Abstract
Substitution of the ribose moiety of various nucleosides and nucleotides with the (N)-methanocarba ring system increases the potency and selectivity as ligands at certain subtypes of adenosine and P2 receptors. We have prepared a key intermediate in the synthesis of these derivatives, ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxybicyclo[3.1.0]hexanecarboxylate (15), starting from L-ribose (8) as a readily available, enantiopure building block. L-ribose was converted to the corresponding 5′-iodo derivative (9), which was cleaved reductively with Zn. Improvements were made in subsequent steps corresponding to a published route to biologically important (N)-methanocarba 5′-uronamido nucleosides, and new steps were added to prepare related 5′-nucleotides.
Keywords: G protein–coupled receptor, Nucleosides, Nucleotides, Purines, Pyrimidines, Iodination
INTRODUCTION
In solution, the conformation of the ribose ring of nucleosides and nucleotides exists in a rapid, dynamic two-state equilibrium between a (N) (Northern, 2′-exo) or (S) (Southern, 2′-endo) pucker conformation.[1,2] It is expected that the target receptors for these small molecules may prefer one of these conformations. It follows logically that when the nucleoside furanose ring is locked in either a (N) or (S) conformation an enhancement of potency and selectivity towards the target receptors may occur. Recently, we have successfully executed this approach by replacing the ribose moiety with the (N)-methanocarba ring system in nucleosides/nucleotides (Chart 1), and demonstrated enhanced selectivity and potency for therapeutically important A3 adenosine receptors (ARs, e.g., 1) and P2 nucleotide receptors (e.g., 2, 3).[3-6]
CHART 1.
Structures of biologically-important (N)-methanocarba nucleoside derivatives 1a, b that act as adenosine A3 receptor ligands and nucleotides 2, 3 that act as P2 receptor ligands.
Both purine and pyrimidine nucleotides have been synthesized bearing the (N)-methanocarba modification of ribose.[7] Our previously reported synthetic route for the above (N)-methanocarba adenine nucleosides is depicted in Scheme 1.[3] By following this synthetic approach and by employing the methanocarba sugar moiety as key synthon we have recently reported a highly A3AR selective series of (N)-methanocarba 5′-N-methyluronamide derivatives, prominent among them were MRS3558 1a and MRS73602 1b.[4] In particular, MRS3558 has a Ki value in binding to the human A3AR of 0.3 nM with at least 900-fold selectivity for the A3AR in comparison to other subtypes, that is, A1, A2A, and A2B ARs. Recently, Matot and coworkers have shown that MRS3558 is effective in attenuating reperfusion lung injury in cats.[8] This result suggests that A3AR agonists have potential as an effective therapy for ischemia/reperfusion-induced lung injury. In addition, Fishman and coworkers have studied extensively A3AR agonists as potential inhibitors in a model of adjuvant-induced arthritis in rats.[9] P2X and P2Y receptors are effectively targeted using related nucleotide derivatives, for example, MRS2339 2 and MRS2365 3, respectively.[10,11] Clearly, the potential therapeutic use of (N)-methanocarba nucleoside and nucleotides that act as receptor ligands demands their easy access in multigram amounts for further biological and clinical studies.
SCHEME 1.
Previously reported initial steps in the synthesis of (N)-methanocarba nucleoside and nucleotide derivatives 1–3.
We previously reported a new synthetic route for the above (N)-methanocarba nucleosides starting from 2,3-O-isopropylidene-D-erythronolactone 4 as depicted in Scheme 1.[3] However, this scheme includes three low temperature steps, an inconsistent, low-yielding Wittig reaction to provide 6 and a subsequent tedious Swern oxidation step to provide 7. The low and inconsistent yields in the Wittig reaction on large scale may be attributed to the quality of potassium t-butoxide and also an excess of Wittig salt in the reaction. In addition, the starting material 2, 3-O-isopropylidene-D-erythronolactone 4 is expensive and not readily available. In this communication we delineate a modified procedure for preparing (N)-methanocarba nucleosides, in particular 1a, on a reasonably large scale. Toward this endeavor we evaluated L-ribose as a less expensive and readily available starting material, using reactions similar to those previously reported using D-ribose.[12,13] Synthesis of 7 from L-ribose avoided problems of the Wittig reaction and Swern oxidation encountered in the previous route. Improvements also were made in steps subsequent to 7 corresponding to our published route to biologically important (N)-methanocarba nucleosides and nucleotides.
METHODS
Chemical Synthesis
1H NMR spectra (300 MHz) were obtained with a Varian Gemini 300 spectrometer (Varian, Inc., Palo Alto, CA, USA). 31P NMR spectra were recorded at room temperature with a Varian XL 300 spectrometer (121.42 MHz); orthophosphoric acid (85%) was used as an external standard. Purity of compounds was checked using a Hewlett-Packard 1100 HPLC equipped with a Luna 5 μm RP-C18(2) analytical column (250 mm × 4.6 mm; Phenomenex, Torrance, CA, USA). System A parameters consisted of the following: linear gradient solvent system of H2O/CH3CN from 95/5 to 20/80 in 20 minutes; flow rate of 1 mL/minute. System B parameters consisted of the following: linear gradient solvent system of 5mm TBAP/CH3CN from 80/20 to 20/80 in 20 minutes followed by isocratic for 2 minutes; flow rate of 1 mL/minute. Peaks were detected by UV absorption with a diode array detector.
