Bilateral renal cystic disease presenting as chronic kidney disease (CKD) in adults is most commonly due to autosomal dominant polycystic kidney disease (ADPKD). Genetic investigation of ADPKD cohorts identifies the underlying PKD1 (77%) or PKD2 mutation (15%) and up to 8% of cases remain as “no PKD mutation detected” (NMD).1 Molecular characterization of NMD-ADPKD cases by genome level sequencing such as whole exome sequencing (WES) may identify individuals misclassified as ADPKD based on clinical and imaging criteria. For example, HANAC (hereditary angiopathy, nephropathy, aneurysms, muscle cramps) syndrome caused by heterozygous COL4A1 mutations can cause bilateral kidney cysts that may phenocopy ADPKD.2, 3, 4
Thin glomerular basement membrane (TBM) disease usually presents with persistent often familial microscopic hematuria with or without CKD progression. A few reports mention the finding of kidney cysts in patients with TBM disease but this association remains largely underrecognized.5,6 Because TBM disease is attributed to heterozygous mutations in COL4A3 and COL4A4,5 we hypothesize that these cysts may be an additional incompletely penetrant consequence of a pathogenic mutation in these type IV collagen proteins. Heterozygous mutations in COL4A3 or COL4A4 may thus explain the presence of kidney cysts not due to ADPKD. We report the WES-based genetic investigation identifying type IV collagen mutations in patients with bilateral kidney cysts who were either NMD-ADPKD or were known to carry a diagnosis of TBM disease. A causal effect of these type IV collagen mutations or their modifier role in the progression of a cystic kidney phenotype remains to be established.
Results
We performed WES on 18 patients with findings of multiple bilateral kidney cysts that were either classified as NMD-ADPKD (13 patients) or carried a diagnosis of TBM disease (5 patients). The 13 patients with NMD-ADPKD were part of National Institutes of Health–sponsored longitudinal ADPKD studies and were known to be negative for underlying mutations in PKD1 or PKD2 (Supplementary MethodsS1–S17). Rare variants with an ethnicity-specific population minor allele frequency cutoff of 0.01% for heterozygous variants and 0.1% for recessive variants qualified as “pathogenic mutations” if they met the criteria of a predicted loss of function or as “likely pathogenic mutations” if they were missense variants deemed deleterious on bioinformatics predictions of MetaSVM and Mutation Taster (Supplementary Methods).
WES identified heterozygous pathogenic COL4A4 mutations in 1 patient with NMD-ADPKD and 3 patients with TBM disease and cystic kidneys (Table 13,4,S1,S3,S17,S18). In addition, 1 female patient with TBM disease and kidney cysts was a carrier for a COL4A5 likely pathogenic missense variant (Table 1). Finally, heterozygous pathogenic or likely pathogenic COL4A1 mutations that can cause kidney cysts as part of HANAC syndrome were present in 3 patients classified as NMD-ADPKD, 2 of whom have been previously reported in individual de-identified case reports.3,4 No mutations were found in other cystic kidney disease genes, including GANAB, DNAJB11, or in a panel of genes associated with polycystic liver disease or with monogenic nephropathy or CKD or focal segmental glomerulosclerosis (Supplementary Methods). There were no COL4A3 mutations in either patients with NMD-ADPKD or patients with TBM. In aggregate, heterozygous pathogenic or likely pathogenic mutations in COL4A1, COL4A4, or COL4A5 were found in 4 of the 13 NMD-ADPKD cases and 4 of the 5 TBM cases with bilateral kidney cysts.
Table 1.
