Abstract
A growing literature associates the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) with affiliative and cognitive outcomes. The majority of this work in humans, however, considers these neuropeptides separately. Also, despite evidence that OT and AVP interact with gonadal hormones, still warranted is an examination of sex and age variations in endogenous neuropeptide levels, their interrelations, and their functional relationships with attachment and cognition in humans. This study measured endogenous plasma OT and AVP levels in generally healthy young (18–31 years) and older (63–81 years) men and women to (i) determine levels of and interrelations between OT and AVP; (ii) explore functional relationships with self-reported attachment (attachment anxiety and avoidance) and performance-based cognition (processing speed, verbal memory); and (iii) identify variations in these effects by sex and age. We observed sex- and age-differential patterns of results: Women had higher plasma OT levels than men and older adults had higher plasma AVP levels than young adults. The two neuropeptides were highly negatively intercorrelated across all groups. Functionally, higher AVP levels were associated with greater attachment anxiety and higher OT and lower AVP levels were associated with faster sensorimotor processing speed, with sex and age moderating these effects. This integrated approach identifies variations in endogenous peripheral neuropeptide levels in humans, supporting their sex- and age-specific role as “difference makers” in attachment and cognition.
Keywords: Oxytocin, Vasopressin, Sex, Age, Attachment Anxiety, Processing Speed
Introduction
Oxytocin (OT) and arginine vasopressin (AVP) are neuropeptides with crucial regulatory functions in a wide spectrum of socioemotional and cognitive processes in humans and animals. These processes include social exploration and recognition (Lopatina, Komleva, Gorina, Higashida, & Salmina, 2018), attachment (C. Sue Carter, 2017b), and anxiety (Neumann & Slattery, 2016). In humans, a direct assessment of neuropeptide levels via brain neurochemistry is not generally possible. Therefore, research often relies on peripheral measurement of endogenous neuropeptide levels or exogenous (e.g., nasal spray) administration to tap into central functions (Grinevich, Knobloch-Bollmann, Eliava, Busnelli, & Chini, 2016). Although not conclusively demonstrated yet, there is growing evidence suggesting that the expression of peripheral and central neuropeptide levels are coordinated (Carson et al., 2014). Previous work mostly considered OT and AVP in isolation and typically examined the neurophysiological, cognitive, and social functions of these two neuropeptides separately. The present study, in contrast, took an integrated approach by determining endogenous OT and AVP levels in the periphery (plasma) and examining sex and age effects in neuropeptides levels, their interrelations, and their functional relationships with affiliative (attachment) and cognitive (processing speed, memory) processes in young and older men and women.
OT and AVP share a distant evolutionary ancestor and differ by only two amino acids. While often their actions have been contrasted, there is considerable evidence of functional overlap (C. Sue Carter, 2017a). OT has an affinity for AVP receptor 1A, the AVP receptor subtype found most commonly in the brain (C. Sue Carter, 2017a), and pre-clinical work suggests that AVP receptors may play a role in some of OT’s central effects (Song, Larkin, Malley, & Albers, 2014). In addition to their functional interplay, OT and AVP interact with other hormonal and neurotransmitter signaling systems (e.g., estrogen, testosterone, dopamine, serotonin; Ebner, Kamin, Diaz, Cohen, & MacDonald, 2014). These interactions are in part due to the coordination of various hormones and the autonomic nervous system by the paraventricular nucleus (Myers, Scheimann, Franco-Villanueva, & Herman, 2017). Awareness of complex interrelations between neuropeptides and other hormone and neurotransmitter systems has grown. Also, there is evidence of sex and age differences in these interacting systems (Ebner et al., 2015). This makes it even more surprising, that only a few studies to date have specifically addressed sex differences and even fewer have considered age-related changes in neuropeptide levels and function, particularly in humans. The present study set out to fill these research gaps.
The existing work on sex and age variations in OT and AVP level and function is not only scarce, but evidence is mixed and difficult to compare across studies (Ebner, Maura, Macdonald, Westberg, & Fischer, 2013). While some studies report comparable endogenous plasma OT levels among men and women (when not pregnant or breastfeeding) (Jokinen et al., 2012), a recent large study observed higher plasma OT levels in men than women (with no differences in OT levels for women breastfeeding or related to contraception intake or menstrual cycle; Weisman, Zagoory-Sharon, Schneiderman, Gordon, & Feldman, 2013).
Higher OT levels in young men were associated with lower trait anxiety and lower attachment anxiety (i.e., anxiolytic function of OT in young men) (Weisman et al., 2013). In contrast, in young women higher OT was correlated with greater attachment anxiety (i.e., anxiogenic function of OT in young women), and in young women extreme OT values were associated with high trait anxiety. Similar support for sex-differential functional relationships of OT comes from Taylor et al. (2010), who found that plasma OT correlated with relational distress in young women (not on birth control) but not in young men. Also, higher plasma OT levels were associated with parenting stress and attachment anxiety only among young women, including women who were breastfeeding (Feldman, Gordon, & Zagoory-Sharon, 2011).
In line with these sexually dimorphic findings pertaining to endogenous neuropeptide levels is evidence that intranasal OT administration in young men reduced amygdala reactivity to general emotional (Quintana et al., 2016) and affective threatening (Kirsch et al., 2005) stimuli, with decreased connectivity between amygdala and brainstem regions involved in fear manifestation (Kirsch et al., 2005). In young women, in contrast, intranasal OT administration increased amygdala reactivity to threatening stimuli (Lischke et al., 2012), but decreased amygdala reactivity to infant crying (Riem et al., 2011). However, the evidence regarding sex differences in amygdala response to OT administration remains mixed (Domes et al., 2010), with other studies reporting increased amygdala reactivity in both males and females after OT administration (Koch et al., 2016). Similarly, there is evidence suggesting sexually dimorphic effects of AVP on emotion and social cognition (de Vries, 2008). For instance, higher endogenous AVP levels were associated with an increase in traits related to negative affect in young men, but not in young women (Taylor et al., 2010).
In addition to evidence of sex variations in OT and AVP, there have been calls for examination of possible age effects in neuropeptide level and function in humans. These proposals were based on psychosocial (e.g., change in social networks; Carstensen, 2006) and biochemical changes with age, including reduced secretion from peripheral glands, dampening of circadian rhythms, and modifications in the central mechanisms controlling hormone release (Conrad & Bimonte-Nelson, 2010).
The literature regarding age effects in endogenous OT and AVP levels is currently small, with some indication that older compared to young adults have more constant levels of AVP-producing neurons throughout a 24-hour cycle (Sannino, Chini, & Grinevich, 2017). However, another study suggested that AVP production was decreased during the day and increased at night in older adults (50–91 years), with a reversed pattern in adults under age 50 (Hofman & Swaab, 1994). Furthermore, an analysis of AVP neuron activity in humans aged 10 to 93 years showed an age-related decrease in AVP peptide production until age 60 followed by a steady increase in AVP peptide production after age 80 (Fliers, Swaab, Pool, & Verwer, 1985).
Existing intranasal OT administration studies are few in number, but generally support the notion that age may play a role in OT level and function, and specifically may be a factor supporting age-by-sex interactions. For example, a single-dose administration of OT relative to placebo improved emotion recognition in older men, while there were no effects in older women or young adults (Campbell, Ruffman, Murray, & Glue, 2014). Similarly, OT administration enhanced attention to feelings in older men and also in young women (Ebner et al., 2015) and increased resting-state connectivity between amygdala and medial prefrontal cortex in young women and with a similar trend in older men (Ebner et al., 2016). A chronic OT administration over 10 days in older men (mean age of 80 years) further suggested improvement in dispositional gratitude and less decline in physical functioning and self-reported fatigue over the study period after OT compared to placebo (Barraza et al., 2013); the study did not include a young adult comparison group.
While there is a growing, convergent body of evidence implicating OT and AVP in social cognition (De Dreu, 2014), non-social cognitive effects have received less attention. This is especially the case with regard to OT, and the directions of the effects are unclear. Some of the early single-dose AVP administration studies suggested improved cognition (e.g., improved memory for sentences, enhanced word recall) in healthy young men, possibly via the neuropeptide’s attention-enhancing properties (Beckwith, Till, & Schneider, 1984); these effects were not detected in young women, again supporting a sexually dimorphic pattern of results. Also 2–3 weeks AVP administration resulted in improved learning and memory (i.e., serial learning, prompted free recall, recall of semantically related words; Weingartner et al., 1981). The only aging study on this topic found that a 3-day dose of AVP slowed response times in older men and women during a secondary response time task, while maintaining performance in a primary task (integer arithmetic); this effect was driven by the oldest individuals among the sample (Jennings, Nebes, & Reynolds, 1986). These results are consistent with an effect of AVP on processing speed or cautiousness (Nephew, 2012; for a review), which may also explain the pronounced effect in very old participants, given slower processing speed and increased cautiousness in this age group.
Regarding OT, there is evidence that the neuropeptide impairs performance in verbal memory (Heinrichs, Meinlschmidt, Wippich, Ehlert, & Hellhammer, 2004), while enhancing social (i.e., faces) but not non-social recognition (Rimmele, Hediger, Heinrichs, & Klaver, 2009). Moreover, a case study documented significant amnesia in a patient with obsessive compulsive disorder after use of intranasal OT over 4 weeks (Ansseau et al., 1987). These findings stand in contrast to work in schizophrenic patients showing improved performance following intranasal OT administration over a period of three weeks; this was seen on several measures of non-social verbal memory, with effects particularly pronounced for short-term recall (Feifel, MacDonald, Cobb, & Minassian, 2012).
Taken together, these studies suggest sex and age variations in level of OT and AVP and in their functional relationships with affiliative and cognitive processes in humans. However, the direction of these effects is currently not clear, and sex and age interactions have not been systematically examined yet. In this context the present study had three central aims: (i) to describe levels of and interrelationships between endogenous plasma OT and AVP; (ii) to examine functional relationships between OT and AVP levels, and socioemotional (attachment anxiety and avoidance) and cognitive (sensorimotor processing speed, short-term verbal memory) outcomes; and (iii) to determine the extent to which sex and age differences modulated these effects. We predicted that plasma OT would be lower in men than women and lower in older than young participants. Specifically, we expected older men would show the lowest plasma OT levels and young women the highest plasma OT levels. For AVP plasma levels, we expected the reverse pattern: higher plasma AVP in men than women and higher plasma AVP in older than young participants, with particularly high levels in older men and particularly low levels in young women. We also predicted a generally negative correlation between OT and AVP. Regarding functional relationships of OT and AVP with adult attachment and cognitive performance, given currently mixed evidence, we did not specify directional hypotheses.
Material and Methods
Participants
One hundred and five generally healthy volunteers participated in the study, comprising 51 young (M = 22.4 years, SD = 2.99, 18–31, 47% female) and 54 older (M = 71.2 years, SD = 4.92, 63–81, 56% female) white, English-speaking adults. Older participants scored ≥ 30 on the Telephone Interview for Cognitive Status (TICS; Brandt, Spencer, & Folstein, 1988). Participants were recruited through mailouts and fliers in the community and on campus and were screened for physical and cognitive health via self-report during an initial phone contact and via blood draw and health review under the supervision of a clinical practitioner at the start of the first study visit. Among the exclusion criteria were pregnancy, as confirmed with pregnancy testing, breastfeeding, psychological disorder, severe or progressive medical illness, excessive smoking or drinking, as well as known allergies to OT or AVP and any contraindication to MRI (to conform with safety precautions for the intranasal OT administration and brain imaging portions of the study reported elsewhere; Ebner et al., 2016; Ebner et al., 2015). Participants were instructed to stay well-hydrated before their visit, but to abstain from smoking, caffeine, alcohol, and use of recreational drugs in the 24 hours, and from food, exercise, or sexual activity in the two hours, leading up to their appointment. All test sessions took place in the mornings, typically starting around 9AM, to control for diurnal variation in neuropeptide levels. Session starting times were quite comparable for the sex-by-age subgroups (young males: M = 8:59AM, SD = 0:29, young females: M = 8:50AM, SD = 0:32, older males: M = 9:11AM, SD = 0:35, older females: M = 9:07AM, SD = 0:22).
Although Weisman et al. (2013) did not find differences in baseline OT related to contraception intake or menstrual cycle, we recorded date of last menstruation, use of oral contraception, and hormone replacement therapy (HRT). All older women were postmenopausal, all young women were premenopausal. Ninety-two percent (22 out of 24) of young women were in the luteal phase of their menstrual cycle. One older man and one older woman were currently on HRT. We excluded the two young women in their follicular phase and the two older adults on HRT, resulting in a sample size of 101 for the present analyses. Seven young women were on oral contraception.1
Table 1 reports means, standard deviations, and range for demographic, health, attachment, and cognitive measures of the final sample (n = 101). There were no differences between the sex-by-age subgroups in years of education or mental or physical self-reported health. Young compared to older participants (F(1,96) = 8.2, p = 0.005, ƞp2 = 0.079) and, marginally, women compared to men reported more attachment anxiety (F(1,96) = 3.6, p = 0.059, ƞp2 = 0.037). There were no differences among the groups for attachment avoidance. Young participants outperformed older participants in sensorimotor processing speed (F(1,97) = 110.0, p < 0.001, ƞp2 = 0.531) and short-term verbal learning memory (F(1,97) = 16.3, p < 0.001, ƞp2 = 0.143).
Table 1.
Means (M), standard deviations (SD), and range for demographic, health, attachment, and cognitive measures in young and older men and women
| Measures | Young Male (n = 27) | Young Female (n = 22) | Older Male (n = 23) | Older Female (n = 29) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | Range | M | SD | Range | M | SD | Range | M | SD | Range | |
| Demographics | ||||||||||||
| Age (yrs.) | 22.1 | 2.91 | 18–31 | 22.9 | 3.22 | 18–30 | 72.4 | 5.11 | 63–81 | 70.5 | 4.60 | 63–80 |
| Education (yrs.) | 15.1 | 1.71 | 12–19 | 16.1 | 2.98 | 12–22 | 16.6 | 3.23 | 12–25 | 16.2 | 3.03 | 10–23 |
| Health | ||||||||||||
| Mental | 8.7 | 1.20 | 5–10 | 8.3 | 1.21 | 6–10 | 8.9 | 0.87 | 7–10 | 8.8 | 1.43 | 3–10 |
| Physical | 8.7 | 0.98 | 6–10 | 8.3 | 1.32 | 6–10 | 8.4 | 1.07 | 6–10 | 8.4 | 1.12 | 5–10 |
| Attachment | ||||||||||||
| ECR-S Anxiety | 2.8 | 1.05 | 1.0–5.0 | 3.4 | 1.21 | 1.3–5.3 | 2.4 | 0.92 | 1.0–4.7 | 2.6 | 1.06 | 1.0–5.8 |
| ECR-S Avoidance | 2.4 | 1.11 | 1.0–5.2 | 2.2 | 0.87 | 1.0–4.0 | 2.3 | 1.03 | 1.0–4.8 | 2.5 | 1.45 | 1.0–6.5 |
| Cognition | ||||||||||||
| Digit Symbol | 63.9 | 9.48 | 44–84 | 64.0 | 10.53 | 51–93 | 42.7 | 5.12 | 34–52 | 46.3 | 10.46 | 19–62 |
| RAVLT | 9.0 | 2.35 | 5–14 | 9.0 | 1.36 | 7–12 | 6.8 | 2.25 | 4–12 | 8.0 | 2.55 | 4–14 |
Note. Excluded from data analysis were two young women in their follicular menstrual cycle and one older man and one older woman on hormone replacement therapy. Mental (Please rate your general physical health.) and physical (Please rate your general mental health/mood.) health were assessed with single items on a response scale from 1 = poor to 10 = excellent. ECR-S = Experiences in Close Relationship Scale (short form; Wei et al., 2007); one young woman did not respond to the ECR-S, reducing the subsample size to 21 for this measure. Digit Symbol = Digit Symbol Substitution Test (Wechsler, 1981); RAVLT = Rey Auditory Verbal Learning Test (Rey, 1964). There were no differences between the sex-by-age subgroups in years of education, mental or physical self-reported health, or attachment avoidance. However, young compared to older participants (F(1,96) = 8.2, p = 0.005, ηp2 = 0.079) and, marginally, women compared to men reported more attachment anxiety (F(1,96) = 3.6, p = 0.059, ηp2 = 0.037). Also, young participants outperformed older participants in sensorimotor processing speed (F(1,97) = 110.0, p < 0.001, ηp2 = 0.531) and short-term verbal learning memory (F(1,97) = 16.3, p < 0.001, ηp2 = 0.143).
Procedure and Measures
Reported data were part of a larger project; only variables analyzed in the present context are described. Test sessions took place at the Institute on Aging, University of Florida medical campus, and were conducted by trained research staff. After written informed consent, participants completed a self-report questionnaire asking about their stress levels on the day of the experiment, the amount of sleep they were able to get the night before, exercise, physical contact (human or pet), sexual activity, food, caffeine, or alcohol consumption within the last 24 hours, current medications, presence of a fever, or had experienced any atypical or upsetting events immediately before coming to the experiment. This was followed by a brief self-report health review covering all major bodily systems (i.e., eyes, ears, nose, throat, pulmonary, genito-urinary, gastrointestinal, central nervous system, mental health, heart/vascular, musculo/skeletal). To assay attachment parameters, participants responded to the Experiences in Close Relationship Scale, Short Form (ECR-S) (Wei, Russell, Mallinckrodt, & Vogel, 2007), which correlates well with the longer version used in prior OT research (Weisman et al., 2013). The ECR-S measures two dimensions: attachment anxiety (6 items; Cronbach’s alpha = 0.76; e.g., “I worry that romantic partners won’t care about me as much as I care about them”) and attachment avoidance (6 items; Cronbach’s alpha = 0.82; e.g., “I want to get close to my partner, but I keep pulling back”). Both measures were assessed on a scale ranging from 1 = disagree strongly to 7 = agree strongly. Participants completed the Digit Symbol-Substitution Task from the Wechsler Adult Intelligence Scale (Digit Symbol; Wechsler, 1981) as measure of sensorimotor processing speed. The Rey Auditory Verbal Learning Test (RAVLT; Rey, 1964) followed to assess short-term verbal learning memory. Participants underwent a blood draw conducted by a professional nurse for screening purposes (basic metabolic panel) and to determine endogenous plasma OT and AVP levels. At the end of the first study visit, a brief consultation with the clinical practitioner took place to go over current health and health history. As part of the larger project, participants came back for a second study visit, reported elsewhere (Ebner et al., 2016; Ebner et al., 2015). Participants were reimbursed at the end of this additional visit. The Institutional Review Board at University of Florida approved the study protocol.
Blood Draw, Processing, and Assays
Six mL blood was drawn into EDTA plasma tubes. Upon filling samples were inverted five times and stored on ice. Samples were immediately centrifuged at 1600 × g for 15 minutes at 4°C, aliquoted, and stored in a −80°C freezer. Samples were thawed at room temperature immediately before assays. Plasma OT and AVP were measured using an Enzyme Immunoassay (EIA) purchased from Enzo Life Sciences, Inc. (Farmingdale, New York), according to the manufacturer’s instructions. The plasma was diluted in assay buffer (at a ratio of 1:8 for the OT assay and 1:2 for the AVP assay) to give results reliably within the linear portion of the standard curve. The EIA has been reported by the manufacturer to be highly sensitive (minimal detection rate = 15.6 picogram per millilitre (pg/ml) for OT and 4.10 pg/ml for AVP) with very little antibody cross-reactivity for other neuropeptides. All samples were run at once and the inter- and intra-assay coefficients of variation were less than 8% for OT and 7.3% for AVP.
We did not extract samples, based on tests for parallelism, spike-recovery, and cross-reactivity/specificity validating the EIA procedure and suggesting that EIA without extraction allows for an accurate assessment of OT in human blood plasma (Carter et al., 2007; MacLean et al., 2019; Lancaster et al., 2017). MacLean et al. (2018) showed that while non-extracted samples yielded considerably higher concentrations than extracted samples, the correlations of OT concentrations when analyzed in the same samples with and without extraction are high, thus determining parallelism of dilutions in non-extracted vs. extracted salivary OT samples. When comparing EIA without extraction to EIA with extraction typically much higher estimates of OT concentrations are found (Szeto et al., 2011) and it has been proposed that removing bound OT during the extraction step may underlie this discrepancy in estimates (Brandtzaeg et al., 2016). As large amounts of OT are quickly bound in blood, extraction may eliminate much of the biologically relevant peptide. The specific mechanisms underlying this process, however, are not fully understood yet. Through disulfide bonds, OT has a high affinity for binding to proteins in blood plasma; breaking these bonds before OT concentration measurement results in estimates that are comparable to levels stemming from unextracted samples (Whittington et al., 2007). It has also been demonstrated that solid-phase extraction can further artificially reduce OT levels in plasma samples (Furman, Chen, & Gotlib, 2011; Kirsch et al., 2005) and can result in low amounts of authentic peptide labelled as OT in extracted samples (Szeto et al., 2011). Validation of these assays is described elsewhere (Carter et al., 2007).
Analyses
To test our hypotheses regarding sex and age variations in plasma OT and AVP levels, we conducted two separate analyses of variance (ANOVAs) with sex (male, female) and age (young, older) as between subject-variables on plasma OT and AVP levels, respectively. To determine the interrelations between plasma OT and AVP levels, we conducted a multiple regression analysis on plasma OT as outcome variable, using plasma AVP as well as sex and age as predictor variables in a full factorial interaction design. Finally, to determine the extent to which plasma OT and AVP levels had functional relationships with affiliative and cognitive outcomes, we conducted two sets of multiple regression analyses on plasma OT and AVP levels as outcome variables. These sets consisted of attachment anxiety, attachment avoidance, sensorimotor processing speed, and short-term verbal learning memory, respectively, as well as sex and age as predictor variables in a full factorial interaction design.
All regression models allowed for non-constant error variance across subgroups and levels of continuous predictors by using robust standard error calculations. We examined linear and nonlinear effects in our data. The linear trend better represented the data.
Results
Sex and Age Variations in Plasma OT and AVP Levels
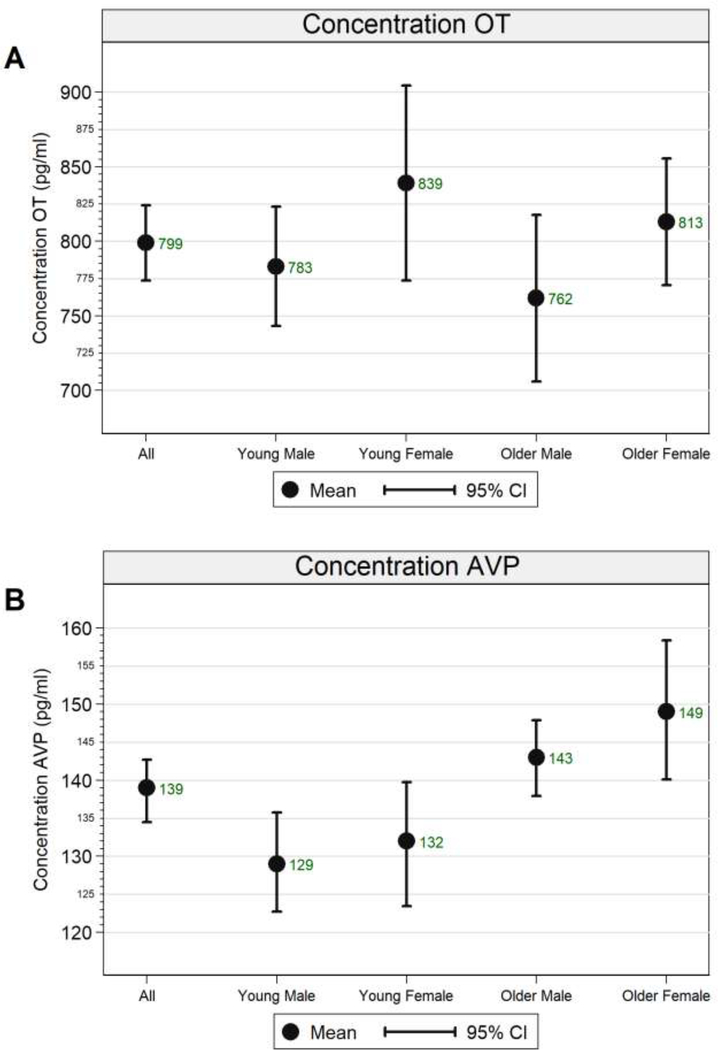
Figure 1 depicts plasma OT and AVP levels in the total sample and in the sex-by-age subgroups.1 Regarding plasma OT levels, the main effect of sex was significant (F(1,97) = 4.423, p = 0.038, partial eta2 = 0.044), while the main effect of age (F(1,97) = 0.875, p = 0.352, partial eta2 = 0.009) and the sex × age interaction (F(1,97) = 0.008, p = 0.927, partial eta2 < 0.001) were not significant. As can be seen in Figure 1A, young women had the numerically highest plasma OT levels (and showed the largest distribution) and older men had the numerically lowest plasma OT levels.
Figure 1.
Means and 90% confidence intervals of A) plasma oxytocin (OT) and B) plasma arginine vasopressin (AVP) levels in picograms per milliliter in the total sample and for the sex-by-age subgroups.
Regarding plasma AVP levels, the main effect of age (F(1,97) = 16.459, p < 0.001, partial eta2 = 0.145) was significant, while the main effect of sex (F(1,97) = 1.268, p = 0.263, partial eta2 = 0.013) and the sex × age interaction (F(1,97) = 0.269, p = 0.605, partial eta2 = 0.003) were not significant. As can be seen in Figure 1B, young men were the subgroup with the numerically lowest plasma AVP levels and older women were the subgroup with the numerically highest plasma AVP levels.
Interrelations between Plasma OT and AVP Levels
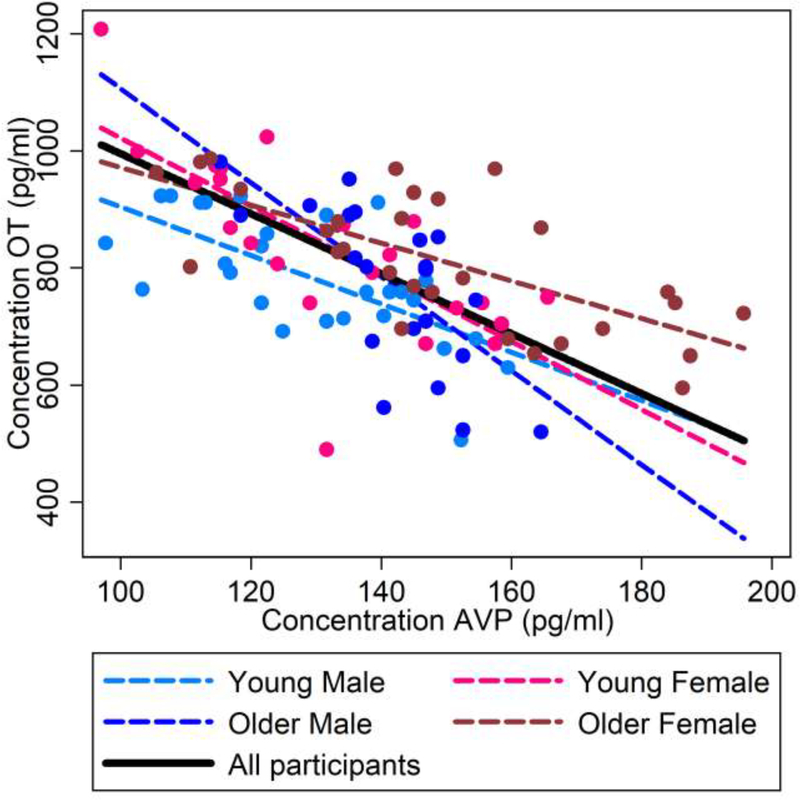
As summarized in Figure 2, there were highly negative, linear effects of plasma AVP levels on plasma OT levels in the total sample (b = −5.122, t(93) = −11.21, p < 0.0001; black solid line), and consistently in young men (b = −4.135, t(25) = −4.42, p < 0.0001; light blue dashed line), young women (b = −5.795, t(20) = −5.78, p < 0.0001; light pink dashed line), older men (b = −8.030, t(21) = −6.69, p < 0.0001; dark blue dashed line), and older women (b = −3.224, t(27) = −6.00, p < 0.0001; dark pink dashed line).
Figure 2.
Interrelations (bivariate correlations) between plasma OT and plasma AVP concentrations (picogram per milliliter) for the total sample (black solid line) and for the sex-by-age subgroups (young males: light blue dashed line; young females: light pink dashed line; older males: dark blue dashed line; older females: dark pink dashed line).
Functional Relations of Plasma OT and AVP Levels with Attachment and Cognition
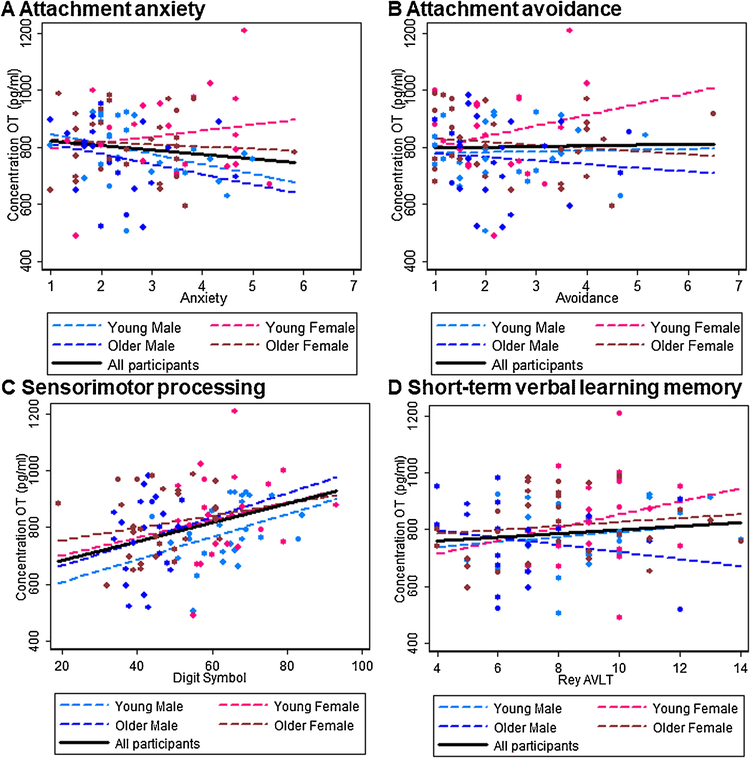
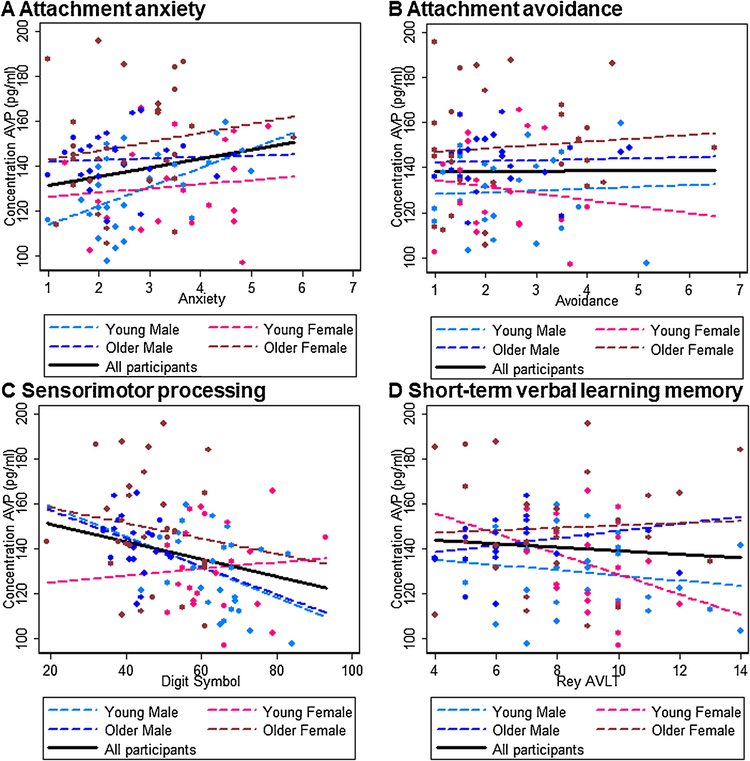
Results are summarized in Figure 3A–D for plasma OT and Figure 4A–D for plasma AVP. There were no significant associations between attachment avoidance for either plasma OT or AVP, but higher plasma AVP levels were associated with higher attachment anxiety (b = 3.978, t(93) = 2.57, p = 0.012) in the total sample. Considering the sex-by-age subgroups, in young men low plasma OT levels were associated with high attachment anxiety (b = −35.125, t(27) = −2.49, p = 0.015) and high plasma AVP levels were associated with high attachment anxiety (b = 8.451, t(27) = 3.96, p < 0.0001). No other effects were significant (ps > 0.05).
Figure 3.
Interrelations (bivariate correlations) between plasma OT concentrations (picogram per milliliter) and A) attachment anxiety, B) attachment avoidance, C) sensorimotor processing, and D) short-term verbal learning memory for the total sample (black solid line) and for the sex-by-age subgroups (young males: light blue dashed line; young females: light pink dashed line; older males: dark blue dashed line; older females: dark pink dashed line).
Figure 4.
Interrelations (bivariate correlations) between plasma AVP concentrations (picogram per milliliter) and A) attachment anxiety, B) attachment avoidance, C) sensorimotor processing, and D) short-term verbal learning memory for the total sample (black solid line) and for the sex-by-age subgroups (young males: light blue dashed line; young females: light pink dashed line; older males: dark blue dashed line; older females: dark pink dashed line).
Regarding cognitive outcomes, higher plasma OT (b = 3.330, t(93) = 2.42, p = 0.018) and lower plasma AVP (b = −0.387, t(93) = −2.01, p = 0.048) levels were associated with faster sensorimotor processing speed in the total sample. Considering the sex-by-age subgroups, in young men high plasma OT levels (b = 3.982, t(27) = 2.00, p = 0.048) and low plasma AVP (b = −0.675, t(25) = −1.81, p = 0.073) were associated with faster sensorimotor processing speed; similarly, in older men low plasma AVP levels were associated with faster sensorimotor processing speed (b = −0.622, t(21) = −2.04, p = 0.044). In young women, finally, low plasma AVP levels were associated with higher short-term verbal learning memory (b = −4.505, t(20) = −2.24, p = 0.027). No other effects were significant (ps > 0.05).
Discussion
The present study provides intriguing evidence of sex and age variations in human OT and AVP endogenous plasma concentrations. Our findings furthermore support a strongly negative association between plasma OT and AVP levels across sex and age and suggest functional links of OT and AVP with (i) self-reported relationship anxiety and (ii) sensorimotor processing speed, with these effects moderated by sex and age. These central findings will be discussed in more detail next.
Plasma OT and AVP Levels Varied by Sex and Age
While sex differences in endogenous OT and AVP concentrations have been reported (Weisman et al., 2013), our findings pertaining to sex and age variations on neuropeptide levels qualify the previous literature and translate evidence from animal work to humans. Sex and age differences in OT and AVP levels and functions can be understood in the context of their long evolutionary history and their role in sexually dimorphic reproductive imperatives and survival strategies. Alterations to the OT and AVP pathways have been implicated in long-term sex-dependent behavioral and developmental changes.
Sex differences
Supporting sex variations in the effects of early life experience on neuropeptides, for example, adult female rats that experienced more maternal licking and grooming as pups showed increased OT receptor (OTR) binding in the bed nucleus of the stria terminalis and central nucleus of the amygdala, whereas adult male rats that experienced more maternal licking and grooming as pups show increased AVP receptor binding in the same areas (Francis, Young, Meaney, & Insel, 2002). Sex differences in AVP receptor expression are observed across multiple species of rodents and non-mammals, with higher density of AVP receptors or receptors for an AVP homologue, vasotocin, in adult males compared to females in the majority of species studied (Smith et al., 2019). Given the considerable consistency of sex differences in AVP distribution across species, it is reasonable to assume some degree of conservation of AVP receptor sex differences and expression that may apply to humans (Smith et al., 2019).
Our findings differed from the Weisman et al. 2013 study. Specifically, Weisman et al. found that men had higher plasma OT levels than women. This is in direct contrast to our study, which found that women had higher plasma OT levels than men. Notable differences between the studies include that Weisman et al. included women at different stages in their menstrual cycles and currently breastfeeding. Whereas, in our study, 22 out of 24 young women were in the luteal phase of their menstrual cycle, and all older women were postmenopausal, and women breastfeeding or individuals on HRT were excluded. The mean age of women included in our young female subgroup (M = 22.4, SD = 2.99) was slightly younger but largely comparable to that of women included in Weisman et al. (M = 26.7, SD = 4.25). However, the Weisman et al. study did not include an older adult subgroup. Additionally, Weisman et al. included 473 participants, with 58.5% young female participants, in their final analysis. Whereas, our study only comprised 101 participants, with 24 young female participants. The collection methods used were similar across both studies, including a blood draw, storage on ice for minimal time, and freezing at −80 C before conducting the assay. Weisman et al. used an OT-ELISA kit by AssayDesign and our study used EIA from Enzo Life Sciences. Different antibodies were used in these assays and are one likely source of between study variation (MacLean et al., 2019). Previous work in our laboratory with the AssayDesigns kit gave basal values consistent with those from Weisman et al. Given evidence of plasma OT fluctuation during each phase of the menstrual cycle (Salonia et al., 2005), both the sampling methods and differences in sample composition may have contributed to the differences in findings. Finally, Weisman et al. found considerably lower plasma OT levels in both the male (M = 399.91 pg/mL, SD = 183.65) and the female (M = 327.13 pg/mL, SD = 164.43 pg/mL) groups compared to our study, which found a mean of 783 pg/mL for young males, 839 pg/mL for young females, 762 pg/mL for older males, and 813 pg/mL for older females (see also Figure 1). One major difference is that the Weisman et al. removed outliers beyond 2.5 SDs (N = 14, 9 women, 5 men) from their final analysis. And, as aforementioned, the average age of their sample was much lower than ours. As a result, methodological differences including age distribution and sample composition may have contributed to the differences in findings across the two studies.
Age differences
Regarding age effects, there is evidence in male mice that systemic OT and OTR levels in satellite cells decline with age (Elabd et al., 2014). This study measured OT plasma levels in young (2- to 4-month-old) and old (18- to 24-month-old) C57/BL6 male mice and found three-fold lower levels in aged mice as compared with young mice, supporting an age-related decline in endocrine levels of OT. Similarly, circulating OT levels declined after ovariectomy, which mimics hormonal aging (Elabd et al., 2008).
Consistent with our findings of higher AVP in older adults compared to younger adults, prior research has shown that AVP increases with age, regardless of sex (Johnson et al., 1994), due to increased activity of magnocellular neurons responsible for production of AVP with age (Fliers, Swaab, & Pool, 1985). Some research has suggested this may be due to decreased kidney function and as compensation for decreased AVP-receptor sensitivity in the kidneys with age (Ishunina & