Abstract
Asthma is an airway epithelium disorder involving allergic lung inflammation. IL-1 receptor–associated kinase M (IRAK-M) is a negative regulator of Toll-like receptor (TLR) signaling on airway epithelial cells and macrophages, and it is known to limit the overproduction of cytokines during the inflammatory process. However, the direct role of IRAK-M in asthma pathogenesis is unclear. In the present study, we found a significant elevation of IRAK-M expression in mouse lungs after ovalbumin (OVA) exposure. Compared with wild-type mice, IRAK-M knockout (KO) mice responded to OVA challenge with significantly worse infiltration of airway inflammatory cells, greater airway responsiveness, higher proinflammatory cytokine levels in lung homogenates, and more prominent T-helper cell type 2 (Th2) and Th17 deviation. OVA exposure also induced higher activities of dendritic cells (DCs) and macrophages from IRAK-M KO mouse lungs. Furthermore, adoptive transfer of either IRAK-M KO bone-marrow–derived DCs or macrophages into wild-type mice aggravated OVA-induced airway inflammation. In vitro experiments showed that IRAK-M KO naive CD4+ T cells were more prone to differentiate into Th17 cells, but not regulatory T cells. Consistently, activation of IκBζ was significantly increased in the absence of IRAK-M, facilitating Th17 polarization. These findings suggest that IRAK-M plays a crucial role in the regulation of allergic airway inflammation by modifying the function of airway epithelia, DCs, and macrophages, and the differentiation of naive CD4+ T cells. Modulation of IRAK-M may provide a novel target for the control of asthma.
Keywords: asthma, dendritic cells, IRAK-M, macrophages, T cell subsets
Clinical Relevance
This work supports the idea that IL-1 receptor–associated kinase M (IRAK-M) regulates allergic airway inflammation in an asthmatic model using IRAK-M knockout mice. IRAK-M deficiency exacerbates ovalbumin-induced airway inflammation and subsequent airway hyperresponsiveness. This is likely the result of an increase in proinflammatory cytokine production by airway epithelia, enhanced activities of dendritic cells and macrophages, and deviated differentiation of naive CD4+ T cells to Th2 and Th17 cells. These findings will provide a basis for further studying IRAK-M as a therapeutic target to control allergic airway inflammation.
Asthma is characterized by chronic airway inflammation. The type 2 immune response, which is abundant in eosinophils and CD4+ T-helper cell type 2 (Th2) cells, is implicated in the pathogenesis of asthma. However, asthmatic airway inflammation is also known to be heterogeneous and regulated by multiple molecular pathways (1).
Over the past decade, emerging evidence has revealed that IL-1 receptor–associated kinases (IRAKs), in particular IRAK-M (also known as IRAK-3), play a role in the regulation of lung inflammation. In healthy human lungs, IRAK-M expression is localized in monocytes, macrophages, airway epithelia, and alveolar cells (2). Activation of IRAK-M signaling is triggered by Toll-like receptors (TLRs) or IL-1 family receptors (IL-1Rs). Upon activation, IRAK-M acts as a negative regulator of NK-κB, which prevents the dissociation of IRAK-1 and -2 from myeloid differentiation factor 88 coupled with TLRs/IL-1Rs, and thus inhibits the downstream inflammatory cascade (3). Therefore, induction of IRAK-M expression may limit the pathological damages associated with cytokine overproduction and innate immune overactivation. For instance, glucocorticoids were shown to inhibit pulmonary inflammation caused by bacterial infection by inducing IRAK-M expression (4). On the other hand, mice deficient in IRAK-M displayed enhanced immune and inflammatory responses to infectious insults (5, 6), partially due to increased activation of NF-κB and mitogen-activated protein kinase (MAPK) (3, 7). IRAK-M deficiency was also shown to promote type 1 diabetes in mice (8). IRAK-M knockout (KO) mice showed increased infiltration of inflammatory cells and elevated expression of cytokines in an experimental model of myocardial infarction (9). In addition, endogenous IRAK-M was reported to attenuate adverse postinfarction remodeling by inhibiting fibroblast-mediated matrix deposition (9).
Moreover, IRAK-M has been found to be related to asthmatic inflammation. Expression of IRAK-M protein was reported to be significantly higher in airway epithelial cells from asthmatic patients, driven by IL-13, a Th2 cytokine that plays an important role in asthma (10). An association between variants of the IRAK-M gene and susceptibility to early-onset persistent asthma was also found in an Italian population (2). Despite the extensive evidence that IRAK-M contributes to lung pathologies induced by infectious or noninfectious insults, the role of this protein in asthmatic inflammation is not well defined.
In this study, we used IRAK-M KO mice to demonstrate that IRAK-M exerts a regulatory effect on airway allergic inflammation induced by ovalbumin (OVA) by modulating the activities of epithelia, dendritic cells (DCs), and macrophages, and by deviating the differentiation of naive CD4+ T cells.
Materials and Methods
Mice
IRAK-M–deficient mice (a gift from Dr. Nikolaos G. Frangogiannis, Albert Einstein College of Medicine) were backcrossed > 10 generations to a C57B/6 genetic background as described elsewhere (3). C57BL/6 wild-type (WT) mice were purchased at the age of 6 weeks (Experimental Animal Center, Beijing, China). All animals were maintained in the mouse facility at Peking Union Medical College Hospital, and 8- to 10-week-old mice were used for all experiments.
All protocols were approved by the Peking Union Medical College Hospital Ethics Committee.
OVA Sensitization and Challenge
The protocol used for OVA immunization and challenge was based on a previous publication (11). Mice were sensitized and challenged with OVA, and killed 24 hours after the last challenge (described in the online supplement).
Airway Responsiveness
Airway responsiveness to inhaled methacholine (MCh) was measured with the use of a flexiVent system (SCIREQ, Montreal, Canada) after the final aerosol challenge as previously described (11). Baseline airway resistance was measured after airway delivery of nebulized vehicle, followed by increasing concentrations of nebulized MCh.
Lung Histology and Immunohistochemistry
Histological and immunohistochemical staining of lung tissue sections was performed as described in the online supplement. Lung histopathological scoring was performed according to previously published methods (11, 12).
Bronchoalveolar Lavage Fluid
Bronchoalveolar lavage (BAL) fluid collection was performed as previously described (11).
Quantitative Real-Time PCR
Total lung RNA was extracted for gene-expression analysis (described in the online supplement).
Analysis of Cytokines and Chemokines
LEGENDplex (BioLegend, San Diego, CA) was used to detect the concentrations of cytokines. The levels of IL-17A, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) in lung homogenates were measured by ELISA (described in the online supplement).
Flow-Cytometry Analysis
To obtain a single-cell suspension, lungs, spleens, and mediastinal lymph nodes (LNs) were digested with collagenase type 1A and type IV bovine pancreatic DNase.
Flow cytometry was used to analyze the expression of costimulatory molecules and signal transduction on mononuclear cells (described in the online supplement).