Clinical characteristics and type IV collagen gene mutations in patients with bilateral kidney cysts without PKD1 or PKD2 mutations
| Patient no. | Cohort | Agea (yr)/sex (ethnicity) | eGFRb/CKD stage/severity classc | Family history | Type IV collagen gene mutation and predicted protein change | Genetic diagnosis | Type IV collagen variant details | ||
|---|---|---|---|---|---|---|---|---|---|
| Variant type | MAFd | Prior report | |||||||
| COL4A4 variants | |||||||||
| 1 | NMD-ADPKD | 25/M (Caucasian) | eGFR:125 (85)/CKD stage 1(2)/class IC | Not available | COL4A4: exon28: c. G2383A: p. G795R | COL4A4-related renal disease | Splice site; exon 28 skipping by minigene assay | 1 × 10–6 | None |
| 2 | TBM | 28/M (Caucasian) | CKD stage 3 | ESRD in father | COL4A4:exon46:c.4503dupA:p.A1502Sfs*17 | COL4A4-related renal disease | Frameshift and premature truncation | Novel | None |
| 3 | TBM | 42/M (Caucasian) | CKD stage 3 | Microhematuria in father; sibling | COL4A4: exon24: c.1697–1G>C | COL4A4-related renal disease | Canonical splice site variant | Not listed | One kindred with familial hematuriaS17 |
| 4 | TBM | 52/F (Caucasian) | CKD stage 4 | Microhematuria in father; sibling | COL4A4: exon 39: c.3704delC: p. P1235Qfs*53 | COL4A4-related renal disease | Frameshift and premature truncation | Novel | None |
| COL4A5 variant | |||||||||
| 5 | TBM | 49/F (Caucasian) | CKD stage 2 | Not available | COL4A5: exon16:c.C899T: p. P300L | COL4A5 carrier | Missense, likely pathogenic | 1 × 10–5 | Nonee |
| COL4A1 variant | |||||||||
| 6 | NMD-ADPKDf | 41/M (Caucasian) | eGFR:77 (78)/ CKD stage 2 (2)/ class IB | Not available | COL4A1: exon31:c.C2351T: p. P784L | HANAC-like syndrome | Missense, likely pathogenic | 1 × 10–5 | Noneg |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; F, female; HANAC, hereditary angiopathy, nephropathy, aneurysms, muscle cramps; M, male; MAF, minor allele frequency; NMD-ADPKD, no PKD mutation detected–autosomal dominant polycystic kidney disease; TBM, thin glomerular basement membrane.
Age (yr) at clinical/imaging or histopathological diagnosis of ADPKD or TBM disease.
Modification of Diet in Renal Disease eGFR in ml/min per 1.73 m2 or CKD stage recorded at the time of inclusion (and at 4-yr follow-up into ADPKD cohort) or at diagnosis of TBM.
Severity class–based age and height-adjusted total kidney volumeS3 at inclusion into CRISP study.S1
Population minor allele frequency as listed in the genome aggregation database (http://gnomad.broadinstitute.org).
This particular variant has not been reported in the Alport syndrome database (http://www.arup.utah.edu). Proline substitutions at this third position of tripeptide repeating unit (G-X-Y) in the collagen triple-helical domain undergo post-translational modification and there are 5 proline substitutions listed as pathogenic in the Alport database.
