ABSTRACT
Background
Developing dietary strategies to prevent excess weight gain during childhood is critical to stem the current obesity epidemic and associated adverse cardiometabolic consequences.
Objectives
We aimed to assess how participation in a family-based weight-management intervention affected nutrient biomarkers and cardiometabolic risk factors (CMRFs) in children (7–12 y old; n = 321) with baseline BMI z score (BMIz) ≥85th percentile.
Methods
This was a secondary analysis from a randomized-controlled, parallel-arm clinical trial. Families of children, recruited from a largely Hispanic population, were assigned to Standard Care (SC; American Academy of Pediatrics overweight/obesity recommendations), or SC + Enhanced Program (SC + EP; 8 skill-building cores, monthly support sessions, targeted diet/physical activity strategies). Nutrient biomarkers (plasma carotenoids, fat-soluble vitamins, RBC fatty acid profiles, desaturase indexes) and CMRFs were measured in archived blood samples collected at baseline and the end of the 1-y intervention.
Results
Children in both groups had significantly lower trans fatty acid and higher pentadecylic acid (15:0), PUFA n–3, and β-carotene concentrations, indicative of decreased hydrogenated fat and increased dairy, vegetable oil, fish, and fruit/vegetable intake, respectively. Similar changes were seen in de novo lipogenesis and desaturase indexes, as well as CMRFs (BMIz, lipid profile, inflammation, adipokines, liver enzymes) in both groups. Using multiple logistic regression, increase in carotenoids and decrease in endogenously synthesized SFA, MUFA, PUFA n–6, and desaturase indexes were associated with improvements in BMIz, blood pressure, lipid profile, glucose metabolism, inflammatory biomarkers, adipokines, and liver enzymes. Trans fatty acids were associated with improvements in BMIz, glucose metabolism, and leptin, with less favorable effects on inflammatory markers and adiponectin.
Conclusions
Providing targeted family-based behavioral counseling, as part of SC, can help overweight/obese children adopt healthier eating patterns that are associated with modest improvements in BMIz and several CMRFs. Limited additional benefit was observed with SC + EP. These results provide critical data to design subsequent interventions to increase the impact of family-based obesity prevention programs.
This trial was registered at clinicaltrials.gov as NCT00851201.
Keywords: childhood obesity, family-based intervention, fatty acids, carotenoids, nutrient biomarkers, cardiometabolic risk factors, adipokines
Introduction
During the past 2 decades the prevalence of overweight and obesity in children has rapidly increased worldwide (1) and it is associated with adverse health outcomes throughout the life span (2, 3). Obesity disproportionally affects children in the lowest socioeconomic category, who have a significantly higher 5-y risk of becoming overweight and developing chronic diseases than those in the middle and highest socioeconomic categories. In addition, obesity rates are higher among Hispanic (22%) and non-Hispanic black (20%) than among non-Hispanic white (15%) children (1). Thus, developing strategies to prevent excess weight gain during childhood in minority communities in order to target health disparities is of critical importance.
Family-based weight management trials, especially lifestyle interventions with a dietary component, have been shown to lower rates of body weight gain and improve cardiometabolic risk factors (CMRFs) (4–6). However, there is a paucity of data reporting dietary intake information and the extent to which dietary modification affects changes in body weight and associated CMRFs (7–9). This is partly due to the challenges associated with accurately measuring dietary intake, especially in children (10). We have previously reported changes in BMI z score (BMIz) and CMRFs for the Family Weight Management Study, a randomized-controlled clinical trial which evaluated the effect of a 12-mo bilingual family-based weight-loss intervention in 7- to 12-y-old children with a BMIz ≥85th percentile, within a safety-net pediatric primary care setting (11). Goals of the dietary intervention component included increasing intakes of highly pigmented fruits/vegetables, exchange of nonfat/low-fat milk for full-fat milk and sugar-sweetened beverages, and reduced-fat for full-fat dairy products, as well as minimizing the intake of fried foods and high-fat savory snacks. These dietary factors were targeted because they have been associated with excess body weight gain in children (12, 13). The present study is a secondary analysis from the Family Weight Management Study (NCT00851201) (11). Our primary aim was to determine the effect of participation in a family-based weight management intervention on objective indicators of dietary intake and endogenous metabolism (nutrient biomarkers) in children with overweight and obesity, and to assess their relation with CMRFs. We hypothesized that greater adoption of the dietary recommendations would be reflected in circulating nutrient biomarker concentrations and lead to an improvement in CMRF profile.
Methods
Study subjects and design
n = 321 children aged 7–12 y with baseline BMIz ≥85th percentile having a plasma/serum and RBC sample at both baseline and the end of the 1-y intervention were included in this study (Supplemental Figure 1). Details of the Family Weight Management Study, including inclusion/exclusion criteria, sample size estimates, and primary endpoints [BMI percentile for age and sex, and selected biomarkers (fasting and 2-h glucose and insulin, lipid profile, and liver enzymes) measured at baseline and 12 mo after randomization], have been described previously (11). Briefly, the study was conducted in a safety-net pediatric primary care setting in Jacobi Medical Center (Bronx, NY, USA) where health services are predominately covered by public funding (Medicaid and the Child Health Insurance Plan), thus providing access to those with limited or no health care. Exclusion criteria included chronic illness, impairments that would affect ability or safety to follow the study protocols, treatment with medications known to affect body weight, and enrollment in another weight management program within 2 y. The study was a 2-arm randomized, controlled, parallel-group trial comparing Standard Care (SC) alone with SC + Enhanced Program (SC + EP). The SC intervention was based on the American Academy of Pediatrics evidence-based recommendations using pilot-tested materials (14). Pediatrician visits were provided quarterly and the procedures followed were similar for both groups. This included an initial comprehensive visit to assess weight-related issues and to engage both the children and parents/guardians in developing intervention goals collaboratively. A 35-item Pediatric Symptom Checklist was used to screen for emotional and behavioral dysfunctions (15), and a 5-item Habits questionnaire was used to assess dietary, physical activity, and sedentary behaviors (16). The follow-up pediatrician appointments were brief visits to review the assessment themes and collaborative goals identified at the initial visit. The pediatricians who provided the SC to both study groups were blinded to treatment allocation.
The EP added a behavioral change component (8 Skill-Building Core sessions and monthly Post-Core Support sessions focused on improving dietary behaviors and increasing engagement in physical activities) provided by bilingual multidisciplinary staff (dietitian, social worker, and fitness instructor). The Skill-Building Core sessions included alternating in-person groups and parent/guardian phone consultations. The in-person core group sessions consisted of food preparation or other skill activities for parents/guardians and children, followed by a physical activity session for the children and discussion session for parents/guardians regarding their role in weight management. The monthly Post-Core Support sessions consisted of engagement activities that were designed to provide ongoing support to parents/guardians and children during the remainder of the 1-y intervention program. All study procedures were approved by the Institutional Review Board of the Albert Einstein College of Medicine. Approval to analyze de-identified samples and data was obtained from Tufts University/Tufts Medical Center Institutional Review Board.
Selection of outcome variables
Nutrient biomarkers
To overcome the inherent limitations associated with subjective assessments of dietary intake (17), and to reflect in vivo nutrient exposure, a consequence of both dietary intake as well as endogenous metabolism (18), an objective multibiomarker approach was used. Dietary biomarkers measured were plasma carotenoid concentrations (pigmented fruit and vegetable intake) (19, 20); fat-soluble vitamins A, D, E, and K (animal foods, fortified foods, supplements, and/or vegetable oils) (21); and RBC fatty acid profiles including linoleic acid (18:2n–6), α-linolenic acid (18:3n–3) (vegetable oil and vegetable oil–based margarines) (22), EPA (20:5n–3), docosapentaenoic acid (22:5n–3), DHA (22:6n–3) (fish) (23, 24), pentadecylic acid (15:0) (dairy products) (25), and trans fatty acids (ruminant/partially hydrogenated fat) (26). Endogenously synthesized SFA and MUFA profiles were also measured, and desaturase enzyme indexes estimated because they reflect de novo lipogenesis (DNL) (27, 28) and have been associated with abdominal adiposity and several metabolic disorders (29).
CMRFs
Available CMRF data from the primary clinical trial included BMIz, lipid profile, glucose metabolism markers, and liver enzymes. Additional CMRF outcomes measured in the present study included proinflammatory [high-sensitivity C-reactive protein (hsCRP), TNF-α, IL-6, IL-1β], vascular adhesion [E-selectin, P-selectin, soluble intercellular adhesion molecule (sICAM)], and coagulation (thrombomodulin) biomarkers. This panel of CMRFs was specifically selected given the association between childhood obesity and chronic low-grade inflammation, which is considered a precursor to several metabolic diseases (30, 31). Adipokines (leptin and adiponectin) were also measured because they have been negatively associated with body fat and insulin sensitivity (32).
Measurement of nutrient biomarkers and estimation of desaturase enzyme activities
Blood specimens were obtained after a minimum 8-h fast and archived aliquots (plasma, serum, RBC) were stored at −80°C until analysis.
Carotenoids and fat-soluble vitamins
Plasma carotenoid and vitamin A and E concentrations were determined by HPLC (33). Carotenoids separated using this method (lutein, zeaxanthin, cryptoxanthin, β-carotene, and lycopene) were quantified by determining peak areas calibrated to known amounts of external standards. Concentrations were corrected for extraction and handling losses by monitoring the recovery of the internal standards and standardized by expressing values per milligram of triglyceride (TG). The intra-assay CV was 4% and interassay CV was 3.9%. Vitamin D (25-hydroxyvitamin D) was measured using a commercially available kit (DiaSorin). The intra-assay CV was 9% and interassay CV was 10%. Vitamin K (phylloquinone) was measured using HPLC (34). Two pooled plasma samples were run as low (CV: 12%) and high (CV: 8%) controls with every batch.
