; p = 0.001) but not in obese patients (n = 33; p > 0.05), the circulating levels of NGF remained similar to pre-exercise intervention levels both in normal weight and obese patients (p > 0.05).
Moradi et al.36 measured NGF in 48 autistic children aged 6–9 years, who were equally assigned to 4 different 2-month protocols, encompassing no intervention, perceptual-motor activities combined with music, vitamin D supplementation alone, and perceptual-motor activities combined with music and vitamin D supplementation. As expected, no variation in NGF levels was observed in the no intervention group, whereas the postintervention concentration of this neurotrophin gradually increased from approximately 3% in the perceptual-motor activities combined with music group to approximately 30% in the vitamin D supplementation group, up to approximately 70% in the perceptual-motor activities combined with music and vitamin D supplementation group.
As regards the other 2 neurotrophins, NT-3 and NT-4, a combined Scopus and PubMed search using the keywords “neurotrophin-3” OR “neurotrophin-4” AND “exercise” OR “sport” identified 93 documents for NT-3 and 48 documents for NT-4, but only one of these was an experimental study involving human subjects.
Domínguez-Sanchez et al.37 carried out a randomized, parallel group clinical study including 51 physically inactive obese men (mean age, 24 ± 3 years) who were randomized to no exercise, high-intensity exercise (4 × 4-min treadmill running at 85%–95% of maximum heart rate (HRmax) alternated with 4-min recovery at 75%–85% of HRmax), resistance training (12–15 repetitions/set at 50%–70% of HRmax alternated with 1-min recovery), or combined high-intensity and resistance exercise. Blood samples were then drawn before exercise and immediately after (i.e., within 1 min) each exercise protocol. Interestingly, BDNF values acutely increased after high-intensity exercise (+7%) and combined training (+12%), but were not modified by resistance training. The values of NT-3 and NT-4 were both found to be increased after resistance training (+13% and +7%, respectively) and combined training (+12% and +9%, respectively), whereas their relative increase (+13% and +1%, respectively) did not reach statistical significance after high-intensity exercise. Notably, the relative variation of BNDF was found to be marginally associated with that of NT-4 (r = 0.59; p = 0.034), but not with that of NT-3, nor were the postexercise changes of NT-3 and NT-4 significantly associated.
3. Discussion
Taken together, the currently available data seemingly confirm the existence of a positive relationship between physical exercise and circulating BDNF levels, both in the short and long term, and also support the beneficial impact of training programs for amplifying the acute BDNF response (Table 2). Notably, an accurate assessment of postexercise variation of BDNF changes requires an appropriate blood sample collection and should be corrected for hemoconcentration.38 Although both aerobic exercise and resistance training seem to be effective in acutely increasing BDNF values, the variation promoted by the former type of exercise seems greater. Controversial information has been reported for exercise intensity and duration. Although most published studies seem to rule out a clear impact of intensity on BDNF concentration, exercise lasting for 30 min or more seems necessary to generate a significant postexercise increase in this molecule (Table 2). Interestingly, the potential role of anaerobic exercise has been highlighted by Schiffer et al.,39 who showed that blood lactate generation may interplay with BDNF blood concentrations, whereby intravenous infusions of approximately 4 mol/L of sodium lactate solution were effective in acutely increasing blood BDNF values by approximately 1.4-fold. Finally, although the impact of exercise on BDNF concentration has occasionally been found to be higher in men than in women, no significant associations have been reported based on age or body mass index. Surprisingly, no effect of physical exercise on circulating BDNF values was found in patients with major depressive disorders, whereas in those with neurologic disorders the effect was globally comparable with that observed in the general population (Table 2).
