Abstract
Introduction
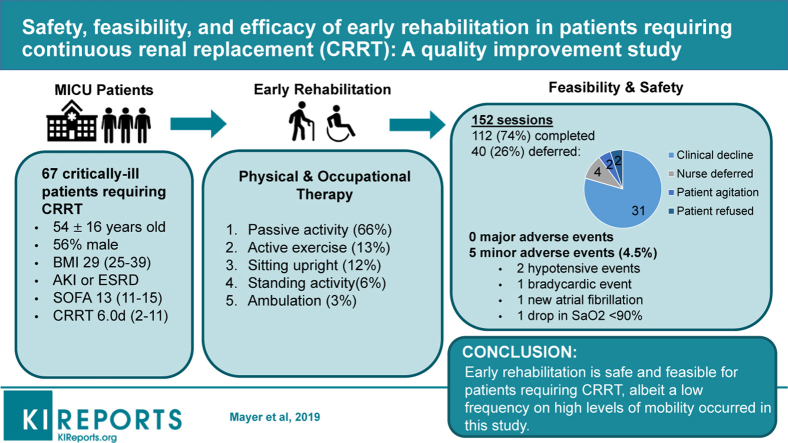
Early rehabilitation in critically ill patients is associated with improved outcomes. Recent research demonstrates that patients requiring continuous renal replacement therapy (CRRT) can safely engage in mobility. The purpose of this study was to assess safety and feasibility of early rehabilitation with focus on mobility in patients requiring CRRT.
Methods
Study design was a mixed methods analysis of a quality improvement protocol. The setting was an intensive care unit (ICU) at a tertiary medical center. Safety was prospectively recorded by incidence of major adverse events including dislodgement of CRRT catheter, accidental extubation, bleeding, and hemodynamic emergency; and minor adverse events such as transient oxygen desaturation >10% of resting. Limited efficacy testing was performed to determine if rehabilitation parameters were associated with clinical outcomes.
Results
A total of 67 patients (54.0 ± 15.6 years old, 44% women, body mass index 29.2 ± 9.3 kg/m2) received early rehabilitation under this protocol. The median days of CRRT were 6.0 (interquartile range [IQR], 2–11) and 72% of patients were on mechanical ventilation concomitantly with CRRT at the time of rehabilitation. A total of 112 rehabilitation sessions were performed of 152 attempts (74% completion rate). No major adverse events occurred. Patients achieving higher levels of mobility were more likely to be alive at discharge (P = 0.076).
Conclusions
The provision of early rehabilitation in critically ill patients requiring CRRT is safe and feasible. Further, these preliminary results suggest that early rehabilitation with focus on mobility may improve patient outcomes in this susceptible population.
Keywords: continuous renal replacement therapy, critical illness, CRRT, early rehabilitation, quality improvement
Graphical abstract
Approximately 50% of patients admitted to the ICU for critical illness are diagnosed with ICU-acquired weakness.1,2 Bedrest and physical inactivity are the primary culprits of physical disability developed during ICU stay.3,4 Patients surviving critical illness suffer significant long-term impairments that affect mobility and quality of life for years after discharge from their initial hospitalization.5,6 Early rehabilitation with focus on mobility is purported to mitigate the short- and long-term physical sequelae of critical illness.7, 8, 9, 10, 11, 12 Implementing early rehabilitation interventions in the ICU is safe and feasible.13,14 Despite the established safety and efficacy, critically ill patients needing CRRT for acute kidney injury or end-stage renal disease are traditionally restricted to the bed.
In the past 5 years, research has demonstrated that early rehabilitation is safe and feasible for patients receiving CRRT in the ICU.15, 16, 17, 18, 19 However, data supporting early rehabilitation and mobility in this population are limited. Therefore, the purpose of this quality improvement study was to elucidate the process of developing and implementing an interdisciplinary protocol to provide early rehabilitation to critically ill patients requiring CRRT in the ICU. We assessed the safety and feasibility of the protocol. Secondarily, we examined if early rehabilitation associates with clinical outcomes in this susceptible population.
Methods
The CRRT and Early Rehabilitation Protocol: Local Problem
The University of Kentucky Albert B. Candler Hospital is an academic medical center with approximately 37,500 admissions in 2017, of which 2600 were admitted to the medical ICU (MICU). According to the Center for Health Services Research at the University of Kentucky, the MICU provides treatment to patients with admission mean sequential organ failure assessment (SOFA) of 8.6, mean ICU length of stay (LOS) of 5.7 days, mean mechanical ventilator (MV) support of 3.7 days, and all-cause hospital mortality of 29%. In 2017, 356 (14.5%) patients admitted to the MICU required CRRT (mean 7.1 days) with increased mortality rate at 50%. Historically at our institution, these patients would have been restricted to bed rest. Therefore, we decided to develop a protocol to provide early rehabilitation to patients receiving CRRT. This quality improvement project occurred in 4 phases: (i) team development, (ii) protocol creation, (iii) protocol implementation with data collection for safety and feasibility, and (iv) exploratory clinical outcome examination.
The CRRT and Early Rehabilitation Protocol: Team Development
To develop the protocol, we first assembled an interdisciplinary team that consisted of nephrologists, intensivists, critical care nurses, physical therapists, and occupational therapists. Team members met to establish the demand and practicality of initiating an early rehabilitation protocol with emphasis placed on teamwork and leadership commitment to early rehabilitation.20, 21, 22 Initial sessions included comprehensive literature review, delineation of stakeholder roles, and discussions on each step of the proposed protocol.
The CRRT and Early Rehabilitation Protocol: Protocol Creation
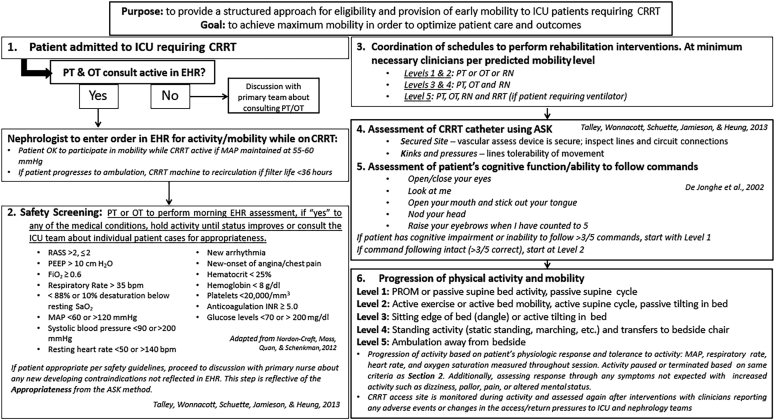
The team created a 2-phase early rehabilitation protocol for patients requiring CRRT. Phase 1 consisted of a multistep assessment of patient appropriateness to engage in the rehabilitation protocol:
-
(i)
Morning review of the patient’s electronic health record to assess hemodynamic status, ventilator settings, and sedation completed by the rehabilitation clinician (Figure 1).15, 23, 24
-
(ii)
If the patient met criteria, a bedside discussion with the patient’s nurse was conducted to assess for recent changes in clinical status or new contraindications.
-
(iii)
Coordination between the interdisciplinary ICU team to schedule and conduct the rehabilitation session.
Figure 1.
Early rehabilitation protocol algorithm for patients requiring continuous renal replacement therapy (CRRT). This figure demonstrates the step-by-step protocol for implementing early rehabilitation in patients requiring CRRT.15, 23, 24 ASK, Appropriateness, Secured Site, and Kink & Pressures; bpm, beats per minute; EHR, electronic health record; ICU, intensive care unit; INR, international normalized ratio; MAP, mean arterial pressure; OT, occupational therapist; PEEP, positive end-expiratory pressure; PROM, passive range-of-motion; PT, physical therapist; RASS, Richmond Agitation and Sedation Scale; RN, registered nurse; RRT, renal replacement therapy; RT, respiratory therapist; SaO2, oxygen saturations. Adapted from Talley CL, Wonnacott RO, Schuette JK, et al. Extending the benefits of early mobility to critically ill patients undergoing continuous renal replacement therapy: the Michigan experience. Critical Care Nursing Quarterly. 2013;36:89–100,15https://journals.lww.com/ccnq/fulltext/2013/01000/Extending_the_Benefits_of_Early_Mobility_to.11.aspx, by permission of Wolters Kluwer Health | Lippincott Williams & Wilkins. Copyright © 2013 Wolters Kluwer Health | Lippincott Williams & Wilkins. Adapted from Nordon-Craft A, Moss M, Quan D, Schenkman M. Intensive care unit–acquired weakness: implications for physical therapist management, Physical Therapy, 2012;92:1494–1506,23 by permission of Oxford University Press. Copyright © 2012 American Physical Therapy Association.
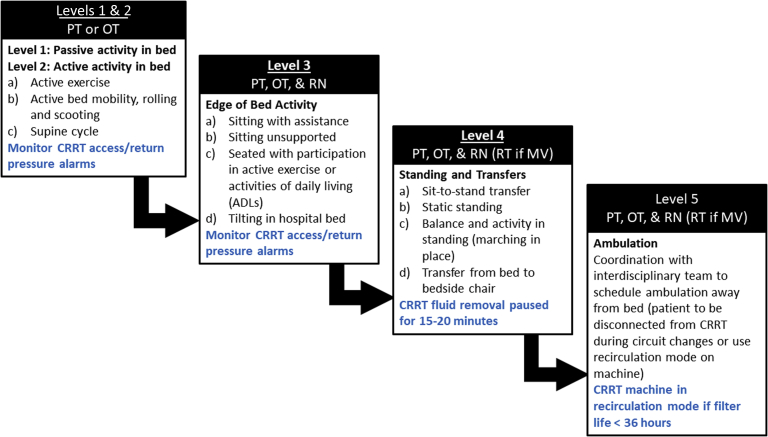
Phase 2 of this early rehabilitation protocol was developed to standardize implementation and progression of physical activity and mobility. Progression of physical activity was driven by the rehabilitation specialist by assessing the patient’s hemodynamic response to increased activity. In addition, clinicians assessed and monitored changes in CRRT deliverables and functionality (Figure 2). This also included review of the “ASK” method to assess Appropriateness, Secured Site, and Kink & Pressures developed by Talley et al.15
Figure 2.
Mobility progression scheme of the early rehabilitation protocol for patients requiring continuous renal replacement therapy (CRRT). Progression to higher level of mobility is based on the patient’s tolerance to activity as assessed by the interdisciplinary team. Figure also describes necessary monitoring of CRRT circuit/access at each level (blue text). Level 5 requires additional communication to coordinate CRRT machine recirculation. Interdisciplinary team includes physical therapist (PT), occupational therapist (OT), registered nurse (RN), respiratory therapist (RT), and physician. MV, mechanical ventilator.
The CRRT and Early Rehabilitation Protocol: Patient Eligibility
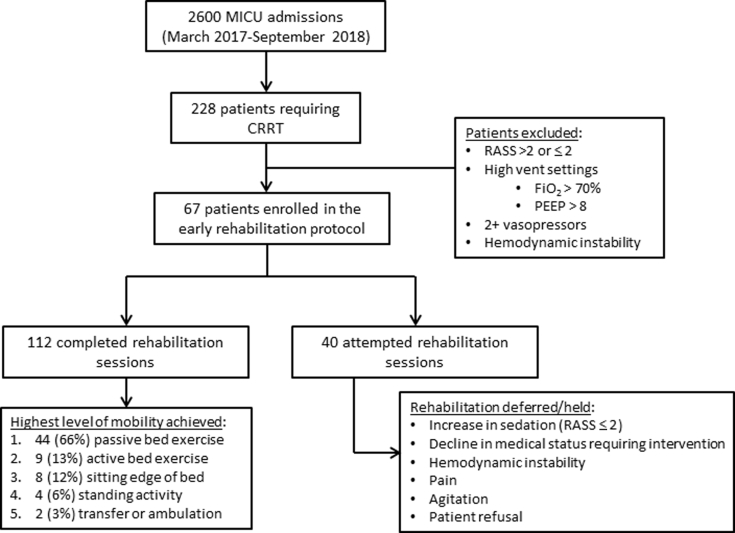
Adult patients admitted to the MICU from March 2017 to September 2018 requiring CRRT were eligible for the protocol. Patients in deep sedation (Richmond Agitation and Sedation Scale < −2), severe agitation (Richmond Agitation and Sedation Scale >2), and high ventilator settings (FiO2 >0.60 and/or positive end-expiratory pressure >10 cm H2O) were excluded, as they would not be appropriate for early rehabilitation regardless of CRRT. This protocol constituted a quality improvement initiative to promote early rehabilitation as standard of care, consequently informed consent was waived. We received approval from the University of Kentucky Institutional Review Board, Office of Research Integrity, Institutional Review Board number 47751 for data collection and analysis. Figure 3 provides description of the number of eligible and participating patients.
Figure 3.
Flowchart of patient selection for participation in the continuous renal replacement therapy (CRRT) early rehabilitation protocol. MICU, medical intensive care unit; PEEP, positive end-expiratory pressure; RASS, Richmond Agitation and Sedation Scale.
The CRRT and Early Rehabilitation Protocol: Protocol Implementation and Data Collection
Demographic and clinical data were collected from the electronic health record, including age, sex, race, body mass index, severity of illness at ICU admission (SOFA score), preexisting comorbidities, Charlson comorbidity index, ICU LOS, hospital LOS, MV days, CRRT days, and cumulative fluid balance. Data of incident major adverse events, including dislodgement of CRRT or other catheter, accidental extubation (if intubated), bleeding, and hemodynamic emergency requiring immediate medical intervention (e.g., i.v. fluids and/or vasopressor administration) were collected. Minor adverse events were defined as transient ≥10% oxygen desaturation below resting SaO2, increase in respiratory rate >35 breaths per minute, mean arterial pressure drop below 60 or elevation above 120 mm Hg, systolic blood pressure <90 or >200 mm Hg, new arrhythmia or significant bradycardia <40 beats per minute or tachycardia >180 beats per minute, and/or new onset of chest pain/angina. Feasibility was assessed according to the 8 areas proposed by Bowen et al. in 2009.25 Specifically, we assessed feasibility by the implementation rate as a percentage of completed rehabilitation sessions in relation to the total number of attempts. We performed a limited efficacy analysis to determine if early rehabilitation interventions associated with clinical outcomes. Selected clinical outcomes (dependent variables) included hospital mortality, MV days, CRRT days, ICU LOS, and hospital LOS. Independent variables related to rehabilitation implementation included the following: (i) the number of completed rehabilitation sessions per patient, (ii) the ratio of completed rehabilitation sessions per CRRT days, (iii) the time from ICU admission to first rehabilitation session (days), and (iv) the highest achieved 5-level mobility category (Figure 2).
Statistical Analysis
Continuous variables were reported as median and IQR or mean and SD according to data distribution. Categorical variables were reported as counts and proportions. Data were assessed for normality (Shapiro-Wilk) and appropriate parametric or nonparametric tests were used. Spearman rho correlation analysis was performed to assess if rehabilitation variables correlated with continuous clinical outcomes (CRRT days, MV days, ICU LOS, and hospital LOS). Chi square and logistic regression were used to examine if rehabilitation variables were associated with all-cause hospital mortality. Candidate variables for multivariable models were selected according to univariable associations and clinical relevance and included SOFA score, Charlson comorbidity index, and presence of liver disease. Statistical significance was set as P < 0.05. All analyses were performed using Sigmaplot 14 (Systat Software Inc, San Jose, CA).