Analysis of Polarization of Naive CD4+ T Cells
Naive CD4+ T cells were isolated from spleens using a commercial kit (Miltenyi Biotec, Bergisch-Gladbach, Germany). The purity of naive CD4+CD62L+CD44− T cells was verified by flow cytometry. The protocols used for polarization and differentiation of naive CD4+ T cells are described in the online supplement.
Bone marrow–derived DCs (BMDCs) and macrophages (BMDMs) were purified, cultured, and stimulated with OVA (described in the online supplement). The purity of the BMDCs and BMDMs was confirmed by flow cytometry.
Naive CD4+ T cells were cocultured with OVA-treated BMDCs/BMDMs. Flow-cytometric analysis was performed to detect T cell polarization (described in the online supplement).
Adoptive Transfer Experiments
Purified BMDCs and BMDMs (1 × 106) were pulsed with OVA for 24 hours and then administered by intratracheal injection to the OVA-sensitized mice 1 day before OVA challenge. The mice were killed 1 day after the last challenge for further evaluation.
Statistical Analysis
Data are expressed as means ± SEM. Multiple comparisons were performed using one-way ANOVA with Tukey’s post hoc test. Comparisons between two groups were done using Student’s t test (GraphPad Prism version 6.0; GraphPad, San Diego, CA). P < 0.05 was considered significant.
Results
Role of IRAK-M in Airway Inflammation and Airway Responsiveness Induced by OVA Exposure
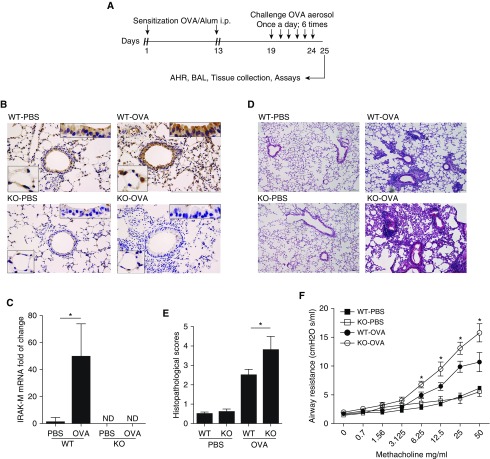
Using an established murine asthmatic model, we examined the change of IRAK-M expression in mouse lungs exposed to OVA (Figure 1A). In comparison with the faint immunohistochemical staining of IRAK-M in the airway and alveolar epithelial cells in PBS-exposed WT mice, OVA stimulation significantly enhanced expression of IRAK-M protein in WT lungs. In IRAK-M KO mice, IRAK-M protein was undetectable by immunohistochemical staining in the airways exposed to either PBS or OVA (Figure 1B). Consistently, expression of IRAK-M mRNA was much higher in OVA-treated WT mouse lungs as compared with the PBS controls (P < 0.05; Figure 1C).
Figure 1.
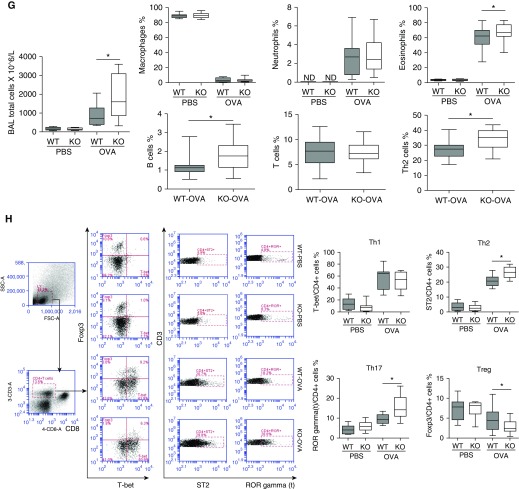
Effect of IL-1 receptor–associated kinase M (IRAK-M) on ovalbumin (OVA)-induced airway inflammation and airway hyperresponsiveness (AHR). (A) Schematic representation of the OVA exposure protocol. (B) Representative images of immunohistochemical staining of IRAK-M in PBS- or OVA-treated wild-type (WT, upper panel) and IRAK-M knockout (KO, lower panel) mouse lungs. (C) IRAK-M mRNA levels in OVA- or PBS-treated WT or IRAK-M KO mouse lungs. IRAK-M gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase; n = 10 in each group; *P < 0.05. (D) Representative lung histological images and (E) semiquantitative histopathological scores from PBS- or OVA-treated WT and IRAK-M KO mice. n = 10 mice in each group; *P < 0.05. (F) Airflow resistance to methacholine stimulation in PBS- or OVA-treated WT and IRAK-M KO mice. n = 10–12 mice in each group; *P < 0.05 WT versus KO-OVA group. (G) Total number of inflammatory cells in bronchoalveolar (BAL) fluid of PBS- or OVA-treated WT and IRAK-M KO mice, and flow cytometry analysis for BAL cells using specific antibodies against different groups of leukocytes. n = 10–30 mice in each group; *P < 0.05. (H) FACS analysis of lung T-helper cell type 1 (Th1), Th2, Th17, and Treg cells using specific antibodies in PBS- or OVA-treated WT and IRAK-M KO mice. The percentages of Th1, Th2, Th17, and Treg cells are plotted. IRAK-M KO mice had a significantly higher percentage of Th2 cells and lower percentage of Treg cells in the lung than WT mice. n = 10–20 mice in each group; *P < 0.05. FSC, forward scatter; i.p., intraperitoneal; ND, not determined; SSC, side scatter.
To investigate the role of IRAK-M in the pathogenesis of allergic asthma, we further characterized airway inflammation and subsequent contractile responses of IRAK-M KO mice after OVA exposure. We found that both WT and IRAK-M KO mice responded to OVA stimulation with typical allergic airway inflammation, as evidenced by thickened airway epithelia and increased inflammatory cells in the peribronchial and perivascular areas (Figure 1D, right panel). Moreover, infiltration of inflammatory cells was more prominent in IRAK-M KO mice, as reflected by higher semiquantitative histopathological scores for OVA-treated IRAK-M KO mice compared with similarly treated WT mice (3.8 ± 0.7 versus 2.5 ± 0.3; P < 0.05; Figure 1E).
As shown in Figure 1F, WT and IRAK-M KO mice showed similar airway resistance to MCh provocation at baseline. OVA treatment increased MCh responses in both WT and IRAK-M KO mice. Notably, the airway resistance was significantly higher in IRAK-M KO mice as compared with the WT mice upon exposure to the same dose of MCh (P < 0.05).
OVA treatment increased the inflammatory cell counts in BAL fluid from both WT and IRAK-M KO mice. Compared with WT mice, IRAK-M KO mice exhibited higher total inflammatory cell counts and higher percentages of eosinophils, Th2 cells, and B cells in BAL fluid after OVA treatment. The percentage of macrophages and neutrophils did not differ between WT and KO mice (Figure 1G). In addition, the total number of inflammatory cells isolated from OVA-treated IRAK-M KO mouse lungs was significantly higher than that obtained from WT mouse lungs (Figure E1A in the online supplement).
