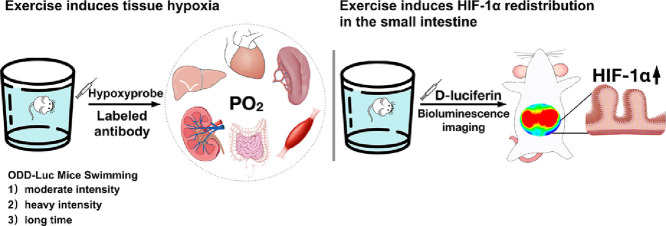
Highlights
-
•
Exercise induces tissue hypoxia redistribution in the small intestine.
-
•
Exercise increases the hypoxia-inducible factor-1α level in the small intestine.
-
•
The post-exercise hypoxia-inducible factor-1α level changes in a time-dependent manner.
Keywords: Blood flow redistribution, Hypoxia-inducible factor-1α, ODD-Luc, Pimonidazole HCl, Swimming
Abstract
Background
Exercise induces blood flow redistribution among tissues, leading to splanchnic hypoperfusion. Intestinal epithelial cells are positioned between the anaerobic lumen and the highly metabolic lamina propria with an oxygen gradient. Hypoxia-inducible factor (HIF)-1α is pivotal in the transcriptional response to the oxygen flux.
Methods
In this study, the pimonidazole hydrochloride staining was applied to observe the tissue hypoxia in different organs, which might be affected by the blood flow redistribution. The HIF-1α luciferase reporter ROSA26 oxygen-dependent degradation domain (ODD)-Luc/+ mouse model (ODD domain-Luc; female, n = 3–6/group) was used to detect the HIF-1α expression in the intestine. We used 3 swimming models: moderate exercise for 30 min, heavy-intensity exercise bearing 5% bodyweight for 1.5 h, and long-time exercise for 3 h.
Results
We found that 1 session of swimming at different intensities could induce tissue hypoxia redistribution in the small intestine, colon, liver and kidney, but not in the spleen, heart, and skeletal muscle. Our data showed that exercise exacerbated the extent of physiological hypoxia in the small intestine. Next, using ODD-Luc mice, we found that moderate exercise increased the in vivo HIF-1α level in the small intestine. The post-exercise HIF-1α level was gradually decreased in a time-dependent manner. Interestingly, the redistribution of tissue hypoxia and the increase of HIF-1α expression were not related to the exercise intensity and duration.
Conclusion
This study provided evidence that the small intestine is the primary target organ for exercise-induced tissue hypoxia and HIF-1α redistribution, suggesting that HIF-1α may be a potential target for the regulation of gastrointestinal functions after exercise.
Graphical abstract
1. Introduction
The lower gastrointestinal (GI) system is the center of nutrient absorption and the first line of defense against pathogens in the lumen.1 Previous studies from our laboratory and others have shown that moderate exercise has a protective effect against stress or GI disease-induced gut barrier dysfunction.2, 3 However, other studies have shown that heavy or strenuous exercise disrupts the intestinal immune homeostasis, thus increasing circulating bacteria, which can lead to whole-body inflammation.4, 5 Exercise decreases the splanchnic blood flow in both humans and rodents.6, 7 Splanchnic ischemia induces GI hypoperfusion, which increases the level of tissue hypoxia in the abdominal organs.8
Hypoxia-inducible factor (HIF) is pivotal in the transcriptional response to hypoxia.9 HIF is composed of a hypoxia-inducible α subunit and a constitutively expressed β subunit. In normoxic conditions, the HIFα is degraded through the prolyl hydroxylases (PHDs). Under hypoxic conditions, the activity of PHDs is inhibited, resulting in the accumulation of HIFα and initiation of downstream transcription. HIFα has 3 isoforms: the ubiquitous HIF-1α, the tissue-specific HIF-2α and HIF-3α.10 HIF-1α activates several important signaling pathways related to metabolism and inflammation.11 However, the HIF-1α protein is unstable in normoxic conditions, with a short half-life of 5 min,12 which increases the difficulty of in situ and in vivo detection of tissue hypoxia and HIF-1α expression.
Using HIF-1α luciferase reporter mice, we recently found that a single bout of moderate exercise could increase the abdominal HIF-1α level.2 In the present study, we hypothesized that exercise could induce tissue hypoxia and HIF-1α accumulation in the lower GI system. Pimonidazole hydrochloride (HCl) was used to detect in situ tissue hypoxia in the organs, which might be affected by the blood flow redistribution. Oxygen-dependent degradation domain (ODD)-Luc mice were also used in this study for detailed in vivo examination of HIF-1α expression. Using 3 exercise models, we find that exercise induced the tissue hypoxia redistribution and an increase in HIF-1α in the small intestine, but these results are not affected by the exercise intensity or duration. These observations, which suggest that HIF-1α may be a potential target for the regulation of GI functions after moderate exercise, may contribute to understanding of the role of exercise interventions in the protection against GI diseases.
2. Methods
2.1. Ethical approval
All experiments were performed in compliance with and approved by the Shanghai University of Sport Ethical Review Board (2014003). Animal care was performed in accordance with the China Laboratory Animal Management Regulations and the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, Washington, DC, USA).
2.2. Mice
HIF-1α luciferase reporter ROSA26 ODD-Luc/+ mice (ODD-Luc, #006206, Jackson Laboratory, Bar Harbor, ME, USA) and their background control Friend Virus B NIH Jackson (FVB/NJ) mice (FVB, #001800, Jackson Laboratory) were used in this study. ODD-Luc mice ubiquitously express a bioluminescent reporter consisting of firefly luciferase fused to the ODD region of HIF-1α.13 Because female mice are less aggressive than males during the heavy-intensity and long-time exercise interventions, we used 8- to 10-week-old female ODD-Luc mice and FVB mice for all studies (body weight = 26–28 g, n = 3–6/group). The mice were kept on a 12-h light/dark cycle (temperature 22°C ± 1°C with 55% ± 10% humidity) and given ad libitum access to food and water.
2.3. Exercise protocols
Different intensities and durations of swimming were selected as the exercise interventions in this study, in accordance with Resource Book for the Design of Animal Exercise Protocols (American Physiological Society, 2006). The mice were placed in tanks (42 cm × 26 cm × 18 cm) filled with warm sterile water (37°C ± 1°C) with a depth of 15–20 cm. Three exercise models were used: (1) Moderate Exercise (ME, SWIM): mice voluntarily swimming for 30 min, (2) Heavy-intensity Exercise (HE): mice swimming for 1.5 h with 5% body-weight loads attached to their tails, and (3) Long-time Exercise (LE): mice voluntarily swimming for 3 h or until fatigued. The mice were determined to be fatigued when they failed to rise to the surface of the water to breathe within 5 s. Sedentary mice (SED) were used as controls (CON).
2.4. Tissue hypoxia detection
Pimonidazole HCl is a hypoxia marker and forms stable protein adducts under in vivo conditions with a partial pressure of oxygen (PO2) of 10 mmHg or lower.14 These adducts are detectable with a specific monoclonal antibody (Hypoxyprobe-1, Hypoxyprobe Inc., Burlington, MA, USA). One hour after intraperitoneal (i.p.) injection of pimonidazole HCl solution (60 mg/kg body weight) in phosphate-buffered saline buffer (Sigma, St. Louis, MO, USA) and after exercise, the ODD-Luc mice were anesthetized with isoflurane (Yipin Pharmaceutical Co. Ltd., Shijiazhuang, Hebei, China) and humanely killed by cervical dislocation. Intestine (jejunum), colon, skeletal muscle, heart, liver, spleen and kidney specimens were fixed in 4% paraformaldehyde (Sigma). Then, immunohistochemical staining was performed following the manufacturer's instructions (Hypoxyprobe Inc.; 3 slices per animal). The results were observed with a microscope and recorded with imaging software (DP80, Olympus, Tokyo, Japan).
2.5. PX-478 treatment
S-2-Amino-3- [4′-N,N,-bis(2-chloroethyl) amino] phenyl propionic acid N-oxide dihydrochloride (PX-478; Selleck Chemicals, Houston, TX, USA) is a small-molecule inhibitor. PX-478 decreases HIF-1α mRNA levels and inhibits the translation and protein expression of HIF-1α.15, 16 The mice received an i.p. injection of PX-478 at a dose of 100 mg/kg body weight in a phosphate-buffered saline buffer.
2.6. Bioluminescence imaging
Immediately after exercise, the fur of the ODD-Luc mice was dried with a towel, and the mice were given an i.p. injection containing of d-luciferin (Promega, Madison, WI, USA) in phosphate-buffered saline buffer (150 mg/kg body weight). The mice were kept warm until the experiment was completed. Fifteen min later, the mice were anesthetized with isoflurane. Fluorescence imaging was performed using the IVIS Lumina system with Living Image software 4.5 (Caliper Life Sciences, Hopkinton, MA, USA). The positive control (9% O2) mice were placed in a hypoxic chamber containing 9% O2 for 4 h.13 Meanwhile, the normoxic control (SED) mice and FVB mice were exposed to 21% O2.
2.7. Statistical analysis
Data are presented as the mean ± SEM. Data were analyzed using Prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA). The Hypoxyprobe values were obtained in arbitrary units, and the mean optical density represented the intensity of staining in specific immunoreactive areas, calculated by the Image J image analysis software Version 1.52 (National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/) and associated plugins. Levene's test was performed to evaluate the homogeneity of variance, and one-way analysis of variance (ANOVA) with the Bonferroni post hoc test was used when the variance was homogeneous. The nonparametric Mann-Whitney U test or Kruskal-Wallis test was used to analyze data without a normal distribution. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Exercise-induced tissue hypoxia redistribution in the small intestine
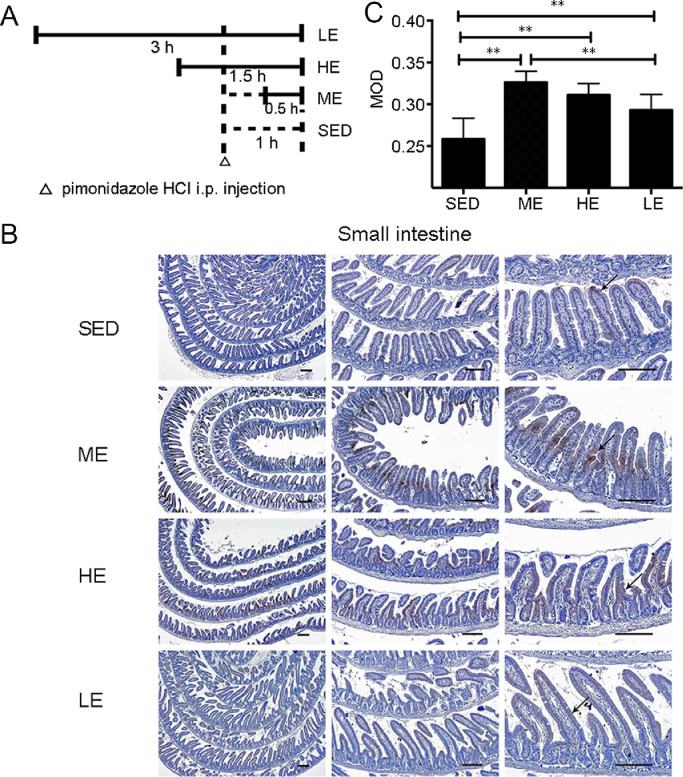
The hypoxia probe pimonidazole HCl was used to determine whether exercise could induce tissue hypoxia redistribution in the small intestine. Three swimming models were conducted to examine the effect of exercise intensity and duration on tissue hypoxia distribution (Fig. 1A). In the control SED group, the top of the villi in the small intestine were intensely stained, whereas the crypt was weakly stained, showing that the small intestine has a physiological O2 gradient from the top of villi to the crypt (Fig. 1B). One session of exercise altered the tissue hypoxia distribution in the small intestine. Positive staining in the crypts was markedly increased after exercise (Fig. 1B). However, the staining in the crypts of the LE group was less intense than that of the HE and ME groups (Fig. 1B and 1C). Taken together, the results show that moderate- and heavy-intensity exercise can induce tissue hypoxia redistribution in the small intestine, indicating that the small intestine is susceptible to exercise-induced tissue hypoxia.