Fatty acid profiles
RBC fatty acid profiles, an indicator of relatively long-term dietary intake (35), were quantified using an established GC method (36). Peaks of interest were identified by comparison with authentic fatty acid standards (Nu-Check-Prep) and the data expressed as molar percentage (mol%) proportions of fatty acids relative to the internal standard. A pooled plasma (control) sample was run monthly and an external standard run daily with every batch of samples to ensure instrument precision. The interassay CVs ranged from 0.5% to 4.3% for fatty acids present at levels >5 mol%, 1.8% to 7.1% for fatty acids present at levels between 1 mol% and 5 mol%, and 2.8% to 11.1% for fatty acids present at levels <1 mol%.
Estimated desaturase enzyme activities
These were calculated as product to precursor ratios of individual fatty acids and included stearoyl-CoA-desaturase [SCD1: palmitoleic acid/palmitic acid (16:1n–7/16:0) and SCD2: oleic acid/stearic acid (18:1n–9/18:0)], δ-6-desaturase [D6D: dihomo-γ-linolenic acid/linoleic acid (20:3n–6/18:2n–6)], and δ-5-desaturase [D5D: arachidonic acid (20:4n–6)/dihomo-γ-linolenic acid] (28).
Measurement of CMRFs
CMRF data were collected at baseline and the end of the 1-y intervention. Height, weight, and blood pressure were measured as previously described (11). BMIz was based on the CDC growth charts for children by age and sex (37). Fasting total cholesterol (TC), LDL cholesterol, HDL cholesterol, TG, insulin, glucose, and liver enzymes [aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP)] were assessed using standard methods, as described for the primary study. A glucose load of 1.75 g/kg body weight (Glucola™) was administered for the 2-h oral-glucose-tolerance test. HOMA-IR, a measure of insulin resistance, was calculated as follows: glucose (mg/dL) × immunoreactive insulin (mmol/L)/22.5 (38).
Additional CMRFs
Markers of inflammation (TNF-α, IL-6, IL-1β), vascular adhesion (E-selectin, P-selectin, sICAM), and coagulation (thrombomodulin), and adipokines (leptin, adiponectin) were measured in serum by commercially available multiplex assays (electrochemiluminescence detection sandwich immunoassay: V-PLEX Human Cytokine Assays; V-PLEX Human Biomarker Assays; Human Metabolic Assays) from Meso Scale Discovery (MSD) using a Meso Scale Discovery SECTOR Imager 2400. The mean intra-assay CVs were <5% and interassay CVs were <7%. hsCRP was measured by solid-phase, 2-site chemiluminescent immunometric assay using the IMMULITE 2000 (Siemens Healthcare Diagnostics). The intra- and interassay CVs were 3.0% and 5.0%, respectively.
Statistical analyses
Sample size estimates for the primary clinical trial which provided the samples for the present study have been reported previously (11). There were 160 children in the SC group and 161 children in the SC + EP group with an archived blood sample to perform the nutrient biomarker and additional CMRF panel analyses proposed in the present study. We conservatively assumed a difference in the 1-y change in RBC fatty acid and carotenoid concentrations between the SC and SC + EP groups of 0.36 SD. With the prespecified sample size of 160 per group, we had 80% power to detect differences with a 2-sided type I error rate of 5%. In addition, to account for multiple comparisons, with a type I error rate = 0.005 under the most conservative Bonferroni adjustment with 80% power, the minimum detectable standardized effect size was 0.41 SD, considered a medium (0.3–0.5) effect size (39).
Analysis methods
The analysis was based upon the intention-to-treat approach. Only children with nutrient biomarker and CMRF data at both baseline and 1 y were included in the analyses. Data from each child were analyzed as per his/her initial assignment in the primary clinical trial to the SC or SC + EP group regardless of actual compliance to the regimen. The data were checked to identify and resolve reasons for missing values, inconsistencies, and out-of-range values. Descriptive analyses of the baseline characteristics of the SC and SC + EP groups were summarized using either mean ± SD, median (IQR) for skewed variables, or proportions. The nutrient biomarkers and CMRF data at baseline and the end of the 1-y intervention were summarized for each group using geometric means and SDs estimated from log-transformed values (40). The effect of the intervention on the nutrient biomarkers and CMRFs (dependent variables) was assessed using a mixed-effects random intercept linear model with group, time, and group-by-time interaction as factors. Robust SEs were used to account for possible model misspecification. Subject was included as a random effect within the model and P values were presented from the corresponding F test for each fixed effect. Dependent variables were log transformed to facilitate reporting differences as mean percentage differences (95% CIs) and were calculated from back-transformed model-based least-square means as {2.72⋀[LSMEANS(1 y − baseline)] − 1}×100%. False discovery rate (FDR) correction was applied to tests of the intervention effect among the nutrient biomarkers, and separately for the CMRFs. An FDR threshold of 0.25 was used for adjusted P values. To evaluate any difference by sex, the 3-way interaction term (sex by group by time) was included in the mixed-effects model. In addition, given the lack of intervention effect, pooled data are presented from the mixed-effects model without the group, and group-by-time terms, to provide an overall summary of the 1-y change in nutrient biomarkers and CMRFs for all children. Associations of nutrient biomarkers with CMRFs were estimated using multiple linear regression models with CMRF at 1 y as the dependent variable and adjusted for sex, age, group, baseline BMIz, and baseline CMRF value. Nutrient biomarkers and CMRFs were standardized (mean: 0; SD: 1) and children in both groups were pooled for use in the association models. Each combination of nutrient biomarker and CMRF was estimated in a separate model. The coefficients from the estimated models represent the mean change in the CMRF (in SD units) associated with an increase in 1 SD of the biomarker. Some of the CMRFs (hsCRP, IL-1, IL-6, leptin, E-selectin, P-selectin, ALT, and 2-h insulin) were log transformed owing to nonnormal distribution. No other violations of the linear regression models were detected after variable transformations. Sensitivity analysis was run for the association models incorporating a random effect for family to account for the nested structure of the data (children with the same parent/guardian). However, the additional random effect did not improve the predictive quality of the regression coefficients, thus the model assuming independent observations was reported throughout. All statistical analyses were performed with SAS software version 9.4 (SAS Institute Inc.).
Results
Baseline characteristics
The median age (9.3 compared with 9.4 y), mean BMIz (2.0 compared with 1.9), and sex distribution (47% compared with 50% females) were similar between the groups at baseline (Table 1). The race/ethnicity distribution was also similar, with >70% of children self-identified as Hispanic/Latino and >70% of the parents self-identified as low income.
TABLE 1.
Baseline characteristics of the children1
| Variables | SC (n = 160) | SC + EP (n = 161) |
|---|---|---|
| Age, y | 9.3 (8–11) | 9.4 (8–11) |
| Sex (%, females/males) | 47/53 | 50/50 |
| BMI z score | 2.0 ± 0.4 | 1.9 ± 0.4 |
| Race/ethnicity, % | ||
| Hispanic | 72.5 | 75.8 |
| Non-Hispanic | 20.0 | 15.2 |
| White, Asian, and other | 7.5 | 9.0 |
| Parent/guardian, % | ||
| Education ≤high school | 81.2 | 75.7 |
| Income <$30,000 | 74.5 | 72.8 |
Values are percentages, medians (IQRs), or means ± SDs. EP, enhanced program; SC, standard care.
Nutrient biomarkers and desaturase enzyme indexes
Overall, children in the SC and SC + EP groups showed similar changes in nutrient biomarker concentrations after the 1-y intervention (Table 2). After FDR adjustment, no significant differences were observed between groups, with the exception of higher RBC DHA and lower trans-vaccenic acid (18:1n–7t) and vitamin E concentrations in the SC + EP than in the SC group.
TABLE 2.
Nutrient biomarker concentrations and desaturase enzyme indexes at baseline and the end of the 1-y intervention by study group1
| SC | SC + EP | P value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomarkers | Baseline3 | 1 Y3 | Mean percentage difference4 | Baseline3 | 1 Y3 | Mean percentage difference4 | Group | Time | Group×time |
| Carotenoids, μg/dL | |||||||||
| Lutein | 8.5 ± 5.1 | 7.8 ± 3.7 | −7.8** (−14.6, −0.4) | 8.2 ± 3.4 | 7.8 ± 3.4 | −4.9 (−11.2, 1.8) | 0.737 | 0.012** | 0.559 |
| Zeaxanthin | 3.2 ± 1.8 | 3.3 ± 1.5 | 2.4 (−5.0, 10.5) | 3.1 ± 1.4 | 3.2 ± 1.4 | 4.3 (−2.2, 11.3) | 0.482 | 0.188 | 0.713 |
| Cryptoxanthin | 7.7 ± 6.5 | 7.9 ± 6.3 | 0.01 (−8.1, 8.9) | 7.6 ± 7.6 | 7.7 ± 7.8 | 0.5 (−10.2, 10.2) | 0.783 | 0.939 | 0.938 |
| β-Carotene | 11.3 ± 12.6 | 13.4 ± 16.8 | 17.5** (4.1, 32.6) | 10.9 ± 8.8 | 13.7 ± 14.1 | 25.7** (11.8, 41.2) | 0.976 | <0.001** | 0.431 |
| Trans-Lycopene | 19.5 ± 15.5 | 18.9 ± 15.0 | −2.6 (−12.0, 7.8) | 19.8 ± 9.7 | 20.4 ± 11.2 | 3.5 (−5.0, 12.7) | 0.362 | 0.911 | 0.367 |
| Fat-soluble vitamins | |||||||||
| Vitamin A, μg/dL | 40.8 ± 10.7 | 40.7 ± 9.1 | −0.4 (−4.7, 4.1) | 39.7 ± 8.2 | 39.0 ± 8.1 | −1.4 (−5.1, 2.5) | 0.257 | 0.001** | 0.875 |
| Vitamin D, ng/mL | 14.9 ± 5.2 | 15.6 ± 5.7 | 3.9 (−2.4, 10.7) | 16.3 ± 6.0 | 15.5 ± 5.7 | −4.8 (−10.6, 1.4) | 0.318 | 0.812 | 0.052 |
| Vitamin E, μg/dL | 165.0 ± 89.1 | 160.9 ± 96.9 | −3.3 (−10.9, 4.9) | 155.6 ± 86.4 | 124.7 ± 74.4 | −19.6** (−26.3, −12.4) | 0.316 | <0.001** | 0.034** |
| Vitamin K, nM/L | 0.35 ± 0.45 | 0.43 ± 0.65 | 23.5 (−4.4, 46.1) | 0.36 ± 0.46 | 0.40 ± 0.44 | 12.1 (−5.8, 33.4) | 0.571 | 0.076 | 0.765 |
| Fatty acids, mol% | |||||||||
| SFA | 41.2 ± 1.9 | 39.9 ± 1.8 | −3.1** (−4.0, −2.2) | 41.1 ± 1.8 | 39.7 ± 1.6 | −3.2** (−4.2, −2.3) | 0.398 | <0.001** | 0.854 |
| 12:0 | 0.13 ± 0.10 | 0.12 ± 0.12 | −12.8 (−25.5, 2.0) | 0.13 ± 0.11 | 0.11 ± 0.11 | −10.5 (−23.9, 5.2) | 0.469 | 0.031** | 0.817 |
| 14:0 | 0.42 ± 0.17 | 0.51 ± 0.18 | 23.4** (14.5, 33.0) | 0.41 ± 0.15 | 0.51 ± 0.17 | 23.7** (15.6, 32.3) | 0.843 | <0.001** | 0.962 |
| 15:0 | 0.35 ± 0.08 | 0.38 ± 0.10 | 8.1** (2.1, 14.5) | 0.36 ± 0.08 | 0.37 ± 0.10 | 4.2 (−1.5, 10.2) | 0.834 | 0.004** | 0.366 |
| 16:0 | 21.4 ± 2.0 | 19.7 ± 1.8 | −8.4** (−9.9, −6.8) | 21.2 ± 1.9 | 19.4 ± 1.6 | −8.6** (−10.1, −7.0) | 0.243 | <0.001** | 0.856 |
| 18:0 | 16.9 ± 0.9 | 17.1 ± 1.0 | 1.8** (0.7, 2.9) | 16.9 ± 0.9 | 17.2 ± 1.0 | 1.6** (0.5, 2.7) | 0.672 | <0.001** | 0.824 |
| 20:0 | 0.18 ± 0.03 | 0.21 ± 0.05 | 15.0** (9.8, 20.4) | 0.18 ± 0.04 | 0.21 ± 0.06 | 16.4** (10.7, 22.4) | 0.130 | <0.001** | 0.720 |
| 22:0 | 0.51 ± 0.12 | 0.54 ± 0.11 | 5.2** (0.2, 10.5) | 0.52 ± 0.09 | 0.54 ± 0.12 | 3.9 (−0.5, 8.4) | 0.548 | 0.007** | 0.704 |
| 24:0 | 1.10 ± 0.22 | 1.15 ± 0.29 | 4.7 (−0.3, 10.1) | 1.12 ± 0.25 | 1.18 ± 0.28 | 5.4** (0.7, 10.4) | 0.258 | 0.004** | 0.847 |
| MUFA | 15.6 ± 1.2 | 15.8 ± 1.2 | 1.3 (−0.1, 2.7) | 15.5 ± 1.4 | 15.6 ± 1.3 | 0.9 (−0.5, 2.3) | 0.223 | 0.031** | 0.659 |
| 16:1n–7 | 0.46 ± 0.15 | 0.52 ± 0.18 | 14.3** (8.7, 20.3) | 0.45 ± 0.18 | 0.50 ± 0.17 | 9.7** (3.0, 16.8) | 0.371 | <0.001** | 0.311 |
| 16:1n–9 | 0.09 ± 0.03 | 0.11 ± 0.03 | 18.7** (12.4, 25.2) | 0.10 ± 0.03 | 0.11 ± 0.03 | 17.3** (10.5, 24.4) | 0.434 | <0.001** | 0.770 |
| 18:1n–7 | 1.72 ± 0.77 | 1.74 ± 0.73 | 1.5 (−6.2, 9.9) | 1.74 ± 0.86 | 1.66 ± 0.66 | −4.8 (−12.4, 3.5) | 0.641 | 0.556 | 0.274 |
| 18:1n–9 | 11.8 ± 1.1 | 11.9 ± 0.9 | 0.04 (−1.2, 1.2) | 11.6 ± 1.2 | 11.7 ± 1.1 | 1.0 (−0.4, 2.4) | 0.260 | 0.306 | 0.265 |
| 20:1n–9 | 0.20 ± 0.04 | 0.23 ± 0.05 | 14.7** (10.9, 18.6) | 0.21 ± 0.04 | 0.24 ± 0.05 | 15.7** (11.9, 19.7) | 0.149 | <0.001** | 0.719 |
| 22:1n–9 | 0.05 ± 0.01 | 0.05 ± 0.02 | 1.0 (−6.9, 9.5) | 0.05 ± 0.02 | 0.05 ± 0.02 | −1.5 (−8.0, 5.4) | 0.542 | 0.912 | 0.640 |
| 24:1n–9 | 1.05 ± 0.19 | 1.11 ± 0.23 | 5.3** (0.7, 10.1) | 1.05 ± 0.20 | 1.13 ± 0.24 | 7.6** (2.8, 12.7) | 0.521 | <0.001** | 0.511 |
| PUFA n–6s | 36.2 ± 1.9 | 37.2 ± 2.0 | 2.5** (1.6, 3.4) | 36.7 ± 1.8 | 37.6 ± 1.8 | 2.7** (1.8, 3.6) | 0.019** | <0.001** | 0.803 |
| 18:2n–6 | 14.1 ± 1.4 | 14.3 ± 1.4 | 0.8 (−0.6, 2.3) | 14.3 ± 1.60 | 14.5 ± 1.55 | 1.0 (−0.8, 2.8) | 0.078 | 0.133 | 0.909 |
| 18:3n–6 | 0.07 ± 0.03 | 0.08 ± 0.03 | 20.0** (11.1, 29.6) | 0.07 ± 0.04 | 0.07 ± 0.04 | 9.5 (−0.4, 20.3) | 0.188 | <0.001** | 0.139 |
| 20:2n–6 | 0.32 ± 0.06 | 0.36 ± 0.07 | 13.0** (10.4, 15.5) | 0.33 ± 0.06 | 0.38 ± 0.07 | 14.5** (11.8, 17.3) | 0.049** | <0.001** | 0.412 |
| 20:3n–6 | 1.84 ± 0.45 | 2.13 ± 0.49 | 15.1** (11.8, 18.5) | 1.90 ± 0.48 | 2.13 ± 0.50 | 13.3** (9.5, 17.3) | 0.315 | <0.001** | 0.483 |
| 20:4n–6 | 15.0 ± 1.2 | 15.2 ± 1.4 | 1.4** (0.1, 2.8) | 15.0 ± 1.3 | 15.2 ± 1.4 | 1.7** (0.2, 3.2) | 0.940 | 0.002** | 0.809 |
| 22:2n–6 | 0.08 ± 0.03 | 0.09 ± 0.06 | 2.2 (−8.7, 14.4) | 0.08 ± 0.04 | 0.08 ± 0.05 | −4.6 (−14.2, 6.2) | 0.287 | 0.753 | 0.387 |
| 22:4n–6 | 3.48 ± 0.59 | 3.88 ± 0.69 | 11.6** (8.9, 14.3) | 3.59 ± 0.61 | 4.00 ± 0.60 | 12.2** (9.2, 15.3) | 0.084 | <0.001** | 0.764 |
| 22:5n–6 | 1.11 ± 0.34 | 0.94 ± 0.28 | −15.8** (−20.5, −10.9) | 1.08 ± 0.32 | 0.98 ± 0.25 | −9.5** (−14.5, −4.3) | 0.652 | <0.001** | 0.078 |
| PUFA n–3s | 5.75 ± 0.94 | 5.99 ± 1.02 | 4.7** (2.3, 7.1) | 5.55 ± 1.03 | 5.97 ± 1.01 | 8.1** (5.4, 11.0) | 0.228 | <0.001** | 0.065 |
| 18:3n–3 | 0.18 ± 0.06 | 0.22 ± 0.06 | 23.6** (17.6, 30.0) | 0.18 ± 0.05 | 0.21 ± 0.06 | 17.7** (12.0, 23.7) | 0.520 | <0.001** | 0.170 |
| 20:5n–3 | 0.32 ± 0.11 | 0.35 ± 0.12 | 11.6** (6.6, 16.8) | 0.31 ± 0.12 | 0.33 ± 0.12 | 9.2** (3.5, 15.4) | 0.120 | <0.001** | 0.555 |
| 22:5n–3 | 2.03 ± 0.30 | 2.15 ± 0.33 | 6.1** (3.2, 9.0) | 2.00 ± 0.35 | 2.11 ± 0.29 | 6.8** (3.6, 10.1) | 0.335 | <0.001** | 0.748 |
| 22:6n–3 | 3.11 ± 0.81 | 3.15 ± 0.88 | 2.1 (−0.8, 5.0) | 2.96 ± 0.81 | 3.21 ± 0.82 | 8.1** (4.7, 11.5) | 0.420 | <0.001** | 0.009** |
