Abstract
Evidence-based weight-loss treatments for children with autism spectrum disorder (ASD) are lacking. Therefore, a parent-based weight-loss treatment for children with ASD (PBT-ASD) was developed. A pilot study was conducted to test the initial efficacy, feasibility, and acceptability of this intervention. Parents of 20 children with ASD and overweight/obesity (mean age = 9.90 (SD = 2.31) years; 90% male; 40% Hispanic) participated in a 16-session PBT-ASD. The PBT-ASD program was found to be feasible and acceptable. Both children and parents lost weight from pre- to post-treatment (p’s < .05). Parent-reported child physical activity and vegetable consumption increased at post-treatment (p’s < .05). This pilot study provides a proof-of-concept for PBT-ASD. Randomized controlled trials with larger samples and follow-up are needed.
Keywords: Autism spectrum disorder, Obesity, Weight-loss, Parent training
Approximately 1 in 59 children in the United States have autism spectrum disorder (ASD) (Baio et al. 2018), a pervasive neurodevelopmental disorder characterized by deficits in social communication and includes restrictive, repetitive and stereotyped patterns of behavior, specific interests, or activities (American Psychiatric Association 2013). Among children with ASD, estimates of overweight and obesity range from 19% (Barnhill et al. 2017) to as high as 55% (Curtin et al. 2005), with the majority of studies reporting rates equal to or greater than typically developing children (Matheson and Douglas 2017). Overweight and obesity are associated with a multitude of negative health sequelae in children and adolescents, including cardiovascular disease risk factors (Friedemann et al. 2012; Goran et al. 2003), type 2 diabetes mellitus and insulin resistance (Goran et al. 2003; Hannon 2005), decreased health-related quality of life (Griffiths et al. 2010; Tsiros et al. 2009), and neurocognitive functioning deficits in areas related to attention, executive functioning, visuospatial and motor skills (Liang et al. 2014). In addition to these negative outcomes on an individual level, health care costs are estimated at $14.1 billion extra annually for children with overweight/obesity (i.e., prescription drugs, emergency room costs, outpatient visit expenditures) (Trasande and Chatterjee 2009). Thus, designing and improving weight-loss interventions in children with ASD is crucial.
There are many factors that contribute to the high rates of overweight and obesity in children with ASD (Matheson and Douglas 2017), including increased sedentary behaviors, reduced exercise patterns, fewer structured physical activities, and increased computer and screen time (MacDonald et al. 2011; Mazurek et al. 2012; Obrusnikova and Cavalier 2011). Moreover, children with ASD often have unusual dietary patterns and consume more energy-dense foods, sugar-sweetened beverages and snack foods, but less fruits and vegetables, compared to typically developing peers (Bandini et al. 2010; Evans et al. 2012). Children with ASD frequently have sensory integration difficulties that extend into the food realm (Cermak et al. 2010), which may limit the variety of foods a child is willing to consume. Genetic studies suggest at least 36 genes overlap between ASD and obesity (Sharma et al. 2012), with several microdeletions that are implicated in both disorders (Bochukova et al. 2010; Shinawi et al. 2011; Walters et al. 2010). Although the propensity towards weight gain in children with ASD is multi-faceted, there is clearly a need for interventions designed to help children with ASD lose weight and live healthier lives.
Despite the individual health consequences and rising public health costs of pediatric obesity, very few studies have targeted weight loss in children with ASD. Currently, the gold-standard behavioral intervention for weight loss in children is family-based behavioral treatment (FBT) (Epstein et al. 2007), which is provided to both the parent and child and provides nutrition and physical activity education in addition to parenting skills and behavior modification strategies. More recently, studies show that parent-based treatments (PBT) are as effective as parent and child treatments in promoting weight loss in the target child (Boutelle et al. 2011; Boutelle et al. 2017; Golan and Crow 2004). PBT programs are more cost-effective and potentially easier to disseminate (Boutelle et al. 2017). Similarly, parent training interventions (also known as parent-mediated interventions) for children with ASD have shown increased child learning by utilizing parents as co-facilitators in the initial learning, generalization, and maintenance of behavior changes (Casagrande and Ingersoll 2017; Ingersoll and Wainer 2013; Koegel et al. 1982; Lovaas et al. 1973; Reichow 2012).
However, evidence-based PBT programs for behavioral weight loss have not yet been adapted for children with ASD nor have they been conducted among parents of children with ASD. Therefore, the authors designed a PBT program specifically tailored to the unique needs and concerns parents of children with ASD (PBT-ASD). Following treatment development, a pilot study of PBT-ASD was conducted to test the initial efficacy, feasibility, and acceptability of this weight-loss intervention called transforming eating, activity, and motivation, utilizing parents (TEAM UP). The main goal of the TEAM UP study was to evaluate feasibility and acceptability with this population. The TEAM UP study also evaluated the preliminary efficacy on child body weight, fruit and vegetable consumption, and physical activity.