Fig. 1.
Exercise-induced hypoxia redistribution in the small intestine. Pimonidazole HCl was used to detect the tissue hypoxia in the small intestine of ODD-Luc mice. (A) Horizontal bars indicate the time-points of experiments. (B) The immunostaining (arrows) show low PO2 in the villus of the SED mice. Exercise markedly increased the staining in the crypts. However, the intensified staining in the crypts was observed in the ME and HE groups but not in the LE group. (C) The MOD indicated the intensity of staining in specifically immunoreactive areas in the intestine of the SED, ME, HE, and LE groups. n = 6/group; Scale bars = 100 μm. ** p < 0.01. HCl = hydrochloride; HE = heavy-intensity exercise; i.p. = intraperitoneal; LE = long-time exercise; ME = moderate exercise; MOD = mean optical density; ODD = oxygen-dependent degradation domain; PO2 = partial pressure of oxygen; SED = sedentary control.
3.2. Exercise-induced tissue hypoxia redistribution in other organs
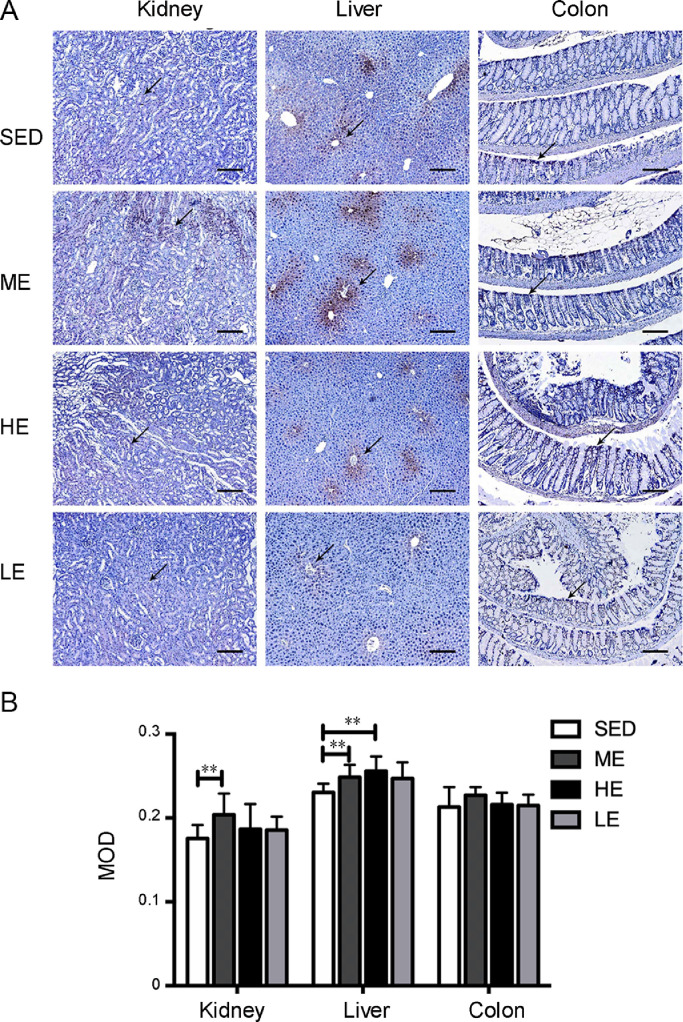
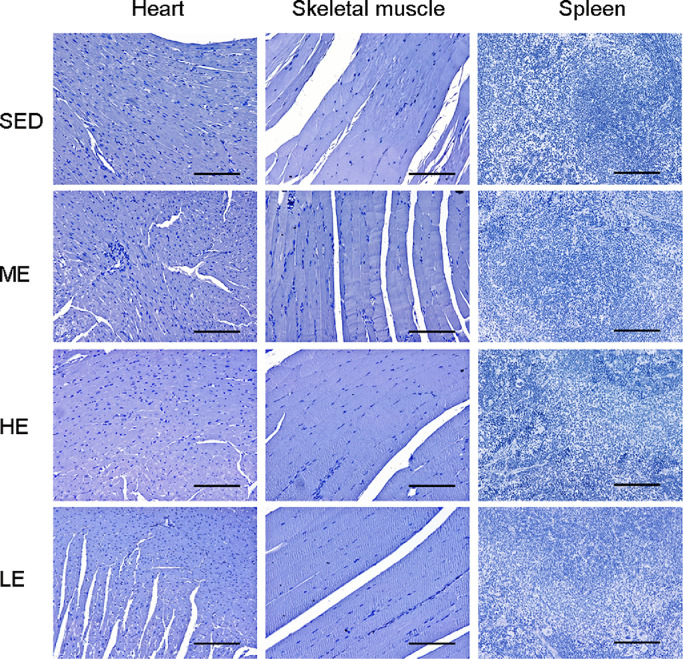
The post-exercise distributions of pimonidazole HCl immunostaining were enhanced in other abdominal organs. As shown in Fig. 2, the ME group displayed a greater extent of positive staining in the kidney, liver, and colon than the control SED group. The renal tubules of the kidney showed positive staining for hypoxia. In the liver, the positive staining radiated outward from the central veins. In the colon, positive staining was retained from the crypt to the villi. Interestingly, the enhancement of pimonidazole HCl staining in the HE and LE groups was not as strong as we expected, suggesting that the exercise-induced tissue hypoxia redistribution is not affected by the exercise intensity or duration. We also examined the tissue hypoxia distribution in the heart, skeletal muscle, and spleen, which are organs thought to maintain or increase blood volume during exercise. No positive staining in these organs was found in either the exercised and sedentary mice (Fig. 3).
Fig. 2.
Moderate but not heavy-intensity or long-time exercise induced tissue hypoxia in the kidney, liver, and colon. (A) Pimonidazole HCl was used to detect the tissue hypoxia in the kidney, liver, and colon of ODD-Luc mice. We found increased immunostaining in the kidney, liver, and colon of the ME group but not in the HE or LE group, compared to the SED group; The arrows show the