Two additional patients from this 17-patient NMD-ADPKD cohort have been previously reported as individual de-identified cases with HANAC syndrome; variant (COL4A1: exon25:c.C1612T: p. R538W)3; variant (COL4A1: exon13:c.C739T: p. Q247X).4
Proline-to-lysine substitution at another residue 352 in COL4A1 is previously reported as pathogenic and tested in a cell culture–based secretion assay.S18
Heterozygous Pathogenic COL4A4 Mutations in 4 Patients With Bilateral Kidney Cysts Without PKD1 or PKD2 Mutations
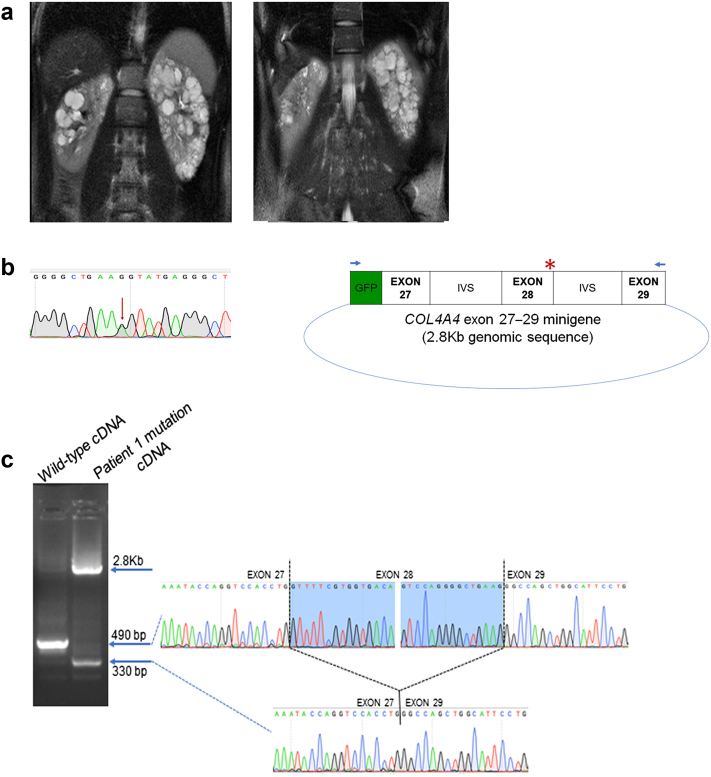
Patient 1 (Table 1) from the NMD-ADPKD group showed multiple bilateral kidney cysts and a relatively well-preserved kidney contour with little increase in total kidney volume (TKV) over time. The baseline height-adjusted TKV of 422 ml/m was stable at 461 ml/m at the time of 4-year follow-up (Figure 1a). WES showed a COL4A4 variant that was predicted to result in a glycine to arginine amino acid change at position 795. This variant is a guanine to adenine nucleotide change at the last base of exon 28, and in silico splice site prediction algorithms predicted the potential loss of the splice donor site for exon 28. We investigated this variant using an in vitro minigene splice assay (Supplementary Methods) and found that it resulted in aberrant splicing by this assay. Reverse-transcription polymerase chain reaction on the mRNA isolated from human embryonic kidney cells transfected with COL4A4 minigene carrying this patient’s mutation showed altered splicing and complete skipping of exon 28 in addition to inefficient splicing resulting in significant persistence of unspliced transcript (Figure 1b and c). Normal splicing pattern was observed with the expression of the wild-type COL4A4 minigene in this assay.
Figure 1.
(a) Magnetic resonance image of the abdomen from patient 1 showing both kidneys with several small to moderate cysts. Imaging at enrollment into the CRISP study (left image) and at 4-year follow-up into the CRISP study (right image) is shown. (b) (Left) Sanger sequence tracing showing the heterozygous substitution variant (red arrow) COL4A4: exon28: c. G2383A in patient 1. (Right) Minigene plasmid containing green fluroescent protein (GFP) contiguous with the 2.8-Kb COL4A4 genomic sequence that includes exons 27–29 and the intervening intronic sequences (IVSs). The red asterisk denotes the position of the COL4A4 variant in patient 1. Blue arrows indicate the primer locations used for reverse-transcription polymerase chain reaction that selectively amplify the minigene sequence and avoid amplification of the native COL4A4 transcript. (c) (Left) Reverse-transcription polymerase chain reaction products from the wild-type splice form (490-base pair [bp]) and from the mutation (2.8-Kb unspliced product and 330-bp product resulting from complete skipping of exon 28). (Right) Sanger sequencing of the wild-type and 330-bp mutated cDNA showing that exon 27 is spliced directly to exon 29 as result of the mutation.
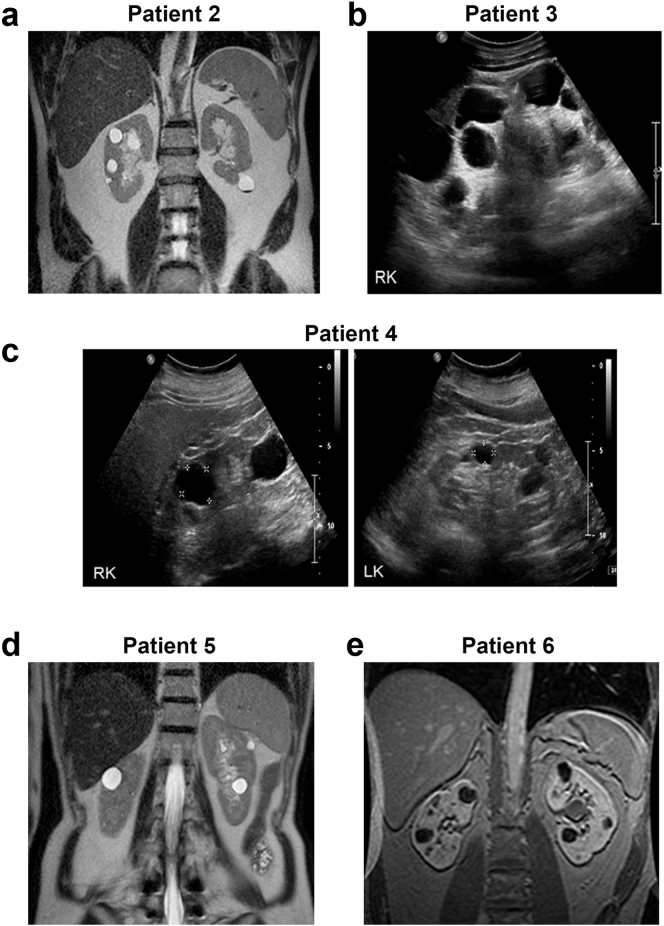
WES analysis on 3 patients presenting with microscopic hematuria and TBM disease showed loss of function heterozygous COL4A4 mutations (Table 1, patients 2–4). Kidney biopsy showed TBM in patient 2 and focal segmental glomerulosclerosis in patient 4 and was not performed in patient 3. Imaging in all 3 individuals showed bilateral multiple well-formed kidney cysts without much increase in kidney size with right and left kidney lengths recorded as 11.8, 11.3 cm; 16.7, 17.0 cm; and 11.0, 10.5 cm for patients 2, 3, and 4, respectively. (Figure 2a–c). Patient 3 had large cysts located at the renal pole of each kidney contributing to the increased sagittal renal dimension. The clinical course, but not the genetic analysis, of patient 2 and patient 3 has been previously reported.6
Figure 2.
Kidney imaging (magnetic resonance [MR] imaging/ultrasound) for patients 2 to 6 with bilateral kidney cysts and type IV collagen gene variants. Images are labeled by patient number as in Table 1. (a) Patient 2: MR imaging showing both of the kidneys with few bilateral well-formed cysts. (b) Patient 3: ultrasound imaging of the right kidney (RK) showing multiple cysts. Left cystic kidney not shown. (c) Patient 4: ultrasound imaging of the RK and left kidney (LK) showing multiple cysts. (d) Patient 5: MR imaging showing both of the kidneys with few bilateral well-formed cysts. (e) Patient 6: MR imaging showing both of the kidneys with bilateral well-formed cysts.
A Likely Pathogenic Missense Variant in COL4A5 Identified in 1 Patient With TBM Disease and Bilateral Kidney Cysts
A female patient (Table 1, patient 5) with persistent microscopic hematuria and TBM disease on kidney biopsy in addition to the imaging finding of bilateral kidney cysts with right and left kidney lengths of 10.5 and 9.5 cm each (Figure 2d) was a carrier for a rare COL4A5 variant that meets pathogenicity criteria (Supplementary Methods) and results in the substitution of a well-conserved proline to lysine at amino acid position 300 in the collagen triple-helical domain.
HANAC Syndrome Causing Heterozygous COL4A1 Mutations in the NMD-ADPKD Group
Two patients from the NMD-ADPKD group have been previously reported to carry HANAC syndrome causing COL4A1 mutations.3,4 In the current study, we found a third NMD-ADPKD patient with a heterozygous COL4A1 missense mutation that results in a proline-to-lysine substitution that met criteria for being likely pathogenic (Table 1, patient 6; Supplementary Methods). A review of imaging for this patient after the molecular finding of a COL4A1 mutation showed minimally enlarged kidneys with height-adjusted TKV of 284 and 294 ml/m at enrollment and 4-year follow-up, respectively, with fewer cysts and well-preserved normal-appearing intervening renal parenchyma consistent with those seen in HANAC syndrome rather than ADPKD (Figure 2e).