| Trans | 1.02 ± 0.25 | 0.89 ± 0.27 | −13.7** (−17.6, −9.6) | 1.02 ± 0.25 | 0.85 ± 0.21 | −17.6** (−21.2, −13.7) | 0.354 | <0.001** | 0.164 |
| 16:1n–7t | 0.08 ± 0.02 | 0.07 ± 0.02 | −12.2** (−16.0, −8.4) | 0.08 ± 0.02 | 0.07 ± 0.02 | −8.4** (−12.5, −4.1) | 0.502 | <0.001** | 0.178 |
| 16:1n–9t | 0.02 ± 0.01 | 0.02 ± 0.01 | −14.1** (−19.7, −8.2) | 0.03 ± 0.01 | 0.02 ± 0.01 | −20.3** (−25.5, −14.8) | 0.969 | <0.001** | 0.120 |
| 18:1n–7t | 0.17 ± 0.05 | 0.16 ± 0.07 | −7.4** (−14.1, −0.2) | 0.17 ± 0.06 | 0.15 ± 0.06 | −16.7** (−22.5, −10.5) | 0.259 | <0.001** | 0.046** |
| 18:1n–9t | 0.33 ± 0.11 | 0.27 ± 0.11 | −16.9** (−21.3, −12.4) | 0.33 ± 0.11 | 0.26 ± 0.08 | −19.8** (−23.5, −15.9) | 0.804 | <0.001** | 0.335 |
| 18:1n–10–12t | 0.20 ± 0.09 | 0.17 ± 0.06 | −16.5** (−23.1, −9.3) | 0.20 ± 0.08 | 0.16 ± 0.05 | −19.7** (−25.8, −13.2) | 0.034** | <0.001** | 0.495 |
| 18:2t | 0.11 ± 0.05 | 0.10 ± 0.05 | −13.6** (−22.0, −4.2) | 0.11 ± 0.06 | 0.09 ± 0.05 | −13.8** (−22.5, −4.0) | 0.324 | <0.001** | 0.976 |
| 18:2CLA | 0.08 ± 0.02 | 0.07 ± 0.03 | −9.9** (−14.3, −5.2) | 0.08 ± 0.02 | 0.07 ± 0.02 | −14.7** (−19.2, −9.9) | 0.434 | <0.001** | 0.145 |
| Desaturase enzyme indexes5 | |||||||||
| SCD1 (16:1n–7/16:0) | 0.020 ± 0.01 | 0.022 ± 0.01 | 9.6** (4.3, 15.1) | 0.020 ± 0.01 | 0.021 ± 0.01 | 5.4 (−0.7, 12.0) | 0.337 | <0.001** | 0.332 |
| SCD2 (18:1n–9/18:0) | 0.70 ± 0.09 | 0.69 ± 0.08 | −1.9** (−3.6, −0.1) | 0.69 ± 0.09 | 0.68 ± 0.09 | −0.6 (−2.7, 1.5) | 0.303 | 0.069 | 0.362 |
| D6D (20:3n–6/18:2n–6) | 0.13 ± 0.03 | 0.15 ± 0.03 | 14.3** (10.7, 18.1) | 0.13 ± 0.03 | 0.15 ± 0.03 | 12.2** (8.2, 16.2) | 0.834 | <0.001** | 0.432 |
| D5D (20:4n–6/20:3n–6) | 8.16 ± 2.35 | 7.13 ± 1.95 | −11.8** (−14.5, −9.0) | 7.91 ± 2.39 | 7.15 ± 2.05 | −10.3** (−13.5, −6.9) | 0.364 | <0.001** | 0.477 |
Number of children with both baseline and 1-y values: SC (n = 125) and SC + EP (n = 128).
CLA, conjugated linolenic acid; D5D, δ-5-desaturase; D6D, δ-6-desaturase; EP, enhanced program; mol%, molar percentage; SC, standard care; SCD, stearoyl co-A desaturase; trans, trans fatty acids. **Denotes significant difference.
F tests on fixed effects of group, time, and group-by-time interaction from a mixed-effects random intercept linear model.
Values are geometric means ± SDs; SDs estimated from log-transformed values.
Values are mean percentage differences (95% CIs) calculated from model-based least-square means.
Values are fatty acid product:precursor ratios.
Given the minimal effect of the SC + EP intervention, nutrient biomarker data from children in both groups were combined and the pooled change over 1 y is summarized in Figure 1. Results indicate a significant increase in plasma concentrations of β-carotene (22%) from yellow/orange fruits and vegetables and decrease in lutein (−6%) from eggs, corn, and green leafy vegetables. A significant decrease in vitamin A (−10%) and vitamin E (−20%) concentrations was observed, which may be due to an overall decrease in intake of animal foods and fortified sugary cereals, and fried foods prepared with vegetable oils, major dietary sources of vitamins A and E, respectively (41). No significant differences were observed in plasma vitamin D or K concentrations. Among the fatty acids, total SFA was significantly decreased in both groups (−3%), primarily due to lower proportions of lauric acid (12:0) (−12%) and palmitic acid (−9%), with compensatory higher proportions of the minor SFAs [2–24% for myristic acid (14:0), stearic acid, arachidic acid (20:0), and lignoceric acid (24:0)]. MUFAs, especially those in the DNL pathway [7–18% for palmitoleic acid, 7-hexadecenoic acid (16:1n–9), gondoic acid (20:1n–9), and nervonic acid (24:1n–9)], as well as total PUFA n–6s, eicosadienoic acid (20:2n–6), dihomo-γ-linolenic acid, arachidonic acid, and adrenic acid (22:4n–6), were significantly increased (2–15%), with the exception of Osbond acid (22:5n–6) which was significantly decreased (−13%), after the 1-y intervention period in both groups. All PUFA n–3s including α-linolenic acid (21%) from vegetable oils and EPA, docosapentaenoic acid, and DHA (5–10%) from fish, were significantly increased in children in both groups. Trans fatty acids, indicators of ruminant fat [trans-palmitoleic acid (16:1n–7t), trans-7-hexadecenoic acid (16:1n–9t), trans-vaccenic acid, and 18:2 conjugated linolenic acid], and of partially hydrogenated fat typically found in traditional margarines and in commercially prepared fried foods and savory snacks [elaidic acid (18:1n–9t), trans-octadecenoic acid (18:1n–10–12t)], were significantly decreased (−10% to −18%) in both groups by the end of the study. Of the desaturase enzyme indexes, SCD1 (8%) and D6D (13%) were significantly increased, whereas D5D (−11%) was significantly decreased, in both groups. A trend toward a decrease in SCD2 was also observed (−2%; P = 0.069).
FIGURE 1.
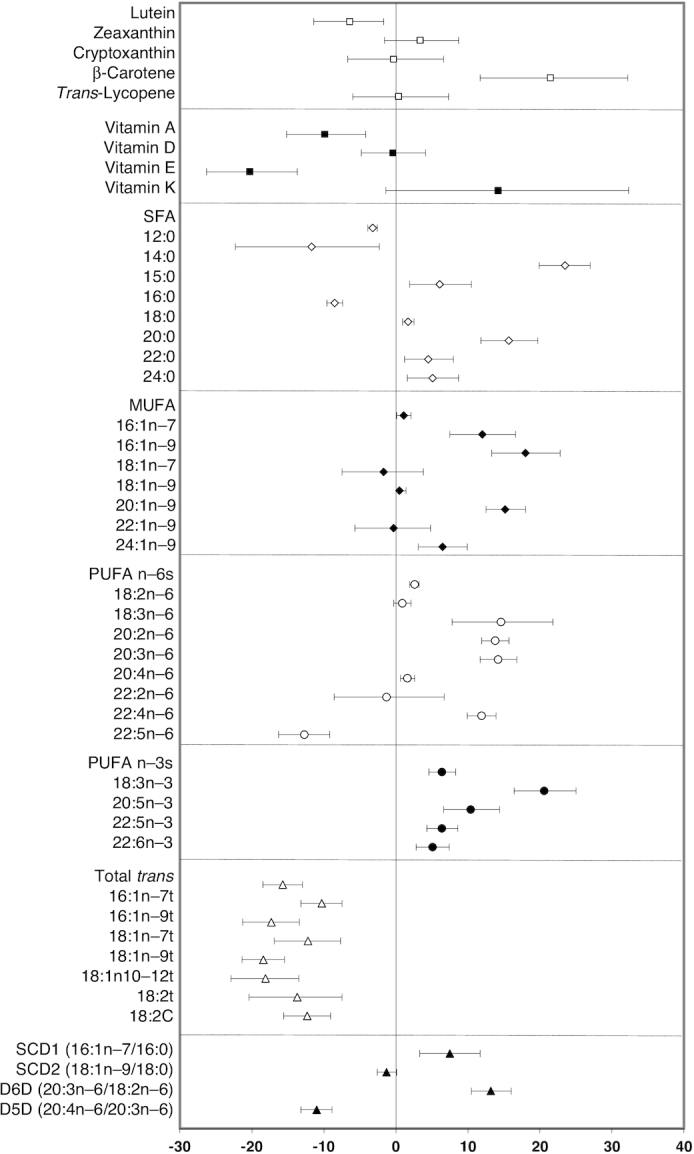
Pooled 1-y change in nutrient biomarker concentrations and desaturase enzyme activities. For each individual nutrient biomarker, the mean percentage difference is plotted as the symbol and the 95% CI is displayed as the bar. The mean percentage difference values and 95% CIs were derived from least-square means calculated from a mixed-effects random intercept model with time (baseline or 1 y) as a fixed effect and a random intercept for subject correlations. A separate model was fitted for each log-transformed outcome. n = 253 and included children in the SC and SC + enhanced program groups with both a baseline and 1-y nutrient biomarker value. D5D, δ-5-desaturase; D6D, δ-6-desaturase; SC, standard care; SCD, stearoyl co-A desaturase.