Results
The CRRT and Early Rehabilitation Protocol: Patient Data
A total of 67 patients receiving CRRT in the MICU participated in at least 1 rehabilitation session during the 18 months of protocol implementation. Patients engaging in the protocol had mean age of 54.0 ± 15.6 years (44.1% women, 92.5% white) and had a median SOFA score of 13 (IQR, 11–15) at the time of admission to the ICU. Of the 67 patients participating, 96% required MV at some point during their ICU stay. Concomitant CRRT and MV occurred during 81 of the 112 (72.3%) rehabilitation sessions performed. The overall demographics and clinical data are presented in Table 1.
Table 1.
Patient characteristics
| Characteristics of patients | PT/OT intervention |
|---|---|
| n = 67 | |
| Demographics | |
| Age, yr ± SD | 54.0 ± 15.6 |
| Women, n (%) | 30 (44.1) |
| White race, n (%) | 62 (92.5) |
| BMI, kg/m2, (median IQR) | 29.2 (25.2–38.5) |
| Kidney function | |
| eGFR at ICU admission, ml/min per 1.73 m2, median (IQR) | 18.4 (10.9–39.0) |
| SCr at ICU admission, mg/dl, median (IQR) | 2.73 (1.30–4.95) |
| End-stage kidney disease, n (%) | 13 (19) |
| Comorbidity | |
| Diabetes, n (%) | 29 (42.6) |
| Hypertension, n (%) | 41 (60.3) |
| Congestive heart failure, n (%) | 20 (29.4) |
| COPD, n (%) | 18 (26.5) |
| Liver disease, n (%) | 40 (58.8) |
| Anemia, n (%) | 8 (12.0) |
| Cancer, n (%) | 1 (1.47) |
| Charlson Index, median (IQR) | 5.0 (3.0–7.0) |
| CRRT characteristics | |
| Peak SCr, mg/dl, median (IQR) | 5.19 (3.55–7.42) |
| Time from ICU admission to CRRT initiation, d, median (IQR) | 3.0 (1–9) |
| CRRT d, median (IQR) | 6.0 (2–11) |
| CRRT modality, n (%) | |
| CVVHDF | 61 (91) |
| SCUF | 8 (9) |
| CRRT access, n (%) | |
| Internal jugular vein | 52 (78) |
| Femoral vein | 13 (9) |
| Subclavian vein | 2 (3) |
| Critical illness parameters | |
| CFB, liters, median (IQR) | 2.22 (−8.8 to 26.0) |
| Pressor or inotrope, n (%) | 63 (92.6) |
| Mechanical ventilation, n (%) | 65 (95.6) |
| Mechanical ventilation d, median (IQR) | 10.2 (5.6–13.3) |
| PRBC transfusion, n (%) | 52 (77) |
| SOFA score, median (IQR) | 13 (11–15) |
| ICU length of stay, median (IQR) | 13 (10–21) |
| Hospital length of stay, median (IQR) | 25.0 (16.7–13.3) |
| Hospital mortality, n (%) | 27 (39.7) |
| Discharge destination, n (%) | |
| Home | 9 (13.4) |
| Rehabilitation (acute and subacute) | 15 (22.4) |
| LTAC | 13 (19.4) |
| Hospice | 5 (7.4) |
| Hospital mortality | 25 (37.3) |
BMI, body mass index; CFB, cumulative fluid balance, represents net difference between fluid in/out from hospital admission to initiation of CRRT; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CVVHDF, continuous veno-venous hemodiafiltration; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; LTAC, long-term acute care; OT, occupational therapy; PRBC, packed red blood cells; PT, physical therapy; SCr, serum creatinine; SCUF, slow continuous ultrafiltration; SOFA, sequential organ failure assessment.
The CRRT and Early Rehabilitation Protocol: Safety Assessment
No major adverse events or unintended stoppage of CRRT occurred during the study period. Rehabilitation clinicians reported 5 minor adverse events (4.5%) in 5 distinct patients (2 hypotensive events with mean arterial pressure <60 mm Hg, 1 bradycardic event <40 beats per minute, and 1 event of new-onset atrial fibrillation). The fifth event occurred while the patient was sitting at the edge of the bed when a CRRT “low flow rate alarm” was triggered that coincided with a transient drop in oxygen saturation (SaO2 98% to 87%). Accordingly, the physical therapist selected to return the patient to supine position and such CRRT functionality and oxygenation returned to baseline. A sixth event was reported with the bedside nurse preventing the patient from sitting at the edge of the bed because of concerns with risk of accidentally dislodging the CRRT catheter. A review of the event by the implementation team determined that this preemptive termination of rehabilitation was related to nurse fear of mobility and not related to the protocol itself.
The CRRT and Early Rehabilitation Protocol: Feasibility by 8 Domains
The following are the 8 domains.25
-
(i)
Acceptability: There were no reports of resistance to this protocol at any time. Nurse deferral of rehabilitation session not related to exclusion criteria occurred only 4 times of 152 total attempts. These deferrals occurred in 4 different patients: 2 occurred because the nurse requested rehabilitation clinicians to return later, as the nephrology team planned to transition the patient off CRRT; 1 deferral occurred to allow the patient to sleep, and 1 occurred because of patient anxiety.
-
(ii)
Demand: The protocol was delivered and perceived as an important change in culture of early rehabilitation and remains active.
-
(iii)
Implementation: A total of 152 rehabilitation sessions were attempted and 112 were completed (73.6%). Of the 40 deferred sessions, the primary reason for deferral of rehabilitation was a recent decline in clinical status (n = 18, 45%) such that the patient no longer met criteria for appropriateness listed in Figure 1 (Safety Screening). Additional reasons for deferral included increased sedation and are listed in Table 2. Of the 112 completed sessions, the median number of sessions per patient was 1.0 (IQR, 1.0–2.0), with a median rate of sessions per CRRT days of 0.21 (IQR, 0.15–0.33) and a median time from ICU admission to first rehabilitation of 5.0 (IQR, 2.0–8.5) days.
-
(iv)
Practicality: The protocol was routinely performed over an 18-month period by 2 full-time physical therapists and 1.5 occupational therapists (46 MICU beds). Moreover, patients refused only twice to engage in this protocol demonstrating, that these individuals can participate in rehabilitation.
-
(v)
Adaptation: No changes were made to the protocol during the 18-month period of implementation.
-
(vi)
Integration: Protocol implementation occurred without adding staff, demonstrating low-cost feasibility of this project.
-
(vii)
Expansion: This study was implemented only in the MICU, but the protocol is currently being expanded to the trauma ICU.
-
(viii)
Limited efficacy testing: An exploratory analysis was performed to determine if early rehabilitation with emphasis on mobility associated with clinical outcomes.
Table 2.
Rehabilitation characteristics
| Rehabilitation characteristics | PT/OT intervention |
|---|---|
| n = 67 | |
| Time to initial PT/OT evaluation, d, median (IQR) | 5.0 (2.0–8.5) |
| Number of rehabilitation sessions per patient, median (IQR) | 1.0 (1.0–2.0) |
| Ratio of rehabilitation sessions per CRRT d, median (IQR) | 0.21 (0.15–0.33) |
| Total number of completed sessions, n (%) | 112 (74) |
| Total number of attempted sessions, n (%) | 40 (26) |
| Reasons for deferral/unable to complete sessions, n (%) | |
| Sedated | 13 (32.5) |
| Recent change or decline in clinical status | 18 (45) |
| Patient refused | 2 (5) |
| Agitation | 2 (5.0) |
| Pain | 1 (2.5) |
| Nurse deferred | 4 (10) |
CRRT, continuous renal replacement therapy; IQR, interquartile range; OT, occupational therapy; PT, physical therapy.
The CRRT and Early Rehabilitation Protocol: Limited Efficacy Testing
There were no associations between age, sex, race, body mass index, Charlson comorbidity index, and SOFA score at ICU admission and the number of completed rehabilitation sessions. The number of completed rehabilitation sessions directly correlated with MV days, hospital LOS, ICU LOS, and CRRT days (r = 0.392, 0.254, 0.384, and 0.467, respectively). The number of completed rehabilitation sessions was not associated with hospital mortality. The ratio of completed rehabilitation sessions per CRRT days directly correlated with MV days and CRRT days (r = 0.345 and 0.640, respectively). Time from ICU admission to first rehabilitation also directly correlated with MV days, hospital LOS, ICU LOS, and CRRT days (r = 0.425, 0.289, 0.444, and 0.399, respectively) (Table 3), but was not associated with hospital mortality. Patients achieving a higher level of mobility were more likely to be alive at time of hospital discharge, although statistically not significant (χ2 = 9.96, P = 0.076) (Supplementary Figure S1). In addition, shorter time from ICU admission to first rehabilitation session was associated with achieving higher mobility status (r = −0.292, P = 0.017).
Table 3.
Correlations of CRRT and critical illness parameters with selected rehabilitation parameters
| Selected critical illness parameters | Completed rehabilitation sessions | Ratio of completed rehabilitation sessions to CRRT d | Time (d) to first rehabilitation session | Highest mobility achieved |
|---|---|---|---|---|
| MV, d | 0.392, P = 0.001 | 0.345, P = 0.004 | 0.425, P = 0.004 | −0.004, P = 0.974 |
| Hospital LOS | 0.254, P = 0.038 | −0.014, P = 0.908 | 0.289, P = 0.018 | 0.125, P = 0.311 |
| ICU LOS | 0.384, P = 0.001 | −0.190, P = 0.123 | 0.444, P < 0.001 | 0.182, P = 0.141 |
| CRRT, d | 0.467, P < 0.001 | 0.640, P < 0.001 | 0.399, P < 0.001 | −0.013, P = 0.92 |
CRRT, continuous renal replacement therapy; ICU, intensive care unit; LOS, length of stay; MV, mechanical ventilator.
