Abstract
Background:
Adverse childhood experiences (ACEs) are a potent risk factor. Despite these findings, studies have also recognized the importance of considering additional sources of genetic and environmental influence that cluster within families.
Objective:
To properly control for latent sources of genetic and within-family environmental influences and isolate the association between ACEs and the following outcomes in adulthood: physical health, depressive symptoms, educational attainment, income attainment, alcohol problems, and antisocial behavior.
Participants and Setting:
Two independent samples of twins and siblings from the United States: the Midlife Development in the United States (MIDUS) study (N = 862) and the National Longitudinal Study of Adolescent to Adult Health (Add Health; N = 3,112).
Methods:
Sibling comparison models, which control for latent sources of genetic and within-family environmental influences, were estimated to examine whether differential exposure to ACEs was associated with the examined outcomes.
Results:
Families that experienced more adversity also experienced more deleterious outcomes. However, siblings that experienced more adversity were no more likely to experience deleterious outcomes than their co-siblings. However, greater exposure to ACEs was associated with increases in depressive symptoms (Add Health). Additional models revealed that the similarity between siblings from the same family stemmed from latent sources of within-family environmental influences not captured by traditional ACEs measures.
Conclusions:
Considering genetic influences and additional latent sources of within-family influences is crucial in isolating the effects of ACEs. Currently employed ACEs measures may not adequately capture the full range of impactful sources of family-level environmental influence.
Keywords: adverse childhood experiences, development, sibling comparison model
Children exposed to greater levels of adverse childhood experiences (ACEs) are significantly more likely to experience internalizing (Anda et al., 2006; Chapman et al., 2004; Lindert et al., 2014) and externalizing problems (Anda et al., 2006; Duke, Pettingell, McMorris, & Borowsky, 2010; Whitfield, Anda, Dube, & Felitti, 2003), poorer socioeconomic outcomes (Egan, Daly, Delaney, 2015; Herman, Susser, Struening, & Link, 1997; Liu et al., 2013), and more physical health problems (Danese & McEwen, 2012; Danese et al., 2009; Koss & Gunnar, 2018) relative to children without such problems. In addition, the overall prevalence with which children are exposed to ACEs is troubling. Nearly half (46%) of children in the United States will experience at least one adverse experience during their lifetime, with 11% experiencing three or more ACEs between birth and age 17 (Sacks, Murphey, & Moore, 2014). While economic disadvantage is often observed as the most common adverse experience reported in the United States, other ACEs are also alarmingly common. For example, 9.1 out of every 1,000 children in the United States experienced maltreatment during 2016, with nearly 75% of those children experiencing neglect and nearly 30% experiencing abuse (U.S. Department of Health and Human Services, 2018).
While ACEs are widely acknowledged as important sources of risk, methodological issues surrounding the evaluation of associations involving ACEs and later life outcomes persist. Multiple recent attempts have been made to more clearly define and operationalize ACEs to identify a more comprehensive set of adverse experiences (Cronholm et al., 2015; Finkelhor et al., 2013; 2015; Wade et al., 2016). ACEs are typically assessed using 10 categories of childhood adversity outlined in the U.S. Centers for Disease Control (CDC) Adverse Childhood Experiences Study (Felitti, et al., 1998), but recent studies have advocated for the addition of more domains that tap additional sources of risk. For example, Finkelhor and colleagues (2013; 2015) noted that the inclusion of additional items increased the predictive validity of the ACEs scale and advocated for the inclusion of domains tapping low socioeconomic status (SES), peer rejection and victimization, community violence exposure, and poor school performance. The authors also acknowledge the possibility of even more domains of childhood adversity that might further increase predictive validity, indicating that additional latent sources of within-family influences not captured by conventional measures of ACEs may also warrant consideration.
Alongside calls to integrate additional environmental sources of within-family influence into existing ACEs measures, recent studies have also noted the importance of considering genetic influences. For example, Finkelhor and colleagues (2013) noted that an “alternative explanation for many of the ACE study findings is that inherited genes for health problems or some temperamental qualities create a spurious connection between abuse and neglect by parents or other family context variables and mental and physical health conditions in their offspring” (p. 74). In line with these observations, previous studies have also noted the importance of controlling for genetic and additional latent sources of environmental influences that cluster within families to better isolate the impact of more specific sources of childhood adversity on deleterious outcomes (Alemany et al., 2013; Brown, Craig, Harris, Handley, & Harvey, 2007; Forsman & Långström, 2012; Laporte, Paris, Guttman, & Russell, 2011; Kendler et al., 2000; Nelson et al., 2002; Young-Wolff, Kendler, Ericson, & Prescott, 2011). The majority of these studies have compared siblings from the same family to examine the extent to which greater exposure to various forms of adversity increases the likelihood of negative outcomes. This design is powerful as it controls for all sources of influence (including both genetic and environmental influences) shared by siblings from the same household and isolates differences between siblings (D’Onofrio, Lahey, Turkheimer, & Lichtenstein, 2013; Turkheimer & Harden, 2014). While some of these studies have found support for the association between childhood adversity and later deleterious outcomes after comparing twins or siblings from the same family (Alemany et al., 2013; Brown et al., 2007; Kendler et al., 2000; Nelson et al., 2002), others have failed to find support (Forsman & Långström, 2012; Laporte et al., 2011; Young-Wolff et al., 2011).
Despite these mixed findings, there is at least preliminary evidence suggesting that a combination of environmental and genetic influences that cluster within families may contribute to observed associations between ACEs and deleterious outcomes. This is concerning, as these sources of influence are latent, making this issue difficult to address in observational studies and potentially resulting in biased findings (D’Onofrio et al., 2013; Johnson, Turkheimer, Gottesman, & Bouchard, 2009). This possibility is underscored by previous studies reporting the comorbidity of various forms of adversity (Dong, Anda, Dube, Giles, & Felitti, 2003), a phenomenon that some have referred to as the “adversity package” (Jirapramukpitak, Harpham, & Prince, 2011; Rossman, 2000). A developed literature has also recognized the role of genetic influences in shaping familial interactions, wherein genetic influences partially contribute to phenotypic similarity across generations (D’Onofrio et al., 2013; Scarr & McCartney, 1983). Studies have also recognized that offspring phenotypes, which are, in part, genetically influenced, may contribute to variability in parental phenotypes, eliciting responses based on offspring behavior (Larsson, Viding, Rijsdijk, & Plomin, 2008). The combined focus on both sets of influences is further underscored by previous studies examining the potential moderating role of genetic influences in the association between childhood adversity and deleterious outcomes (Caspi et al., 2002; Kim-Cohen et al., 2006). In perhaps the most well-known example, Caspi et al. (2002) examined the association between childhood maltreatment and antisocial behavior. The results indicated a nonsignificant association, but they did find evidence of a gene-environment interaction, wherein those individuals who experienced maltreatment and also possessed the a low-activity variant of the monoamine oxidase A (MAOA-uVNTR) gene engaged in significantly greater levels of antisocial behavior. These findings, coupled with those from additional studies identifying significant gene-environment interactions (Kim-Cohen et al., 2006), provide evidence suggesting that a closer examination of genetic influences may result in a better understanding of the ways in which childhood adversity may be associated with deleterious outcomes in adulthood.