Less solid and clear information can be garnered from the limited number of studies that investigated the impact of physical exercise on circulating values of the 3 other major neurotrophins (NGF, NT-3, and NT-4). Although definitive conclusions cannot be made, the current evidence suggests that—unlike BDNF—the impact of physical exercise on circulating values of NGF remains largely uncertain. Significant increases were observed in the several interventional studies,28,31,32,34,36 although the results of the remaining studies do not support the existence of any significant interaction. In the only interventional study that evaluated the postexercise variation of both NT-3 and NT-4, the concentration of these 2 biomarkers increased after resistance and combined training.37
Thus, given that physical exercise is capable of both acutely and chronically increasing the circulating values of some neurotrophins, especially NBDF, understanding how this phenomenon would translate into tangible benefits in humans becomes essential. Some studies have combined the assessment of BDNF variation and neurocognitive improvement after exercise. Kimhy et al.40 carried out a single-blind randomized clinical study that included 33 patients with schizophrenia who were randomized either to a 12-week aerobic whole body exercise program (n = 16) or to standard psychiatric treatment (n = 17). At the end of the study, the concentration of BDNF increased by 11% in the aerobic exercise group compared to 2% in the cohort that received standard psychiatric treatment. Neurocognitive functioning was also substantially improved in the aerobic exercise group, and approximately 15% of this variation was explained by postaerobic exercise BDNF variation. In a subsequent study, Heisz et al.41 explored the impact of physical exercise and cognitive training on neurotrophic factors and neurocognitive functioning in 95 healthy young adults (mean age, 21 ± 3 years; 58 women and 37 men). The participants were randomized to no intervention, 6 weeks of exercise training (20 min of high-intensity interval training 3 times/week), or 6 weeks of the previously mentioned exercise training combined with cognitive training. Individuals with greater aerobic fitness improvements in response to exercise training showed greater increases in BDNF levels (r = 0.25; p < 0.05), as well as better high-interference memory performance as a result of the combined exercise and cognitive training compared with exercise alone (r = 0.42; p < 0.05). More recently, Leckie et al.42 explored the effect of aerobic training on executive function and BDNF levels in 90 older adults (mean age, 67 ± 6 years; 59 women and 33 men). The subjects were randomized either to a no exercise protocol or to an intervention involving 1 year of walking (3 days/week for 40 min at 60%–75% of HRmax reserve). In members of the walking group aged more than 65 years, the serum concentration of BDNF significantly increased by approximately 25%, and this variation was significantly associated with improvement in executive function and task-switch performance. Encouraging results were also published by Hakansson et al.,43 who recruited 19 healthy older adults 65–85 years of age. All of them performed a single session of 3 different types of intervention: 35 min of whole body aerobic exercise at moderate intensity, 35 min of cognitive training, and 35 min of mindfulness practice. Blood samples for BDNF analysis were collected immediately before, immediately after, and at 20 min and 60 min after each exercise. In agreement with previous findings, physical exercise generated an immediate and significant increase in circulating BDNF values (+17%; p = 0.004), although no significant changes were observed after cognitive training and mindfulness practice. Notably, the postexercise BDNF variation was significantly associated with working memory function (r = 0.89; p = 0.02).
These converging findings, along with evidence that the brain is the major contributor of exercise-induced circulating BDNF,44 convincingly support the hypothesis that acute or chronic postexercise increases in BDNF translates into an improvement of some executive and cognitive functions, thus carrying substantial clinical implications. First, both disease- and age-related impairment in cognitive function may be attenuated by participation in exercise programs targeting the neurotrophin pathways (i.e., moderate-intensity aerobic exercise lasting for ≥30 min). Repeated training is also advisable, since each session of exercise seems to be effective in generating BDNF production, thus amplifying the magnitude of this phenomenon over time. Interestingly, exercise duration but not its intensity was found to be associated with BDNF variations,18 thus supporting the hypothesis that regular performance of light exercise may be sufficient to trigger beneficial effects on BDNF. This concept has been recently confirmed by Park et al.,45 who assessed the effects of an exercise intervention based on 20 min of daily low- to moderate-intensity gardening (i.e., approximately 3.5 metabolic equivalents, involving digging, fertilizing, raking, planting, cleaning, and watering) in 41 elderly subjects (mean age, 77 ± 6 years). The authors reported that the circulating levels of BDNF significantly increased by approximately 8% (p = 0.038) immediately after this low to moderate level of physical activity.
Given the evidence that physical exercise has a favorable impact on BDNF concentration in patients with neurologic disorders, it is reasonable to suggest that regular performance of aerobic exercise should be an active part of a rehabilitation program in these patients, thus modulating the interplay of neurotrophins, especially BDNF, with the pathogenesis of these conditions. This conclusion is supported by results of 2 very recent meta-analyses showing that BDNF concentration was significantly reduced in patients with Parkinson's disease (SMD = –1.03; 95%CI: –1.83 to –0.23; p = 0.012)46 and Alzheimer's disease (Hedges’ g = –0.339; 95%CI: –0.572 to –0.106; p = 0.004)47 compared to healthy populations.