Rare Likely Pathogenic Collagen IV Gene Mutations in Patients With ADPKD and in Population Controls
The 13-patient NMD-ADPKD cohort is composed of 9 Caucasian patients, 1 of whom had a COL4A4 mutation and 2 had COL4A1 mutations. Non-Caucasian patients with NMD-ADPKD include 3 African American individuals of whom 1 carries a COL4A1 mutation. The final individual with NMD-ADPKD has Mexican ethnicity. The COL4A4/COL4A5/COL4A1 mutations in the 161 PKD gene mutation–defined ADPKD cohort (Table 2S1,S3) were all present in the 140 Caucasian patients and none in the 16 African American, 4 Mexican, and 1 Asian individual.
Table 2.
Type IV collagen gene mutations in 161 patients with ADPKD with an underlying PKD1 or PKD2 mutation
| Patient ID | Agea (yr)/sex (ethnicity) | eGFRb/CKD stage/severity classc | ADPKD gene mutation | Type IV collagen gene variant | Type IV collagen variant details | |
|---|---|---|---|---|---|---|
| Variant type | MAFd | |||||
| COL4A4 variant | ||||||
| CR89B3 | 34/M (Caucasian) | 100 (90)/CKD stage 1/class 1C | PKD2 splice site exon12: c.2358+1G>A | COL4A4: exon46:c.C4513T: p. Q1505X | Premature truncation | Novel |
| COL4A1 variants | ||||||
| CR89C2 | 20/M (Caucasian) | 100 (79)/CKD stage 1(2)/class 1B |
PKD2 frameshift deletion exon1: c.424delG: p. G142fs |
COL4A1: exon42: c. A3623G: p. E1208G | Missense, likely pathogenic | 10–6 |
| CR90D10 | 19/M (Caucasian) | 186 (113)/CKD stage 1(1)/class 1C |
PKD1 frameshift insertion exon 11: 2477_2478insCGGGT: Ile827fs72X |
COL4A1: exon27:c.C1933T: p. P645S | Missense, likely pathogenic | Novel |
ADPKD, autosomal dominant polycystic kidney disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; M, male; MAF, minor allele frequency.
Age at ADPKD study entry.
Modification of Diet in Renal Disease eGFR in ml/min per 1.73 m2 or CKD stage recorded at the time of inclusion (and at 4-yr follow-up into ADPKD cohort).
Severity class–based age and height-adjusted total kidney volumeS3 at inclusion into CRISP study.S1
Population minor allele frequency as listed in the genome aggregation database (http://gnomad.broadinstitute.org).
We tested and found no significant difference in the total burden of COL4A4/COL4A5/COL4A1 mutations in the 149 Caucasian patients from the ADPKD cohort that included 9 NMD patients and 140 patients with PKD mutation ADPKD versus those in the European (non-Finnish) individuals in the gnomAD v2.1.1 database: 6 of 292 versus 665 of 62,183 alleles, respectively (P = 0.14). However, in a subgroup analysis, we found enrichment of these mutations in 9 NMD-ADPKD patients of Caucasian ethnicity when compared with 140 PKD mutation–defined Caucasian patients with ADPKD: 3 of 15 versus 3 of 277 alleles had type IV collagen mutations, respectively (P = 0.003) or to the European (non-Finnish) individuals in the gnomAD v2.1.1 database: 3 of 15 versus 665 of 62,183 alleles in gnomAD (P = 0.0008). For P value calculations, we compared deleterious alleles/normal alleles of the total tested (Supplementary Methods).
Discussion
Mutations in type IV collagen proteins that are an integral component of the glomerular and tubular basement membranes in the kidney underlie a spectrum of mild to progressive kidney disease. Six genetically distinct α-helical isoforms (α1–α6) of type IV collagen encoded by their respective COL4A1–A6 genes form a triple helix with the α1α1α2 and α3α4α5 heterotrimers being the most predominant in the human kidney.7 Although recessive or biallelic mutations in COL4A3 or COL4A4 or a hemizygous state for COL4A5 results in Alport syndrome with an early-onset progressive nephropathy, the clinical spectrum of heterozygous COL4A3 or COL4A4 mutations or a COL4A5 female carrier state is more varied and less well described. Heterozygous COL4A3 (OMIM#120070) or COL4A4 (OMIM#120131) mutations may present with TBM disease. Female carriers of COL4A5-associated X-linked Alport syndrome most commonly show microscopic hematuria and an uncommon but variable risk of CKD progression. They may manifest ultrastructural abnormalities of varied glomerular basement membrane thickness and lamellation over time.8
The finding of kidney cysts has been mentioned in some clinical reports of TBM disease.5,6 Genetic investigation of these cases has been limited and none systematically excluded mutations in genes known to cause a polycystic kidney phenotype. Pierides et al.5 report multiple renal cysts in 4 families presenting with persistent hematuria and focal segmental glomerulosclerosis with underlying heterozygous glycine substitution mutations in COL4A3. Sevillano et al.6 noted bilateral kidney cysts in 9 (56%) of the 16 patients with TBM disease and subnephrotic proteinuria, whereas no kidney cysts were reported in the 16 patients with TBM without proteinuria; none of the patients were genetically investigated. Two patients with TBM with renal cystic disease from this latter series are included in our present report (Table 1, patients 2 and 3). It is similarly interesting to note the finding of multiple kidney cysts in a female patient with the rare 2q36 deletion syndrome that involves deletion of 24 genes; COL4A4 and COL4A3 were suggested to be the most plausible candidates for the nephropathy.9 Although the cystic kidney phenotype may not predominate the clinical presentation of type IV collagen mutation–related TBM disease, this finding strengthens the likely association between type IV collagen mutations and renal cysts. The observation that all 3 patients with TBM with COL4A4-associated kidney cysts described here had advanced CKD, and the previously reported association of proteinuria with cystic kidneys in TBM disease by Sevillano et al.,6 is supportive for investigating cystic kidney phenotype as a marker of CKD progression and severity in TBM disease. It is also likely that collagen IV mutation–associated kidney cysts have a glomerular or a tubular origin depending on the isoform involved. For example, whereas the α1 isoform is the most abundant constituent of basement membranes in the renal tubules, the α3α4α5 isoform is predominantly expressed in the mature glomerular basement membrane with some distal tubular expression.7
We found heterozygous loss of function COL4A4 mutations in 4 patients with CKD and bilateral multiple kidney cysts in the absence of other pathogenic mutation in genes known to associate with cystic kidneys. The association of pathogenic COL4A4 mutations with renal cystic disease, although mechanistically or causally unclear, is worth noting for several reasons. The clinical significance is to prevent misclassification of these patients as ADPKD, which is the most common genetic cause of polycystic kidneys and CKD in adults. Of note, the renal imaging findings of patient 1 who was misclassified as ADPKD showed a limited height-TKV and kidney function–based progression than would be expected in ADPKD due to either PKD1 or PKD2 mutation. Similarly, although TKV as a more accurate measure of kidney size was not done for the patients with TBM, none of them had a notable increase in kidney size, thus differentiating the imaging findings from that of a typical ADPKD. Because genetic testing for diagnosis is used only for a subset of ADPKD cases in clinical practice, it may be useful to reinforce that clinically milder or atypical cases of molecularly uncharacterized ADPKD may be more likely to represent phenocopies. As has been previously described, renal cysts are a well-recognized component of HANAC syndrome caused by heterozygous mutations in COL4A1 encoding for the α1 isoform that is predominantly expressed in the renal tubular and embryonic kidney basement membrane.2 Patient 6 in the present report in addition to the prior reported patients with HANAC syndrome from the same NMD-ADPKD group emphasizes that HANAC syndrome may phenocopy ADPKD. The recognition of type IV collagen mutations in patients with cystic kidneys may thus allow an accurate molecular classification for prognostic information, to enable correct application of available therapies and for inclusion of these patients into clinical trials.