Materials
All reagents were from standard commercial sources and of analytical grade.L-ribose was purchased from V-Labs, Inc. (Covington, LA, USA).
Ethyl (4S,5S)-3-[2,2-dimethyl-5-vinyl(1,3-dioxolan-4-yl)]-3-oxopropanoate (10).
Concentrated HCl (5 mL) was added to a suspension of L-ribose 8 (150 g, 1 mol) in acetone (400 mL) and methanol (400 mL) at room temperature. The resulting reaction mixture was refluxed for 3 hours and cooled to room temperature. It was then neutralized with pyridine and concentrated in vacuo. The residue was partitioned between water (300 mL) and ethyl acetate (300 mL three times). The combined organic phase was washed with a saturated copper sulfate solution followed by brine, dried over sodium sulfate, and concentrated. The crude residue was used in the next step without further treatment.
The above crude methyl ethers (164 g) were dissolved in toluene (750 mL) and the solution was treated with imidazole (85 g, 1.25 mol), triphenylphosphine (243 g, 0.93 mol), and acetonitrile (200 mL). The resulting reaction mixture was heated to 70°C, and iodine (253 g, 1 mol) was added carefully in small portions till the reaction mixture maintained a dark brown appearance. The reaction temperature was held constant at 70°C for 1 hour. The reaction mixture was filtered, and the residue was washed with toluene (500 mL). The combined organic layer was washed with 5% sodium thiosulfate solution, water, and brine and then concentrated. The crude residue was first treated with a mixture of hexanes and ethyl acetate (3:1). Solid triphenylphosphine oxide precipitated, and the mixture was filtered and the filtrate concentrated. This process was repeated to remove most of the triphenylphosphine oxide. A further purification of the concentrated filtrate by flash column chromatography (silica gel, ethyl acetate:hexanes, 1:10) afforded the iodo derivative 9 as the mixture of anomers (216 g, colorless oil, 69% in two steps). αD25 + 3.1° (c = 2.7, EtOH).
Powdered Zn (9.75 g, 150 mmol) was added to the solution of the iodides 9 (31.4 g, 100 mmol) in isopropanol (100 mL). To this resulting reaction slurry, acetic acid (10.5 g, 175 mmol) was slowly added in 30 minutes (cold water bath was used to control the reaction temperature), and the reaction mixture was maintained at 30°C and stirred for an additional 2 hours. The solution was decanted and concentrated under reduced pressure. The residue obtained was partitioned with ethyl acetate (200 mL), and water (100 mL) and stirred for 30 minutes. The organic layer was separated, and the aqueous layer was extracted with ethyl acetate (100 mL). The combined organic layer was washed with brine, dried and concentrated to afford crude aldehyde 7 as a colorless oil (12.8 g), which was carried forward for the next step.
Crude aldehyde 7 in dichloromethane (25 mL) was treated with anhydrous SnCl2 (1.9 g, 10 mmol) and ethyldiazoacetate (9.3 g, 82 mmol) in dichloromethane (80 mL) was added drop wise over a period of 1 hour at 0°C. The mixture was then allowed to stir at room temperature for 1 hour. The reaction mixture was filtered through a pad of Celite and concentrated. The desired keto ester was purified by column chromatography (silica gel, hexanes: ethyl acetate, 95:5) to afford 10, (16.3 g, 68% based on the iodide derivative) as an oil. 1H NMR (CDCl3) δ 11.92 (s, 0.1H, D2O exchangeable, enolic OH), 5.93–6.08 (m, 1H), 5.39–5.56 (m, 2H), 4.46–4.72 (m, 1H), 4.13–4.38 (m, 3H), 3.65 (AB q, 2H, J = 17.4 Hz), 1.64–1.57 (m, 6H, 2), 1.34–1.41 (m, 3H); HRMS (M − 1)− : calculated 241.1076, found 241.1084.
Ethyl (4S,5S)-3-[2,2-dimethyl-5-vinyl(1,3-dioxolan-4-yl)]-2-diazo-3-oxopropanoate (11).