4. Conclusion
Several lines of evidence now attest to the idea that physical exercise is essential for brain health and that the repeated or chronic release of neurotrophins—especially BDNF—triggered by motor activities may be a key mediator of this effect. Therefore, promotion of adequate volumes and intensities of physical exercise (i.e., approximately 3 months of moderate-intensity aerobic exercise, with 2–3 sessions/week lasting not less than 30 min) represents an inexpensive and safe strategy for boosting BDNF release that may preserve or restore cognitive function. Additional studies should be conducted that further explore the interplay between physical exercise, neurotrophins, and cognitive function.
Acknowledgments
Acknowledgment
Fabian Sanchis-Gomar is supported by a postdoctoral contract granted by Subprograma Atracció de Talent - Contractes Postdoctorals de la Universitat de València.
Authors’ contributions
GL and FSG reviewed the literature and participated in drafting, writing, and revising this review manuscript; CM revised the manuscript and provided meaningful edits and comments. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Pareja-Galeano H., Mayero S., Sanchis-Gomar F. Exercise, neuroplasticity, and growth factors in adolescence. In: Farooqui TFaAA., editor. Diet and exercise in cognitive function and neurological diseases. Wiley Blackwell; Hoboken, NJ: 2015. pp. 323–337. [Google Scholar]
- 2.Bothwell M. Recent advances in understanding neurotrophin signaling. F1000Res. 2016;5 doi: 10.12688/f1000research.8434.1. pii: F1000 Faculty Rev-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilar M., Mira H. Regulation of neurogenesis by neurotrophins during adulthood: expected and unexpected roles. Front Neurosci. 2016;10:26. doi: 10.3389/fnins.2016.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu B., Nagappan G., Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Pareja-Galeano H., Brioche T., Sanchis-Gomar F., Montal A., Jovani C., Martinez-Costa C. Impact of exercise training on neuroplasticity-related growth factors in adolescents. J Musculoskelet Neuronal Interact. 2013;13:368–371. [PubMed] [Google Scholar]
- 7.Warburton D.E.R., Bredin S.S.D. Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol. 2017;32:541–556. doi: 10.1097/HCO.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 8.Vina J., Sanchis-Gomar F., Martinez-Bello V., Gomez-Cabrera M.C. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pareja-Galeano H., Mayero S., Perales M., Garatachea N., Santos-Lozano A., Fiuza-Luces C. Biological rationale for regular physical exercise as an effective intervention for the prevention and treatment of depressive disorders. Curr Pharm Des. 2016;22:3764–3775. doi: 10.2174/1381612822666160322144537. [DOI] [PubMed] [Google Scholar]
- 10.Ploughman M. Exercise is brain food: the effects of physical activity on cognitive function. Dev Neurorehabil. 2008;11:236–240. doi: 10.1080/17518420801997007. [DOI] [PubMed] [Google Scholar]
- 11.Lee M.C., Byun K., Kim J.S., Lee H., Kim K. Trends in exercise neuroscience: raising demand for brain fitness. J Exerc Rehabil. 2019;15:176–179. doi: 10.12965/jer.1938046.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Sayes J., Harasym D., Turco C.V., Locke M.B., Nelson A.J. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 2019;25:65–85. doi: 10.1177/1073858418771538. [DOI] [PubMed] [Google Scholar]
- 13.Pareja-Galeano H., Sanchis-Gomar F., Mayero S. Autism spectrum disorders: possible implications of BDNF modulation through epigenetics. Acta Psychiatr Scand. 2013;128:97. doi: 10.1111/acps.12071. [DOI] [PubMed] [Google Scholar]
- 14.Premack D. Human and animal cognition: continuity and discontinuity. Proc Natl Acad Sci U S A. 2007;104:13861–13867. doi: 10.1073/pnas.0706147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sherwood C.C., Subiaul F., Zawidzki T.W. A natural history of the human mind: tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szuhany K.L., Bugatti M., Otto M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinoff A., Herrmann N., Swardfager W., Liu C.S., Sherman C., Chan S. The effect of exercise training on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF): a meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinoff A., Herrmann N., Swardfager W., Lanctot K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: a meta-analysis. Eur J Neurosci. 2017;46:1635–1646. doi: 10.1111/ejn.13603. [DOI] [PubMed] [Google Scholar]
- 19.Feter N., Alt R., Dias M.G., Rombaldi A.J. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci Sports. 2019;34:293–304. [Google Scholar]
- 20.Dinoff A., Herrmann N., Swardfager W., Gallagher D., Lanctot K.L. The effect of exercise on resting concentrations of peripheral brain-derived neurotrophic factor (BDNF) in major depressive disorder: a meta-analysis. J Psychiatr Res. 2018;105:123–131. doi: 10.1016/j.jpsychires.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Kurebayashi Y., Otaki J. Does physical exercise increase brain-derived neurotrophic factor in major depressive disorder? A meta-analysis. Psychiatr Danub. 2018;30:129–135. doi: 10.24869/psyd.2018.129. [DOI] [PubMed] [Google Scholar]
- 22.Mackay C.P., Kuys S.S., Brauer S.G. The effect of aerobic exercise on brain-derived neurotrophic factor in people with neurological disorders: a systematic review and meta-analysis. Neural Plast. 2017;2017 doi: 10.1155/2017/4716197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch M.A., van Wegen E.E.H., Newman M.A., Heyn P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson's disease: a systematic review and meta-analysis. Transl Neurodegener. 2018;7:7. doi: 10.1186/s40035-018-0112-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enette L., Vogel T., Fanon J.L., Lang P.O. Effect of interval and continuous aerobic training on basal serum and plasma brain-derived neurotrophic factor values in seniors: a systematic review of intervention studies. Rejuvenation Res. 2017;20:473–483. doi: 10.1089/rej.2016.1886. [DOI] [PubMed] [Google Scholar]
- 25.Gold S.M., Schulz K.H., Hartmann S., Mladek M., Lang U.E., Hellweg R. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138:99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 26.Schulz K.H., Gold S.M., Witte J., Bartsch K., Lang U.E., Hellweg R. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. 2004;225:11–18. doi: 10.1016/j.jns.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Rokling-Andersen M.H., Reseland J.E., Veierod M.B., Anderssen S.A., Jacobs D.R., Jr, Urdal P. Effects of long-term exercise and diet intervention on plasma adipokine concentrations. Am J Clin Nutr. 2007;86:1293–1301. doi: 10.1093/ajcn/86.5.1293. [DOI] [PubMed] [Google Scholar]
- 28.Bonini M., Fioretti D., Sargentini V., Del Giacco S., Rinaldi M., Tranquilli C. Increased nerve growth factor serum levels in top athletes. Clin J Sport Med. 2013;23:228–231. doi: 10.1097/JSM.0b013e31827ee6d5. [DOI] [PubMed] [Google Scholar]
- 29.Bansi J., Bloch W., Gamper U., Kesselring J. Training in MS: influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Mult Scler. 2013;19:613–621. doi: 10.1177/1352458512458605. [DOI] [PubMed] [Google Scholar]
- 30.Kim Y.I. The impact of exercise training on basal BDNF in athletic adolescents. J Phys Ther Sci. 2016;28:3066–3069. doi: 10.1589/jpts.28.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azali Alamadari K., Choobineh S. Integrated effects of aerobic training on metabolic risk factors, circulatory neurotrophins, testosterone and cortisol in midlife males with metabolic syndrome. Med Sport. 2016;69:228–239. [Google Scholar]
- 32.Cho S.Y., So W.Y., Roh H.T. Effects of aerobic exercise training and cranial electrotherapy stimulation on the stress-related hormone, the neurotrophic factor, and mood states in obese middle-aged women: a pilot clinical trial. Salud Mental. 2016;39:249–256. [Google Scholar]