Our analysis demonstrates an association between type IV collagen mutations and kidney cysts of varying severity; however, justifying a true causal relationship will require validation in larger cohorts and biological investigation for underlying pathophysiological mechanism. Our assessment of rare variant pathogenicity included in silico predictions and a minigene assay that classifies a variant listed as missense in the gnomAD database (http://gnomad.broadinstitute.org) functionally as a splice site variant based on an in vitro splice assay. Our analysis showed no significant enrichment of type IV collagen mutations in the entire ADPKD cohort (including NMD and PKD-mutation–defined patients) compared with population controls. Although our cohort numbers are small for direct comparisons, there is a significant enrichment of type IV collagen mutations in patients with NMD-ADPKD when compared with ethnically matched patients with ADPKD with an underlying PKD1 or PKD2 mutation or to general population controls in the gnomAD database. Larger ADPKD cohorts will need to be investigated to demonstrate if patients with NMD-ADPKD substantially contribute to the enrichment of type IV collagen mutations in such cohorts. The finding of a few pathogenic collagen IV gene variants in the PKD mutation–defined ADPKD group and the independent association of kidney cysts with collagen IV mutations is permissive for these proteins fulfilling a gene modifier role in ADPKD that has long been known for its phenotypic variability in families carrying the same PKD1 or PKD2 mutation. Analysis of much larger ADPKD cohorts of varying disease severity would be required for testing this hypothesis.
Disclosure
SS is a founder, shareholder, consultant, and scientific advisory board member for Goldfinch Bio. Patients previously reported have been cited in the text. All the other authors declared no competing interests.
Acknowledgments
The authors thank the patients for their participation in the study. The authors are grateful for the support from the Yale Center for Mendelian Genomics (National Institutes of Health [NIH] U54 HG006504). This work was supported by Polycystic Kidney Disease foundation grants to AG (207F17a) and WB (217G18a and 190F15a) and NIH grants for the George M. O’Brien Kidney Center at Yale (P30 DK079310) and DK100592 to SS; and Department of Pediatrics, Yale University. The data and samples from the CRISP and HALT study conducted by the CRISP and HALT investigators and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with investigators of the CRISP study and does not necessarily reflect the opinions or views of the CRISP study, the NIDDK Central Repositories, or the NIDDK.
Footnotes
Contributor Information
Ashima Gulati, Email: ashima.gulati@yale.edu, agulati2@childrensnational.org.
Stefan Somlo, Email: stefan.somlo@yale.edu.
Supplementary Material
References
- 1.Hwang Y.H., Conklin J., Chan W. Refining genotype-phenotype correlation in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27:1861–1868. doi: 10.1681/ASN.2015060648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaisier E., Gribouval O., Alamowitch S. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687–2695. doi: 10.1056/NEJMoa071906. [DOI] [PubMed] [Google Scholar]
- 3.Gulati A., Bae K.T., Somlo S., Watnick T. Genomic analysis to avoid misdiagnosis of adults with bilateral renal cysts. Ann Intern Med. 2018;169:130–131. doi: 10.7326/L17-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornec-Le Gall E., Chebib F.T., Madsen C.D. The value of genetic testing in polycystic kidney diseases illustrated by a family with PKD2 and COL4A1 mutations. Am J Kidney Dis. 2018;72:302–308. doi: 10.1053/j.ajkd.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierides A., Voskarides K., Athanasiou Y. Clinico-pathological correlations in 127 patients in 11 large pedigrees, segregating one of three heterozygous mutations in the COL4A3/ COL4A4 genes associated with familial haematuria and significant late progression to proteinuria and chronic kidney disease from focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2009;24:2721–2729. doi: 10.1093/ndt/gfp158. [DOI] [PubMed] [Google Scholar]
- 6.Sevillano A.M., Gutierrez E., Morales E. Multiple kidney cysts in thin basement membrane disease with proteinuria and kidney function impairment. Clin Kidney J. 2014;7:251–256. doi: 10.1093/ckj/sfu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heidet L., Cai Y., Guicharnaud L. Glomerular expression of type IV collagen chains in normal and X-linked Alport syndrome kidneys. Am J Pathol. 2000;156:1901–1910. doi: 10.1016/S0002-9440(10)65063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagher H., Buzza M., Colville D. A comparison of the clinical, histopathologic, and ultrastructural phenotypes in carriers of X-linked and autosomal recessive Alport's syndrome. Am J Kidney Dis. 2001;38:1217–1228. doi: 10.1053/ajkd.2001.29217. [DOI] [PubMed] [Google Scholar]
- 9.Doco-Fenzy M., Landais E., Andrieux J. Deletion 2q36.2q36.3 with multiple renal cysts and severe mental retardation. Eur J Med Genet. 2008;51:598–607. doi: 10.1016/j.ejmg.2008.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.