Collectively, these observations underline the importance of accounting for latent sources of genetic and within-family environmental influences when examining associations between ACEs and later outcomes. The current study aims to accomplish this objective by examining the association between ACEs and a range of deleterious outcomes in adulthood that span multiple developmental domains in two separate samples of twin and sibling pairs. The use of twin and sibling pairs allows for the estimation of sibling comparison models, a methodological approach that isolates environmental differences between siblings from the same family while eliminating latent sources of within-family influence stemming from both genetic and environmental sources (D’Onofrio et al., 2013; Turkheimer & Harden, 2014).
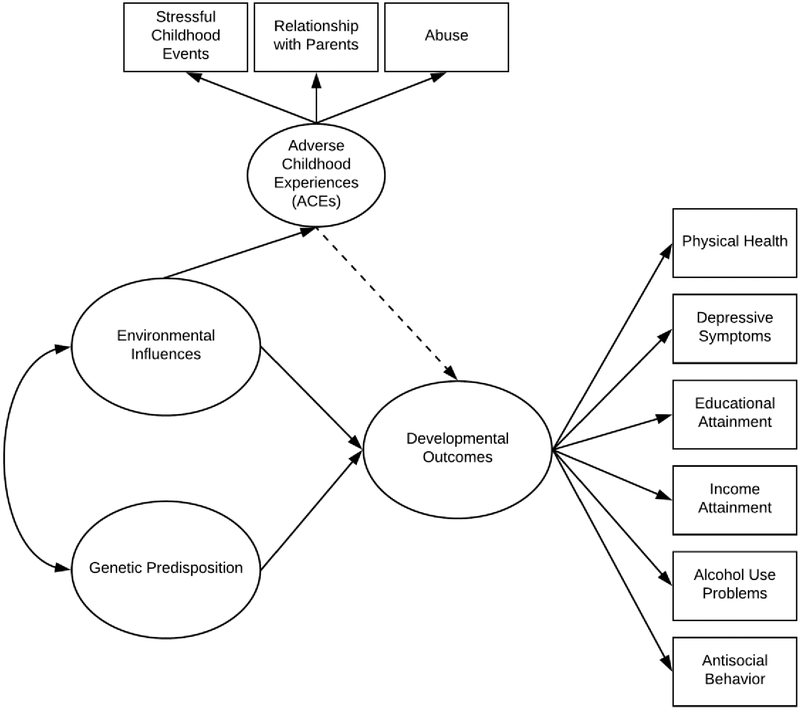
The current study offers the most comprehensive examination of the impact of ACEs on developmental outcomes to date. In an effort to better elucidate the goals of the current study, a conceptual model outlining the examined associations and the role both environmental and genetic influences in the development of both ACEs and the examined developmental outcomes is offered in Figure 1. Directly in line with findings flowing from the existing literature, the double headed arrow between latent sources of family environmental influences and genetic predisposition represents gene-environment interplay, in which genetic influences may result in differences in family environments (i.e., gene-environment correlation) and that family environments may moderate the association between genetic influences and developmental outcomes (i.e., gene-environment interactions). The single headed arrow running from family environmental influences to ACEs indicates that ACEs represent one source of family environmental influences. The single headed arrows running from environmental influences and genetic predisposition to developmental outcomes represent the combined influence of environmental and genetic influences on developmental outcomes. In turn, the dashed single headed arrow represents the influence of ACEs, as a specific source of environmental influence, on developmental outcomes. The arrow is dashed to indicate that the association may not be significant after controlling for additional latent sources of environmental and genetic influence.
Figure 1. Conceptual Model for Adverse Childhood Experiences and Developmental Outcomes.
Note: The presented model acknowledges the simultaneous contribution of environmental and genetic influences on the examined developmental outcomes. The double headed arrow between latent sources of family environmental influences and genetic predisposition represents gene-environment interplay, in which genetic influences may result in differences in family environments (i.e., gene-environment correlation) and that family environments may moderate the association between genetic influences and developmental outcomes (i.e., gene-environment interactions). The single headed arrow running from family environmental influences to adverse childhood experiences (ACEs) indicates that ACEs represent one source of family environmental influences. The single headed arrows running from environmental influences and genetic predisposition to developmental outcomes represent the combined influence of environmental and genetic influences on developmental outcomes. In turn, the dashed single headed arrow represents the influence of ACEs, as a specific source of environmental influence, on developmental outcomes. The arrow is dashed to represent the fact that the association may not be significant after controlling for additional latent sources of environmental influences and genetic predisposition.
In line with these observations, the current study expands on the existing literature in three ways. First, the current study examines a wide range of deleterious outcomes experienced in adulthood. Previous research has typically focused on a single outcome (e.g., externalizing behaviors; Anda et al., 2006; Duke et al., 2010; Whitfield et al., 2003) or a narrow set of related outcomes (e.g., socioeconomic disadvantage; Egan et al., 2015; Herman et al., 1997; Liu et al., 2013). The outcomes examined in the current study are intended to represent a cross-section of the consequences that have been previously linked to ACEs. The six examined outcomes in no way represent an exhaustive list of such consequences, but do provide a relatively comprehensive overview of such consequences. By examining outcomes that span multiple developmental domains, the current study aims to provide a more comprehensive investigation of the detrimental impact of ACEs later in the life course. Second, the current study employs sibling comparison models, a highly conservative and powerful analytic approach, to control for latent sources of genetic and environmental influences that cluster within families and better isolate the effect of ACEs on the examined outcomes. While previous studies have employed similar methodological approaches (Alemany et al., 2013; Brown et al., 2007; Forsman & Långström, 2012; Laporte et al., 2011; Kendler et al., 2000; Nelson et al., 2002; Young-Wolff et al., 2011), the majority of these studies have examined a much narrower set of adverse experiences (e.g., childhood maltreatment) and outcomes. The use of a more comprehensive measure of ACEs and the examination of a broader set of outcomes overcomes these limitations. Third, the current study employs two similar, yet distinct, samples in an effort to replicate the results from one sample with the other. While there are important similarities between the two employed samples, there are also important differences in the demographic composition of the two samples, in the employed measures, and in the composition of the examined family dyads (i.e., twin pairs versus sibling pairs). These differences provide a unique opportunity to examine the robustness of the findings, as a similar pattern of results across the two samples would provide substantial evidence suggesting that the findings are reliable and valid.
Directly in line with these observations, the two samples examined in the current study were selected for four reasons. First, the National Survey of Midlife Development in the United States (MIDUS) is comprised of a sample with a wider age range (20 to 75 at Wave 1 and 28 to 84 at Wave 2) than the National Longitudinal Study of Adolescent to Adult Health (Add Health) sample (12 to 21 at Wave I and 24 to 32 at Wave IV), resulting in different recall periods pertaining to childhood adversity. Second, the MIDUS sample contains a subsample of twins, while the Add Health contains a subsample of twins and singleton siblings. This difference between the two samples allows for the examination of the extent to which findings may differ across varying levels of genetic relatedness. Third, and relatedly, since the Add Health contains both twin and sibling pairs, the resulting sample provides increased levels of statistical power (N = 3,112) compared to the MIDUS sample (N = 862). This difference between the two samples will demonstrate whether findings are sensitive to differences in statistical power. Fourth, and finally, while the measures (both the ACEs and outcome measures) used for both samples likely tap the same latent constructs, they do differ in operationalization (e.g., depressive symptoms are measured using the Screening Version of the World Health Organization Composite Interview Diagnostic Interview in the MIDUS and the Center for Epidemiologic Studies Depression Scale in the Add Health). These differences will, again, demonstrate the robustness of the findings from each individual sample if replicated in the other. In this way, the two samples are largely complementary but also are characterized by important differences that can be leveraged to examine the robustness of the resulting findings, wherein, convergence in results from both samples would provide strong support for the findings.
DATA
National Survey of Midlife Development in the United States (MIDUS)
The MIDUS is a longitudinal and nationally representative sample of adults from the United States that spans three waves of data collection and nearly 18 years, with MIDUS I collected between 1995–1996 and MIDUS III collected in 2013 (Radler & Ryff, 2010; Ryff et al., 2016). Extensive phone interviews were conducted during each wave and covered a wide variety of topics. During MIDUS II data collection procedures, a subsample was selected to participate in the Biomarker Project, which was carried out over two days and included the collection of 12-hour urine samples, fasting blood draws, and the collection of whole saliva (Love et al., 2010). Also nested within the full sample is a subsample of monozygotic (MZ) and dizygotic (DZ) twin pairs (n = 1,914 or 988 pairs), which were identified by screening sampled households and oversampling households that contained twins. The final analytic sample (N = 862) was limited to MZ (n = 464) and same-sex DZ (n = 398) twin pairs.
National Longitudinal Study of Adolescent to Adult Health (Add Health)
The Add Health is a prospective nationally representative longitudinal sample of youth from the United States that spans four waves of data collection and nearly 12 years of development with youth aged between 12 and 21 at Wave I (collected between 1994–1995) and between 24 and 32 at Wave IV (collected between 2007–2008; Harris, 2013). During each wave of data collection, participants were interviewed in their home by a trained member of the research team. During Wave IV, trained interviewers also collected anthropomorphic and cardiovascular measures as well as dried blood spots to examine biomarkers related to physical health (Entzel et al., 2009). Nested within the overall Add Health Sample is a subsample of nearly 3,000 twin, sibling (both full and half siblings), cousin, and unrelated (i.e., step-siblings) pairs (Harris et al., 2006). The final analytic sample for the current study (N = 3,112) draws from this subsample of twin and sibling pairs and is comprised of MZ twins (n = 564), as well as same-sex DZ twins (n = 490), full siblings (n = 1,212), half-siblings (n = 370), cousins that lived in the same household (n = 168), and step- (or unrelated) siblings (n = 308).
MEASURES
Outcomes
Physical Health Problems.
Additional information regarding the description of each outcome (including the physical health problems measure) within both samples is presented in the online supplement. Means, prevalence rates, and other descriptive information for all measures (including physical health) across both samples are presented in Table 1. Sex-specific descriptive statistics are also presented for both samples in the supplemental online documentation. For the MIDUS sample, physical health was measured using 24 biomarkers collected during the Biomarker Project of MIDUS II and tapping the following seven biological systems: 1) cardiovascular functioning (e.g., resting heart rate); 2) glucose consumption (e.g., blood glucose); 3) lipid metabolism (e.g., low density lipoprotein cholesterol levels); 4) inflammation (e.g., C-reactive protein); 5) hypothalamic-pituitary-adrenal axis activity (e.g., urinary cortisol); 6) sympathetic nervous system (e.g., urinary epinephrine); and 7) parasympathetic nervous system (e.g., high-frequency heart rate variability). A complete list of all included biomarkers (for both samples) is included in the online supplement. The biomarkers tapping each of these seven biological systems were combined using a two-step measurement strategy used previously (Gruenewald et al., 2012; Schwartz, 2017) to create an allostatic load index tapping the accumulated physiological wear and tear following prolonged exposure to environmental stressors. First, all 24 biomarkers were recoded into quartiles and dummy indicator variables were used to identify participants that fell within the highest risk quartile. Second, these indicators were then averaged within each system and then summed to reflect an overall measure of allostatic load. Since only a subset of the twin subsample participated in the Biomarker Project, the resulting sample size for models examining the physical health measure was reduced (n = 290 [145 pairs]).
Table 1.
Descriptive Statistics for both Study Samples
| Measures | MIDUS | Add Health |
|---|---|---|
| Outcomes | ||
| Physical Health Problems (mean[SD]) | 1.65[1.07] | .92[.71] |
| Depressive Symptoms (mean[SD]) | .45[1.53] | 5.24[5.11] |
| Educational Attainment (%) | ||
| At least High School | 93.48 | 91.10 |
| Less than High School | 6.52 | 8.90 |
| Income Attainment (%) | ||
| At or above National Poverty Line | 59.13 | 82.26 |
| Below National Poverty Line | 40.87 | 17.74 |
| Alcohol Problems (mean[SD]) | .30[.71] | .84[1.48] |
| Antisocial Behavior (mean[SD]) | 5.31[1.83] | .29[.85] |
| Adverse Childhood Experiences | ||
| Adverse Childhood Experiences Scale (mean[SD]) | .02[.33] | .06[.69] |
| Statistical Covariates | ||
| Physical Activity in the Past Week (%) | ||
| At least five weekly activities | -- | 52.67 |
| Less than five weekly activities | -- | 47.33 |
| Fast Food Consumption in Past Week (mean[SD]) | -- | 2.39[2.70] |
| Diet to Control Weight (mean[SD]) | 1.52[1.08] | -- |
| Smoked Cigarettes in the Past Week (%) | ||
| Yes | 14.04 | 36.25 |
| No | 85.96 | 63.75 |
| Daily Stressful Experiences (mean[SD]) | 1.06[1.22] | 4.82[2.96] |
| Verbal Ability (mean[SD]) | 18.17[5.96] | 99.34[15.80] |
| Age (mean[SD]) | 54.20[11.74] | 16.08[1.73] |
| Sex (%) | ||
| Male | 42.69 | 50.00 |
| Female | 57.31 | 50.00 |
| Race (%) | ||
| Caucasian | 94.78 | 63.21 |
| All other Races | 5.22 | 36.79 |
| N | 862 | 3,112 |
For the Add Health sample, 11 biomarkers tapping cardiometabolic risk collected from all participants during Wave IV interviews and were used to assess overall physical health. The 11 biomarkers tapped functioning in the following four biological systems: 1) cardiovascular functioning (e.g., pulse pressure); 2) glucose metabolism (e.g., blood glucose); 3) lipid metabolism (e.g., high density lipoprotein cholesterol levels); and 4) inflammation (e.g., C-reactive protein). A complete list of the biomarkers can be found in the online supplement. The same two-step measurement strategy employed for the MIDUS sample was also used for the Add Health sample (Hatzenbuehler et al., 2014; Wickrama et al., 2015).
Depressive Symptoms.
For the MIDUS sample, depressive symptoms were measured during the MIDUS II interview using the Screening Version of the World Health Organization Composite International Diagnostic Interview (CIDI; Kessler et al., 1998). The CIDI is a validated self-reported scale comprised of seven items asking participants to report whether they experienced each of the seven items (e.g., felt sad, blue, or depressed) all day or most of the day nearly every day for two weeks at any time during the past year. The resulting items were coded dichotomously (0 = no and 1 = yes) and then summed to reflect overall depressive symptoms. For the Add Health sample, depressive symptoms were assessed during Wave IV using a 10-item version of the Center for Epidemiologic Studies Depression (CESD) Scale (Radloff, 1977). Participants were asked to report the frequency in which they experienced each of the 10 items over the past seven days (e.g., couldn’t shake off the blues) with response categories ranging between 0 (never or rarely) and 3 (most of the time or all of the time), which were then summed to create an overall index of depressive symptoms.
Educational Attainment.
For the MIDUS sample, educational attainment was assessed using a single, self-reported item from the MIDUS II interview in which participants were asked to indicate the highest level of education they had completed, with responses dichotomized (0 = less than high school and 1 = high school or greater). For the Add Health sample, educational attainment was also measured using a single self-reported item from Wave IV in which participants were asked to indicate their highest level of educational attainment. Responses were coded similar to the educational attainment measure for the MIDUS sample.
Income Attainment.
For the MIDUS sample, income attainment was measured using a single item from the MIDUS II interview in which participants were asked to report their total household income from the past calendar year. Responses were coded to reflect whether answers fell above or below the Federal Poverty Line for a family of four in 2005 (the mid-point of data collection for MIDUS II) and coded such that 0 = $19,999 or less and 1 = $20,000 or more. For the Add Health sample, income attainment was measured similarly, with a single self-reported measure of total household income from the previous calendar year collected during Wave IV interviews. Directly in line with the Federal Poverty Line for a family of four during 2008 (the year in which Wave IV of data collection was carried out), the final income measure was coded such that 0 = $24,999 or less and 1 = $25,000 or more.
Alcohol Use Problems.
For both samples, alcohol use problems were measured using a six-item version of the Alcohol Screening Test (Selzer, 1971). Participants were asked to indicate whether they had experienced each item related to alcohol use within the past 12 months (e.g., did you have a strong desire or urge to use alcohol that you could not resist or could not think of anything else?). Responses were coded dichotomously (0 = no and 1 = yes) and summed. The Alcohol Screening Test was administered during the MIDUS II interviews and the Wave IV interview for the Add Health sample.
Antisocial Behavior.
For the MIDUS sample, antisocial behavior was assessed using the aggression scale of the Multidimensional Personality Questionnaire (MPQ; Patrick et al., 2002). The aggression scale asks participants to indicate how well each of four items reflects their feelings (e.g., when I get angry I am often ready to hit someone), with responses ranging between 1 (false) and 4 (true of you), which were then summed to reflect overall levels of aggression. For the Add Health, an 11-item variety index of self-reported criminal acts over the past 12 months was used. The items included both violent (e.g., taking part in a physical fight, using a weapon to get something from another person, hurting someone bad enough to need physical care) and nonviolent (e.g., damaging property, entering a house to steal something, buying or selling stolen property) behaviors, which were coded dichotomously (0 = no and 1 = yes), and then summed to reflect the number of offenses committed over the past 12 months.
Adverse Childhood Experiences (ACEs)
More detailed information on the ACEs measures for both the MIDUS and Add Health samples, including the coding, timing of measurement, and specific wording of each item, is included in the online supplement. For the MIDUS sample, ACEs was measured using 17 retrospective indicators from both the MIDUS I and II interviews, which tapped three domains: 1) stressful events during childhood (nine items; e.g., parental alcohol or drug problems, low household income); 2) relationships with parents while growing up (two items; self-reported relationship with mother and father); and 3) abuse in childhood (six items; maternal and paternal items from the conflict tactics scale [CTS]). Following the lead of previous studies analyzing the MIDUS sample (Slopen et al., 2010), the stressful events during childhood items were coded dichotomously, while the items from the other domains were coded categorically on a Likert scale (see online supplement for the specific coding of each individual item). Due to these differences in response categories, confirmatory factor analysis (CFA) was used to examine the factor structure of the examined items and construct a factor score tapping the underlying latent construct of ACEs. A single factor solution provided an acceptable fit to the data (comparative fit index [CFI] = .92; Tucker-Lewis Index [TLI] = .91; root mean square error of approximation [RMSEA] = .07) and factor scores from the CFA were extracted to create the ACEs measure, with greater values indicating greater exposure to ACEs.
For the Add Health, ACEs were measured following a previously developed procedure (Brumley et al., 2017). The final measure was comprised of 11 retrospective measures from multiple raters collected across Waves I, III, and IV tapping the same three domains as the ACEs measure from the MIDUS sample (stressful life events during childhood, seven items, e.g., parental alcohol problems, family financial problems; relationships with parents, one item, parental warmth composite score; abuse in childhood, three items, e.g., physical and sexual abuse in childhood). Following procedures outlined previously (and described in more detail in the online supplement), all 11 items were dichotomized (Brumley et al., 2017; Evans et al., 2013). The items were then used to create a Rasch scale, which weights individual items based on their overall prevalence in the analytic sample, more appropriately weighting rare events than a summative index (Osgood et al., 2002). The resulting ACEs scale was coded such that higher scores indicated greater exposure to ACEs.
Statistical Covariates
Due to the broad range of examined outcomes examined, a diverse and comprehensive set of control variables were included in the estimated models. Correlation matrices containing all study measures for both samples are presented in the accompanying supplemental material. For the MIDUS sample, a total of seven covariates were included in the estimated models as control variables. First, two lifestyle measures tapping the frequency in which each participant had used a diet to control their weight over the past 12 months (with responses ranging between 1 [never] and 5 [a lot]) and a self-reported measure of the regular use of cigarettes currently (coded 0 = no and 1 = yes). Second, daily stress was measured by asking participants to indicate whether seven stressful incidents had occurred in the past 24 hours (e.g., did you have an argument or disagreement with anyone since yesterday?). Each item was coded dichotomously (0 = no, 1 = yes) and then summed to indicate the number of stressful incidents experienced in the past 24 hours. Third, verbal ability was measured using the fluency score on the Brief Test of Adult Cognition by Telephone (BTACT; Tun & Lachman, 2006). Finally, three demographic covariates were also included in the estimated models: 1) age measured in years during MIDUS II interviews; 2) sex measured dichotomously (0 = female and 1 = male); and 3) race also measured dichotomously (0 = Caucasian and 1 = all other races).
The covariates included in models analyzing the Add Health sample were similar to those listed above with a few exceptions. In addition to a smoking indicator variable, participants were also asked how many times they ate fast food in the past week (this item replaces the dieting measure from the MIDUS), and how often they engaged in physical activity over the past week with the resulting measure dichotomized at the median and coded such that 0 = less than five times and 1 = five or more times. To assess everyday stressors, a four-item version of the Perceived Stress Scale (PSS; Cohen et al., 1983) was included. Verbal ability was assessed using a revised version of the Peabody Picture Vocabulary Test Revised (PPVT-R), which was administered during Wave III interviews. Finally, the same three demographic measures assessed in the MIDUS were also included.
PLAN OF ANALYSIS
All statistical models (aside from the unconditional models) included all covariates and were estimated in Mplus 7.4 (Muthén & Muthén, 2012) using full information maximum likelihood (FIML) to handle missing values. Models for continuous outcomes (physical health and depressive symptoms in both samples; antisocial behavior in the MIDUS sample) were estimated using linear regression, models for dichotomous outcomes (educational attainment and income attainment in both samples) were estimated using logistic regression, and models for overdispersed count outcomes (alcohol problems in both models and antisocial behavior in the Add Health) were estimated using negative binomial regression. To account for the nested nature of the data (i.e., twin and/or sibling pairs nested within families), multilevel models were estimated with robust standard errors. The first set of models were unconditional models aimed at examining the extent to which the examined ACEs and outcome measures varied within and between families from both samples. The second set of models examined the baseline association between ACEs and the examined outcomes after controlling for the included covariates. The third set of models involved the estimation of sibling comparison models, in which exposure to ACEs for siblings from the same dyad are compared to model the extent to which differences in exposure results in differences in the examined outcomes. This modeling approach is highly conservative, as the resulting coefficients are adjusted for all factors shared by siblings from the same household including genetic factors and additional unmeasured family-level covariates (i.e., within-family influences; D’Onofrio et al., 2013; Turkheimer & Harden, 2014). The fourth set of models examine the extent to which genetic factors and unmeasured familial influences contributed to the associations examined in the sibling comparison models estimated in the previous step (Turkheimer & Harden, 2014).
RESULTS
Prior to estimating the sibling comparison models, it was first necessary to examine the extent to which the employed ACEs measures and the outcome measures vary within and between families. Unconditional multilevel models were estimated and the intraclass correlation coefficient (ICC) was calculated to reflect the proportion of variance in the examined measure that varied between families, with the residual variance explained by within-family influences. For the MIDUS sample, the unconditional model examining ACEs measure had a corresponding ICC of .60 (95% CI = .54-.66), indicating that 60% of the variance in ACEs was explained by factors that vary between families and the remaining 40% of the variance was explained by within family factors. For all of the examined outcomes, the corresponding ICC ranged between .44 (95% CI = .29-.61, income attainment) and .29 (95% CI = .22-.39, depressive symptoms; and 95% CI = .20-.41, alcohol use problems), indicating that the majority of the variance in each of the outcomes was explained by within-family influences. A similar pattern of findings was present for the Add Health sample, with only two minor exceptions. First, a majority of the variance in the ACEs measure was explained by within family influences (ICC = .44, 95% CI = .41-.49). Second, the majority of the variance in the educational attainment measure was explained by between-family influences (ICC = .61, 95% CI = .46-.74). The ICC for the remaining outcome measures ranged between .46 (95% CI = .35-.57, income attainment) and .20 (95% CI = .15-.26, alcohol use problems; and 95% CI = .15-.27, antisocial behavior). Collectively, the results of the unconditional models for both samples revealed the examined ACEs measures, as well as the outcomes, vary substantively within and between families.
The results from the baseline multilevel models are presented in Table 2. For the MIDUS sample, increased exposure to ACEs resulted in significantly greater levels of depressive symptoms (b = .47, p = .005), a lower likelihood of obtaining a high school diploma (b = −1.99, p < .001), a greater prevalence of alcohol problems (b = .61, p = .038), and greater overall levels of antisocial behavior (b = 1.08, p < .001) in adulthood. However, greater exposure to ACEs was not significantly associated with physical health problems (b = −.02, p = .895) or income attainment (b = .55, p = .115). For the Add Health sample, greater exposure to ACEs was associated with more physical health problems (b = .11, p < .001), increased levels of depressive symptoms (b = .38, p < .001), a decreased likelihood of obtaining a high school diploma (b = −.71, p < .001), and increased levels of antisocial behavior (b = .46, p < .001) in adulthood.
Table 2.
Results from Baseline Multilevel Models
| Physical Health Problemsa | Depressive Symptomsa | Educational Attainmentb | Income Attainmentb | Alcohol Problemsc | Antisocial Behaviord | |
|---|---|---|---|---|---|---|
| MIDUS ACEs | −.02 | .47** | −1.99** | .55 | .61* | 1.08** |
| (−.38 to.33) | (.15 to .79) | (−2.99 to −.99) | (−.14 to 1.24) | (.03 to 1.19) | (.62 to 1.54) | |
| Add Health ACEs | .11** | .38** | −.71** | −.56** | .03 | .46** |
| (.05 to .17) | (.17 to .59) | (−.93 to −.50) | (−.75 to −.36) | (−.09 to .14) | (.31 to .62) |
Note: All models account for nested family structure of both samples.
Unstandardized regression coefficients presented with accompanying 95% confidence intervals presented in parentheses.
Models estimated using the MIDUS data include the following covariates: diet to control weight; smoked cigarettes in the past week; daily stressful experiences; verbal ability (BTACT); age; sex; and race. Models estimated using the Add Health data include the following covariates: physical activity in the past week; fast food consumption in the past week; smoked cigarettes in the past week; daily stressful experiences (perceived stress scale); verbal ability (Picture Vocabulary Test); age; sex; and race.
Missing values were handled using full information maximum likelihood (FIML) estimation for all estimated multivariate models.
Model estimated using a multilevel linear regression model.
Model estimated using a multilevel logistic regression model.
Model estimated using multilevel negative binomial regression model.
Model estimated using a multilevel linear regression model for the MIDUS sample and a multilevel negative binomial model for the Add Health sample.
p < .05;
p < .001
The results for the sibling comparison models are presented in Table 3, with the resulting associations parsed into between- and within-family effects. The former reflect average familial levels of ACEs on the examined outcome and the latter reflect the effect of differential exposure to ACEs between siblings from the same family on the outcomes. The within-family effects are expected to reflect a casual effect, as they are adjusted for all covariance between siblings from the same family (and all covariates). For the MIDUS sample, twins exposed to greater levels of ACEs were more likely to engage in antisocial behavior (b = 1.29, p = .001) and had fewer physical health problems (b = −.56, p < .001) compared to their co-twins. The latter finding is not in the expected direction and is likely a result of limited statistical power (n = 290) for the MIDUS biomarker twin subsample. For the Add Health sample, siblings with greater exposure to ACEs experienced greater levels of depressive symptoms (b = .53, p = .003). The results from both samples indicate that factors other than those captured in the employed ACEs measures (e.g., genetic influences or unmeasured family-level influences) are implicated in the associations observed in the baseline models.
Table 3.
Results from Sibling-Comparison Models
| Physical Health Problemsa | Depressive Symptomsa | Educational Attainmentb | Income Attainmentb | Alcohol Problemsc | Antisocial Behaviord | |
|---|---|---|---|---|---|---|
| MIDUS ACEs | ||||||
| Between-Family Effect | .23 | .45* | −1.88** | .45 | .60 | 1.00** |
| (−.26 to .72) | (.07 to .83) | (−2.77 to −.99) | (−.31 to 1.21) | (−.31 to 1.31) | (.44 to 1.55) | |
| Within-Family Effect | −.56* | .51 | −1.55 | .96 | .81 | 1.29** |
| (−1.09 to −.03) | (−.08 to 1.10) | (−3.99 to .46) | (−.64 to 2.55) | (−.29 to 1.92) | (.53 to 2.06) | |
| Add Health ACEs | ||||||
| Between-Family Effect | .14** | .30* | −.90** | −.73** | .01 | .63** |
| (.07 to .22) | (.05 to .55) | (−1.15 to −.65) | (−.97 to −.48) | (−.06 to .08) | (.42 to .83) | |
| Within-Family Effect | .06 | .53* | −.29 | −.31 | .10 | .16 |
| (−.04 to .16) | (.18 to .88) | (−.64 to .07) | (−.64 to .02) | (−.09 to .28) | (−.12 to .45) |
Note: All models account for nested family structure of both samples.
Unstandardized regression coefficients presented with accompanying 95% confidence intervals presented in parentheses.
Models estimated using the MIDUS data include the following covariates: diet to control weight; smoked cigarettes in the past week; daily stressful experiences; verbal ability (BTACT); age; sex; and race. Models estimated using the Add Health data include the following covariates: physical activity in the past week; fast food consumption in the past week; smoked cigarettes in the past week; daily stressful experiences (perceived stress scale); verbal ability (Picture Vocabulary Test); age; sex; and race.
Missing values were handled using full information maximum likelihood (FIML) estimation for all estimated multivariate models.
Between-family effects provide an estimation of the association between adverse childhood experiences (ACEs) and each outcome across families. Within-family effects reflect differences in the same association between siblings from the same family.
Model estimated using a multilevel linear regression model.
Model estimated using a multilevel logistic regression model.
Model estimated using multilevel negative binomial regression model.
Model estimated using a multilevel linear regression model for the MIDUS sample and a multilevel negative binomial model for the Add Health sample.
p < .05;
p < .001
In order to more closely examine this possibility, two additional sets of sibling comparison models were estimated, with the results presented in Table 4. The first set of models included an interaction term between the within-family effect and genetic relatedness (i.e., zygosity) for each examined twin or sibling pair. A significant interaction term would indicate that the examined within-family effect varies across levels of genetic relatedness, providing evidence that genetic influences may account for discrepancies between within- and between-family effects. Alternatively, a nonsignificant interaction term would provide evidence indicating that the observed discrepancies are the result of additional unmeasured family-level influences that are not genetic in origin (Turkheimer & Harden, 2014). As can be seen from the results from the MIDUS sample, only the interaction term examining depressive symptoms was significant (b, −1.30, p = .028), indicating that the observed differences in between- and within-family effects are due to genetic influences. However, no other interaction terms were significant for either sample, indicating that the associations observed in the baseline models are likely driven by additional, latent sources of family-based environmental (as opposed to genetic) influences. The second set of sibling comparison models were aimed at examining the extent to which the estimated within-family effects varied across gender and included an interaction term between the estimated within-family effect and gender. The results are presented in the online supplemental material and failed to reveal any significant moderating effects, indicating that the estimated within-family effects do not systematically vary across gender.
Table 4.
Results from Sibling-Comparison Models Testing for Genetic Confounding
| Physical Health Problemsa | Depressive Symptomsa | Educational Attainmentb | Income Attainmentb | Alcohol Problemsc | Antisocial Behaviord | |
|---|---|---|---|---|---|---|
| MIDUS | ||||||
| Main Effects | ||||||
| Between-Family Effect | .23 | .44* | −2.01** | .43 | .60 | 1.00** |
| (−.24 to .71) | (.06 to .83) | (−2.78 to −1.24) | (−.33 to 1.19) | (−.12 to 1.32) | (.45 to 1.55) | |
| Within-Family Effect | .17 | 2.50* | −2.53 | .77 | 1.27 | −.58 |
| (−1.39 to 1.73) | (.59 to 4.41) | (−10.16 to 5.11) | (−3.95 to 5.49) | (−1.91 to 4.46) | (−2.84 to 1.68) | |
| Zygosity | .29* | −.14 | −.89** | −.32 | −.25 | .05 |
| (.03 to .56) | (−.36 to .08) | (−1.12 to −.65) | (−.96 to .32) | (−.89 to .39) | (−.24 to .33) | |
| Interaction Effects | ||||||
| Zygosity × Within-Family Effect | −.53 | −1.30* | .45 | .14 | −.28 | 1.21 |
| (−1.69 to .62) | (−2.46 to −.14) | (−3.93 to 4.84) | (−3.01 to 3.28) | (−2.48 to 1.91) | (−.26 to 2.69) | |
| Add Health | ||||||
| Main Effects | ||||||
| Between-Family Effect | .13** | .36* | −.88** | −.67** | −.01 | .61** |
| (.06 to .21) | (.10 to .62) | (−1.14 to −.61) | (−.92 to −.43) | (−.09 to .07) | (.41 to .81) | |
| Within-Family Effect | .10 | .38 | −.35 | −.09 | .11 | −.29 |
| (−.13 to .33) | (−.43 to 1.19) | (−1.07 to .38) | (−.84 to .67) | (−.33 to .55) | (−.91 to .33) | |
| Zygosity | .03 | −.17* | −.11 | −.16* | .04 | .02 |
| (−.01 to .07) | (−.29 to −.04) | (−.20 to −.01) | (−.29 −.03) | (.00 to .07) | (−.12 to .16) | |
| Interaction Effects | ||||||
| Zygosity × Within-Family Effect | −.02 | .06 | .03 | −.08 | −.01 | .17 |
| (−.11 to .08) | (−.24 to .36) | (−.92 to −.43) | (−.33 to .18) | (−.16 to .15) | (−.07 to .40) |
Note: All models account for nested family structure of both samples.
Unstandardized regression coefficients presented with accompanying 95% confidence intervals presented in parentheses.
Models estimated using the MIDUS data include the following covariates: diet to control weight; smoked cigarettes in the past week; daily stressful experiences; verbal ability (BTACT); age; sex; and race. Models estimated using the Add Health data include the following covariates: physical activity in the past week; fast food consumption in the past week; smoked cigarettes in the past week; daily stressful experiences (perceived stress scale); verbal ability (Picture Vocabulary Test); age; sex; and race.
Missing values were handled using full information maximum likelihood (FIML) estimation for all estimated multivariate models.
Between-family effects provide an estimation of the association between adverse childhood experiences (ACEs) and each outcome across families. Within-family effects reflect differences in the same association between siblings from the same family.
A significant multiplicative interaction term between zygosity and the within-family effect would provide evidence of genetic confounding, while a nonsignificant interaction term would provide evidence of familial confounding.
Model estimated using a multilevel linear regression model.
Model estimated using a multilevel logistic regression model.
Model estimated using multilevel negative binomial regression model.
Model estimated using a multilevel linear regression model for the MIDUS sample and a multilevel negative binomial model for the Add Health sample.
p < .05;
p < .001
DISCUSSION
Childhood adversity has occupied a significant portion of the developmental literature with previous studies linking greater exposure to adversity in childhood to a wide range of negative outcomes later in life (Anda et al., 2006; Chapman et al., 2004; Danese & McEwen, 2012; Duke et al., 2010; Egan et al., 2015; Koss & Gunnar, 2018; Lindert et al., 2014; Whitfield et al., 2003). More recently, studies have acknowledged the importance of examining a broader set of within-family experiences when examining the association between childhood adversity and deleterious outcomes, with such studies pointing to additional sources of both environmental (Finkelhor et al., 2013; 2015; Cronholm et al., 2015; Wade et al., 2016) and genetic (Finkelhor et al., 2013; McCrory et al., 2010) influences. Directly in line with these observations, previous studies have examined the association between various forms of childhood adversity and developmental outcomes while controlling for latent sources of within-family influence. The results of these studies have been decidedly mixed, with some studies reporting significant associations (Alemany et al., 2013; Brown et al., 2007; Kendler et al., 2000; Nelson et al., 2002) and others reporting nonsignificant results (Forsman & Långström, 2012; Laporte et al., 2011; Young-Wolff et al., 2011). The current study contributed to this literature by examining the associations between a broader measure of adversity—ACEs—and a wide range of outcomes in two complementary but independent samples, while taking into account latent sources of within-family influences. The longitudinal analyses revealed two findings.