Methods
Participants
Interested families were recruited using flyers, local email mailing lists, and physician referrals. Parents completed an initial phone screen with study staff to determine eligibility. Children were eligible to participate in the study if they were between 5 and 14 years old with a confirmed diagnosis of ASD and a body mass index (BMI) equal to or greater than the 85th percentile. Exclusion criteria were child or parent participation in another weight-loss treatment program, co-occurring diabetes, or a recent change (previous 3 months) in medication known to affect weight loss (children only). All participants completed inform consent procedures compliant with the University of California, San Diego Institutional Review Board. Parents received a $25 gift card at baseline and a $50 gift card at post-treatment as compensation for completing assessment measures. Questionnaires were administered through an online survey system and completed at home.
Measures
Anthropometric measurements were conducted at baseline, mid-treatment (week 8), and post-treatment. All questionnaires measures were completed at the baseline and post-treatment time points only.
Anthropometrics
Height and weight were measured for children and parents at each time point. Height was measured using a portable Schorr height board in triplicate. Weight was measured Tanita Digital Scale (model WB-110A) in duplicate. Weight was converted to body mass index (BMI = [kg/m2]) for parents and children. BMI-for-age percentile scores and standardized BMI scores (BMI-z) were also calculated for children using the Center for Disease Control and Prevention 2000 growth charts (Kuczmarski et al. 2002).
Acceptability Questionnaire
Parents responded to questions regarding their satisfaction with the program on the post-treatment online survey. Parents rated the program on general helpfulness as well as how helpful the program was with regard to child weight management. Questions included, “Has the TEAM UP program made you feel better about your child?”, “Has the TEAM UP program helped your child’s weight get better?”, “Has the TEAM UP program made things worse in you/your child’s life,” and “Overall, how much has the TEAM UP program helped you and your child?”. Parents responded to these acceptability questions using a four-point response scale that ranged from “yes, a lot” to “no, not at all.”
Fruit and Vegetable Intake
Parents completed the Block Food Frequency Questionnaire (Block et al. 1986), a parent-report questionnaire about their child’s food intake and portion sizes over the past week. The number of fruit and vegetable servings over the past week were totaled for each child.
Child Physical Activity
Parents completed the Finnish Leisure Time Physical Activity questionnaire (Telama et al. 1985) about their child’s physical activity. This measure assesses physical activity level and sedentary behaviors over the past 7 days.
Demographics including age, sex, ethnicity, medication use, and therapy services were reported by parents about their child as well as themselves at baseline.
Treatment Program
The TEAM UP program consisted of 16-weekly, hour-long, parent-only sessions. At week 8, both the parent and the child attended the group to promote adherence. An advanced graduate student trained in PBT (BM) delivered the treatment. Weekly supervision was provided by a licensed clinical psychologist (KB) with expertise in pediatric obesity treatment and PBT clinical trials. Treatment focused on four target areas: dietary recommendations/calorie reduction, physical activity, behavior change, and parenting strategies. Consistent with traditional FBT, behavior therapy targets included self-monitoring, stimulus control, portion control, goal setting, planning for high-risk situations, utilizing motivation systems, and relapse prevention. The core curriculum was adapted specifically for use with children with ASD. Adaptations included using social stories to plan for high-risk situations (e.g., holidays, parties, buffets), token economies to reward progress towards behavioral weight-loss goals, visual supports (such as labeling food in the house with stickers) to promote increased fruit and vegetable intake, and food chaining to increase consumption of non-preferred lower calorie food choices.
Calorie goals were set individually for each child based on shaping down 20% of total intake until the child reached 1000–1200 kcals for 5 out of 7 days, consistent with other behavioral weight-loss treatment protocols for youth (Boutelle et al. 2015, 2017). Parents were instructed to track their child’s intake during the first week of the study without making modifications or changes to provide a baseline intake estimate. Then, parents were asked to reduce calories by 20% each following week until the child was consuming between 1000 and 1200 kcals a day for 5 out of 7 days. Parents were provided with handouts to assist in accurately reading nutrition labels and were provided with website resources as well as a detailed reference guide that listed serving sizes and calories for hundreds of food items. It was also recommended that children consume at least five fruits/vegetable servings every day. Parents were charged with utilizing behavioral principles, such as stimulus control, portion control, shaping, and reinforcement strategies to help their children reach these goals. Parents were instructed to complete daily self-monitoring logs of food intake for themselves and their child. Parents were encouraged to self-monitor their own caloric intake and physical activity throughout the program in order to model behavioral skills and healthy eating recommendations for their children. Children who were able to participate in completing self-monitoring logs were allowed to do so with parental guidance and support.
Parents were encouraged to help their children achieve at least 60 minutes per day of moderate-to-vigorous physical activity. They were also asked to track their child’s physical activity in a weekly self-monitoring log and prompted to increase activity gradually through shaping and positive reinforcement strategies. Often, physical activity became a target behavior in a reinforcement system or token economy. If parents did not already have a pre-existing reinforcement system in place, they were encouraged to construct one and add in the TEAM UP goals to help promote behavior change. Parents reported on the number of times per week that their child engaged in mild, moderate, or strenuous physical activities.
A parent-and-child physical activity class, led by individuals with expertise and certification in movement-based instruction for children with ASD, was held at week 8 to encourage physical activity participation in families. This visit occurred in conjunction with the mid-treatment assessment and anthropometric measurements. Children were present for the baseline assessment, week 8 mid-treatment assessment and physical activity class, and week 16 graduation ceremony and post-treatment assessment.
Statistical Analysis
Parents who completed the baseline survey and attended at least one treatment session were included in this study. Treatment attendance was taken each session and calculated by the group leader (BM). Child anthropometric results are presented for completers (n = 17) as well as the entire sample (n = 20) using the last observation carried forward for participants without mid-treatment or post-treatment data points. Parent-completed survey data is also presented for completers (n = 16) and the entire sample (n = 20) using data imputation techniques to account for missing survey data at post-treatment. Paired samples t test were conducted to analyze pre and post-treatment differences. Analyses were conducted in SPSS Version 25 (IBM Corp. 2017). Statistical significance for all analyses was set at the p < .05 level.
Results
Participants were 20 parents (90% female; Mean ± SD: 42.65 ± 6.64 years) and children (90% male; 2 females) between the ages of 6 and 13 years old (9.90 ± 2.31 years; Table 1). The racial/ethnic breakdown for parents in this study was 35% Hispanic, 30% Non-Hispanic Caucasian, 20% Asian, 10% Native Hawaiian or Pacific Islander, and 5% American Indian. The racial/ethnic breakdown for children in this study was similar, with 45% Non-Hispanic Caucasian, 40% Hispanic, 20% Asian, 5% Native Hawaiian or Pacific Islander, and 5% multi-racial. All children that participated in the study had a confirmed diagnosis of ASD. At baseline, 4 children (20%) were taking at least one prescription medication (including Ritalin, Vyvanse, Fludrocortistone, Clonidine, or Seroquel) and 13 children (65%) were concurrently in therapeutic services, such as speech therapy, occupational therapy, physical therapy, school counseling, therapeutic horseback riding, and Applied Behavioral Analysis (ABA). Approximately half of the parents (55%) were taking prescription medication at baseline (including Fluoxetine, Lexapro, Levothyroxine, Novolog, Citalopram, Lisinopril, Enbrel, Amlodopine, Levomir, Crestar, Lunesta, and Nortryptol). None of the children or parents were engaged in a weight-loss treatment program or diet plan outside of the TEAM UP study.