Neat triethylamine (28.3 g, 280 mmol) was added dropwise to a solution of keto ester 10 (34.0 g, 140 mmol) and tosyl azide (27.6 g, 140 mmol) in acetonitrile (150 mL) at 0°C. The reaction mixture was stirred for 30 minutes at 0°C and then a further 30 minutes at room temperature followed by evaporation. The residue was dissolved in chloroform (100 ml) and the resulting precipitate was removed by filtration. The filtrate was diluted with hexane (100 mL), filtered again, and evaporated. The residue was purified by column chromatography (silica gel, 5% to 10% ethyl acetate-hexane) to afford the title compound as pale yellow oil (36.0 g, 133 mmol, 95%). 1HNMR (CDCl3) δ 6.01 (ddd, 1H, J = 16.3, 10.4, 7.4 Hz), 5.70 (d, 1H, J = 7.6 Hz), 5.60 (d, 1H, J = 17.1 Hz), 5.41 (d, 1H, J = 10.2 Hz), 4.97 (t, 1H, J = 7.5 Hz), 4.30 (q, 2H, J = 7.1 Hz), 1.65(s, 3H), 1.42 (s, 3H), 1.32 (t, 3H, J = 7.1 Hz); FAB MS m/z (relative intensity) 269 (MH+, 86). αD25 + 6.1° (c = 1.1).
Ethyl (1S,3S,4S,5S)-3,4-O-isopropylidene-2-oxoMcydo[3.1.0]hexanecarboxylate (12).
A solution of diazo compound 11 (26.9 g, 100 mmol) in dry toluene (100 mL) was added through a dropping funnel over a period of 4 hours to a refluxing mixture of toluene (25 mL) and CuI (3.8 g, 20 mmol). The reaction mixture was refluxed for 1 hour. The reaction mixture was cooled to room temperature, concentrated and purified by column chromatography (silica gel; hexanes:ethyl acetate, 70:30) to provide bicylic compound 12 as a colorless oil (12.24 g, 51%) and compound 13 as a pale yellow oil (3.36 g, 14%).
12: 1H NMR (CDCl3) δ 5.13 (ddd, 1H, J = 9.3, 5.6, 1.2 Hz), 4.37 (d, 1H, J = 8.3 Hz), 4.21 (q, 2H, J = 7.3 Hz), 2.75 (dt, 1H, J = 8.7, 5.4 Hz), 2.10 (dd, 1H, J = 8.6, 5.1 Hz), 1.81(t, 1H, J ~ 5.6 Hz), 1.52 (s, 3H), 1.31(s, 3H), 1.29 (t, 3H, J = 7.1 Hz); HRMS (M + 1)+ : calculated 241.1076, found 241.1092; αD25 + 9.5° (c = 1.1, EtOH). 13: 1H NMR (CDCl3) δ 4.87 (d, 1H, J = 5.1 Hz), 4.48 (dd, 1H, J = 4.6, 1.6 Hz), 4.31 (dq, 2H, J = 7.1, 1.5 Hz), 2.92 (dd, 1H, J = 8.7, 5.8 Hz), 2.31 (ddd, 1H, J = 8.8, 5.6, 1.7 Hz), 1.56 (s, 3H), 1.41 (s, 3H), 1.32 (t, 3H, J = 4.1 Hz), 182 (t, 1H, J = 5.1 Hz); HRMS (M + 1)+: calculated 241.1076, found 241.1082; αD25 + 4.9° (c = 0.65, EtOH).
Ethyl (1S,2R,3S,4S,5S)-3,4-O-isopropylidene-2-hydroxybicyclo[3.1.0]hexane-carboxylate (14).
NaBH4 (0.38 g, 10 mmol) was added to a stirred solution of 12 (2.4 g, 10 mmol) in ethanol (30 mL) at room temperature, and stirring was continued for an additional 1 hour. The reaction mixture was then treated with acetone (4 mL) and concentrated to dryness. The residue was purified by column chromatography (silica gel; hexanes:ethyl acetate, 70:30) to give compound 14 (1.68 g, 69%) as a white solid. 14: m.p. 111°C (cyclohexane); 1H NMR (CDCl3) δ 5.05 (t, 1H, J = 7.5 Hz), 4.91 (t, 1H, J = 6 Hz), 4.66 (t, 1H, J = 7 Hz), 4.14–4.30, (m, 2H), 2.46 (d, 1H, J = 12 Hz, OH), 2.16–2.29(m, 1H), 1.38–1.54(m, 5H), 1.31 (s, 3H), 1.24 (t, 3H, J = 6.5 Hz). HRMS (M + 1)+ : calculated 243.1232, found 243.1233.
Ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxybicyclo[3.1.0]hexanecarboxylate (15).
A solution of 14 (0.96 g, 4.0 mmol) and trifluoroacetic acid (0.68 g, 6 mmol) in acetone (20 mL) was stirred at 25°C for 6 hours. The reaction mixture was then concentrated under reduced pressure. The residue was dissolved in CHCl3 (50 mL) and washed with 10% sodium bicarbonate solution. The organic layer was concentrated to furnish a mixture of isomerized alcohols 14 and 15 in a 6:4 ratio based on NMR. This crude mixture was further purified by crystallization from cyclohexane (20 mL), in which alcohol 14 separates out as crystals. The mother liquor was concentrated to afford the requisite 15 (0.340 g, 35%) as a colorless solid with purity >80%. The analytical sample of alcohol 15 can be obtained by repeated recrystallization from cyclohexane. 15: m.p. 83°C (cyclohexane). 1H NMR (CDCl3) δ 5.35 (d, 1H, J = 5.8 Hz), 4.38–4.56 (m, 2H), 4.04–4.19 (m, 2H), 2.36–2.41 (m, 2H), 1.41–1.62 (m, 5H), 1.33 (s, 3H), 1.13 (t, 2H, J = 3.2 Hz); HRMS (M + 1)+ : calculated 243.1232, found 241.1245.