- 33.Roh H.T., So W.Y. The effects of aerobic exercise training on oxidant-antioxidant balance, neurotrophic factor levels, and blood-brain barrier function in obese and non-obese men. J Sport Health Sci. 2017;6:447–453. doi: 10.1016/j.jshs.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roh H.T., Cho S.Y., Yoon H.G., So W.Y. Effect of exercise intensity on neurotrophic factors and blood-brain barrier permeability induced by oxidative-nitrosative stress in male college students. Int J Sport Nutr Exerc Metab. 2017;27:239–246. doi: 10.1123/ijsnem.2016-0009. [DOI] [PubMed] [Google Scholar]
- 35.Mokhtarzade M., Motl R., Negaresh R., Zimmer P., Khodadoost M., Baker J.S. Exercise-induced changes in neurotrophic factors and markers of blood-brain barrier permeability are moderated by weight status in multiple sclerosis. Neuropeptides. 2018;70:93–100. doi: 10.1016/j.npep.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Moradi H., Sohrabi M., Taheri H., Khodashenas E., Movahedi A. The effects of different combinations of perceptual-motor exercises, music, and vitamin D supplementation on the nerve growth factor in children with high-functioning autism. Complement Ther Clin Pract. 2018;31:139–145. doi: 10.1016/j.ctcp.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Domínguez-Sanchez M.A., Bustos-Cruz R.H., Velasco-Orjuela G.P., Quintero A.P., Tordecilla-Sanders A., Correa-Bautista J.E. Acute effects of high intensity, resistance, or combined protocol on the increase of level of neurotrophic factors in physically inactive overweight adults: the Brainfit Study. Front Physiol. 2018;9:741. doi: 10.3389/fphys.2018.00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pareja-Galeano H., Alis R., Sanchis-Gomar F., Cabo H., Cortell-Ballester J., Gomez-Cabrera M.C. Methodological considerations to determine the effect of exercise on brain-derived neurotrophic factor levels. Clin Biochem. 2015;48:162–166. doi: 10.1016/j.clinbiochem.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Schiffer T., Schulte S., Sperlich B., Achtzehn S., Fricke H., Struder H.K. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci Lett. 2011;488:234–237. doi: 10.1016/j.neulet.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Kimhy D., Vakhrusheva J., Bartels M.N., Armstrong H.F., Ballon J.S., Khan S. The impact of aerobic exercise on brain-derived neurotrophic factor and neurocognition in individuals with schizophrenia: a single-blind, randomized clinical trial. Schizophr Bull. 2015;41:859–868. doi: 10.1093/schbul/sbv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heisz J.J., Clark I.B., Bonin K., Paolucci E.M., Michalsk Bi, Becker S. The effects of physical exercise and cognitive training on memory and neurotrophic factors. J Cogn Neurosci. 2017;29:1895–1907. doi: 10.1162/jocn_a_01164. [DOI] [PubMed] [Google Scholar]
- 42.Leckie R.L., Oberlin L.E., Voss M.W., Prakash R.S., Szabo-Reed A., Chaddock-Heyman L. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. doi: 10.3389/fnhum.2014.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hakansson K., Ledreux A., Daffner K., Terjestam Y., Bergman P., Carlsson R. BDNF responses in healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: associations with working memory function. J Alzheimers Dis. 2017;55:645–657. doi: 10.3233/JAD-160593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill T., Polk J.D. BDNF, endurance activity, and mechanisms underlying the evolution of hominin brains. Am J Phys Anthropol. 2019;168(Suppl. 67):S47–62. doi: 10.1002/ajpa.23762. [DOI] [PubMed] [Google Scholar]
- 45.Park S.A., Lee A.Y., Park H.G., Lee W.L. Benefits of gardening activities for cognitive function according to measurement of brain nerve growth factor levels. Int J Environ Res Public Health. 2019;16 doi: 10.3390/ijerph16050760. pii: E760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang L., Zhang H., Wang C., Ming F., Shi X., Yang M. Serum level of brain-derived neurotrophic factor in Parkinson's disease: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2019;88:168–174. doi: 10.1016/j.pnpbp.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Qin X.Y., Cao C., Cawley N.X., Liu T.T., Yuan J., Loh Y.P. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer's disease: a meta-analysis study (N=7277) Mol Psychiatry. 2017;22:312–320. doi: 10.1038/mp.2016.62. [DOI] [PubMed] [Google Scholar]