CMRFs
CMRFs were divided into 7 broad categories: BMIz; blood pressure (systolic and diastolic); glucose metabolism (fasting and 2-h glucose and insulin, HOMA-IR); lipid profile (TC, LDL cholesterol, HDL cholesterol, TG); markers of inflammation (hsCRP, TNFα, IL-1, IL-6), vascular adhesion (E-selectin, P-selectin, sICAM), and coagulation (thrombomodulin); adipokines (adiponectin, leptin); and liver enzymes (ALT, AST, and ALP). At the end of the 1-y intervention period, BMIz was significantly decreased in both the SC and SC + EP groups, with no significant difference between groups (Table 3). Both groups had favorable decreases in LDL cholesterol and TNF-α, and increases in P-selectin and leptin concentrations at the end of 1 y. However, unfavorable increases in insulin, HOMA-IR, TG, P-selectin, and sICAM concentrations were observed in both groups. Children in the SC + EP group had a modest additional improvement in hsCRP and liver enzyme activities (AST, ALT, and ALP). After FDR adjustment, only differences in hsCRP and ALT remained significant in the SC + EP compared with the SC group.
TABLE 3.
CMRFs at baseline and the end of the 1-y intervention by study group1
| SC | SC + EP | P value2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CMRFs | Baseline3 | 1 Y3 | Mean percentage difference4 | Baseline3 | 1 Y3 | Mean percentage difference4 | Group | Time | Group×time |
| BMIz | 1.96 ± 0.43 | 1.76 ± 0.97 | −7.7 (−11.0, −4.4) | 1.89 ± 0.49 | 1.70 ± 0.62 | −9.8 (−12.9, −6.6) | 0.098 | <0.001** | 0.362 |
| Blood pressure, mm Hg | |||||||||
| Diastolic blood pressure | 59 ± 5.6 | 59 ± 6.0 | −0.02 (−1.8, 1.8) | 58 ± 5.6 | 58 ± 5.7 | −0.04 (−1.9, 1.8) | 0.102 | 0.963 | 0.984 |
| Systolic blood pressure | 106 ± 10.3 | 106 ± 12.8 | 0.06 (−1.3, 2.5) | 106 ± 10.8 | 107 ± 12.4 | 1.4 (−0.4, 3.1) | 0.755 | 0.137 | 0.556 |
| Glucose metabolism | |||||||||
| Fasting glucose, mg/dL | 83.4 ± 19.0 | 86.3 ± 6.8 | 1.6** (0.01, 3.2) | 84.9 ± 7.8 | 86.4 ± 7.1 | 1.4 (−0.3, 3.1) | 0.318 | 0.011** | 0.872 |
| Fasting insulin, μU/mL | 14.2 ± 10.9 | 17.3 ± 14.9 | 22.3** (11.6, 34.1) | 13.5 ± 11.3 | 16.9 ± 14.8 | 24.0** (12.9, 36.3) | 0.441 | <0.001** | 0.835 |
| 2-h glucose, mg/dL | 95.0 ± 43.9 | 99.9 ± 18.7 | 2.9 (−0.4, 6.4) | 95.1 ± 18.0 | 95.7 ± 16.9 | 0.9 (−2.3, 4.2) | 0.801 | 0.110 | 0.393 |
| 2-h insulin, μU/mL | 69.1 ± 85.8 | 88.3 ± 125 | 30.2** (14.9, 47.5) | 57.7 ± 98.6 | 79.6 ± 104 | 35.2** (17.5, 55.5) | 0.061 | <0.001** | 0.694 |
| HOMA-IR | 2.93 ± 2.75 | 3.68 ± 3.50 | 24.9** (12.9, 38.1) | 2.82 ± 2.58 | 3.59 ± 3.32 | 26.1** (14.2, 39.2) | 0.585 | <0.001** | 0.892 |
| Lipid profile1 | |||||||||
| Total cholesterol, mg/dL | 154.1 ± 56.1 | 158.3 ± 29.7 | 0.6 (−1.4, 2.7) | 150.4 ± 27.2 | 146.8 ± 27.0 | −1.8 (−3.9, 0.4) | 0.198 | 0.443 | 0.119 |
| LDL cholesterol, mg/dL | 92.9 ± 25.0 | 90.2 ± 26.0 | −2.9** (−5.5, −0.2) | 86.4 ± 23.7 | 81.1 ± 21.4 | −5.5** (−8.3, −2.5) | 0.003** | <0.001** | 0.197 |
| HDL cholesterol, mg/dL | 45.6 ± 9.97 | 46.8 ± 10.5 | 1.1 (−1.4, 3.7) | 45.9 ± 10.1 | 46.4 ± 11.2 | 1.8 (−0.4, 4.1) | 0.654 | 0.087 | 0.678 |
| Triglycerides, mg/dL | 78.3 ± 47.3 | 86.3 ± 53.6 | 11.5** (2.6, 21.3) | 72.5 ± 44.6 | 78.9 ± 45.1 | 8.4** (1.2, 16.1) | 0.057 | 0.001** | 0.602 |
| Inflammatory, vascular adhesion, and coagulation markers2 | |||||||||
| hsCRP, mg/L | 1.77 ± 6.53 | 1.87 ± 8.24 | 8.4 (−12.1, 33.8) | 1.56 ± 8.26 | 1.12 ± 5.33 | −27.9** (−40, −14) | 0.015** | 0.079 | 0.004** |
| TNF-α, pg/mL | 6.30 ± 3.87 | 4.83 ± 2.01 | −22.8** (−30, −15) | 6.15 ± 2.09 | 4.92 ± 1.97 | −19.9** (−26, −14) | 0.830 | <0.001** | 0.531 |
| IL-1, pg/mL | 0.25 ± 0.19 | 0.26 ± 0.22 | 3.7 (−10.0, 19.4) | 0.26 ± 0.18 | 0.24 ± 0.15 | −4.9 (−17.1, 9.1) | 0.621 | 0.888 | 0.390 |
| IL-6, pg/mL | 0.99 ± 1.83 | 0.91 ± 1.92 | −8.0 (−22.4, 9.2) | 0.89 ± 1.27 | 0.79 ± 1.88 | −11.4 (−30.5, 13.0) | 0.216 | 0.177 | 0.802 |
| E-selectin, pg/mL | 4.88 ± 4.19 | 5.44 ± 4.05 | 12.1 (−1.9, 28.2) | 5.12 ± 4.32 | 5.04 ± 3.72 | −1.6 (−14.4, 13.2) | 0.768 | 0.316 | 0.185 |
| P-selectin, pg/mL | 36.1 ± 19.7 | 45.2 ± 24.4 | 25.7** (13.5, 39.4) | 36.6 ± 19.9 | 47.0 ± 27.0 | 28.5** (14.9, 43.6) | 0.609 | <0.001** | 0.782 |
| sICAM, ng/mL | 0.55 ± 0.37 | 0.61 ± 0.31 | 11.1 (−2.2, 26.2) | 0.53 ± 0.30 | 0.64 ± 0.30 | 21.9** (7.5, 38.3) | 0.900 | 0.001** | 0.309 |
| Thrombomodulin, ng/mL | 2.10 ± 1.07 | 2.25 ± 0.75 | 7.1 (−2.6, 17.8) | 2.08 ± 0.87 | 2.26 ± 0.84 | 8.5 (−1.7, 19.8) | 0.913 | 0.032** | 0.852 |
| Adipokines2 | |||||||||
| Adiponectin, mg/mL | 112.9 ± 53.1 | 93.2 ± 136.3 | −17.2 (−29.3, 2.9) | 117.4 ± 55.5 | 111.7 ± 56.3 | −4.9 (−11.4, 2.1) | 0.068 | 0.007** | 0.117 |
| Leptin, ng/mL | 16.2 ± 28.7 | 18.8 ± 33.0 | 16.3** (0.8, 34.1) | 12.4 ± 34.7 | 14.6 ± 32.7 | 17.9** (0.02, 39.1) | 0.033** | 0.005** | 0.899 |
| Liver enzymes1 | |||||||||
| AST, U/L | 29.9 ± 7.0 | 29.2 ± 10.7 | −2.9 (−8.1, 2.7) | 31.0 ± 8.4 | 28.5 ± 6.9 | −8.1** (−12.0, −4.0) | 0.783 | 0.002** | 0.125 |
| ALT, U/L | 23.4 ± 9.2 | 24.7 ± 11.9 | 5.5 (−1.2, 12.5) | 24.2 ± 11.8 | 21.9 ± 9.4 | −9.6** (−15.0, −3.9) | 0.235 | 0.290 | 0.001** |
| ALP, U/L | 276.5 ± 86.8 | 264.4 ± 103.9 | −3.9 (−7.9, 0.3) | 282.9 ± 90.2 | 266.2 ± 102.7 | −6.6** (−10.5, −2.6) | 0.819 | <0.001** | 0.345 |
Number of children with both baseline and 1-y values: BMIz (n = 159 in SC and n = 159 in SC + EP); blood pressure (n = 137 in SC and n = 133 in SC + EP); glucose metabolism, lipid profile, and hsCRP (n = 132 in SC and n = 131 in SC + EP); other inflammatory, vascular adhesion, and coagulation markers and adipokines (n = 113 in SC and n = 108 in SC + EP); and liver enzymes (n = 130 in SC and n = 132 in SC + EP). ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMIz, BMI z score; CMRF, cardiometabolic risk factor; EP, enhanced program; hsCRP, high-sensitivity C-reactive protein; SC, standard care; sICAM, soluble intercellular adhesion molecule. **Denotes significant difference.
F tests on fixed effects of group, time, and group-by-time interaction from a mixed-effects random intercept linear model.
Values are geometric means ± SDs; SDs estimated from log-transformed values.
Values are mean percentage differences (95% CIs) calculated from model-based least-square means.
When both groups were combined (Figure 2), an overall improvement in CMRFs was observed including a significant decrease in BMIz (−10%), LDL cholesterol (−4%), TNF-α (−21%), and liver enzymes (−2% to −6%) and an increase in HDL-cholesterol (2%) concentrations. However, unfavorable increases in fasting (23%) and 2-h insulin (33%), HOMA-IR (26%), TG (10%), P-selectin (27%), sICAM (16%), and leptin (17%) were also observed over the 1-y intervention period.
FIGURE 2.
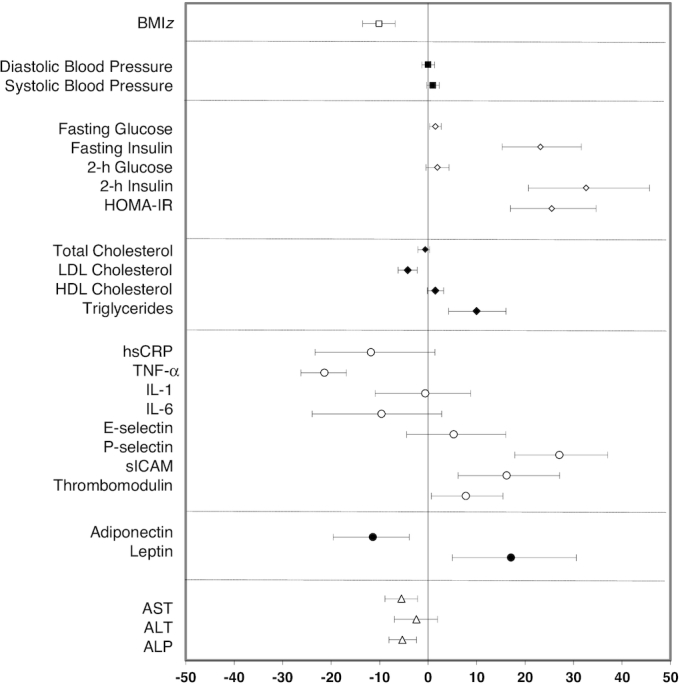
Pooled 1-y change in CMRFs. For each individual CMRF, the mean percentage difference is plotted as the symbol and the 95% CI is displayed as the bar. The mean percentage difference values and 95% CIs are derived from least-square means calculated from a mixed-effects random intercept model with time (baseline or 1 y) as a fixed effect and a random intercept for subject correlations. A separate model is fitted for each log-transformed outcome. Numbers of children in the SC and SC + enhanced program groups with both baseline and 1-y values were as follows: BMIz (n = 317); blood pressure (n = 270); glucose metabolism (n = 264); lipid profile (n = 265); hsCRP (n = 261); other inflammatory, vascular adhesion, and coagulation markers, and adipokines (n = 221); and liver enzymes (n = 268). ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMIz, BMI z score; CMRF, cardiometabolic risk factor; hsCRP, high-sensitivity C-reactive protein; sICAM, soluble intercellular adhesion molecule.
Association between biomarkers and CMRFs
A heat map of the association between the nutrient biomarkers and CMRFs is presented in Figure 3 with the β-coefficient for each association in Supplemental Figure 2. Of note, no difference in either nutrient biomarkers or CMRFs was observed between sexes.
FIGURE 3.
Heat map of the associations between nutrient biomarkers, desaturase enzyme activities, and CMRFs. The associations between biomarkers and CMRFs were estimated using multiple linear regression models adjusted for sex, age, study group, and baseline CMRF value. Each pixel represents the β-coefficients from the estimated models (mean change in the CMRF associated with an increase in 1 SD of the biomarker). Blue indicates a negative association, whereas red indicates a positive association, with the darkness of each color corresponding to the magnitude of the β-coefficient. *Significant association. The number of children included in the association models ranged from 208 to 253. Adipo, adiponectin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMIz, BMI z score; CLA, conjugated linolenic acid; CMRF, cardiometabolic risk factor; CRP, C-reactive protein; DBP, diastolic blood pressure; D5D, δ-5-desaturase; D6D, δ-6-desaturase; E-sel, E-selectin; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; P-sel, P-selectin; SBP, systolic blood pressure; SCD, stearoyl co-A desaturase; sICAM, soluble intercellular adhesion molecule; TC, total cholesterol; TG, triglyceride; Throm, thrombomodulin.
Carotenoids
Increase in plasma carotenoids was generally associated with an improvement in CMRFs including a decrease in BMIz (cryptoxanthin), diastolic blood pressure (zeaxanthin), TC (β-carotene), TG, glucose, insulin, HOMA-IR, leptin (cryptoxanthin), and liver enzymes (β-carotene and lycopene) and an increase in HDL cholesterol and adiponectin (lutein, cryptoxanthin, lycopene).
Fat-soluble vitamins
Increase in vitamin A was associated with a favorable decrease in fasting glucose, hsCRP, and leptin. Increases in vitamins E and K were associated with an unfavorable increase in BMIz, TC, TG, fasting and 2-h insulin, HOMA-IR, and leptin. No significant associations were observed between vitamin D and any of the CMRFs.
RBC fatty acids
Among the SFAs, associations with CMRFs varied by fatty acid type. Total SFA and palmitic acid, the predominant SFA in the diet, were associated with an unfavorable increase in TNFα, IL-1, E- and P-selectin, sICAM, thrombomodulin, ALT, and AST and decrease in HDL cholesterol and 2-h glucose. Longer-chain SFAs (stearic acid to lignoceric acid) were generally associated with an increase in BMIz and leptin (lignoceric acid) and a decrease in TC [behenic acid (22:0)], TNF-α (stearic acid, arachidic acid), IL-1 and IL-6 (behenic acid), P-selectin (stearic acid, behenic acid), sICAM (stearic acid, arachidic acid), thrombomodulin (arachidic acid), and liver enzymes (stearic acid). Interestingly, shorter-chain SFAs (lauric acid and myristic acid) were associated with favorable decreases in blood pressure and lipids (TC, LDL cholesterol, HDL cholesterol), hsCRP, and adipokines. The odd-chain fatty acid, pentadecylic acid (a biomarker of dairy fat), was associated with a favorable CMRF profile (decrease in systolic blood pressure, glucose, IL-1, and P-selectin).
Among the MUFAs, those produced by DNL were associated with an adverse CMRF profile. Specifically, palmitoleic acid was strongly associated with an increase in BMIz, TC, TG, glucose metabolism, hsCRP, IL-6, and leptin and a decrease in adiponectin. Total MUFA, predominantly oleic acid, was associated with an increase in ALT, whereas erucic acid (22:1n–9) was associated with a decrease in ALP. Conversely, gondoic acid was strongly associated with a decrease in TC, LDL cholesterol, TNF-α, E-selectin, thrombomodulin, and leptin, and along with vaccenic acid a decrease in systolic blood pressure.
PUFA n–6s had differential associations with the CMRFs. Increases in γ-linolenic acid (18:3n–6) and dihomo-γ-linolenic acid were associated with increases in BMIz, systolic blood pressure, TC, LDL cholesterol, TG, glucose metabolism, and leptin and a decrease in TNF-α. However, total PUFA n–6s, arachidonic acid, and adrenic acid were associated with a decrease in TNF-α, IL-6, P-selectin, sICAM, thrombomodulin, and liver enzymes. Linoleic acid was associated with an increase in liver enzymes.
PUFA n–3s, either plant derived (α-linolenic acid) or from marine sources, were not strongly associated with many of the CMRFs; the exception was EPA that was associated with an increase in BMIz, systolic blood pressure, TC, LDL cholesterol, hsCRP, and leptin. Favorable effects of total PUFA n–3s and DHA were observed with regard to the liver enzymes, namely a decrease in ALT.
Increases in trans fatty acids were associated with a favorable decrease in BMIz, blood pressure, glucose, insulin, HOMA-IR, and leptin and an increase in adiponectin, but were also associated with unfavorable increases in inflammatory markers.
Desaturase enzyme indexes
Unfavorable changes were observed in desaturase enzyme activities, namely increases in SCD1 and D6D which were associated with increases in most of the CMRFs (BMIz, TC, TG, glucose, insulin, HOMA-IR, CRP, IL-6, leptin), whereas increases in SCD2 were primarily associated with an increase in 2-h insulin, AST, and ALT. In contrast, increase in D5D was associated with a favorable decrease in 2-h glucose and insulin, and ALT.
Discussion
Assessing changes in dietary intake resulting from a lifestyle intervention is an important aspect of evaluating the effectiveness of weight-loss programs in children and helps to refine dietary recommendations to promote healthy weight. However, challenges associated with measuring dietary intake, particularly in children, along with the inherent limitations of subjective dietary assessment tools have contributed to the dearth of information in this area. The present study is the first to our knowledge to use a combination of nutrient biomarkers to objectively assess the extent to which a family-based weight-loss intervention shifted dietary behaviors and affected CMRFs in high-risk children. Overall, results indicated a shift toward healthier eating patterns, characterized by higher intakes of fruits and vegetables (carotenoids), dairy (pentadecylic acid), vegetable oils/margarines (α-linolenic acid), and fish (EPA, docosapentaenoic acid, DHA) and lower intakes of ruminant and partially hydrogenated fat (trans fatty acids), in both groups. These changes were associated with modest improvements in BMIz and several CMRFs. With the exception of a few nutrient biomarkers (vitamin E, DHA, trans-vaccenic acid) and CMRFs (hsCRP, ALT), limited additional benefit was observed with SC + EP.
On a cautionary note, increases were observed in fatty acids resulting from DNL, as well as SCD1 and D6D enzyme activities. DNL is the enzymatic pathway for converting excess dietary carbohydrate into fatty acids (28), modulated by SCD1 which converts the SFA palmitic acid to the MUFA 16:1 (palmitoleic acid) , and SCD2 which converts the SFA stearic acid to the MUFA 18:1 (oleic acid). D6D and D5D are the rate-limiting desaturase enzymes in the conversion of the essential diet-derived fatty acids, linoleic acid and α-linolenic acid, via a series of intermediates to the long-chain PUFAs arachidonic acid and EPA, respectively (42). Parallel activation of DNL and SCD1 activities has been reported in response to low-fat, high-carbohydrate diets (43). In addition, in vitro studies have shown that type of dietary fat can affect D6D activities, with dietary trans fatty acids decreasing D6D activities in rat liver liposomes (44), and dietary PUFAs (linoleic acid and α-linolenic acid) being associated with an increase in D6D activities in human peripheral blood mononuclear cells (45). Based on these data, we speculate that the decrease in dietary fat, especially trans fat intake, was at the expense of increasing dietary carbohydrate, potentially accounting for the upregulation of DNL, SCD1, and D6D activities observed in the children at the end of the 1-y intervention. The decreased conversion of dihomo-γ-linolenic acid to arachidonic acid is most likely due to an increase in long-chain PUFA n–3 intake, given competition between PUFA n–6 and n–3 for D5D (46).