areas of the renal tubules, the central veins and the crypt, which showed positive staining after 1 session of moderate exercise. (B) The MOD indicated the intensity of staining in specifically immunoreactive areas of the kidney, liver, and colon in the SED, ME, HE, and LE groups. n = 6/group; Scale bars = 100 μm. **p < 0.01. HC1 = hydrochloride; HE = heavy-intensity exercise; LE = long-time exercise; ME = moderate exercise; MOD = mean optical density; ODD = oxygen-dependent degradation domain; SED = sedentary control.
Fig. 3.
Exercise did not induce hypoxia in the heart, skeletal muscle or spleen tissue. Pimonidazole HCl was used to detect the tissue hypoxia. No positive staining was observed in the heart, skeletal muscle, and spleen of the ODD-Luc mice with or without exercise intervention. n = 6/group; Scale bars = 100 μm. HCl = hydrochloride; HE = heavy-intensity exercise; LE = long-time exercise; ME = moderate exercise; ODD = oxygen-dependent degradation domain; SED = sedentary control.
3.3. Exercise increased the HIF-1α level in the small intestine
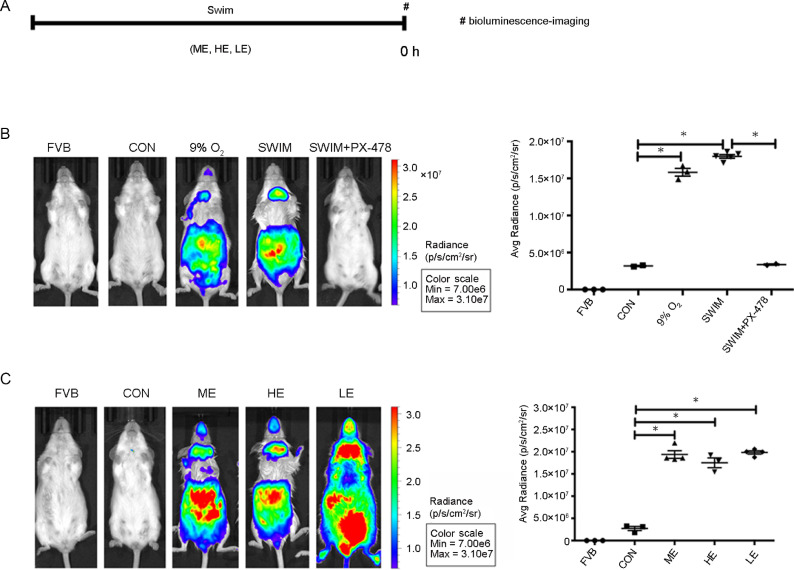
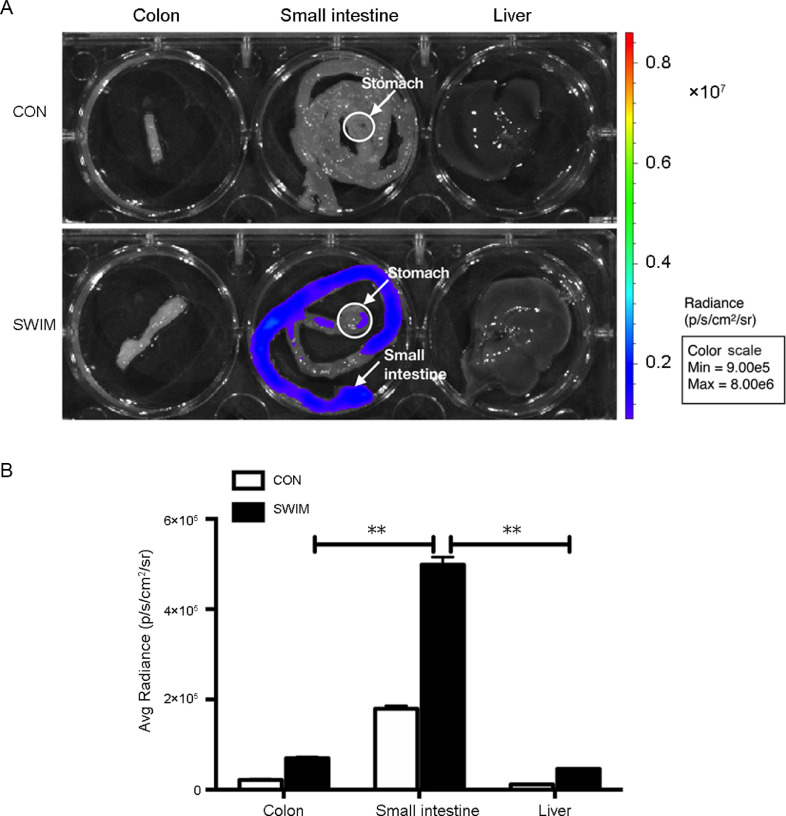
We examined in vivo HIF-1α expression in the abdominal area of ODD-Luc mice to confirm that the HIF-1α level was increased by the exercise-induced tissue hypoxia (Fig. 4A). In Fig. 4B, greater abdominal luciferase activity was found in the positive control ODD-Luc mice which were placed in 9% O2 hypoxic chambers for 4 h than that in the normoxia control ODD-Luc mice (9% O2 vs. CON, p = 0.006). No luciferase activity was detected in the background control FVB/NJ mice. The mice that were i.p. injected with the HIF-1α inhibitor PX-478 showed no luciferase activity after moderate exercise (SWIM+PX-478 vs. SWIM, p = 0.0003). In Fig. 4C, the luciferase activity was increased after exercise (ME vs. CON, p = 0.0053; HE vs. CON, p = 0.0223; LE vs. CON, p = 0.0005). However, no differences were found among the 3 exercise groups (ME vs. HE, p > 0.99; ME vs. LE, p > 0.99; HE vs. LE, p > 0.99). Next, we observed the expression of HIF-1α in the abdominal organs to determine which organs were most susceptible to exercise-induced tissue hypoxia. As shown in Fig. 5, the stomach, small intestine, colon, and liver of ODD-Luc mice were immediately harvested after one session of moderate exercise and luciferase activity was then detected. We found that the small intestine had a higher photon level than the colon and liver, suggesting that the small intestine is the target organ of exercise-induced tissue hypoxia and HIF-1α redistribution (p < 0.01).