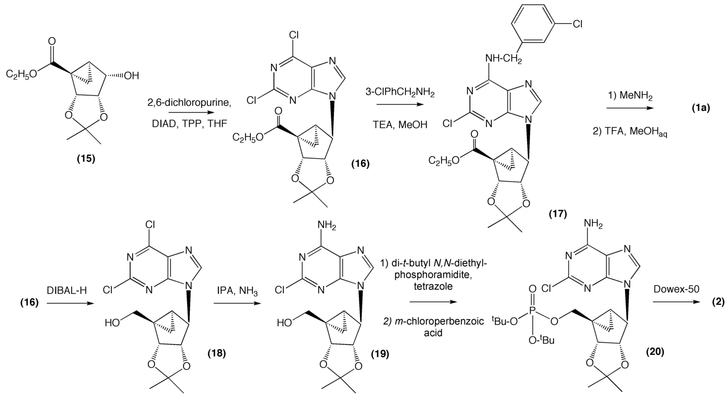
Ethyl (1′S,2′R,3′S,4′R,5′S)-4′-(2,6-dichloropurin-9-yl]-2′,3′-O-(isopropylidene)-bicyclo[3.1.0]hexanecarboxylate (16).
A mixture of triphenyl phosphine (1.04 g, 4.0 mmol) and 2,6-dichloropurine (0.756 g, 4.0 mmol) in dry THF (5 mL) was treated with diisopropyl azodicarboxylate (0.80 g, 4.0 mmol) at room temperature. After stirring for 20 minutes, a solution of the methanocarba sugar 15 (0.48 g, 2.0 mmol) in THF (5 mL) was added and the mixture was stirred an additional 8 hours. Concentration and purification of the residue by repeated column chromatography (silica gel; ethyl acetate:hexanes, 3:1) provided provided pure 16 (0.346 g, 42%) as a colorless amorphous solid.
16: 1H NMR (CDCl3) δ 8.06 (s, 1H), 5.81 (d, 1H, 6.7 Hz), 4.92 (s, 1H), 4.68 (d, 1H, J = 5.5Hz), 4.05–4.36 (m, 2H), 2.14–2.2 (m, 1H), 1.75–1.82 (m, 1H), 1.52–1.62 (m, 4H), 1.11–1.35 (m, 6H); HRMS (M + 1)+ : calculated 413.0783, found 413.0780; αD25 + 2.6° (c = 1.4, EtOH).
(1′S,2′R,3′S,4′S,5′S)-4′-[6-(3-Chlorobenzylamino)-2-chloropurin-9-yl]-2′,3′-O-isopropylidenebicyclo[3.1.0]hexane-1′-carboxylic Acid Ethyl Ester (17).
3-Chlorobenzylamine (0.705 g, 5.0 mmol) was added to a solution of 16 (0.413 g, 1.0 mmol) and triethylamine (2 mL) in methanol (5 mL). The mixture was stirred at room temperature for 3 hours, and then concentrated in vacuo to dryness. The residue was purified by column chromatography (silica gel; chloroform:methanol, 10:1) to give 17 as a pale yellow oil (0.316 g, 61%). 1H NMR (CDCl3) δ 1.18–1.37 (m, 3H), 1.41–1.85 (m, 8H), 2.18–2.29 (m, 1H), 4.05–4.41 (m, 2H), 4.78–4.87 (m, 3H), 5.39 (d, J = 4.8 Hz, 1H), 5.82 (d, J = 7.6 Hz, 1H), 6.24 (br s, 1H), 7.21–7.42 (m, 4H), 7.68 (s, 1H). HRMS (M + 1)+: calculated 518.1362, found 518.1357; αD25 + 3.0° (c = 0.4, EtOH).
(1′S,2′R,3′S,4′S,5′S)-4′-[6-(3-Chlorobenzylamino)-2-chloropurin-9-yl]-2,3′-di-hydroxybicyclo[3.1.0]hexane-1′-carboxylic Acid Methylamide (1a).