The changes in DNL and desaturase enzyme activities may also partially explain the unfavorable effects observed in glucose metabolism markers and TG concentrations. Although we cannot exclude the possibility that pubertal changes might have influenced these results (47), it has been documented that a shift toward a lower-fat, higher-refined-carbohydrate dietary pattern can upregulate hepatic DNL and increase VLDL production, resulting in higher circulating TG concentrations and over time in insulin resistance (48). Although the SC dietary guidance provided to the children emphasized increasing intake of highly pigmented fruits and vegetables, substituting nonfat/low-fat for full-fat milk and dairy products, and decreasing intake of commercially fried foods/savory snacks and sugary beverages, no specific instructions were provided with regard to type of dietary carbohydrate. Additional guidance to replace refined-carbohydrate foods with whole-grain foods may have mitigated the adverse effects observed on glucose metabolism and TG concentrations.
We also observed increases in adhesion molecules (P-selectin and sICAM) that have been associated with endothelial dysfunction in obese children (30, 31). This suggests that vascular injury may already be present in this group of high-risk children and that the study intervention was unable to slow or reverse this trajectory. In contrast, there was a significant benefit of the intervention on liver enzymes, indicators of nonalcoholic fatty liver disease, the prevalence of which is markedly increasing in obese children (49). We observed an increase in leptin and no significant change in adiponectin, consistent with the modest reductions in body weight after the intervention (50).
The association between nutrient biomarkers and CMRFs indicated that increases in plasma carotenoids resulted in the most favorable CMRF profile, consistent with previous reports (51). Interestingly, trans fatty acids were associated negatively with BMIz, blood pressure, and glucose metabolism and positively with inflammatory biomarkers. Dietary trans fatty acids represent a mixture of isomers, the relative proportion of which differs between the 2 major dietary sources: partially hydrogenated fat and ruminant fat. It has been shown that trans fatty acids resulting from partial hydrogenation of vegetable oils (margarines/shortening) result in a more unfavorable CMRF profile than those found in dairy products (52). The children in this study decreased consumption of high-fat dairy products; however, the increases observed in α-linolenic acid and 18:2 trans fatty acids are indicative of higher vegetable oil–based margarine intake (27), which might account for the mixed CMRF results observed. Of note, in the United States and many other countries the use of partially hydrogenated fat has now been phased out (53), but it was a significant dietary contributor at the time of this study. Despite an increase in fish consumption indicated by higher long-chain PUFA n–3s, there were no major associations with CMRFs, in contrast to previous work (54). In the current study, EPA was associated with unfavorable effects on some CMRFs. This is probably due to the fact that the sources of fish consumed were fried, which has been shown to have less beneficial effects than non–fried fish sources on CMRFs (55).
This study has several strengths, including the first report of dietary changes in a family-based weight loss intervention using an objective multibiomarker approach. Nutrient biomarkers were specifically selected to be validated indicators of the foods targeted in the SC recommendations. However, these biomarkers are unable to capture total energy intake/balance or quantity of the food consumed. Also, carotenoids are stored in adipose tissue and may be released into the circulation in response to weight loss. This was partially addressed during data analysis by expressing carotenoids per milligram of TG. The desaturase indexes were estimated from RBC fatty acid profiles, so offer an indirect rather than direct measure of enzyme activity. The similar trajectories in nutrient biomarkers observed between the groups may reflect the experience of the study pediatricians providing the quarterly consultations, as well as the increased interaction with research staff. However, we cannot exclude the possibility that crosstalk between the groups may have diminished the magnitude of the differences observed. Finally, generalizability of our findings is limited to our study population: high-risk children with overweight and obesity living in minority communities.
In conclusion, our data suggest that the SC intervention, based on American Academy of Pediatrics evidence-based recommendations, and provided by primary care pediatricians with expertise in weight management and family-based behavioral counseling, can help overweight and obese children and improve theireating patterns, BMIz scores, and several CMRFs. The addition of a more intensive lifestyle intervention program resulted in limited additional dietary and metabolic benefits. Neither intervention could reverse the unfavorable trajectories in glucose metabolism, TGs, and vascular injury markers. These results provide critical data with which to design subsequent interventions to increase the impact of family-based obesity prevention programs and emphasize the public health importance of such efforts to stem the tide of childhood obesity in high-risk groups.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the assistance of the Cardiovascular Nutrition and Nutrition Evaluation Laboratories. The authors’ responsibilities were as follows—NRM, AHL, and JW-R: contributed to designing and conducting the present study; JW-R, AEG-P, PMD, MG, and YM-R: contributed to designing and conducting the primary intervention; XX, QG, and KB: assumed responsibility for data analysis; NRM: wrote the manuscript with editing from AHL, JW-R, AEG-P, PMD, XX, QG, KB, MG, and YM-R; and all authors: read and approved the final manuscript.
Notes
Supported by NIH grant R01 HL101236 (to AHL), National Institute of Diabetes and Digestive and Kidney Diseases grant NIDDK R18 DK075981 (to JW-R), New York Regional Center for Diabetes Translational Research grant DK111022 (to JW-R), and the USDA under agreement no. 58-1950-4-401 (to NM).
Author disclosures: The authors report no conflicts of interest.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors, and do not necessarily reflect the view of the USDA.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Data described in the article, code book, and analytic code will be made available upon request. Data sharing will require a signed agreement that addresses expenses for data transfer and protects participant confidentiality by exchanging de-identified data in protected formats.
Abbreviations used: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMIz, BMI z score; CMRF, cardiometabolic risk factor; DNL, de novo lipogenesis; D5D, δ-5-desaturase; D6D, δ-6-desaturase; EP, enhanced program; FDR, false discovery rate; hsCRP, high-sensitivity C-reactive protein; mol%, molar percentage; SC, standard care; SCD, stearoyl co-A desaturase; sICAM, soluble intercellular adhesion molecule; TC, total cholesterol; TG, triglyceride.
References
- 1. CDC. Childhood obesity facts. [Internet] Atlanta (GA): CDC; [updated October 2017; cited 2019 Apr 18]. Available from: https://www.cdc.gov/obesity/data/childhood.html. [Google Scholar]
- 2. Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes. 2012;36:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–19. [DOI] [PubMed] [Google Scholar]
- 4. Ho M, Garnett SP, Baur L, Burrows T, Stewart L, Neve M, Collins C. Effectiveness of lifestyle interventions in child obesity: systematic review with meta-analysis. Pediatrics. 2012;130:e1647–71. [DOI] [PubMed] [Google Scholar]
- 5. Brown CL, Perrin EM. Obesity prevention and treatment in primary care. Acad Pediatr. 2018;18:736–45. [DOI] [PubMed] [Google Scholar]
- 6. Psaltopoulou T, Tzanninis S, Ntanasis-Stathopoulos I, Panotopoulos G, Kostopoulou M, Tzanninis IG, Tsagianni A, Sergentanis TN. Prevention and treatment of childhood and adolescent obesity: a systematic review of meta-analyses. World J Pediatr. 2019;15:350–81. [DOI] [PubMed] [Google Scholar]
- 7. Burrows T, Golley RK, Khambalia A, McNaughton SA, Magarey A, Rosenkranz RR, Alllman-Farinelli M, Rangan AM, Truby H, Collins C. The quality of dietary intake methodology and reporting in child and adolescent obesity intervention trials: a systematic review. Obes Rev. 2012;13:1125–38. [DOI] [PubMed] [Google Scholar]
- 8. Hilger-Kolb J, Bosle C, Motic I, Hoffman K. Association between dietary factors and obesity-related biomarkers in healthy children and adolescents—a systematic review. Nutr J. 2017;16:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, Olajide J, Mainardi GM, Corpeleijn E, O'Malley C et al.. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst Rev. 2017;6:CD012651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magarey A, Watson J, Golley RK, Burrows T, Sutherland R, McNaughton SA, Denney-Wilson E, Campbell K, Collins C. Assessing dietary intake in children and adolescents: considerations and recommendations for obesity research. Int J Pediatr Obes. 2011;6:2–11. [DOI] [PubMed] [Google Scholar]
- 11. Wylie-Rosett J, Groisman-Perelstein AE, Diamantis PM, Jimenez CC, Shankar V, Conlon BA, Mossavar-Rahmani Y, Isasi CR, Martin SN, Ginsberg M et al.. Embedding weight management into safety-net pediatric primary care: randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zheng M, Rangan A, Olsen NJ, Andersen LB, Wedderkopp N, Kristensen P, Grøntved A, Ried-Larsen M, Lempert SM, Allman-Farinelli M et al.. Substituting sugar-sweetened beverages with water or milk is inversely associated with body fatness development from childhood to adolescence. Nutrition. 2015;31:38–44. [DOI] [PubMed] [Google Scholar]
- 13. Morenga LT, Montez JM. Health effects of saturated and trans-fatty acid intake in children and adolescents: systematic review and meta-analysis. PLoS One. 2017;12:e0186672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spear BA, Barlow SE, Ervin C, Ludwig DS, Saelens BE, Schetzina KE, Taveras EM. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S254–88. [DOI] [PubMed] [Google Scholar]
- 15. Murphy JM, Reede J, Jellinek MS, Bishop SJ. Screening for psychosocial dysfunction in inner-city children: further validation of the pediatric symptom checklist. J Am Acad Child Adoles Psychiatry. 1992;31:1105–11. [DOI] [PubMed] [Google Scholar]
- 16. Wright ND, Groisman-Perelstein AE, Wylie-Rosett J, Vernon N, Diamantis PM, Isasi CR. A lifestyle assessment and intervention tool for pediatric weight management: the HABITS questionnaire. J Hum Nutr Diet. 2011;24:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prentice RL, Sugar E, Wang CY, Neuhouser M, Patterson R. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr. 2002;5:977–84. [DOI] [PubMed] [Google Scholar]
- 18. Jenab M, Slimani N, Bictash M, Ferrari P, Bingham SA. Biomarkers in nutritional epidemiology: applications, needs and new horizons. Human Genetics. 2009;125:507–25. [DOI] [PubMed] [Google Scholar]
- 19. Al-Delaimy WK, Ferrari P, Slimani N, Pala V, Johansson I, Nilsson S, Mattisson I, Wirfalt E, Galasso R, Palli D et al.. Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr. 2005;59:1387–96. [DOI] [PubMed] [Google Scholar]
- 20. Burrows TL, Warren JM, Colyvas K, Garg ML, Collins CE. Validation of overweight children's fruit and vegetable intake using plasma carotenoids. Obesity. 2009;17:162–8. [DOI] [PubMed] [Google Scholar]
- 21. Johnson EJ, Mohn ES. Nutrition for health: fat-soluble vitamins. In: Bier DM, Mann J, Alpers DH, Vorster HHE, Gibney MJ, editors. Nutrition for the primary care provider. World Rev Nutr Diet vol 111 Basel: Karger; 2015. pp. 38–44. [DOI] [PubMed] [Google Scholar]
- 22. Katan MB, vanBirgelen A, Deslypere JP, Penders M, vanStaveren WA. Biological markers of dietary intake, with emphasis on fatty acids. Ann Nutr Metab. 1991;35:249–52. [DOI] [PubMed] [Google Scholar]
- 23. Arab L. Biomarkers of fat and fatty acid intake. J Nutr. 2003;133:925S–32S. [DOI] [PubMed] [Google Scholar]
- 24. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. [DOI] [PubMed] [Google Scholar]
- 25. Golley RK, Hendrie GA. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children's dairy fat intake. Ann Nutr Metab. 2014;65:310–6. [DOI] [PubMed] [Google Scholar]
- 26. Brouwer IA, Wanders AJ, Katan MB. Effect of animal and industrial trans fatty acids on HDL and LDL cholesterol levels in humans – a quantitative review. PLoS One. 2010;5:e9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venalainen T, Schwab U, Agren JJ, de Mello V, Lindi D, Eloranta A-M, Kiiskinen S, Laaksonen DE, Lakka TA. Cross-sectional associations of food consumption with plasma fatty acid composition and estimated desaturase activities in Finnish children. Lipids. 2014;49:467–79. [DOI] [PubMed] [Google Scholar]
- 28. Vessby B, Gustafson I-B, Tengblad S, Berglund L. Indices of fatty acid desaturase activity in healthy human subjects: effects of different types of dietary fat. Br J Nutr. 2013;110:871–9. [DOI] [PubMed] [Google Scholar]
- 29. Paillard F, Catheline D, Duff FL, Bouriel M, Deugnier Y, Pouchard M, Daubert JC, Legrand P. Plasma palmitoleic acid, a product of stearoyl-CoA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr Metab Cardiovasc Dis. 2008;18:436–40. [DOI] [PubMed] [Google Scholar]
- 30. Elnashar NA, Elhosary AA, Elnashar MA, Mohamed MA. Some inflammatory and endothelial dysfunction biomarker levels in obese pre-pubertal children. J Biotech Biochem. 2017;3:71–7. [Google Scholar]
- 31. Glowinska B, Jadwiga MU, Florys B. Soluble adhesion molecules (sICAM-1, sVCAM-1) and selectins (sE selectin, sP selectin, sL selectin) levels in children and adolescents with obesity, hypertension and diabetes. Metabolism. 2005;54:1020–6. [DOI] [PubMed] [Google Scholar]
- 32. Cambuli VM, Musiu MC, Incani M, Paderi M, Serpe R, Marras V, Cossu E, Cavallo MG, Mariotti S, Loche S et al.. Assessment of adiponectin and leptin as biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab. 2008;93:3051–7. [DOI] [PubMed] [Google Scholar]
- 33. Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, Krinsky NI, Russell RM. Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr. 1996;64:594–602. [DOI] [PubMed] [Google Scholar]
- 34. Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol. 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 35. Fuhrman BJ, Barba M, Krogh V, Micheli A, Pala V, Lauria R, Chajes V, Riboli E, Sieri S, Berrino F et al.. Erythrocyte membrane phospholipid composition as a biomarker of dietary fat. Ann Nutr Metab. 2006;50:95–102. [DOI] [PubMed] [Google Scholar]
- 36. Matthan NR, Ip B, Resteghini N, Ausman LM, Lichtenstein AH. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J Lipid Res. 2010;51:2826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. CDC. CDC growth chart. [Internet] Atlanta (GA): CDC; 2017; [cited 2019 Apr 18]. Available from: https://www.cdc.gov/growthcharts/clinical_charts.htm. [Google Scholar]
- 38. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. [DOI] [PubMed] [Google Scholar]
- 39. Cohn JS. A power primer. Psychol Bull. 1992;112:155–9. [DOI] [PubMed] [Google Scholar]
- 40. Quan H, Zhang J. Estimate of standard deviation for a log-transformed variable using arithmetic means and standard deviations. Stat Med. 2003;22:2723–36. [DOI] [PubMed] [Google Scholar]
- 41. NIH, Office of Dietary Supplements. Vitamin E fact sheet for consumers. [Internet] Bethesda (MD): Office of Dietary Supplements; 2016; [updated 10 July, 2019; cited 2019 Oct 10]. Available from: https://ods.od.nih.gov/factsheets/vitamine-healthprofessional/. [Google Scholar]
- 42. Wolters M, Schlenz H, Bornhorst C, Rise P, Galli C, Moreno LA, Pala V, Siani A, Veidebaum T, Tornaritis M et al.. Desaturase activity is associated with weight status and metabolic risk markers in young children. J Clin Endocrinol Metab. 2015;100:3760–9. [DOI] [PubMed] [Google Scholar]
- 43. Chong MF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87:817–23. [DOI] [PubMed] [Google Scholar]
- 44. Mahfouz MM, Smith TL, Kummerow FA. Effect of dietary fats on desaturase activities and the biosynthesis of fatty acids in rat-liver microsomes. Lipids. 1984;19:214–22. [DOI] [PubMed] [Google Scholar]
- 45. Xiang M, Rahman MA, Ai H, Li X, Harbige LS. Diet and gene expression: delta-5 and delta-6 desaturases in healthy Chinese and European subjects. Ann Nutr Metab. 2006;50:492–8. [DOI] [PubMed] [Google Scholar]
- 46. Tosi F, Sartori F, Guarini P, Olivieri O, Martinelli N. Delta-5 and delta-6 desaturases: crucial enzymes in polyunsaturated fatty acid-related pathways with pleiotropic influences in health and disease. In: Camps J, editor. Oxidative stress and inflammation in non-communicable diseases—molecular mechanisms and perspectives in therapeutics. Advances in Experimental Medicine and Biology vol 824 Cham: Springer; 2014. pp. 61–81. [DOI] [PubMed] [Google Scholar]
- 47. Eissa MA, Mihalopoulos NL, Holubkov R, Dai S, Labarthe DR. Changes in fasting lipids during puberty. J Pediatr. 2016;170:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parks EJ, Krauss RM, Christiansen MP, Neese RA, Hellerstein MK. Effects of a low-fat, high-carbohydrate diet on VLDL-triglyceride assembly, production, and clearance. J Clin Invest. 1999;104:1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Baidal JAW, Elbel EE, Lavine JE, Rifas-Shiman SL, Gillman MW, Oken E, Taveras EM. Associations of early to mid-childhood adiposity with elevated mid-childhood alanine aminotransferase levels in the Project Viva cohort. J Pediatr. 2018;197:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murer SB, Knöpfli BH, Aeberli I, Jung A, Wildhaber J, Wildhaber-Brooks J, Zimmermann MB. Baseline leptin and leptin reduction predict improvements in metabolic variables and long-term fat loss in obese children and adolescents: a prospective study of an inpatient weight-loss program. Am J Clin Nutr. 2011;93:695–702. [DOI] [PubMed] [Google Scholar]
- 51. Gust JL, Logomarsino JV. The association between carotenoid status and body composition in children 2–18 years of age – a systematic review. Int J Vit Nutr Res. 2016;86:91–120. [DOI] [PubMed] [Google Scholar]
- 52. Gebauer SK, Chardigny JM, Jakobsen MU, Lamarche B, Lock AL, Proctor SD, Baer DJ. Effects of ruminant trans fatty acids on cardiovascular disease and cancer: a comprehensive review of epidemiological, clinical, and mechanistic studies. Adv Nutr. 2011;2:332–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. US FDA. Final determination regarding partially hydrogenated oils (removing trans fat). [Internet] Silver Spring (MD): FDA;[updated 18 May, 2018; cited 2019 Oct 10]. Available from: https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm449162.htm. [Google Scholar]
- 54. Gray B, Steyn F, Davies PSW, Vitetta L. Omega-3 fatty acids: a review of the effects on adiponectin and leptin and potential implications for obesity management. Eur J Clin Nutr. 2013;67:1234–42. [DOI] [PubMed] [Google Scholar]
- 55. He K, Liu KL, Daviglus ML, Jenny NS, Mayer-Davis E, Jiang R, Steffen LM, Siscovick D, Tsai MY, Herrington D. Associations of dietary long chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol. 2009;103:1238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