Fig. 4.
Exercise increased HIF-1α in the abdominal area. Bioluminescent imaging was used to detect HIF-1α expression in vivo. Bioluminescent imaging was used to detect HIF-1α expression in vivo. (A) Horizontal bars show the time-points of experiments. (B) Photons in the abdominal area of mice (left). FVB = FVBN/J mice were exposed to normoxic conditions, n = 3/group; CON = ODD-Luc mice were exposed to normoxic conditions, n = 3/group; 9% O2 = ODD-Luc mice were exposed to 9% O2 for 4 h, n = 3/group; SWIM = ODD-Luc mice swam for 30 min, n = 5/group; SWIM+PX-478 = ODD-Luc mice injected with PX-478 and swam for 30 min, n = 3/group. The average photon radiance of the region of interest was also calculated (right). (C) Photons in the abdominal area of mice that underwent different exercise interventions (left). FVB = FVBN/J mice were exposed to normoxic conditions, n = 3/group; CON = ODD-Luc mice were exposed to normoxic conditions, n = 3/group; ME = ODD-Luc mice swam for 30 min, n = 4/group; HE = heavy-intensity exercise of ODD-Luc mice, n = 3/group; LE = long-time exercise of ODD-Luc mice, n = 4/group. The average photon radiance of the region of interest was also calculated (right). The color bar indicates the photon level (cm2/s/steradian) and the minimum and maximum threshold values. *p < 0.05. HE = heavy-intensity exercise; HIF = hypoxia-inducible factor; LE = long-time exercise; ME = moderate exercise; ODD = oxygen-dependent degradation domain.
Fig. 5.
Moderate exercise-induced HIF-1α expression in the small intestine. (A) ODD-Luc mice were immediately anesthetized and sacrificed after a bout of 30 min of swimming, n = 3/group. Their abdominal cavity viscera were removed for bioluminescence imaging to detect HIF-1α expression. The small intestine generated a greater level of photons (arrows) than the colon, liver, and stomach. (B) The photon levels in the colon, small intestine, and liver of ODD-Luc mice were recorded after a moderate exercise bout, n = 3/group. **p < 0.01. The color bar indicates the photon level (cm2/s/steradian) and the minimum and maximum threshold values. CON = ODD-Luc mice were exposed to normoxic conditions; HIF = hypoxia-inducible factor; ODD = oxygen-dependent degradation domain.
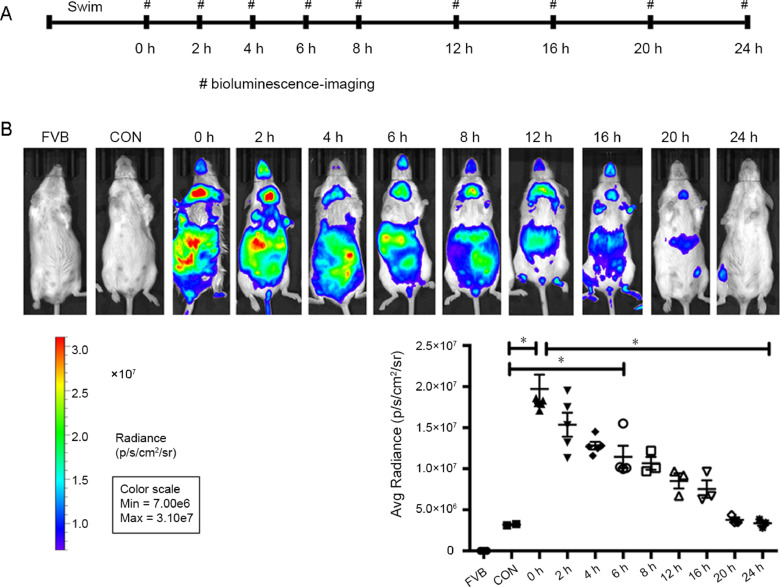
3.4. The post-exercise HIF-1α level was altered in a time-dependent manner
To understand the expression pattern of HIF-1α in the small intestine after exercise, we measured the in vivo HIF-1α expression in the abdominal area of the ODD-Luc mice at rest, to obtain the baseline values, and at the 0th, 2nd, 4th, 6th, 8th, 12th, 16th, 20th, and 24th h after a moderate exercise bout. As shown in Fig. 6, a time-course analysis of photons indicated that HIF-1α expression significantly increased after exercise and gradually decreased to the baseline level. The photon level was increased at the 0th h after exercise in the ODD-Luc mice compared to the sedentary group (p < 0.0001). The photon level then decreased over time, and the levels at the 2nd, 4th and 6th post-exercise hours were still greater than those of the control group (2nd h vs. CON, p < 0.0001; 4th h vs. CON, p = 0.0037; 6th h vs. CON, p = 0.0326) and returned to the baseline in the next 24 h (24th h vs. CON, p > 0.99). In summary, these findings demonstrate that exercise altered the HIF-1α distribution in the small intestine in a time-dependent manner.
Fig. 6.
The post-exercise HIF-1α level changed in a time-dependent manner. Bioluminescent imaging was applied to detect HIF-1α expression in vivo. (A) Horizontal bars show the time-points of detection in the SWIM group. (B) The photon levels in the abdominal area of ODD-Luc mice were recorded at the 0th, 2nd, 4th, 6th, 8th, 12th, 16th, 20th, and 24th h after a moderate exercise bout of swimming. 0th h: n = 4/group; 2nd h: n = 5/group; 4th h: n = 4/group; 6th h: n = 4/group; 8th h: n = 3/group; 12th h: n = 3/group; 16th h: n = 3/group; 20th h: n = 3/group; 24th h: n = 3/group. The scattergram indicates the individual expression of HIF-1α at different time-points. Luciferase activity was increased at the 0th h post-exercise and gradually returned to the baseline value during the next 24 h. The color bar indicates the photon level (cm2/s/steradian) and minimum and maximum threshold values. *p < 0.05. CON = ODD-Luc mice were exposed to normoxic conditions; FVB = FVBN/J mice were exposed to normoxic conditions; HIF = hypoxia-inducible factor; ODD = oxygen-dependent degradation domain.
4. Discussion
Exercise results in a remarkable redistribution of blood flow, which increases in active skeletal muscles but decreases in the splanchnic circulation.17 The regional blood flow in the kidney, spleen, stomach, and intestine was measured by using the microsphere technique in rats. Regional vascular resistance of the intestine was 29.5 mmHg/mL/min/g before exercise and increased to 84.5 mmHg/mL/min/g after exercise, and the results showed that the intestinal blood flow was decreased by exercise.18, 19 The effect of exercise on gastric mucosal perfusion adequacy has been investigated using air tonometry in athletes, with the results suggesting that GI system ischemia was present in all athletes during maximum intensity exercise and in 50% of the athletes during submaximal exercise.7 Athletes with GI symptoms, such as stomach pain, diarrhea, and constipation, had an increased susceptibility to developing ischemia during exercise.7 However, the relationship between exercise-induced lower blood perfusion and hypoxia has rarely been studied in mouse models.
Sufficient blood perfusion is important to maintain GI system function, deliver oxygen, and nutrients, and remove the products of metabolism. The small intestine has a unique oxygenation characteristic, that is, regular fluctuations in blood perfusion.20 Additionally, in the small intestine, a steep O2 gradient is present from the villi to the crypt and from the anaerobic lumen to the epithelial mucosa, leading to graded hypoxia.20 Intestinal epithelial cells are positioned between the anaerobic lumen and the highly metabolic lamina propria and therefore are located in a physiologically hypoxic environment with a steep O2 gradient.21 In the lumen, PO2 is less than 1 mmHg.20
In the present study, we investigated tissue hypoxia distribution in the internal organs of exercised mice and sedentary mice. The local PO2 was detected using pimonidazole HCl.22 In the control group, the pimonidazole HCl staining results were consistent with those of other studies23, 24 and showed an oxygen gradient in the small intestine (Fig. 1) and tissue hypoxia in the liver and the kidney (Fig. 2). A single bout of moderate exercise exacerbated the hypoxia in small intestine the kidney, liver, and colon (Figs. 1 and 2). In the small intestine, both the location and extent of hypoxic tissue were altered (Fig. 1). However, positive staining was not found before or after exercise in the heart, skeletal muscle, and spleen tissues (Fig. 3).
HIF-1α is pivotal for survival, metabolism, and oxygen homeostasis.25 PHDs hydroxylate a prolyl residue in the amino- and the carboxy-terminal ODD domains.26 Factor-inhibiting HIF hydroxylates an asparagine in the carboxy-terminal activation domain. The regulation of both PHDs and factor-inhibiting HIF results in the destruction of the HIFα subunit and inactivation of transcriptional activity.27 During hypoxia, these processes are inhibited, and a transcriptionally active complex is formed. Under normoxic conditions, PHDs, which are HIF hydroxylases, are activated. The HIF-1α protein level in the brains of mice exposed to 6% O2 for 75 min was half of its maximum level 15 min after the mice were returned to normoxic conditions and decreased to normoxic levels 60 min later, indicating a rapid degradation rate of HIF-1α in vivo.28 Another study showed that the HIF-1α protein is unstable because it has a short half-life of 5 min,12 which increases the difficulty of detecting HIF-1α under normoxic conditions. Therefore, we used ODD-Luc mice that provided a bioluminescent reporter consisting of firefly luciferase fused to a region of HIF-1α. Many applications of HIF-1α have been described, including gene regulation, tumor growth, and inflammation.29, 30
In this study, a HIF-1α reporter mouse was used to further study the influence of exercise on HIF-1α distribution. We found that moderate exercise increased HIF-1α in the abdominal area in vivo (Fig. 4), and we further confirmed that the expression of HIF-1α was increased in the small intestine (Figs. 5A and 5B). We evaluated the expression of HIF-1α in the small intestine by using 3 exercise models. However, the increase in HIF-1α level was not affected by the exercise intensity (Fig. 4). Additionally, we measured the photon output at different times after exercise and found a gradual reduction. The level of photons emitted at the 0th h, 2nd h, 4th h, and 6th h was greater in the ME group than that in the control group (Fig. 6). Previous studies have shown that the duration of hypoxia influences the reoxygenation response.31 The delay in HIF-1α protein degradation after exercise may reflect the necessity of retaining this protein until its target genes are upregulated,32 whereas the HIF-1α and HIF-1β mRNA levels were unaffected by changes in oxygen tension.33 Considering the role of PHDs in HIF-1α degradation under normoxic conditions, one possible reason for the time-dependent change in HIF-1α level after an increases in PO2 could be the recovery rate of PHD activity.
There are, however, a few limitations to our study. The observed alterations in our study could be the effect of exercise intensity, exercise duration, or the collective effect of exercise duration and intensity. Because the duration and exercise intensities are different among the 3 groups, determining the exact cause for the observed alterations is problematic. This limitation could be addressed in future research.
5. Conclusion
Our study demonstrated that the small intestine is the primary target organ for exercise-induced tissue hypoxia and HIF-1α redistribution. These observations suggest that exercise affects HIF-1α and the regulation of GI functions. The study may help us understand the protective role that moderate exercise plays in the prevention of stress or GI symptom-induced gut barrier dysfunction.
Acknowledgments
Acknowledgments
We thank Shennian Ge and Professor Yexiong Tan, Eastern Hepatobiliary Surgery Hospital, Yanqiong Cheng and Professor Xiaoyuan Zi, Changhai Hospital, Second Military Medical University (SMMU), for bioluminescent imaging, and Changchen Liu, Yongchao Cai, and Professor Zhiying He, Shanghai East Hospital, Tongji University, for technical help and animal studies.
This research was supported by National Natural Science Foundation of China (Grant number: 31471135, 31701040, and 31801003), Shanghai Sailing Program (Grant number: 17YF1418000), and Shanghai Municipal Education Commission (Grant number: Chenguang Program 16CG57).
Authors’ contributions
While conducting this study, DW played a role in performing the experiment, collecting the data, interpreting the data, and drafting the manuscript; WC played a role in performing the experiment and collecting the data; DX played a role in performing the experiment, collecting the data, drafting the manuscript, and revising the manuscript critically for important intellectual content; YPH played a role in revising the manuscript critically for important intellectual content; BL played a role in performing the experiment, interpreting the data, drafting the manuscript, and revising the manuscript critically for important intellectual content; PC played a role in revising the manuscript critically for important intellectual content. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Contributor Information
Beibei Luo, Email: bluo@sus.edu.cn.
Peijie Chen, Email: chenpeijie@sus.edu.cn.
References
- 1.Ley R.E., Peterson D.A., Gordon J.I. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Luo B., Xiang D., Wu D., Liu C., Fang Y., Chen P. Hepatic PHD2/HIF-1α axis is involved in postexercise systemic energy homeostasis. FASEB J. 2018;32:4670–4680. doi: 10.1096/fj.201701139R. [DOI] [PubMed] [Google Scholar]
- 3.Allen J.M., Mailing L.J., Cohrs J., Salmonson C., Fryer J.D., Nehra V. Exercise training-induced modification of the gut microbiota persists after microbiota colonization and attenuates the response to chemically-induced colitis in gnotobiotic mice. Gut Microbes. 2018;9:115–130. doi: 10.1080/19490976.2017.1372077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mach N., Fuster-Botella D. Endurance exercise and gut microbiota: a review. J Sport Health Sci. 2017;6:179–197. doi: 10.1016/j.jshs.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland A.M., Hyatt H.W., Smuder A.J., Sollanek K.J., Morton A.B., Roberts M.D. Influence of endurance exercise training on antioxidant enzymes, tight junction proteins, and inflammatory markers in the rat ileum. BMC Res Notes. 2015;8:514. doi: 10.1186/s13104-015-1500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Otte J.A., Oostveen E., Geelkerken R.H., Groeneveld A.B., Kolkman J.J. Exercise induces gastric ischemia in healthy volunteers: a tonometry study. J Appl Physiol. 2001;91:866–871. doi: 10.1152/jappl.2001.91.2.866. [DOI] [PubMed] [Google Scholar]
- 7.ter Steege R.W., Geelkerken R.H., Huisman A.B., Kolkman J.J. Abdominal symptoms during physical exercise and the role of gastrointestinal ischaemia: a study in 12 symptomatic athletes. Br J Sports Med. 2012;46:931–935. doi: 10.1136/bjsports-2011-090277. [DOI] [PubMed] [Google Scholar]
- 8.van Wijck K., Lenaerts K., Grootjans J., Wijnands K.A., Poeze M., van Loon L.J. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol. 2012;303:G155–G168. doi: 10.1152/ajpgi.00066.2012. [DOI] [PubMed] [Google Scholar]
- 9.Semenza G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzo F.R., Huff C., Myllymäki M., Olenchock B., Swierczek S., Tashi T. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46:951–956. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nizet V., Johnson R.S. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L.E., Gu J., Schau M., Bunn H.F. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safran M., Kim W.Y., O'Connell F., Flippin L., Günzler V., Horner J.W. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci U S A. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ljungkvist A.S., Bussink J., Rijken P.F., Raleigh J.A., Denekamp J., Van Der Kogel A.J. Changes in tumor hypoxia measured with a double hypoxic marker technique. Int J Radiat Oncol Biol Phys. 2000;48:1529–1538. doi: 10.1016/s0360-3016(00)00787-2. [DOI] [PubMed] [Google Scholar]
- 15.Welsh S., Williams R., Kirkpatrick L., Paine-Murrieta G., Powis G. Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxia-inducible factor-1α. Mol Cancer Ther. 2004;3:233–244. [PubMed] [Google Scholar]
- 16.Koh M.Y., Spivak-Kroizman T., Venturini S., Welsh S., Williams R.R., Kirkpatrick D.L. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α. Mol Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 17.Laughlin M.H., Armstrong R.B., White J., Rouk K. A method for using microspheres to measure muscle blood flow in exercising rats. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:1629–1635. doi: 10.1152/jappl.1982.52.6.1629. [DOI] [PubMed] [Google Scholar]
- 18.Reinhardt C.P., Dalhberg S., Tries M.A., Marcel R., Leppo J.A. Stable labeled microspheres to measure perfusion: validation of a neutron activation assay technique. Am J Physiol Heart Circ Physiol. 2001;280:H108–H116. doi: 10.1152/ajpheart.2001.280.1.H108. [DOI] [PubMed] [Google Scholar]
- 19.Maeda S., Miyauchi T., Iemitsu M., Tanabe T., Irukayama-Tomobe Y., Goto K. Involvement of endogenous endothelin-1 in exercise-induced redistribution of tissue blood flow: an endothelin receptor antagonist reduces the redistribution. Circulation. 2002;106:2188–2193. doi: 10.1161/01.cir.0000038362.16740.a2. [DOI] [PubMed] [Google Scholar]
- 20.Albenberg L., Esipova T.V., Judge C.P., Bittinger K., Chen J., Laughlin A. Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology. 2014;147:1055–1063. doi: 10.1053/j.gastro.2014.07.020. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuta G.T., Turner J.R., Taylor C.T., Hershberg R.M., Comerford K., Narravula S. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizukami Y., Jo W.S., Duerr E.M., Gala M., Li J., Zhang X. Induction of interleukin-8 preserves the angiogenic response in HIF-1α-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 23.Samoszuk M.K., Walter J., Mechetner E. Improved immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. J Histochem Cytochem. 2004;52:837–839. doi: 10.1369/jhc.4B6248.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semenza G.L. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 26.Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF‐1α in normoxia. EMBO J. 2003;22:4082–4090. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schofield C.J., Ratcliffe P.J. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 28.Stroka D.M., Burkhardt T., Desbaillets I., Wenger R.H., Neil D.A., Bauer C. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 29.Li F., Sonveaux P., Rabbani Z.N., Liu S., Yan B., Huang Q. Regulation of HIF-1α stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada H., Itasaka S., Kizaka-Kondoh S., Shibuya K., Morinibu A., Shinomiya K. Akt/mTOR pathway assures the synthesis of HIF-1alpha protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J Biol Chem. 2009;284:5332–5342. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 31.Berra E., Richard D.E., Gothié E., Pouysségur J. HIF-1-dependent transcriptional activity is required for oxygen-mediated HIF-1α degradation. FEBS Lett. 2001;491:85–90. doi: 10.1016/s0014-5793(01)02159-7. [DOI] [PubMed] [Google Scholar]
- 32.Jewell U.R., Kvietikova I., Scheid A., Bauer C., Wenger R.H., Gassmann M. Induction of HIF-1α in response to hypoxia is instantaneous. FASEB J. 2001;15:1312–1314. [PubMed] [Google Scholar]
- 33.Huang L.E., Arany Z., Livingston D.M., Bunn H.F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its α subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]