First, the results of the baseline models largely replicated previous studies and revealed that greater exposure to ACEs resulted in a significant increase in negative developmental outcomes during adulthood. The results of the sibling comparison models, however, revealed that the majority of the significant associations observed in the baseline models were confounded by latent sources of within-family influences, with only the association between ACEs and physical health remaining significant in the MIDUS sample and the association between ACEs and depressive symptoms remaining significant in the Add Health sample. To be clear, these findings do not indicate that ACEs have no meaningful impact on the examined outcomes. Rather, the between-family effects from the sibling comparison models indicate that children from families that are differentially exposed to ACEs are significantly more likely to experience deleterious outcomes in adulthood, pointing to ACEs as an important marker of risk. However, these findings are tempered by the within-family effects which compare siblings from the same family and reveal that siblings exposed to a greater level of ACEs are not consistently more likely to experience deleterious outcomes relative to their co-sibling. These results indicate that additional, latent sources of either genetic or environmental influence that cluster within families with greater levels of ACEs are largely responsible for the observed between-family effects. While similar findings have been reported by a handful of studies employing similar analytic strategies to examine more restrictive measures of adversity and a narrower set of outcomes (Forsman & Långström, 2012; Laporte et al., 2011; Young-Wolff et al., 2011), such studies have not attempted to disentangle shared genetic and environmental sources of influence in an effort to better understand the underlying factors contributing to this pattern of results.
The second finding from the current study was aimed at unpacking the disparity in within- and between-family effects observed in the sibling comparison models. The results of additional sibling comparison models examining whether the observed within-family effects are significantly moderated by genetic relatedness revealed that the vast majority of the discrepancies between within- and between-family effects are the result of environmental (as opposed to genetic) influences not captured by the employed ACEs measures that cluster within families. While these findings align with recent calls to expand measures of ACEs to include additional domains of adversity (Finkelhor et al., 2013; 2015; Cronholm et al., 2015; Wade et al., 2016), they also directly align with previous studies noting the comorbidity between various forms of adversity (Dong et al., 2003) ultimately culminating into an “adversity package” (Jirapramukpitak et al., 2011; Rossman, 2000). This pattern of findings calls into question the strategy of continuously expanding the number of domains captured in measures of ACEs, as it remains likely that, regardless of how expansive such measures are, additional latent sources of meaningful environmental influence will remain omitted. While it may not be feasible to exhaustively measure childhood adversity, the continued refinement of measurement strategies aimed at balancing comprehension and parsimony would be beneficial.
The only exception to this pattern of results was for the association involving depressive symptoms in the MIDUS sample, which revealed that genetic influences contributed to observed differences between within- and between-family effects. This finding aligns with observations from previous research highlighting the importance of considering genetic influences when examining the association between childhood adversity and deleterious outcomes (Finkelhor et al., 2013; McCrory et al., 2010). These observations also further underscore recent calls for the increased use of genetically informed research strategies when examining broader associations in the behavioral sciences (D’Onofrio et al., 2013; Johnson et al., 2009), pointing to the importance of an increased use of such methodologies in future research.
Despite these contributions, the current study is not without limitation, with at least three limitations worth noting. First, it remains unclear as to whether the findings observed in the current study will generalize to larger populations. While both the MIDUS and Add Health are comprised of nationally representative samples, the twin and sibling subsamples of each are not necessarily representative of the population of the United States, pointing to the need for additional research employing samples that more effectively generalize to larger populations. Second, the biomarker twin sample from the MIDUS is relatively underpowered (n = 290), potentially contributing to the null results observed in the majority of the estimated models. Third, and finally, the majority of the items used to construct the ACEs measures employed in the current study relied on retrospective reports. The results of a recent study note that the use of retrospective, compared to prospective, measures may increase Type II error (particularly when paired with subjective outcome measures) while also noting systematic differences in reporting across various personality traits (Reuben et al., 2016). In this way, the findings from the current study would be bolstered if replicated with prospective measures of adversity.
While families that experienced greater overall levels of ACEs also experienced a greater concentration of deleterious outcomes, these associations appear to be primarily driven by additional latent sources of environmental influence clustered within families that experience greater overall levels of adversity. These findings underscore the importance of considering the adversity package that accompanies specific forms of adversity experienced early in the life course. Future research aimed at a continued refinement of measurement strategies surrounding ACEs with an eye toward balancing the competing objectives of comprehension and parsimony would also contribute to a better understanding of the association between early life adversity and various developmental outcomes. The results of the current study also point to the importance of the continued consideration of latent sources of genetic and environmental influence that systematically cluster within families when examining the intergenerational transmission of phenotypes. Continued research in this area will be useful in identifying, and prioritizing, those sources of childhood adversity that are most consistently linked with subsequent negative outcomes.
Supplementary Material
Acknowledgments
The MIDUS 1 study (Midlife in the U.S.) was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development. The MIDUS 2 research was supported by a grant from the National Institute on Aging (P01-AG020166) to conduct a longitudinal follow-up of the MIDUS 1 investigation. The research was further supported by the following grants M01-RR023942 (Georgetown), M01-RR00865 (UCLA) from the General Clinical Research Centers Program and UL1TR000427 (UW) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health. This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
References
- Alemany S, Goldberg X, van Winkel R, Gastó C, Peralta V, & Fananas L (2013). Childhood adversity and psychosis: Examining whether the association is due to genetic confounding using a monozygotic twin differences approach. European Psychiatry, 28, 207–212. [DOI] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield CH, Perry BD, … & Giles WH, (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256, 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Craig TK, Harris TO, Handley RV, & Harvey AL (2007). Validity of retrospective measures of early maltreatment and depressive episodes using the Childhood Experience of Care and Abuse (CECA) instrument—A life-course study of adult chronic depression—2. Journal of Affective Disorders, 103, 217–224. [DOI] [PubMed] [Google Scholar]
- Brumley LD, Jaffee SR, & Brumley BP (2017). Pathways from childhood adversity to problem behaviors in young adulthood: The mediating role of adolescents’ future expectations. Journal of Youth and Adolescence, 46, 1–14. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, … & Poulton R (2002). Role of genotype in the cycle of violence in maltreated children. Science, 297, 851–854. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, & Anda RF (2004). Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders, 82, 217–225. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, and Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 386–396. [PubMed] [Google Scholar]
- Cronholm PF, Forke CM, Wade R, Bair-Merritt MH, Davis M, Harkins-Schwarz M, … & Fein JA (2015). Adverse childhood experiences: expanding the concept of adversity. American Journal of Preventive Medicine, 49, 354–361. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, & Lichtenstein P (2013). Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. American Journal of Public Health, 103, S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, & McEwen BS (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior, 106, 29–39. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, … & Caspi A (2009). Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of Pediatrics & Adolescent Medicine, 163, 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Anda RF, Dube SR, Giles WH, & Felitti VJ (2003). The relationship of exposure to childhood sexual abuse to other forms of abuse, neglect, and household dysfunction during childhood. Child abuse & Neglect, 27, 625–639. [DOI] [PubMed] [Google Scholar]