Compound 17 (0.518 g, 1 mmol) was dissolved in methanol (5 mL) and treated with an aqueous solution of methylamine (2 mL, 40%). This mixture was stirred at room temperature for 12 hours, then evaporated to dryness. This crude intermediate was treated directly with a solution of trifluoroacetic acid in MeOH (15 mL, 10%) and H2O (0.5 mL), and the mixture was heated at 70°C for 3 hours. The solution was cooled, and the solvent was removed to dryness by coevaporation with acetonitrile in vacuo. The white residue was purified by flash column chromatography (silica gel, chloroform:methanol, 8:2) to give the final product 1a as a white powder (0.250 g, 54%). M.p. 122°C (decomp). 1H NMR (CDCl3) δ 1.18–1.39 (m, 1H), 1.46–1.63 (m, 1H), 2.04–2.09 (m, 1H), 2.83 (d, J = 4.8 Hz, 1H), 3.87–4.08 (m, 2H), 4.78–5.05 (m, 5H), 6.73 (br s, 1H), 7.04 (br s, 1H), 7.13–7.31 (m, 4H), 7.75(s, 1H). HRMS (M + 1)+: calculated 463.1052, found 463.1053. HPLC (system A) 14.8 minutes (99%), (system B) 12.5 minutes (99%). αD25 + 1.2° (c = 1.0, EtOH).
(1′S,2′R,3′S,4′R,5′S)-4-(2,6-dichloro-purin-9-yl)-1-[hydroxymethyl]bicyclo-[3.1.0]-hexane-2,3-w(O-isopropylidine) (18).
A stirred solution of ester 16 (0.207 g, 0.5 mmol) in CH2Cl2 (10 mL) cooled to −78°C was treated dropwise with DIBAL-H (1.5 mL, 1.5 M solution in toluene) and stirred at that temperature for 1 hour. Methanol (1.5 mL) and 1 M cold sulfuric acid (10 mL) were added carefully to adjust the reaction mixture to pH 4–5. The reaction mixture was filtered through a pad of Celite, and the filtrate was separated. The aqueous phase was extracted with dichloromethane, and the combined organic layer was evaporated and concentrated under reduced pressure. The residue was purified by flash column chromatography (silica gel, CHCl3:MeOH, 10:1) to give alcohol 18 (0.103 g, 56%) as a solid. 1H NMR (CDCl3) δ 1.05–1.08 (m, 1H), 1.21–138 (m, 7H), 1.70–1.74 (m, 1H), 3.51(d, J = 12 Hz, 1H), 4.21–4.27 (m, 1H), 4.63 (d, J = 7.5 Hz, 1H), 4.96 (s, 1H), 5.51 (d, J = 12 Hz, 1H), 8.32 (s, 1H). HRMS (M + 1)+: calculated 371.0678, found 371.0669.
(1′S,2′R,3′S,4′R,5′S)-4-(6-amino-2-chloro-purin-9-yl)-1-[hydroxymethyl]bicyclo-[3.1.0]hexane-2,3-(O-isopropylidine) (19).
Compound 18 (0.186 g, 0.5 mmol) was treated with 2 M ammonia in isopropyl alcohol (2 mL) and stirred for 8 hours. The resulting reaction mixture was concentrated and the residue was purified by silica gel column chromatography (CHCl3 : MeOH, 80:20) to afford 19 (0.08 g, 46%) as a colorless solid. 1H NMR (CDCl3) δ 7.80 (s, 1H), 5.89 (bs, 2H), 5.56 (d, 1H, J = 6.4 Hz), 4.76 (s, 1H), 4.66 (d, 1H, J = 7.4 Hz), 4.26 (d, 1H, J = 11.5 Hz), 3.37 (d, 1H, J = 11.5 Hz), 1.78–1.70 (m, 1H), 1.55 (s, 3H), 1.26 (s, 3H), 1.14–1.20 (m, 1H), 0.96–1.20 (m, 1H). HRMS (M + 1)+: calculated 352.1176, found 353.1163.
(1′S,2′R,3′S,4′R,5′S)-4-(6-Amino-2-chloro-9H-purin-9-yl)-1-[(di-tert-butyl phosphate)methyl]bicyclo[3.1.0]hexane-2,3-(O-isopropylidene) (20).
Neat di-t-butyl N,N-diethylphosphoramidite (0.075 g, 0.3 mmol) was added to a stirred solution of 19 (0.035 g, 0.1 mmol) in anhydrous THF (2 mL) at room temperature followed by addition of tetrazole (0.063 g, 0.9 mmol). After 1 hour the reaction mixture was cooled to −78°C, and m-chloroperbenzoic acid (0.050 g, 77%) was added. The reaction mixture was warmed to 0°C and stirred for 15 minutes and then treated with triethylamine (0.5 mL). Purification was accomplished by preparative thin-layer chromatography using 4:1 chloroform:methanol to give 20 as a solid (0.025 g, 45%). 1H NMR (CDCl3): δ 1H NMR (CHCl3) δ 7.93 (s, 1H), 5.98 (bs, 2H), 5.35 (d, 1H, J = 6.1 Hz), 4.94 (d, 1H, J = 5.7 Hz), 4.65 (d, 1H, J = 6.1 Hz), 4.30–4.45 (m, 2H), 1.76–1.84 (m, 1H), 1.22–1.61 (m, 25H), 1.37–1.42 (m, 1H), 1.13–1.19 (m, 1H). HRMS (M + 1 + Na)+: calculated 566.1911, found 566.1916. αD25 + 0.6° (c = 0.15, EtOH).