Airway inflammation in asthma is characterized by imbalanced T cell subsets in favor of the Th2 type (13). To determine whether IRAK-M deficiency contributed to this imbalance, we used flow cytometry to analyze the composition of T cells isolated from lungs of OVA-treated mice. OVA treatment increased the percentages of Th2 and Th17 cells in both WT and IRAK-M KO mice. Moreover, the increment in the percentages of Th2 and Th17 cells in IRAK-M KO mice was significantly greater than that observed in WT mice (Figure 1H). Similarly, higher percentages of Th2 and Th17 cells were noted in the splenocytes from OVA-treated IRAK-M KO mice compared with similarly treated WT mice (Figure E1B). Conversely, the percentage of lung regulatory T (Treg) cells decreased after OVA treatment in WT mice, and this reduction was even greater in OVA-exposed IRAK-M KO mice (Figure 1H). There was no difference in the downregulation of Tregs in splenocytes between the two genotypes of OVA-exposed mice (Figure E1B).
To further investigate the influence of IRAK-M deficiency on the activation of different subsets of lung T cells, we measured the mRNA expression of specific nuclear transcription factors for activation of various T cell subsets, including T-bet for Th1 cells, GATA3 for Th2 cells, RORC for Th17 cells, and Foxp3 for Tregs. We found that the mRNA expression of T-bet, GATA3, and RORC was significantly higher in OVA-treated IRAK-M KO lungs compared with similarly treated WT lungs. There was no significant difference in mRNA expression of Foxp3 between the two groups (Figure E1C).
Effect of IRAK-M Deficiency on Production of Cytokines
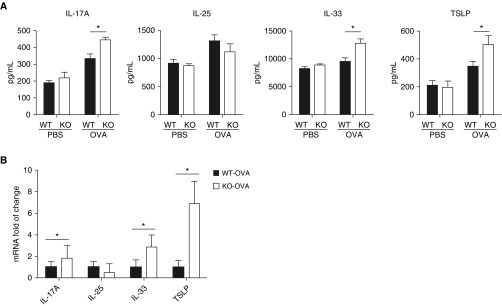
The airway epithelia serve as the first barrier of defense against environmental insults (14). Upon inhaled allergen challenge, the airway epithelial cells are activated and release innate immune cytokines such as IL-25, IL-33, and TSLP, which can activate Th2 cells (15). Because the levels of IL-17A, IL-25, IL-33, and TSLP were not detectable in BAL fluids, we measured the levels of these epithelium-derived cytokines in supernatants of lung homogenates by ELISA. The concentrations of IL-17A, IL-33, and TSLP were significantly higher in lung homogenates from OVA-treated IRAK-M KO mice compared with those obtained from WT mice (Figure 2A). Consistently, significantly higher mRNA expression of these cytokines was seen in the lungs of OVA-treated IRAK-M KO mice (Figure 2B). No difference in IL-25 at the mRNA or protein level was noted between IRAK-M KO and WT mice (Figures 2A and 2B). Additionally, OVA treatment induced significantly higher mRNA expression of TGF-β and IL-10 in IRAK-M KO mice compared with WT mice (Figure E2). These data suggested that the ubiquitous loss of IRAK-M over-tuned the immune responses to Th2/Th17 dominant inflammation by inhaled allergen.
Figure 2.
Influence of IRAK-M deficiency on OVA-induced cytokine production in mouse lungs. (A) IL-17A, thymic stromal lymphopoietin (TSLP), IL-25, and IL-33 in supernatant of PBS- or OVA-treated WT and IRAK-M KO lung homogenates by ELISA. n = 10 mice in each group; *P < 0.05. (B) Quantitative real-time PCR analysis for mRNA expression of IL-17A, TSLP, IL-25, and IL-33 in OVA-treated WT or IRAK-M KO mouse lungs. n = 10 animals in each group; *P < 0.05.
Effect of IRAK-M Deficiency on In Vitro Function of BMDCs
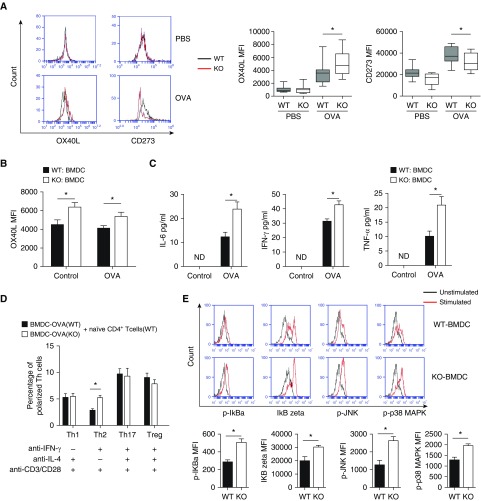
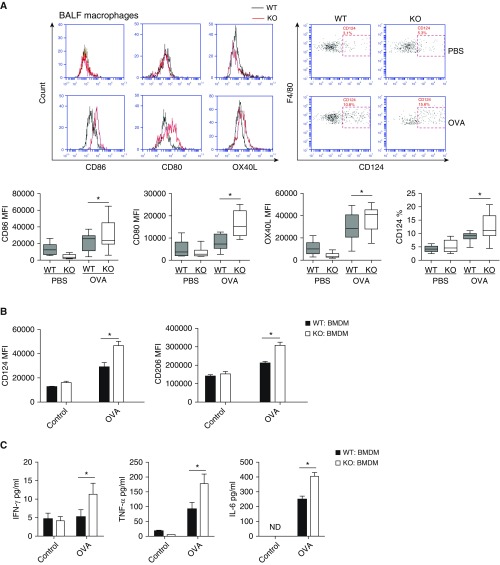
DCs are the most efficient antigen-presenting cells (APCs) in the immune system. Airway DCs capture the antigens and bridge innate and adaptive immune cells during the inflammatory process in asthma (16, 17). As T cell polarization is primarily driven by costimulatory molecules on APCs, we examined the influence of IRAK-M deficiency on the expression of costimulatory molecules on DCs from OVA-exposed mouse lungs. We found that lung DCs from IRAK-M KO mice expressed significantly higher levels of OX40L and lower levels of CD273 as compared with those from WT mice (P < 0.05, respectively; Figure 3A). Similarly, BMDCs isolated from IRAK-M KO mice expressed significantly higher levels of OX40L than those obtained from WT mice at baseline and after OVA treatment in vitro (Figure 3B).
Figure 3.