Table 1.
Participant characteristics
| N = 20 | |
|---|---|
| Child | |
| Sex (% male) | 90% |
| Mean age (SD) | 9.90 years (2.31) |
| Race/Ethnicity | |
| Non-Hispanic Caucasian | 45% |
| Hispanic | 40% |
| Asian | 20% |
| Native Hawaiian/Pacific Islander | 5% |
| Multi-racial | 5% |
| Medication | 20% |
| Therapeutic services | 65% |
| Baseline BMI (SD) | 27.57 kg/m2 (5.46) |
| Baseline BMI %tile (SD) | 97.26% (3.16) |
| Baseline BMI-z (SD) | 2.17 (0.46) |
| Parent | |
| Sex (% female) | 90% |
| Mean age (SD) | 42.65 years (6.64) |
| Marital status (% currently married) | 65% |
| Education (% college graduates) | 55% |
| Race/Ethnicity | |
| Hispanic | 35% |
| Non-Hispanic Caucasian | 30% |
| Asian | 20% |
| Native Hawaiian/Pacific Islander | 10% |
| American Indian | 5% |
| Baseline BMI (SD; N = 19) | 29.43 kg/m2 (5.67) |
Feasibility and Acceptability
Recruitment and Enrollment
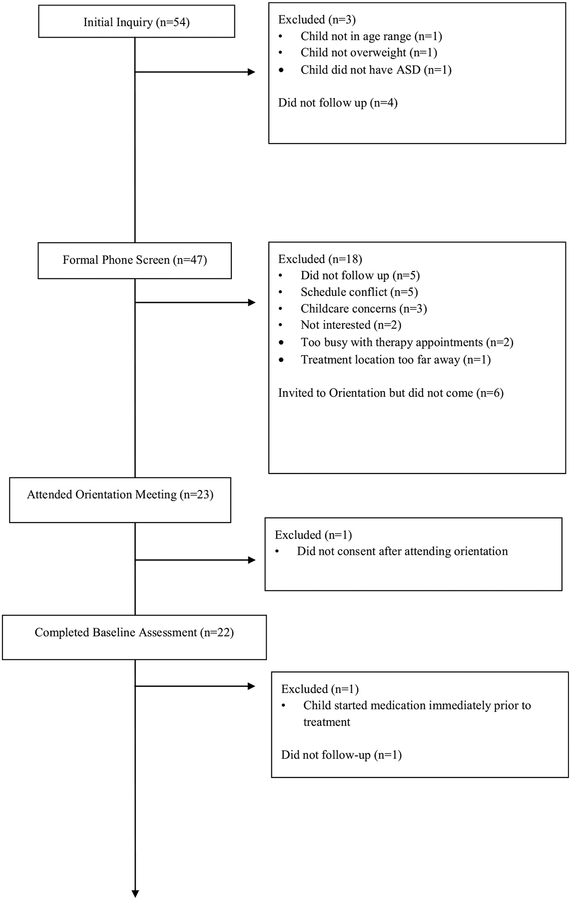
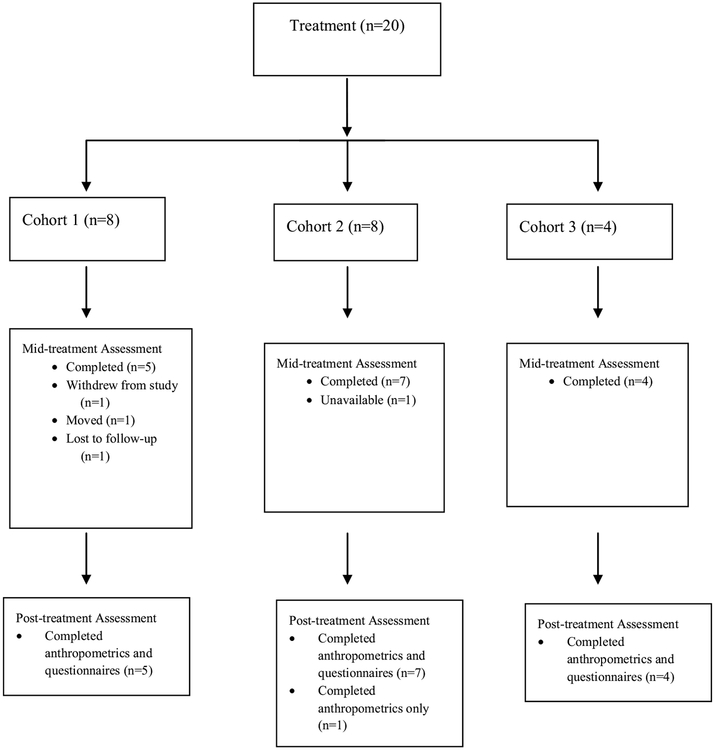
A total of 54 families were initially interested in learning more about the TEAM UP study. Of those, 47 parents completed a formal phone screen, which resulted in 23 families attending an in-person orientation meeting the week prior to the start of treatment. Twenty-two parents initially enrolled in the study and signed informed consent. Three cohorts were run over a three-year period. The consort diagram (Fig. 1) contains additional information regarding recruitment and enrollment for this study.
Fig. 1.
TEAM UP consort diagram
Completion Rates
Twenty parents completed the baseline assessment and attended at least one treatment session. Post-treatment questionnaires were completed by 16 of the 20 parents that completed the baseline assessment and attended at least one treatment session. One family attended three of the first four sessions and then was lost to follow-up. Another parent attended only two treatment sessions before moving out of the country. One family decided to withdraw from the study prior to the week 8 mid-treatment assessment. A fourth parent attended treatment sessions throughout the study and completed the anthropometric measurements at post-treatment but did not complete the online questionnaires. Post-treatment anthropometric data was collected on 17 children (85% of sample that initiated treatment).
Attendance
Weekly treatment sessions were well attended across the three cohorts. Twelve parents (63% of sample) attended at least 13 out of 16 treatment sessions (80% of treatment sessions). Two parents attended all 16 sessions. The remainder of the sample attended sessions as follows: 2 parents between 8 and 12 sessions, 3 parents between 4 and 7 sessions, and 3 parents attended 3 or fewer sessions. These weekly attendance rates are compelling, particularly considering that groups were parent-only and child care was not provided by the study during the group time.
Treatment Satisfaction
Sixteen parents completed the post-treatment survey. The majority of parents (93%) reported that participation in the program made them feel better about their child. All parents reported that the TEAM UP program had helped to improve their child’s weight (100%). Specifically, almost one-third (36%) of the sample reported that the TEAM UP program improved their child’s weight “a lot” whereas another half of the sample (50%) reported that the program had “somewhat” improved their child’s weight. Importantly, all parents reported that the TEAM UP program helped themselves and their child, with 79% of parents reporting that the program helped “a lot” and 21% of parents identifying that the program had helped “somewhat”, with no parents choosing “a little” or “not at all” for this question.
Treatment Outcomes
Child Weight Loss
At baseline, child BMI for this sample ranged from 19.00 to 38.00 kg/m2, with a mean BMI of 27.57 kg/m2 (SD = 5.46). When using statistical techniques to account for missing data and participant drop out (i.e., last observation carried forward), the mean BMI of the sample remained largely unchanged halfway through treatment (8-week mid-treatment assessment: 27.04 kg/m2 ± SD = 5.44) and decreased overall by the end of treatment (post-treatment assessment: 26.35 kg/m2 ± 5.21). The change in BMI from pre to post-treatment reached statistical significance for this sample (t = 3.58; p < .01). Effect size estimates of the change in BMI from baseline to post-treatment suggest a small effect (Cohen’s d = 0.23; CI: − 2.16 to 2.52; Table 2). BMI-z analyses paralleled these results, with baseline scores of 2.17 (SD = 0.46) decreasing to 2.03 (SD = 0.49) at the week 8 mid-treatment time point and 1.92 (SD = 0.59) by the end of treatment for the entire sample. This change from pre to post-treatment in BMI-z scores was also significant (t = 3.31, p < .01) and reflective of an approximately moderate effect size (Cohen’s d = 0.48; CI: 0.28 to 0.74). Similar results were obtained when analyzing the data with completers only (p’s < .01).
Table 2.
TEAM UP treatment outcomes
| Baseline M (SD) | Week 8 M (SD) | Post-treatment M (SD) | T-statistic (baseline to post-treatment) | Cohen’s d | |
|---|---|---|---|---|---|
| Child | |||||
| BMI | 27.57 (5.46) | 27.04 (5.44) | 26.35 (5.21) | t = 3.58** | d = 0.23 (CI: − 2.16 to 2.52) |
| BMI-z | 2.17 (0.46) | 2.03 (0.49) | 1.92 (0.59) | t = 3.31** | d = 0.48 (CI: 0.28 to 0.74) |
| Physical activity (episodes per week) | 5.82 (5.77) | – | 8.53 (6.91) | t = − 4.26** | d = − 0.44 (CI: − 3.18 to 2.85) |
| Fruit intake (servings per week) | 10.2 (6.84) | – | 11.75 (7.89) | t = − 1.41 | – |
| Vegetable intake (servings per week) | 4.6 (5.13) | – | 6.9 (7.13) | t = − 2.15* | d = − 0.38 (CI: − 2.63 to 2.74) |
| Parent | |||||
| BMI | 29.43 (5.67) | 29.04 (5.63) | 28.64 (5.54) | t = 2.69* | d = 0.15 (CI: − 2.40 to 2.62) |
BMI body mass index, BMI-z standardized BMI scores, CI confidence interval, M mean, SD standard deviation
p < .05,
p < .01
Weight loss varied greatly across cohort and among individuals. By the end of the 16-week treatment, 6 children lost 9.5 pounds or more, with one child losing over 20 pounds in this time frame. At post-treatment, two children were in the healthy weight range (one child went from obese to healthy weight range and one child went from overweight to healthy weight range).
Child Physical Activity
There was significant variability in the responses reported for physical activity at baseline (range: 0 episodes to 60 episodes in the previous week). Two parents reported over 40 episodes of child physical activity over the past week at baseline. When these two outliers were removed, the average number of episodes of child physical activity at baseline was 5.82 times (SD = 5.77) per week. At post-treatment, physical activity rose to an average of 8.53 times per week (SD = 6.91), almost three additional times per week compared to baseline. This change from pre to post-treatment was statistically significant (t = − 4.26; p < .01) and reflective of a small effect size (Cohen’s d = − 0.44; CI: − 3.18 to 2.85).
Child Fruit and Vegetable Intake
Parents reported that children consumed a higher number of fruit servings on average compared to vegetable servings at both baseline and post-treatment. At baseline, children consumed on average 10.20 servings (SD = 6.84) of fruit per week compared to 4.60 servings (SD = 5.13) of vegetables. Compared to baseline, child intake of fruit servings/week at post-treatment increased to 11.75 servings (SD = 7.89) and vegetable servings/week increased to an average of 6.90 servings (SD = 7.13) per week. Whereas changes in fruit servings for the entire sample were not significant (p > .05), changes in reported vegetable consumption were significantly increased from baseline to post-treatment (t = − 2.15, p = .045; Cohen’s d = − 0.38; CI: − 2.63 to 2.74). These results remained significant when analyzing completers only (p < .05).
Parent Weight Loss
Parent weight was collected on nineteen of the twenty participating parents. One participating parent was wheelchair bound and unable to provide anthropometric measurements. At baseline, parent BMI ranged from 19.70 to 38.20 kg/m2 (29.43 kg/m2 ± 5.67). Although parents were not required to be overweight at the start of the study, nine of the nineteen participating parents (47%) had overweight or obesity at baseline. Data imputation techniques were utilized to account for missing data at the 8-week and post-treatment assessments.
Parent BMI decreased slightly at the 8-week mid-treatment assessment (29.04 kg/m2 ± 5.63). At post-treatment, parent weight continued to decrease (28.64 kg/m2 ± 5.51). This decrease from pre to post-treatment was statistically significant (t = 2.69; p = .02). Effect size estimates suggest a small effect (Cohen’s d = .15; CI: − 2.40 to 2.62). Results remained largely unchanged when analyzing completers only (t = 2.78; p = .01). Almost half (40%) of participating parents lost 5 pounds or more, with 3 parents losing 10 or more pounds following the 16-week intervention.
Discussion
This is the first study to date to adapt a parent-based weight-loss treatment specifically for children with ASD and overweight or obesity (PBT-ASD). This pilot study found that PBT-ASD is acceptable and feasible to parents and children. Almost two-thirds of the sample attended at least 80% of treatment sessions. Furthermore, the majority of participants completed the post-treatment assessment, with only three families lost to follow-up. There was only one family-initiated withdrawal during the entire study. Parent satisfaction with the intervention was high, with the majority of parents indicating that they found the TEAM UP program helpful.
The treatment outcome data for this pilot study supports the initial effectiveness of PBT-ASD. Children in the study lost weight from baseline to post-treatment, although significant individual variability existed in terms of weight-loss outcomes. Impressively, two children ended the study in the healthy weight range after the 16-week intervention. Children in the study also increased their physical activity and vegetable consumption. Furthermore, parents in the study lost a significant amount of weight from baseline to post-treatment, although parent weight was never explicitly targeted in the group treatment sessions. Thus, the initial evidence suggests that PBT-ASD may promote weight loss among children with ASD and their parents.
Similar to other PBT programs, children did not need to be present in TEAM UP in order to successfully lose weight (Boutelle et al. 2017). PBT interventions are also less costly and require fewer staff, thus saving money and resources. Additionally, many parents also lost weight by participating in this intervention. Although parent weight loss was not a target of the TEAM UP intervention, parents were encouraged to model healthy eating and activity behaviors for their child. Restructuring the home environment and providing additional opportunities for physical and lifestyle activity may promote positive health behavior change in family members beyond the identified patient. Future research studies should continue to assess the impact that family-based behavioral weight-loss programs have on all household members, regardless of whether those individuals presented to treatment.
There are a number of strengths and limitations that should be considered when interpreting findings from this study. First, the study utilized and adapted an evidence-based intervention for pediatric obesity and found promising results for children with ASD. The sample was relatively diverse with both males and females participating. Parents were utilized as the agents of change to promote weight loss for their child and were not required to follow restrictive diets or eliminate preferred foods from their child’s diet. This was particularly important, as children with ASD often have limited preferred foods and decreased variety compared to children without ASD (Bandini et al. 2010; Cermak et al. 2010; Schreck et al. 2004; Zimmer et al. 2012). However, the study did not include follow-up time points and thus it is unknown whether children maintained their weight-loss results once the study ended. Additionally, the sample was small and heterogeneous, with a wide-range of ages (young children to teenagers), adaptive functioning, medication use, and communication abilities (verbal to non-verbal children). Cognitive abilities of the children in the study were not assessed, and thus it is unknown how well the study results will generalize to children with ASD and accompanying intellectual impairment. These factors should be addressed in larger studies in order to better understand how differences in sample characteristics may impact weight-loss outcomes for children with ASD. Finally, although caloric intake was recorded on self-monitoring logs, these data were not systematically collected nor analyzed, thus it is unknown whether children in the study lost weight due to caloric restriction, expansion of food preferences, increased physical activity, or a combination of these or other factors not accounted for in the present study.
Overall, the TEAM UP study was feasible and acceptable and demonstrated initial efficacy for PBT to promote weight loss in children with ASD. In addition to weight loss, children enrolled in TEAM UP demonstrated positive changes in physical activity and eating patterns, such as increased vegetable intake. Future research should seek to investigate the impact of PBT-ASD in larger samples using randomized clinical trials. Since this is a parent-only intervention, it is possible that the interventions that parents learn in the program could impact other family members. Changing eating and physical activity habits in the target child may have trickle down effects to the whole family. PBT interventions should continue to track the progress of all family members—not just the identified participant—in order to gain additional information regarding how changing healthy habits for one individual may benefit the entire family. It is imperative that clinicians and researchers alike continue to develop and implement weight-loss treatments for children with ASD in order to promote healthier weight trajectories through improved eating behaviors and physical activity habits.