(1′S,2′R,3′S,4′R,5′S)-4-(6-Amino-2-chloro-9H-purin-9-yl)-1-[phosphoryoxy-methyl]bicyclo[3.1.0]hexane-2,3-diol (2).
To a solution containing 20 (0.028 g, 0.05 mmol) in MeOH (3 mL) and water (3 mL) was added Dowex-50 resin (0.050 g). The mixture was stirred for 3 hours at 70°C and the resin removed by filtration. The filtration was then treated with triethylammonium bicarbonate buffer (1 mL, 1 M) and concentrated under reduced pressure. The residue was purified by ion-exchange column chromatography using Sephadex-DEAE A-25 resin with linear gradient (0.01–0.5 M) of 0.5 M ammonium bicarbonate as mobile phase to give 2 (0.005 g, 25%) as a white solid. 1H NMR (D2O) δ 8.44 (s, 1H), 4.72 (s, 1H), 4.42–4.52 (m, 1H), 4.00 (d, 1H, J = 6.6 Hz), 3.62–3.76 (m, 1H), 1.84–1.96 (m, 1H), 1.53–1.62 (m, 1H), 0.96–1.05 (m, 1H). 31P NMR (D2O) 0.677. HRMS (M − 1)+: calculated 390.0357, found 390.0359. HPLC (system B) 8.2 minutes (98%). For optical rotation measurements a crystalline salt with triethylamine was crystallized. M.p. 215°C (decomp); αD25 + 0° (c = 0.16, 80% EtOH).
RESULTS AND DISCUSSION
The key step in our published route to (N)-methanocarba nucleosides involves intramolecular cyclopropanation of the diazo derivative 11 to afford the requisite bicyclo[3.1.0]hexan-2-one derivative 12 as the major isomer (Scheme 2). Isomerization of the isopropylidene group in 14 provided the key (N)-methanocarba bicyclic pseudosugar intermediate, ethyl (1S,2R,3S,4S,5S)-2,3-O-(isopropylidene)-4-hydroxybicyclo[3.1.0]-hexanecarboxylate 15 which was converted into requisite (N)-methanocarba nucleosides by following a standard set of reactions in good yields (Scheme 3).
SCHEME 2.
Improved synthesis of a key (N)-methanocarba bicyclic pseudosugar intermediate 15 starting from L-ribose 8.
SCHEME 3.
Synthesis of the (N)-methanocarba nucleoside derivative MRS3558 1a and nucleotide derivative MRS2339 2.
We explored the use of commercially available L-ribose 8 as an enantiopure building block for the construction of 15. Accordingly, 8 was converted into a 5:1 mixture of iodides 9, which were isolated as a colorless oil in 69% overall yield. An important attribute of this iodide preparation was the trituration of the crude product with hexanes/ethyl acetate (3:1) to precipitate the triphenylphosphine oxide byproduct thereby allowing facile removal by filtration. The mixture of 5′-iodoribose derivatives was initially cleaved reductively with Zn in refluxing methanol according to a literature procedure.[12,13] The purification of the crude aldehyde 7 was attempted by using distillation under reduced pressure,[13] which resulted in polymerization of the product. To overcome this problem the aldehyde was generated in situ and was reacted further without purification. We also employed isopropyl alcohol as a co-solvent along with methanol in this reaction. It is presumed that the formation of the undesired methyl hemiacetal was minimized to almost negligible levels due to the presence of a large excess of isopropyl alcohol. At this stage the reaction mixture was simply filtered through Celite to remove an excess of Zn, and the alcoholic layer containing 7 was concentrated to dryness. The residue was redissolved in dichloromethane and reacted with ethyl diazoacetate to afford the keto ester 10 in 42% yield based on the iodoribose mixture 9. Although we obtained some improvement in yields using this reaction protocol we continued to probe this reaction sequence further. We observed that the resulting aldehyde was very unstable under the present reaction conditions, that is, refluxing in i-PrOH/MeOH. So, our aim was to carry out this reaction sequence at lower temperature to avoid the decomposition of the aldehyde. Accordingly, when the same reaction was carried out in the presence of acetic acid we observed that the Zn-mediated reduction proceeded smoothly at room temperature. We believe that this acid-catalysed reduction occurs upon protonation of the methoxy group followed by the rate-determining reductive elimination of the “MeOH” moiety to afford the aldehyde. We also observed that instead of simply filtering to remove the Zn, an aqueous workup at this stage was necessary to remove the unwanted ZnI from the reaction mixture. The resulting aldehyde was subjected to further reaction with a tin-mediated ethyl diazoacetate addition reaction to afford 10 in 68% yield based on the iodoribose mixture 8. It is pertinent to mention here that longer reaction times with ethyl diazoacetate led to formation of unwanted side products that made the column chromatographic separation of 10 difficult as well as lowering the overall yields. It is to be noted here that the combined yield of 10 by this modified acid catalyzed protocol is 47% based on L-ribose, while our earlier approach had only a 19% overall yield based on expensive 2, 3-O-isopropylidene-D-erythronolactone.
The keto ester 10 was reacted with tosyl azide to afford the diazo derivative 11 in 95% yield. The byproduct p-toluenesulfonamide generated in this reaction separates out easily when the residue was dissolved in chloroform. The diazo derivative 11 then underwent a thermally-induced intramolecular cyclopropanation to give bicyclo[3.1.0]hexan-2-one derivatives 12 and 13 in a combined 48% yield as per our earlier procedure. However we observed that the newly formed sterically strained α-keto cyclopropyl derivatives 12 and 13 were unstable under these reaction conditions. Based on this observation the slow addition (over 4 hours) of the diazo derivative 11 was employed during this reaction. This modification has indeed resulted in considerable improvement in the yields, and a favorable diastereoisomeric ratio (4:1) for the desired isomer 12 (51%) was obtained. The small amount of the starting diazo compound 11 was also recovered from this reaction. This bicyclo derivative 12 was isolated chromatographically and reduced stereoselectively with NaBH4 to give alcohol 14 as a single product in 69% yield. Alcohol 14 was then subjected to trifluoroacetic acid-catalyzed equilibration at room temperature to produce the requisite isomeric acetonide 15. The isolation of 15 was achieved by crystallization from cyclohexane, in which the mother liquor was rich in the requisite isomer 15 with purity >80%. The crystalline isomer 14 could be recycled by re-subjecting it to the above equilibration procedure. Pure 15 could be obtained by repeated recrystallizations from cyclohexane. However, for most applications it was practical to use 15 at ~80% purity in subsequent reactions.
Our initial studies towards nucleophilic substitution of either the 1′-mesylate or 1′-triflate derivative of 15, with the sodium salt of purines under a variety of conditions failed presumably due to steric reasons. The best result was obtained when alcohol 15 was subjected to a Mitsunobu coupling reaction using 2,6-dichloropurine as the base to afford the condensed product 16 (Scheme 3). However, we observed that this condensed product was contaminated with byproducts arising from the Mitsunobu reaction. Repeated purification (at least three times) with silica gel column chromatography afforded the pure compound 16 in 42% yield. We observed that a slightly impure product could be carried forward in reaction with 3-chlorobenzylamine to provide 17 in 61% yield after silica gel column chromatography. Finally, 17 was treated with excess methylamine followed by acetonide deprotection with 10% TFA to produce 1a (MRS 3558) in 54% yield over two steps whose spectral properties matched those of the identical compound reported in the literature.[4]
This modified procedure starting from L-ribose was also used to prepare MRS2339 2, a (N)-methanocarba derivative of 2-chloroadenosine 5′-monophosphate. Recently, Liang and coworkers have shown that compound 2 acts as a P2X receptor agonist and when administered in a mouse model of heart failure it reduces cardiac myocyte hypertrophy and enhances survival.[10] For its preparation we first reduced the ester functionality in 16 using diisobutylaluminum hydride (DIBAL-H) at −78°C, to afford the alcohol 18 in 56% yield. Initially, the chloro group at the 6-position was replaced with an amino functionality by exposure to methanolic ammonia, however the reaction was also accompanied by the formation of the 6-methoxy substituted derivative. This problem was overcome by treating 18 with 2 M ammonia in isopropyl alcohol to afford the 6-amino derivative 19 in moderate yields. (N)-Methanocarba nucleoside 19 was phosphorylated by first reacting with di-t-butyl N,N-diethylphosphoramidite and tetrazole followed by treatment with m-chloroperbenzoic acid to afford the di-t-butyl phosphate derivative 20 in 45% yield. Both t-butyl groups and the acetonide group in 20 were deprotected simultaneously by using Dowex-50 resin in the acid form, to afford the crude (N)-methanocarba nucleotide 2. The final purification of the resulting monophosphate was accomplished by using Sephadex ion exchange column chromatography to afford 2 as the ammonium salt in 25% yield. The spectral properties of 2 were identical to those reported previously.[14]
In conclusion, we have demonstrated the feasibility of employing readily available L-ribose as a precursor in the preparation of (N)-methanocarba nucleosides and nucleotides of biological interest. In this regard the modified synthesis bypasses the pitfalls experienced in our earlier approach thereby allowing a highly efficient and convergent synthesis of purine receptor ligands MRS 3558 and MRS 2339. In addition to certain subtypes of adenosine and P2 receptors, the (N)-methanocarba nucleosides have been explored and found to interact effectively with adenosine deaminase,[15] and the ENT1nucleoside transporter.[16] Furthermore, nucleosides bearing the (N)-methanocarba modification have been shown to be substrates for cellular and viral DNA polymerases.[7, 17] At present, use of this modified synthetic procedure to synthesize (N)-methanocarba nucleosides with potential antiviral activity is underway in our laboratories.