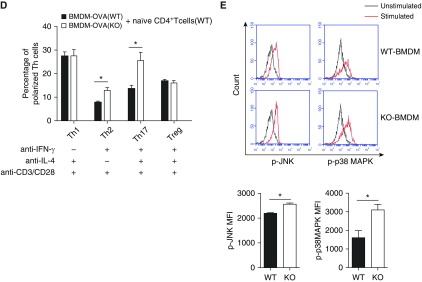
Regulatory role of IRAK-M deficiency in the activities of bone marrow–derived dendritic cells (BMDCs). (A) Flow cytometry analysis of expression of costimulatory molecules on lung DCs from PBS- or OVA-treated WT and IRAK-M KO mice. n = 10–15 mice in each group; *P < 0.05. (B) In vitro flow cytometry analysis of the expression of the costimulatory molecule OX40L on IRAK-M KO or WT BMDCs at baseline or after OVA stimulation; n = 10 in each group; *P < 0.05. (C) ELISA of cytokine production by IRAK-M KO or WT BMDCs after in vitro OVA stimulation. n = 10–15 in each group; *P < 0.05. (D) In vitro differentiation of naive CD4+ T cells incubated with IRAK-M KO or WT BMDCs, with the presence of the indicated costimulatory factors. The percentages of T cell subpopulations were detected by flow cytometry analysis and plotted. n = 10 in each group; *P < 0.05. (E) Flow cytometry analysis of intracellular transcription factors, including p-IκBζ, IκBζ, phosphorylated c-JUN N-terminal protein kinase (p-JNK), and p-p38 mitogen-activated protein kinase (MAPK), in IRAK-M KO or WT BMDCs after in vitro OVA stimulation. n = 10 in each group; *P < 0.05. MFI, mean fluorescent intensity.
We then examined the influence of IRAK-M deficiency on cytokine secretion by BMDCs after a 24-hour incubation with OVA. We found that IRAK-M KO BMDCs secreted more IL-6, IFN-γ, and TNF-α than the WT BMDCs (Figure 3C). Moreover, when cocultured with naive WT CD4+ T cells, these OVA-treated IRAK-M KO BMDCs induced more Th2 cell differentiation than the OVA-treated WT BMDCs (Figure 3D). After stimulation with OVA, expression of p-IκBα, IκBζ, p-JNK, and p-p38 MAPK was higher in IRAK-M KO than in WT BMDCs, as determined by fluorescence-activated cell sorting analysis (Figure 3E).
Adoptive Transfer of OVA-Treated IRAK-M KO BMDCs Enhances Airway Inflammation
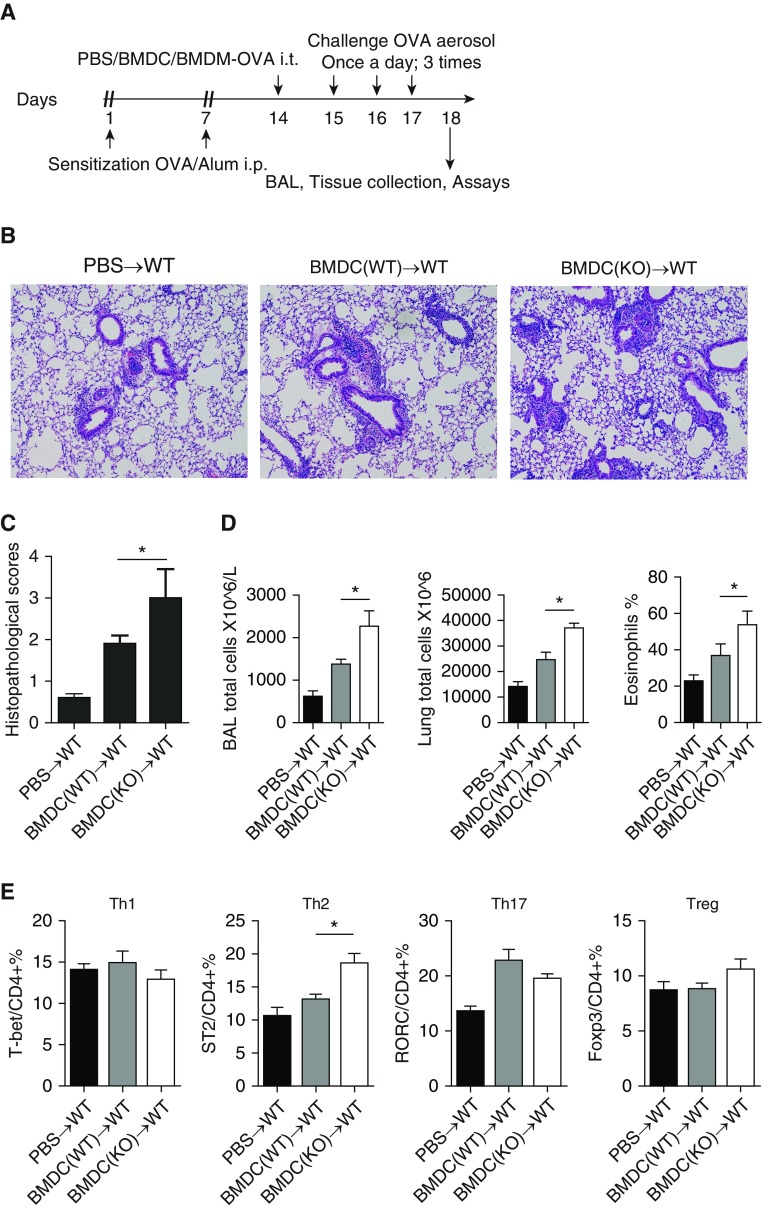
To investigate whether IRAK-M plays a direct role in asthmatic airway inflammation by regulating BMDC function, we conducted adoptive-transfer experiments as shown in Figure 4A. Compared with the transfer of WT BMDCs, transfer of IRAK-M KO BMDCs resulted in worse OVA-induced airway inflammation, as evidenced by more inflammatory cells around the bronchi and vessels, higher semiquantitative histopathological scores (2.7 ± 0.5 versus 1.8 ± 0.2; P < 0.05), and more inflammatory cells in BAL fluid and lungs (Figures 4B–4D). The percentage of lung Th2 cells was higher in mice that received IRAK-M KO BMDCs (Figure 4E). Taken together, these findings indicated that IRAK-M KO BMDCs aggravated asthmatic inflammation by releasing more cytokines and promoting Th2 differentiation.
Figure 4.
Enhanced OVA-induced airway inflammation by IRAK-M KO BMDCs. (A) Schematic representation of the experimental protocol used for adoptive transfer of OVA-treated WT or IRAK-M KO BMDCs to WT mice. (B) Representative images of hematoxylin and eosin staining and (C) semiquantitative histopathological scores of OVA-exposed WT mouse lungs that received PBS or transfer of WT or IRAK-M KO BMDCs. n = 10 mice in each group; *P < 0.05. (D) Total inflammatory cell counts in BAL fluid and lung, and percentages of eosinophils in BAL fluid from OVA-treated WT mice that received PBS or WT or IRAK-M KO bone marrow–derived macrophages (BMDMs). n = 10 animals in each group; *P < 0.05. (E) Flow cytometry analysis of T cell subpopulations in whole-lung tissue from OVA-treated WT mice adoptively transferred with PBS or WT or IRKA-M KO BMDCs. n = 10 animals in each group; *P < 0.05. i.t., intratracheal; RORC, retinoic acid-related orphan receptor C.
Effect of IRAK-M on In Vitro Function of BMDMs
In addition to DCs, macrophages also affect the differentiation of T cells by providing costimulatory molecules and secreting cytokines. Consistent with this, we found that IRAK-M deficiency enhanced the OVA-induced expression of costimulatory molecules (i.e., CD80, CD86, CD124, and OX40L) on macrophages from BAL fluid (Figure 5A). After in vitro OVA incubation, IRAK-M KO macrophages expressed significantly higher levels of CD124 and CD206 than the WT macrophages (Figure 5B).
Figure 5.
Upregulation of BMDM activities by IRAK-M deficiency. (A) Flow cytometry analysis of expression of costimulatory molecules on macrophages from BAL fluid of PBS- or OVA-treated WT and IRAK-M KO mice. n = 10–15 mice in each group; *P < 0.05. (B) Flow cytometry analysis of CD124 and CD206 expression by BMDMs from WT and IRAK-M KO mice after ex vivo stimulation with PBS or OVA. n = 10 in each group; *P < 0.05. (C) ELISA of cytokine production by IRAK-M KO and WT BMDMs after in vitro PBS or OVA stimulation. n = 10 in each group; *P < 0.05. (D) In vitro differentiation of naive CD4+ T cells incubated with IRAK-M KO or WT BMDMs in the presence of the correspondent stimulatory factors. The T cell subpopulations were detected by flow cytometry analysis. n = 10 in each group; *P < 0.05. (E) Flow cytometry analysis of intracellular transcription factors, including p-JNK and p-p38 MAPK, in WT or IRAK-M KO BMDMs after OVA stimulation. n = 10 in each group; *P < 0.05. BALF, BAL fluid.
Upon ex vivo stimulation with OVA, IRAK-M KO BMDMs released significantly higher levels of TNF-α, IFN-γ, and IL-6 than the WT BMDMs (Figure 5C). When coincubated with naive CD4+ T cells, IRAK-M KO BMDMs induced more Th2 and Th17 differentiation than the WT BMDMs (Figure 5D). Furthermore, OVA stimulation induced much higher expression of p–JNK and p-p38 MAPK in IRAK-M KO BMDMs than in WT BMDMs (Figure 5E).
Adoptive Transfer of OVA-Treated IRAK-M KO BMDMs Enhances Airway Inflammation
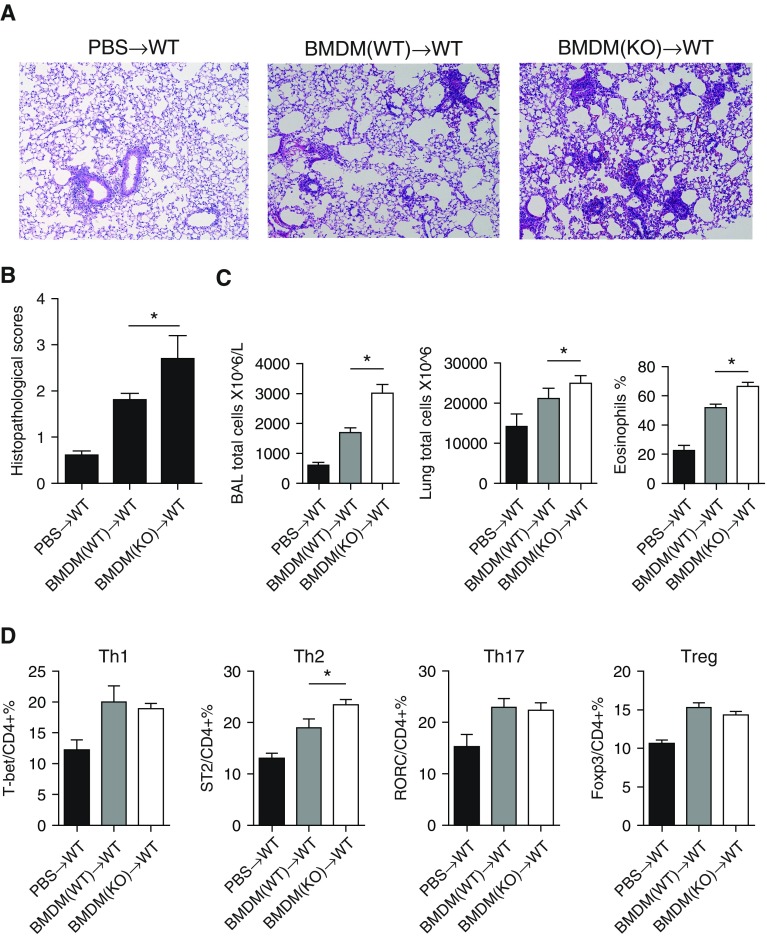
After conducting a similar protocol for adoptive transfer of BMDCs (Figure 4A), we found that mice that received IRAK-M KO BMDMs showed worse airway inflammatory responses to OVA treatment, with more inflammatory cells in BAL fluids and lungs, higher concentrations of proinflammatory cytokines (i.e., TNF-α, IL-4, and IL-10) in BAL fluids, and a higher percentage of lung Th2 cells, compared with the mice that received WT BMDMs (Figures 6A–6D). These data suggested that IRAK-M deficiency could enhance the proinflammatory effect of BMDMs by promoting cytokine production and the differentiation of naive CD4+ T cells to Th2 cells.
Figure 6.
Enhanced OVA-induced airway inflammation by IRAK-M KO BMDMs. (A) Representative images of hematoxylin and eosin staining and (B) semiquantitative histopathological scores of OVA-exposed WT lungs with PBS or transfer of WT or IRAK-M KO BMDMs. n = 10 mice in each group; *P < 0.05. (C) Total inflammatory cell counts in BAL fluid and lung, and the percentage of eosinophils in BAL fluid from OVA-treated WT mice with PBS or transfer of WT or IRKA-M KO BMDMs. n = 10 mice in each group; *P < 0.05. (D) Flow cytometry analysis of T cell subpopulations of whole-lung tissue from OVA-treated mice with PBS or transfer of WT or IRKA-M KO BMDMs. n = 10 mice in each group; *P < 0.05.