- Duke NN, Pettingell SL, McMorris BJ, & Borowsky IW (2010). Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics, 125, e778–e786. [DOI] [PubMed] [Google Scholar]
- Egan M, Daly M, & Delaney L (2015). Childhood psychological distress and youth unemployment: Evidence from two British cohort studies. Social Science & Medicine, 124, 11–17. [DOI] [PubMed] [Google Scholar]
- Entzel P, Whitsel EA, Richardson A, Tabor J, Hallquist S, Hussey J, Halpern CT, Harris KM (2009). Add Health Wave IV documentation: Cardiovascular and anthropometric measures. UNC Chapel Hill: Carolina Population Center; Available at http://www.cpc.unc.edu/projects/addhealth/data/guides/Wave%20IV%20cardiovascular%20and%20anthropometric%20documentation%20110209.pdf. [Google Scholar]
- Evans GW, Li D, & Whipple SS (2013). Cumulative risk and child development. Psychological Bulletin, 139, 1342–1396. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … & Marks JS (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. American Journal of Preventive Medicine, 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Shattuck A, Turner H, & Hamby S (2013). Improving the adverse childhood experiences study scale. JAMA Pediatrics, 167, 70–75. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Shattuck A, Turner H, & Hamby S (2015). A revised inventory of adverse childhood experiences. Child Abuse & Neglect, 48, 13–21. [DOI] [PubMed] [Google Scholar]
- Forsman M, & Långström N (2012). Child maltreatment and adult violent offending: Population-based twin study addressing the ‘cycle of violence’ hypothesis. Psychological Medicine, 42, 1977–1983. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B & Seeman TE (2012). History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine, 74, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM (2013). The Add Health study: design and accomplishments. Chapel Hill, NC: Carolina Population Center, University of North Carolina at Chapel Hill. [Google Scholar]
- Harris KM, Halpern CT, Smolen A, & Haberstick BC (2006). The national longitudinal study of adolescent health (Add Health) twin data. Twin Research and Human Genetics, 9, 988–997. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Slopen N, & McLaughlin KA (2014). Stressful life events, sexual orientation, and cardiometabolic risk among young adults in the United States. Health Psychology, 33, 1185–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman DB, Susser ES, Struening EL, & Link BL (1997). Adverse childhood experiences: Are they risk factors for adult homelessness? American Journal of Public Health, 87, 249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirapramukpitak T, Harpham T, & Prince M (2011). Family violence and its ‘adversity package’: A community survey of family violence and adverse mental outcomes among young people. Social Psychiatry and Psychiatric Epidemiology, 46, 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Turkheimer E, Gottesman II, & Bouchard TJ Jr (2009). Beyond heritability: Twin studies in behavioral research. Current Directions in Psychological Science, 18, 217–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Bulik CM, Silberg J, Hettema JM, Myers J, & Prescott CA (2000). Childhood sexual abuse and adult psychiatric and substance use disorders in women: An epidemiological and cotwin control analysis. Archives of General Psychiatry, 57, 953–959. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews A, Mroczek D, Ustun B, & Wittchen HU (1998). The World Health Organization Composite International Diagnostic Interview Short-Form (CIDI-SF). International Journal of Methods in Psychiatric Research, 7, 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, & Moffitt TE (2006). MAOA, maltreatment, and gene–environment interaction predicting children’s mental health: new evidence and a meta-analysis. Molecular Psychiatry, 11, 903–913. [DOI] [PubMed] [Google Scholar]
- Koss KJ, & Gunnar MR (2017). Annual Research Review: Early adversity, the hypothalamic–pituitary–adrenocortical axis, and child psychopathology. Journal of Child Psychology and Psychiatry, 59, 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte L, Paris J, Guttman H, & Russell J (2011). Psychopathology, childhood trauma, and personality traits in patients with borderline personality disorder and their sisters. Journal of Personality Disorders, 25, 448–462. [DOI] [PubMed] [Google Scholar]
- Larsson H, Viding E, Rijsdijk FV, & Plomin R (2008). Relationships between parental negativity and childhood antisocial behavior over time: A bidirectional effects model in a longitudinal genetically informative design. Journal of Abnormal Child Psychology, 36, 633–645. [DOI] [PubMed] [Google Scholar]
- Liu Y, Croft JB, Chapman DP, Perry GS, Greenlund KJ, Zhao G, & Edwards VJ (2013). Relationship between adverse childhood experiences and unemployment among adults from five US states. Social Psychiatry and Psychiatric Epidemiology, 48, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD, 2010. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. Journal of Aging and Health, 22, 1059–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén B,O (1998–2012). Mplus: Stataistical analysis with latent variables. user’s guide. (7th ed.). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nelson EC, Heath AC, Madden PA, Cooper ML, Dinwiddie SH, Bucholz KK, … & Martin NG (2002). Association between self-reported childhood sexual abuse and adverse psychosocial outcomes: Results from a twin study. Archives of General Psychiatry, 59, 139–145. [DOI] [PubMed] [Google Scholar]
- Osgood DW, McMorris BJ, & Potenza MT (2002). Analyzing multiple-item measures of crime and deviance I: Item response theory scaling. Journal of Quantitative Criminology, 18, 267–296. [Google Scholar]
- Patrick CJ, Curtin JJ, & Tellegen A (2002). Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychological Assessment, 14, 150–163. [DOI] [PubMed] [Google Scholar]
- Radler BT, Ryff CD (2010). Who participates? Accounting for longitudinal retention in the MIDUS national study of health and well-being. Journal of Aging and Health, 22, 307–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS 1977. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychology Measurement, 1, 385–401. [Google Scholar]
- Rossman BR (2000). Time heals all: How much and for whom? Journal of Emotional Abuse, 2, 31–50. [Google Scholar]
- Ryff C, Almeida D, Ayanian J, Binkley N, Carr D, & Williams D (2016). National Survey of Midlife Development in the United States (MIDUS 3), 2013–2014. Inter-university Consortium for Political and Social Research: Ann Arbor, MI. [Google Scholar]
- Sacks V, Murphey D, & Moore K (2014). Adverse childhood experiences: National and state-level prevalence. Publication #2014–18, Child Trends. [Google Scholar]
- Scarr S, & McCartney K (1983). How people make their own environments: A theory of genotype→ environment effects. Child Development, 54, 424–435. [DOI] [PubMed] [Google Scholar]
- Schwartz JA (2017). Long-term physical health consequences of perceived inequality: Results from a twin comparison design. Social Science & Medicine, 187, 184–192. [DOI] [PubMed] [Google Scholar]
- Selzer ML (1971). The Michigan Alcohol Screening Test: The quest for a new diagnostic instrument. American Journal of Psychiatry, 127, 89–94. [DOI] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine, 72, 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, & Lachman ME (2006). Telephone assessment of cognitive function in adulthood: The Brief Test of Adult Cognition by Telephone. Age and Ageing, 35, 629–632. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, & Harden KP (2014). Behavior genetic research methods: Testing quasi-causal hypotheses using multivariate twin data In Reis HT, & Judd CM (Eds.), Handbook of research methods in social and personality psychology. (pp. 159–187). Cambridge, MA: Cambridge University Press. [Google Scholar]
- U.S. Department of Health and Human Services. (2018). Child maltreatment 2016 Washington, DC: Children’s Bureau. [Google Scholar]
- Wade R, Cronholm PF, Fein JA, Forke CM, Davis MB, Harkins-Schwarz M, … & Bair-Merritt MH (2016). Household and community-level adverse childhood experiences and adult health outcomes in a diverse urban population. Child abuse & Neglect, 52, 135–145. [DOI] [PubMed] [Google Scholar]
- Whitfield CL, Anda RF, Dube SR, & Felitti VJ (2003). Violent childhood experiences and the risk of intimate partner violence in adults: Assessment in a large health maintenance organization. Journal of Interpersonal Violence, 18, 166–185. [Google Scholar]
- Wickrama KA, Lee TK, & O’Neal CW (2015). Stressful life experiences in adolescence and cardiometabolic risk factors in young adulthood. Journal of Adolescent Health, 56, 456–463. [DOI] [PubMed] [Google Scholar]
- Young-Wolff KC, Kendler KS, Ericson ML, & Prescott CA (2011). Accounting for the association between childhood maltreatment and alcohol-use disorders in males: a twin study. Psychological Medicine, 41, 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.