Acknowledgments
Mass spectral measurements were carried out by Dr. John Lloyd and NMR measurements by Wesley White (NIDDK). This research was supported in part by the Intramural Research Program of the NIH, National Institute of Diabetes and Digestive and Kidney Diseases. BVJ thanks Gilead Sciences (Foster City, CA) for research support.
REFERENCES
- 1.Altona C; Sundaralingham M Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J. Am. Chem. Soc 1972, 94, 8205–8212. [DOI] [PubMed] [Google Scholar]
- 2.Marquez VE; Siddiqui MA; Ezzitouni A; Russ P; Wang J; Wagner RW; Matteucci MD Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem 1996, 39, 3739–3747. [DOI] [PubMed] [Google Scholar]
- 3.Joshi BV; Moon HR; Fettinger JC; Marquez VE; Jacobson KA A new synthetic route to (N)-methanocarba nucleosides designed as A3 adenosine receptor agonists. J. Org. Chem 2005, 70, 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tchilibon S;Joshi BV; Kim SK; Duong HT; Gao ZG;Jacobson KA (N)-Methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists. J. Med. Chem 2005, 48, 1745–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HS; Ravi RG; Marquez VE; Maddileti S; Wihlborg A-K; Erlinge D; Malmsjö M; Boyer JL; Harden TK; Jacobson KA Methanocarba modification of uracil and adenine nucleotides: High potency of Northern ring conformation at P2Y1, P2Y2, P2Y4 and P2Y11, but not P2Y6 receptors. J. Med. Chem 2002, 45, 208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhatriwala M; Ravi RG; Patel RI; Boyer JL; Jacobson KA; Harden TK Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analogue. J. Pharm. Exp. Therap 2004, 311, 1038–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquez VE; Ezzitouni A; Russ P; Siddiqui MA; Ford H Jr.; Feldman RJ; Mitsuya H; George C; Barchi JJ Jr. HIV-1reverse transcriptase can discriminate between two conformationally locked carbocyclic AZT triphosphate analogues. J. Am. Chem. Soc 1998, 120, 2780–2789. [Google Scholar]
- 8.Matot I; Weininger CF; Zeira E; Galun E; Joshi BV; Jacobson KA Adenosine receptors and mitogen activated protein kinases in lung injury following in-vivo reperfusion. Critical Care 2006, 10, R65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baharav E; Bar-Yehuda S; Madi L; Silberman D; Rath-Wolfson L; Halpren M; Ochaion A; Weinberger A; Fishman P The anti-inflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J. Rheumatol 2005, 32, 469–476. [PubMed] [Google Scholar]
- 10.Shen JB; Cronin C; Sonin D; Joshi BV; Carolina M; Nieto G; Harrison D; Jacobson KA; Liang BT P2X purinergic receptor-mediated ionic current in cardiac myocytes of calsequestrin model of cardiomyopathy. Implications for the treatment of heart failure. Am.J. Physiol 2007, 292, H1077–H1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourdon DM; Boyer JL; Mahanty S;Jacobson KA; Harden TK (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J. Thromb. Haemost 2006, 4, 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallos JK; Koftis TV; Massen ZS; Dellios CC; Mourtzinos IT; Coutouli-Argyropoulou E; Koumbis AK Bicyclo[3.1.0]hexanes from sugar-derived diazo compounds and iodonium ylides. Diastereocontrol and synthetic applications. Tetrahedron 2002, 58, 8043–8053. [Google Scholar]
- 13.Paquette LA; Bailey B Evaluation of D-ribose as an enantiopure building block for construction of the C-ring of taxol and its congeners. J. Org. Chem 1995, 60, 7849–7856. [Google Scholar]
- 14.Ravi RG; Kim HS; Servos J; Zimmermann H; Lee K; S Maddileti S; Boyer JL; Harden TK; Jacobson KA Adenine nucleotides analogues locked in a Northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J. Med. Chem 2002, 45, 2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquez VE; Russ P; Alonso R; Siddiqui MA; Shin KJ; George C; Nicklaus MC; Dai F; Ford H Jr. Conformationally restricted nucleosides. The reaction of adenosine deaminase with substrates built on a bicyclo[3.1.0]hexane template. Nucleosides Nucleotides 1999, 18, 521–530. [DOI] [PubMed] [Google Scholar]
- 16.Lee K; Cass C; Jacobson KA Synthesis using ring closure metathesis and effect on nucleoside transport of a (N)-methanocarba S-(4-nitrobenzyl)thioinosine derivative. Org. Lett 2001, 3, 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marquez VE; Ben-Kasus T; Barchi JJ Jr.; Green KM; Nicklaus MC; Agbaria R Experimental and structural evidence that herpes 1kinase and cellular DNA polymerase(s) discriminate on the basis of sugar pucker. J. Am. Chem. Soc 2004, 126, 543–549. [DOI] [PubMed] [Google Scholar]