Effect of IRAK-M Deficiency on Naive CD4+ T Cell Polarization
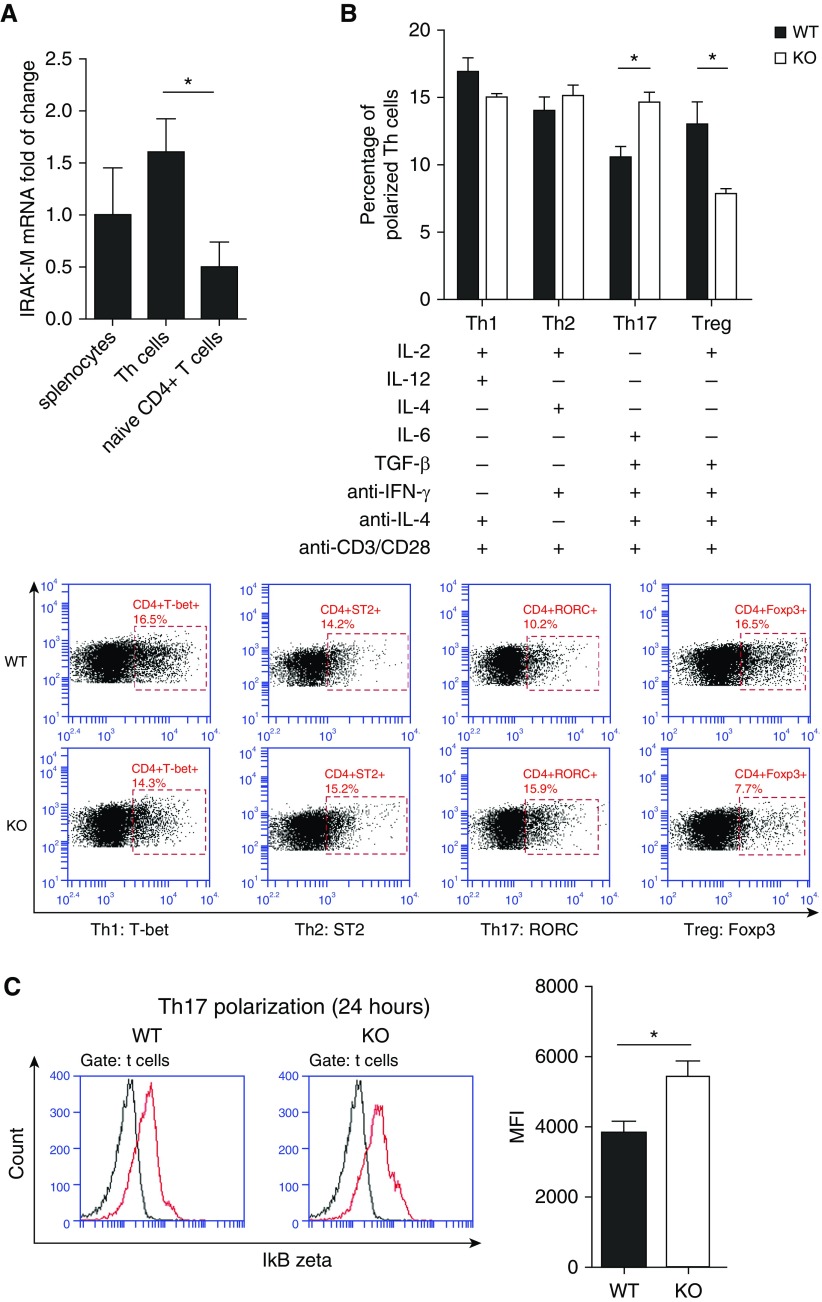
To assess whether IRAK-M has a direct effect on the proliferation and differentiation of naive CD4+ T cells, we compared the activities of spleen cells and lung naive CD4+ T cells in WT and IRAK-M KO mice. We found that anti-CD3/CD28 monoclonal antibodies induced similar increases in the expression of nuclear Ki-67, a marker for cell proliferation, in both splenocytes and lung naive CD4+ T cells from the two genotypes of mice (Figure E3), indicating that IRAK-M deficiency had no direct effect on the proliferation of naive CD4+ T cells. However, the mRNA expression of IRAK-M was higher in differentiated Th cells than in naive CD4+ T cells (Figure 7A), suggesting an association of IRAK-M with T cell differentiation.
Figure 7.
Effect of IRAK-M deficiency on the differentiation of naive CD4+ T cells. (A) Baseline mRNA expression of IRAK-M by splenocytes, Th cells, and naive CD4+ T cells isolated from WT mice. (B) Flow cytometry analysis of in vitro differentiation of WT or IRAK-M KO naive CD4+ T cells to specific T cell subsets after a 5-day incubation with various colony-stimulating factors. (C) Representative flow cytometric images and summary plot of IκBζ expression in Th17 cells from WT or IRAK-M KO mice after activation. n = 10 mice in each group; *P < 0.05.
After a 5-day incubation with the indicated colony-stimulating factors, a higher ratio of Th17 differentiation was observed in IRAK-M KO than in WT naive CD4+ T cells. Conversely, a higher ratio of Treg differentiation was found in WT cells (Figure 7B). The ratio of differentiation to Th1 or Th2 cells did not differ between the two genotypes of mice. These data suggest that a lack of IRAK-M enhanced the differentiation of naive CD4+ T cells to Th17 cells, but not to Tregs, after TCR activation.
IRAK-M has been reported to be a negative regulator of NF-κB and IκB coupling in the TLR and IL-1R signaling pathway (3). IκBζ is a nuclear IκB family member and a transcription factor that is required for Th17 development in mice (18). We found that TCR stimulation induced higher expression of ΙκBζ in IRAK-M KO Th17 cells than in WT Th17 cells (Figure 7C). Therefore, our data suggest that IRAK-M enhances Th17 differentiation from naive CD4+ T cells by activating the IκBζ and NF-κB pathway.
Discussion
Although a growing body of evidence suggests that IRAK-M has a potential role in infectious or noninfectious lung pathophysiology, to our knowledge, this is the first study to examine the role of IRAK-M in the pathogenesis of asthmatic airway inflammation. In this study, we found a low expression of IRAK-M in airway and alveolar epithelial cells of WT mouse lungs with no allergen exposure. Expression of IRAK-M protein was significantly upregulated in the OVA mouse model, consistent with a previous observation of higher expression of IRAK-M in airway epithelia from asthma patients (10). Using the IRAK-M gene KO mouse model, we demonstrated enhanced airway inflammation and airway responsiveness in response to OVA stimulation, likely via an increase in production of proinflammatory cytokines from airway epithelia, enhanced activities of DCs and macrophages, and deviated naive T cell differentiation to Th2 and Th17 cells.
It is increasingly recognized that respiratory epithelial cells serve as important modulators of innate or adaptive immune responses by releasing chemokines and cytokines (15, 19). In the present study, we found that IRAK-M deficiency led to an elevated secretion of several epithelial cytokines, including TSLP, IL-33, and IL-25, at both the mRNA and protein levels. These cytokines have been reported to mediate allergic airway inflammation. For instance, overexpression of TSLP in the lung epithelium was shown to result in the spontaneous development of an asthma-like disease in a mouse model (20). In addition, TSLP was proven to be a powerful stimulus for Th2 polarization in lungs (21, 22), and was found to be expressed at higher levels in lung epithelia and BAL fluid from asthmatic patients (23). The receptor for TSLP on BMDCs was also found to increase in response to allergen exposure (24). Moreover, IL-25 and IL-33, important for asthma, were the upstream regulators of type 2 cytokines (25, 26).
In addition to its influence on epithelial function, we found that IRAK-M deficiency modified the activities of DCs. DCs are the most proficient APCs of the lung and play a critical role in the initiation and maintenance of asthmatic airway inflammation. Airway DCs continuously capture and process inhaled allergens, and thus regulate the Th2-mediated allergic immune response (27). Initiation of airway allergic inflammation was reported to be promoted by antigen uptake and processing by airway APCs, particularly airway DC subsets that highly express the costimulatory molecule CD40 and are capable of inducing CD4+ T cell activation (28). Furthermore, lung DCs showed enhanced antigen presentation and increased antigen-specific CD4+ T cell proliferation in an asthma mouse model induced by OVA (29). We observed that airway APCs (including DCs and macrophages) from IRAK-M KO mice treated with OVA had a higher expression of some costimulatory molecules (OX40L, CD80, and CD86). We also found that IRAK-M KO BMDCs or BMDMs were able to induce T cell differentiation into Th2 or Th17 subsets in vitro. These data indicate that IRAK-M may play a role in the Th2-mediated inflammatory process by influencing the activation status of airway APCs, mainly DCs and macrophages.
An inhibitory effect of IRAK-M on the function of DCs in Helicobacter pylori–induced inflammation was previously reported (30). On the other hand, a lack of IRAK-M was reported to enhance the migration and longevity of DCs, as well as the expression of major histocompatibility complex II and CD80 by DCs upon lipopolysaccharide stimulation (31). Our data showed that IRAK-M deficiency promoted proinflammatory cytokine production and the expression of costimulatory molecules on the cellular surface of DCs to drive Th2 cell differentiation under OVA treatment. It has been reported that IRAK-M is induced in DCs by TLR ligation, and its absence leads to excessive activation of the p38 MAPK and NF-κB pathways (7). This is consistent with our observation of overexpression of p-IκBα, IκBζ, p-JNK, and p-p38 MAPK in stimulated IRAK-M KO DCs. Furthermore, we demonstrated that adoptive transfer of IRAK-M KO DCs enhanced Th2 inflammation upon OVA stimulation. These findings suggest a regulatory role of IRAK-M in Th2 inflammation via DCs.
Lung macrophages also perform a wide range of immunoregulation activities by expressing costimulatory molecules and secreting cytokines (32). A lack of IRAK-M was reported to suppress alternative macrophage activation in bleomycin-induced lung injury (33). In the present study, we demonstrated that IRAK-M deficiency upregulated proinflammatory cytokine production and costimulatory molecule expression by macrophages, which resulted in enhanced differentiation of Th2 and Th17. Adoptive transfer of IRAK-M KO BMDMs exacerbated OVA-induced airway inflammation. Interestingly, despite the promotive effect of IRAK-M KO BMDMs on the differentiation of both Th2 and Th17 in vitro, we found that only the differentiation of Th2, and not Th17, was significantly increased after OVA treatment in mice with adoptive transfer of IRAK-M KO BMDMs. This is likely due to the regulation of Th17 priming by other cell types or IRAK family members in vivo, which minimizes the influence of IRAK-M deficiency in macrophages (34, 35).
In addition to the typical Th1/Th2 imbalance, a Th17/Treg imbalance has been implicated in the pathogenesis of asthmatic inflammation in recent years (36). A predominant expression of Th17 is now used as a biomarker for asthma phenotyping, in particular for severe, poorly controlled asthma (37–39). In the present study, we found higher percentages of Th17 and Th2 cells, and lower percentages of Treg cells in the lungs of OVA-treated IRAK-M KO mice. In addition to the influence of DCs and macrophages on T cell differentiation, in vitro experiments indicated that IRAK-M KO naive CD4+ T cells were more prone to polarize into Th17 cells, but not Tregs, after OVA stimulation. The underlying mechanism may relate to the upregulation of IκBζ, a potent molecule that induces the differentiation of naive CD4+ T cells into Th17 cells in the setting of IRAK-M deficiency. Our adoptive-transfer experiments were not able to demonstrate a significant effect of either IRAK-M KO BMDCs or BMDMs on enhancing Th17 or Treg cell differentiation in vivo. This suggests that Th17 priming and maturation of Treg cells may be regulated by other endogenous factors (34, 35, 40). For instance, IRAK-2 and -4 have also been shown to promote the differentiation of Th17 cells (34, 35), and IRAK-1 has a regulatory role in the differentiation of both Th17 and Treg cells (40).
In conclusion, our data indicate that IRAK-M plays a critical regulatory role in allergic airway inflammation by modulating the function of multiple cell types, ultimately leading to Th2- and Th17-dominant immune responses. Our data indicate that modulation of IRAK-M function may provide an alternative therapy to restore the immune balance and relieve asthmatic airway inflammation.
Acknowledgments
Acknowledgments
The authors thank Professor Nikolaos G. Frangogiannis of the Albert Einstein College of Medicine for providing the IRAK-M knockout mice. We also thank Professor Ying Sun and Dr. Youming Zhang for their critical comments and helpful discussions regarding the manuscript, and Dr. Kewu Huang for help with the flexiVent measurements of airway reactivity.
Footnotes
This work was supported by grants from the National Natural Sciences Foundation of China (Nos. 81170040, 81470229, and 30470767) and the Education Ministry of China New Century Excellent Talent (NCET 06-0156).
Author Contributions: M.Z. performed the experiments; W.C. facilitated the experiments; W.Z. performed the pathological evaluations; Y.B. reviewed the experimental data and edited the manuscript for important intellectual content; J.G. designed and supervised this study, interpreted the experimental findings, and drafted the manuscript. All authors were involved in the experimental design, data interpretation, and manuscript preparation. All authors read and approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0370OC on June 30, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wenzel SE. Emergence of biomolecular pathways to define novel asthma phenotypes. Type-2 immunity and beyond. Am J Respir Cell Mol Biol. 2016;55:1–4. doi: 10.1165/rcmb.2016-0141PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D, et al. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet. 2007;80:1103–1114. doi: 10.1086/518259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kobayashi K, Hernandez LD, Galán JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 4.Miyata M, Lee JY, Susuki-Miyata S, Wang WY, Xu H, Kai H, Kobayashi KS, Flavell RA, Li JD. Glucocorticoids suppress inflammation via the upregulation of negative regulator IRAK-M. Nat Commun. 2015;6:6062. doi: 10.1038/ncomms7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–2542. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, Kunkel SL, Standiford TJ. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J Immunol. 2010;184:1410–1418. doi: 10.4049/jimmunol.0901709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Yu M, Fukuda K, Im J, Yao P, Cui W, Bulek K, Zepp J, Wan Y, Kim TW, et al. IRAK-M mediates Toll-like receptor/IL-1R-induced NFκB activation and cytokine production. EMBO J. 2013;32:583–596. doi: 10.1038/emboj.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Q, Majewska-Szczepanik M, Zhang X, Szczepanik M, Zhou Z, Wong FS, Wen L. IRAK-M deficiency promotes the development of type 1 diabetes in NOD mice. Diabetes. 2014;63:2761–2775. doi: 10.2337/db13-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–2608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Jiang D, Smith S, Thaikoottathil J, Martin RJ, Bowler RP, Chu HW. IL-13 dampens human airway epithelial innate immunity through induction of IL-1 receptor–associated kinase M. J Allergy Clin Immunol. 2012;129:825–833.e2. doi: 10.1016/j.jaci.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Y, Yan H, Xiao Y, Piao H, Xiang R, Jiang L, Chen H, Huang K, Guo Z, Zhou W, et al. Attenuation of antigen-induced airway hyperresponsiveness and inflammation in CXCR3 knockout mice. Respir Res. 2011;12:123. doi: 10.1186/1465-9921-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzaki Y, Hamada K, Nomi T, Ito T, Sho M, Kai Y, Nakajima Y, Kimura H. A small-molecule compound targeting CCR5 and CXCR3 prevents airway hyperresponsiveness and inflammation. Eur Respir J. 2008;31:783–789. doi: 10.1183/09031936.00111507. [DOI] [PubMed] [Google Scholar]
- 13.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Parker D, Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divekar R, Kita H. Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr Opin Allergy Clin Immunol. 2015;15:98–103. doi: 10.1097/ACI.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt PG, Upham JW. The role of dendritic cells in asthma. Curr Opin Allergy Clin Immunol. 2004;4:39–44. doi: 10.1097/00130832-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 17.de Heer HJ, Hammad H, Kool M, Lambrecht BN. Dendritic cell subsets and immune regulation in the lung. Semin Immunol. 2005;17:295–303. doi: 10.1016/j.smim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto K, Iwai Y, Oh-Hora M, Yamamoto M, Morio T, Aoki K, Ohya K, Jetten AM, Akira S, Muta T, et al. IκBζ regulates T(H)17 development by cooperating with ROR nuclear receptors. Nature. 2010;464:1381–1385. doi: 10.1038/nature08922. [DOI] [PubMed] [Google Scholar]
- 19.Wang YH, Liu YJ. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin Exp Allergy. 2009;39:798–806. doi: 10.1111/j.1365-2222.2009.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 21.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleck B, Kazeros A, Bakal K, Garcia-Medina L, Adams A, Liu M, Lee RA, Tse DB, Chiu A, Grunig G, et al. Coexpression of type 2 immune targets in sputum-derived epithelial and dendritic cells from asthmatic subjects. J Allergy Clin Immunol. 2015;136:619–627.e615. doi: 10.1016/j.jaci.2014.12.1950. [DOI] [PubMed] [Google Scholar]
- 24.El-Gammal A, Oliveria JP, Howie K, Watson R, Mitchell P, Chen R, Baatjes A, Smith S, Al-Sajee D, Hawke TJ, et al. Allergen-induced changes in bone marrow and airway dendritic cells in subjects with asthma. Am J Respir Crit Care Med. 2016;194:169–177. doi: 10.1164/rccm.201508-1623OC. [DOI] [PubMed] [Google Scholar]
- 25.Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, Bonser LR, Zhao J, Xu Y, Erle DJ, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Vroman H, van den Blink B, Kool M. Mode of dendritic cell activation: the decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity? Immunobiology. 2015;220:254–261. doi: 10.1016/j.imbio.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 28.von Garnier C, Wikstrom ME, Zosky G, Turner DJ, Sly PD, Smith M, Thomas JA, Judd SR, Strickland DH, Holt PG, et al. Allergic airways disease develops after an increase in allergen capture and processing in the airway mucosa. J Immunol. 2007;179:5748–5759. doi: 10.4049/jimmunol.179.9.5748. [DOI] [PubMed] [Google Scholar]
- 29.Hartwig C, Mazzega M, Constabel H, Krishnaswamy JK, Gessner JE, Braun A, Tschernig T, Behrens GM. Fcγ receptor-mediated antigen uptake by lung DC contributes to allergic airway hyper-responsiveness and inflammation. Eur J Immunol. 2010;40:1284–1295. doi: 10.1002/eji.200939900. [DOI] [PubMed] [Google Scholar]
- 30.Shiu J, Czinn SJ, Kobayashi KS, Sun Y, Blanchard TG. IRAK-M expression limits dendritic cell activation and proinflammatory cytokine production in response to Helicobacter pylori. PLoS One. 2013;8:e66914. doi: 10.1371/journal.pone.0066914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turnis ME, Song XT, Bear A, Foster AE, Gottschalk S, Brenner MK, Chen SY, Rooney CM. IRAK-M removal counteracts dendritic cell vaccine deficits in migration and longevity. J Immunol. 2010;185:4223–4232. doi: 10.4049/jimmunol.0903507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balhara J, Gounni AS. The alveolar macrophages in asthma: a double-edged sword. Mucosal Immunol. 2012;5:605–609. doi: 10.1038/mi.2012.74. [DOI] [PubMed] [Google Scholar]
- 33.Ballinger MN, Newstead MW, Zeng X, Bhan U, Mo XM, Kunkel SL, Moore BB, Flavell R, Christman JW, Standiford TJ. IRAK-M promotes alternative macrophage activation and fibroproliferation in bleomycin-induced lung injury. J Immunol. 2015;194:1894–1904. doi: 10.4049/jimmunol.1402377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staschke KA, Dong S, Saha J, Zhao J, Brooks NA, Hepburn DL, Xia J, Gulen MF, Kang Z, Altuntas CZ, et al. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J Immunol. 2009;183:568–577. doi: 10.4049/jimmunol.0802361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PM, Jacque B, Conner JR, Poltorak A, Stadecker MJ. IRAK-2 regulates IL-1-mediated pathogenic Th17 cell development in helminthic infection. PLoS Pathog. 2011;7:e1002272. doi: 10.1371/journal.ppat.1002272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinks TS, Zhou X, Staples KJ, Dimitrov BD, Manta A, Petrossian T, Lum PY, Smith CG, Ward JA, Howarth PH, et al. Innate and adaptive T cells in asthmatic patients: Relationship to severity and disease mechanisms. J Allergy Clin Immunol. 2015;136:323–333. doi: 10.1016/j.jaci.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 38.Bullens DM, Truyen E, Coteur L, Dilissen E, Hellings PW, Dupont LJ, Ceuppens JL. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res. 2006;7:135. doi: 10.1186/1465-9921-7-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Ramli W, Préfontaine D, Chouiali F, Martin JG, Olivenstein R, Lemière C, Hamid Q. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol. 2009;123:1185–1187. doi: 10.1016/j.jaci.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Maitra U, Davis S, Reilly CM, Li L. Differential regulation of Foxp3 and IL-17 expression in CD4 T helper cells by IRAK-1. J Immunol. 2009;182:5763–5769. doi: 10.4049/jimmunol.0900124. [DOI] [PMC free article] [PubMed] [Google Scholar]