There were no significant correlations between rehabilitation parameters and age, body mass index, sequential organ failure assessment score at intensive care unit admission, and Charlson comorbidity index (data not shown).
Statistical analysis using Spearman rho test. Data presented as correlation coefficient, P value.
In multivariable analysis, none of the rehabilitation parameters was independently associated with hospital mortality. However, higher Charlson comorbidity index and the presence of liver disease were significantly associated with hospital mortality (odds ratio: 3.296; 95% confidence interval: 1.00–10.82; P = 0.049 and odds ratio: 1.29; 95% confidence interval: 1.01–1.66; P = 0.046, respectively) (Supplementary Table S1).
Discussion
The main finding of our study was that the implementation of an early rehabilitation protocol with emphasis on mobility for patients requiring CRRT in the ICU is safe and feasible. This quality improvement project confirms that patients on CRRT can safely engage in early rehabilitation interventions.15, 16, 17, 18, 19 Of interest, no major adverse events occurred and only 5 minor adverse events were reported; all transient in nature with no consequences in the patient’s clinical status or CRRT functionality. No unintended interruptions of CRRT occurred during these early rehabilitation interventions. However, these data should be interpreted cautiously, as the incidence of high levels of mobility was low (34% of sessions achieved active mobility). It is possible that passive activity is not enough physiological stimuli to elicit adverse events. Nonetheless, the achieved levels of mobility in our study are consistent with prior studies in the critically ill population.15,16,18
The safety and feasibility of early rehabilitation, activity, and mobility for patients requiring mechanical ventilation is well-established in the literature.8,9,13,14,26 Furthermore, early mobility has been shown to improve short- and long-term outcomes for patients with critical illness.7, 8, 9, 10, 11 Despite the supporting evidence, early interventions remain relatively low in clinical practice,27, 28, 29, 30 particularly in patients on CRRT. Low incidence of early mobility in clinical practice is thought to be multifactorial, including patient factors, such as heavy sedation, complex catheters and tubes, and hemodynamic instability.31, 32, 33, 34 ICU providers, staff, and culture also play important roles in preventing or advocating for early mobility.35, 36, 37 In the past decade, these concepts have been extended to patients requiring CRRT. In 2010, Pohlman et al.38 reported performing rehabilitation interventions in patients requiring MV and CRRT. Although the focus was on mobility in patients with MV, 9% of the sessions performed included patients requiring both MV and CRRT. Three years later, Talley et al.15 published the first study implementing early mobility in patients on CRRT. In 2014, Hodgson et al.26 published expert consensus stating that CRRT has a low risk of an adverse event during early rehabilitation.
After our protocol creation, the implementation team identified nursing acceptability as a key component to the success of this project. A previous study by Anekwe et al.39 identified that early mobilization is not a top priority (49% of respondents) of ICU clinicians; moreover, 58% felt they were not trained to implement these interventions. Thus, our interdisciplinary team provided educational sessions to the nursing and rehabilitation staff to highlight the protocol and address any concerns. To support the practicality of this protocol, the team provided education at regular staff meetings and through identified clinician champions.40 We were able to integrate the protocol in a cost-effective manner without adding meetings or paying for additional resources. After education, the protocol was implemented and continues to remain active. A mixed methods analysis of the first 18 months of protocol activation was performed to assess safety, feasibility, and limited efficacy. The feasibility of this project, specifically, the rate of completed sessions in reference to the total number of attempts (74% success rate) may be questioned. Clinically, however, this is more an indication of the severity and heterogeneity of our patient population. Most of the deferred sessions were explained by an increase in sedation or a recent decline in clinical status that required interventions (e.g., vasopressor support for hypotension). Furthermore, the current rehabilitation clinician-to-patient ratio (1:22) in the MICU may have played a role in reducing the total number of completed rehabilitation sessions decreasing the number of patients who received more than 1 rehabilitation session while on CRRT. The clinician-to-patient ratio also could have prevented appropriate progression to higher levels of mobility. Prior literature demonstrates that increasing rehabilitation staff can affect outcomes.41
Patients in this study had a high severity of acute illness (median SOFA score of 13), high percentage of concomitant MV, high frequency of receiving blood products, and a high prevalence of liver disease and end-stage kidney disease. Of the 112 rehabilitation sessions, 72% were performed in patients with both CRRT and MV. Therefore, the complexity of this medical ICU population may have limited the progression of active mobility supporting a higher frequency of passive interventions.
The limited efficacy analysis revealed that patients achieving higher levels of mobility were more likely to survive the hospitalization, although not statistically significant. These results should be interpreted cautiously, as the sample size is small and there is lack of a control group. Therefore, it is likely that patients achieving higher levels of mobility were less acutely ill and as such may have had favorable outcomes regardless of rehabilitation interventions. We also observed a relationship between shorter time from ICU admission to first rehabilitation session and higher mobility status. This observation suggests that early rehabilitation may be beneficial for delivery of more effective therapy. However, we did not find an association between shorter time to first rehabilitation and improvement in clinical outcomes such as reduced MV or length of hospital or ICU stay, perhaps limited by the small sample size of the study. Others previously demonstrated that longer time to first physical therapy was associated with longer hospital LOS.42 This is an important relationship that should be examined further to understand if reducing time to first rehabilitation therapy can affect clinical outcomes in these patients.
Our study has limitations. The primary limitation is the low frequency of rehabilitation sessions that included high levels of mobility, as most of these interventions were passive. This can be explained by a combination of reasons, including significant number of patients with high rates of sedation and high acuity of illness and comorbidity that would have prevented high levels of mobility regardless of CRRT. In addition, the culture of early mobility is improving in our institution but remains limited in complex patients in the ICU. Moreover, nursing fear of mobility despite the education provided may have prevented high levels of mobility. Another limitation is the absence of measures of functional outcomes, such as Physical Function ICU Test,43 gait speed, and sit to stand time in the limited efficacy testing facet of the study. Finally, as this was established as a quality improvement project, a control group was not available; consequently, we were not able to examine clinical outcomes in patients who did not receive rehabilitation interventions.
Conclusions
Implementation of an early rehabilitation protocol with focus on physical activity and mobility is safe and feasible in critically ill patients receiving CRRT, albeit only a low proportion of patients achieved high levels of mobility. These data are consistent with prior literature demonstrating that critically ill patients can safely engage in rehabilitation interventions without major adverse events and/or unintended CRRT interruptions. In our study, high comorbidity and acuity of illness may have played a role in preventing high levels of mobility. Further evidence is required to determine the efficacy of early rehabilitation in improving patient outcomes; specifically, interventional trials are warranted.
Disclosure
All the authors declared no competing interests.
Acknowledgments
KPM is currently supported in part by a Promotion of Doctoral Studies, Level II Scholarship from the Foundation for Physical Therapy Research. JAN is currently supported by an Early Career Pilot Grant from the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001998. The authors sincerely acknowledge the clinical physical therapists, occupational therapists, and countless nurses in the MICU that worked to implement this protocol, as well as the entire CRRT Quality Improvement Team.
Footnotes
Figure S1. Highest level of mobility achieved during rehabilitation sessions stratified by hospital mortality. Patients achieving higher levels of mobility were more likely to be alive at discharge, although not statistically significant (χ2 = 9.96, P = 0.076, Cramer’s V = 0.39).
Table S1. Multivariable logistic regression models of hospital mortality as the dependent variable and selected rehabilitation parameters analyzed as the main independent variable.
Supplementary Material
References
- 1.Appleton R.T., Kinsella J., Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc. 2015;16:126–136. doi: 10.1177/1751143714563016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens R.D., Dowdy D.W., Michaels R.K. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med. 2007;33:1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 3.de Jonghe B., Lacherade J.-C., Sharshar T. Intensive care unit-acquired weakness: risk factors and prevention. Crit Care Med. 2009;37:S309–S315. doi: 10.1097/CCM.0b013e3181b6e64c. [DOI] [PubMed] [Google Scholar]
- 4.Hermans G., Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herridge M.S., Cheung A.M., Tansey C.M. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 6.Herridge M.S., Tansey C.M., Matte A. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 7.Schweickert W.D., Pohlman M.C., Pohlman A.S. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Z., Peng X., Zhu B. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94:551–561. doi: 10.1016/j.apmr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Adler J., Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23:5–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Morris P.E., Goad A., Thompson C. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit Care Med. 2008;36:2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 11.Burtin C., Clerckx B., Robbeets C. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 12.Denehy L., Skinner E.H., Edbrooke L. Exercise rehabilitation for patients with critical illness: a randomized controlled trial with 12 months of follow-up. Crit Care. 2013;17:R156. doi: 10.1186/cc12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nydahl P., Sricharoenchai T., Chandra S. Safety of patient mobilization and rehabilitation in the intensive care unit. systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14:766–777. doi: 10.1513/AnnalsATS.201611-843SR. [DOI] [PubMed] [Google Scholar]
- 14.Bailey P., Thomsen G.E., Spuhler V.J. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35:139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 15.Talley C.L., Wonnacott R.O., Schuette J.K. Extending the benefits of early mobility to critically ill patients undergoing continuous renal replacement therapy: the Michigan experience. Crit Care Nurs Q. 2013;36:89–100. doi: 10.1097/CNQ.0b013e3182753387. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y.T., Haines T.P., Ritchie P. Early mobilization on continuous renal replacement therapy is safe and may improve filter life. Crit Care. 2014;18:R161. doi: 10.1186/cc14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownback C.A., Fletcher P., Pierce L.N., Klaus S. Early mobility activities during continuous renal replacement therapy. Am J Crit Care. 2014;23:348–351. doi: 10.4037/ajcc2014889. quiz 352. [DOI] [PubMed] [Google Scholar]
- 18.Toonstra A.L., Zanni J.M., Sperati C.J. Feasibility and safety of physical therapy during continuous renal replacement therapy in the intensive care unit. Ann Am Thorac Soc. 2016;13:699–704. doi: 10.1513/AnnalsATS.201506-359OC. [DOI] [PubMed] [Google Scholar]
- 19.Ragland C., Ochoa L., Hartjes T. Early mobilisation in intensive care during renal replacement therapy: a quality improvement project. Intens Crit Care Nurs. 2019;52:22–27. doi: 10.1016/j.iccn.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Hickmann C.E., Castanares-Zapatero D., Bialais E. Teamwork enables high level of early mobilization in critically ill patients. Ann Intensive Care. 2016;6:80. doi: 10.1186/s13613-016-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiarici A., Serpilli O., Andreolini M. An early tailored approach is the key to effective rehabilitation in the intensive care unit. Arch Phys Med Rehabil. 2019;100:1506–1514. doi: 10.1016/j.apmr.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Phelan S., Lin F., Mitchell M., Chaboyer W. Implementing early mobilisation in the intensive care unit: an integrative review. Int J Nurs Stud. 2018;77:91–105. doi: 10.1016/j.ijnurstu.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Nordon-Craft A., Moss M., Quan D., Schenkman M. Intensive care unit–acquired weakness: implications for physical therapist management. Phys Ther. 2012;92:1494–1506. doi: 10.2522/ptj.20110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Jonghe B., Sharshar T., Lefaucheur J.P. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288:2859–2867. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 25.Bowen D.J., Kreuter M., Spring B. How we design feasibility studies. Am J Prev Med. 2009;36:452–457. doi: 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgson C.L., Stiller K., Needham D.M. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care. 2014;18:658. doi: 10.1186/s13054-014-0658-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nydahl P., Ruhl A.P., Bartoszek G. Early mobilization of mechanically ventilated patients: a 1-day point-prevalence study in Germany. Crit Care Med. 2014;42:1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 28.Berney S.C., Rose J.W., Bernhardt J., Denehy L. Prospective observation of physical activity in critically ill patients who were intubated for more than 48 hours. J Crit Care. 2015;30:658–663. doi: 10.1016/j.jcrc.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Connolly B.A., Mortimore J.L., Douiri A. Low levels of physical activity during critical illness and weaning: the evidence-reality gap. J Intensive Care Med. 2019;34:818–827. doi: 10.1177/0885066617716377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berney S.C., Harrold M., Webb S.A. Intensive care unit mobility practices in Australia and New Zealand: a point prevalence study. Crit Care Resusc. 2013;15:260–265. [PubMed] [Google Scholar]
- 31.Dubb R., Nydahl P., Hermes C. Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc. 2016;13:724–730. doi: 10.1513/AnnalsATS.201509-586CME. [DOI] [PubMed] [Google Scholar]
- 32.Jolley S.E., Regan-Baggs J., Dickson R.P., Hough C.L. Medical intensive care unit clinician attitudes and perceived barriers towards early mobilization of critically ill patients: a cross-sectional survey study. BMC Anesthesiol. 2014;14:84. doi: 10.1186/1471-2253-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leditschke I.A., Green M., Irvine J. What are the barriers to mobilizing intensive care patients? Cardiopulm Phys Ther J. 2012;23:26–29. [PMC free article] [PubMed] [Google Scholar]
- 34.Morris P.E. Moving our critically ill patients: mobility barriers and benefits. Crit Care Clin. 2007;23:1–20. doi: 10.1016/j.ccc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Bailey P.P., Miller R.R., 3rd, Clemmer T.P. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10 Suppl):S429–S435. doi: 10.1097/CCM.0b013e3181b6e227. [DOI] [PubMed] [Google Scholar]
- 36.Green M., Marzano V., Leditschke I.A. Mobilization of intensive care patients: a multidisciplinary practical guide for clinicians. J Multidiscip Healthc. 2016;9:247–256. doi: 10.2147/JMDH.S99811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins R.O., Spuhler V.J., Thomsen G.E. Transforming ICU culture to facilitate early mobility. Crit Care Clin. 2007;23:81–96. doi: 10.1016/j.ccc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Pohlman M.C., Schweickert W.D., Pohlman A.S. Feasibility of physical and occupational therapy beginning from initiation of mechanical ventilation. Crit Care Med. 2010;38:2089–2094. doi: 10.1097/CCM.0b013e3181f270c3. [DOI] [PubMed] [Google Scholar]
- 39.Anekwe DE, Koo KK, de Marchie M, et al. Interprofessional survey of perceived barriers and facilitators to early mobilization of critically ill patients in Montreal, Canada [e-pub ahead of print]. J Intensive Care Med. https://doi.org/10.1177/0885066617696846. Accessed April 20, 2019. [DOI] [PubMed]
- 40.Bruce R., Forry C. Integrating a mobility champion in the intensive care unit. Dimens Crit Care Nurs. 2018;37:201–209. doi: 10.1097/DCC.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 41.Johnson J.K., Lohse B., Bento H.A. Improving outcomes for critically ill cardiovascular patients through increased physical therapy staffing. Arch Phys Med Rehabil. 2019;100:270–277.e1. doi: 10.1016/j.apmr.2018.07.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson A.M., Henning A.N., Morris P.E. Timing and amount of physical therapy treatment are associated with length of stay in the cardiothoracic ICU. Sci Rep. 2017;7:17591. doi: 10.1038/s41598-017-17624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denehy L., de Morton N.A., Skinner E.H. A physical function test for use in the intensive care unit: validity, responsiveness, and predictive utility of the physical function ICU test (scored) Phys Ther. 2013;93:1636–1645. doi: 10.2522/ptj.20120310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.