Acknowledgments
The authors are grateful to the Healthy Weight Research Network for Children with Autism Spectrum Disorder and Developmental Disabilities for providing the funding for this pilot study (HRSA UA3 MC25735). Amy Drahota, Ph.D., also received funding from the National Institutes of Mental Health (K01 MH093477). The authors would like to thank the families that participated in the TEAM UP study as well as the dedicated staff at the Center for Healthy Eating and Activity Research. Initial findings regarding treatment development and preliminary results from this study were presented at the Obesity Society Annual Scientific Meeting and the Association for Behavioral and Cognitive Therapies Convention in 2015.
Funding The study was funded by Healthy Weight Research Network for Children with Autism Spectrum Disorder and Developmental Disabilities (HRSA UA3 MC25735); National Institutes of Mental Health (K01 MH093477; PI: Drahota)
Footnotes
Conflict of interest The authors declare that they have no conflicts of interests.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of autism spectrum disorder among children aged 8 years: Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1–23. 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, et al. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. Journal of Pediatrics, 157(2), 259–264. 10.1016/j.jpeds.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnhill K, Gutierrez A, Ghossainy M, Marediya Z, Marti CN, & Hewitson L (2017). Growth status of children with autism spectrum disorder: A case–control study. Journal of Human Nutrition & Dietetics, 30(1), 59–65. 10.1111/jhn.12396. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, & Gardner L (1986). A data-based approach to diet questionnaire design and testing. American Journal of Epidemiology, 124(3), 453–469. 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, … Farooqi IS (2010). Large, rare chromosomal deletions associated with severe early-onset obesity. Nature, 463(7281), 666–670. 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Braden A, Douglas JM, Rhee KE, Strong D, Rock CL, … Crow S (2015). Design of the FRESH study: A randomized controlled trial of a parent-only and parent-child family-based treatment for childhood obesity. Contemporary Clinical Trials, 45, 364–370. 10.1016/j.cct.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Cafri G, & Crow SJ (2011). Parent-only treatment for childhood obesity: A randomized controlled trial. Obesity, 19(3), 574–580. 10.1038/oby.2010.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutelle KN, Rhee KE, Liang J, Braden A, Douglas J, Strong D, … Crow SJ (2017). Effect of attendance of the child on body weight, energy intake, and physical activity in childhood obesity treatment: A randomized clinical trial. JAMA Pediatrics, 171(7), 622–628. 10.1001/jamapediatrics.2017.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casagrande K, & Ingersoll B (2017). Parent-mediated interventions for social communication in young children with ASD In Leaf J (Ed.), Handbook of social skills and autism spectrum disorder: Assessment, curricula, and interventions. New York, NY: Springer. [Google Scholar]
- Cermak SA, Curtin C, & Bandini LG (2010). Food selectivity and sensory sensitivity in children with autism spectrum disorders. Journal of the American Dietetic Association, 110(2), 238 10.1016/j.jada.2009.10.032.Food. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin C, Bandini LG, Perrin EC, Tybor DJ, & Must A (2005). Prevalence of overweight in children and adolescents with attention deficit hyperactivity disorder and autism spectrum disorders: A chart review. BMC Pediatrics. 10.1186/1471-2431-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Paluch RA, Roemmich JN, & Beecher MD (2007). Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychology, 26(4), 381–391. 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans EW, Must A, Anderson SE, Curtin C, Scampini R, Maslin M, et al. (2012). Dietary patterns and body mass index in children with autism and typically developing children. Research in Autism Spectrum Disorders, 6(1), 399–405. 10.1016/j.rasd.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, & Ward AM (2012). Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta-analysis. BMJ. 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan M, & Crow S (2004). Parents are key players in the prevention and treatment of weight-related problems. Nutrition Reviews. 10.1301/nr.2004.jan.39?50. [DOI] [PubMed] [Google Scholar]
- Goran MI, Ball GDC, & Cruz ML (2003). Cardiovascular endocrinology 2: Obesity and risk of type 2 diabetes and cardiovascular disease in children and adolescents. Journal of Clinical Endocrinology and Metabolism. 10.1210/jc.2002-021442. [DOI] [PubMed] [Google Scholar]
- Griffiths LJ, Parsons TJ, & Hill AJ (2010). Self-esteem and quality of life in obese children and adolescents: A systematic review. International Journal of Pediatric Obesity, 1, 4 10.3109/17477160903473697. [DOI] [PubMed] [Google Scholar]
- Hannon TS (2005). Childhood obesity and type 2 diabetes mellitus. Pediatrics, 116(2), 473–480. 10.1542/peds.2004-2536. [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2017). IBM SPSS statistics for Macintosh, version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Ingersoll B, & Wainer A (2013). Initial efficacy of project ImPACT: A parent-mediated social communication intervention for young children with ASD. Journal of Autism and Developmental Disorders, 43(12), 2943–2952. 10.1007/s10803-013-1840-9. [DOI] [PubMed] [Google Scholar]
- Koegel RL, Schreibman L, Britten KR, Burke JC, & Neill RE (1982). A comparison of parent training to direct child treatment In Koegel AL, Rincover RL, & Egel A (Eds.), Educating and understanding autistic children (pp. 260–279). San Diego, CA: College Hill Press. [Google Scholar]
- Kuczmarski RRJ, Ogden CLC, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, … Johnson CL (2002). 2000 CDC Growth Charts for the United States: Methods and development. Vital and Health Statistics. Series 11, Data from the National Health Survey. [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, & Boutelle KN (2014). Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity, 38(4), 494–506. 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovaas OI, Koegel R, Simmons JQ, & Long JS (1973). Some generalization and follow-up measures on autistic children in behavior therapy1. Journal of Applied Behavior Analysis, 6(1), 1310815 10.1901/jaba.1973.6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Esposito P, & Ulrich D (2011). The physical activity patterns of children with autism. BMC Research Notes. 10.1186/1756-0500-4-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson BE, & Douglas JM (2017). Overweight and obesity in children with autism spectrum disorder (ASD): A critical review investigating the etiology, development, and maintenance of this relationship. Review Journal of Autism and Developmental Disorders, 4(2), 142–156. 10.1007/s40489-017-0103-7. [DOI] [Google Scholar]
- Mazurek MO, Shattuck PT, Wagner M, & Cooper BP (2012). Prevalence and correlates of screen-based media use among youths with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(8), 1757–1767. 10.1007/s10803-011-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrusnikova I, & Cavalier AR (2011). Perceived barriers and facilitators of participation in after-school physical activity by children with autism spectrum disorders. Journal of Developmental and Physical Disabilities, 23(3), 195–211. 10.1007/s10882-010-9215-z. [DOI] [Google Scholar]
- Reichow B (2012). Overview of meta-analyses on early intensive behavioral intervention for young children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(4), 512–520. 10.1007/s10803-011-1218-9. [DOI] [PubMed] [Google Scholar]
- Schreck KA, Williams K, & Smith AF (2004). A comparison of eating behaviors between children with and without autism. Journal of Autism and Developmental Disorders, 34(4), 433–438. 10.1023/B:JADD.0000037419.78531.86. [DOI] [PubMed] [Google Scholar]
- Sharma JR, Arieff Z, & Sagar S (2012). Autism and obesity: Prevalence, molecular basis and potential therapies. Autism Insights. 10.4137/AUI.S9138. [DOI] [Google Scholar]
- Shinawi M, Sahoo T, Maranda B, Skinner SA, Skinner C, Chinault C, … Beaudet AL (2011). 11p14.1 microdeletions associated with ADHD, autism, developmental delay, and obesity. American Journal of Medical Genetics, Part A, 155(6), 1272–1280. 10.1002/ajmg.a.33878. [DOI] [PubMed] [Google Scholar]
- Telama R, Viikari J, Valikmaki I, Siren-Tiusanen H, Åkerblom HK, Uhari M, … Suoninen P (1985). Atherosclerosis precursors in Finnish children and adolescents. X. Leisure-time physical activity. Acta Pædiatrica, 74, 169–180. 10.1111/j.1651-2227.1985.tb10092.x. [DOI] [PubMed] [Google Scholar]
- Trasande L, & Chatterjee S (2009). The impact of obesity on health service utilization and costs in childhood. Obesity, 17(9), 1749–1754. 10.1038/oby.2009.67. [DOI] [PubMed] [Google Scholar]
- Tsiros MD, Olds T, Buckley JD, Grimshaw P, Brennan L, Walkley J, … Coates AM (2009). Health-related quality of life in obese children and adolescents. International Journal of Obesity. 10.1038/ijo.2009.42. [DOI] [PubMed] [Google Scholar]
- Walters RG, Jacquemont S, Valsesia A, De Smith AJ, Martinet D, Andersson J, … Beckmann JS (2010). A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature, 463(7281), 671–675. 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, & Summer S (2012). Food variety as a predictor of nutritional status among children with autism. Journal of Autism and Developmental Disorders, 42(4), 549–556. 10.1007/s10803-011-1268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]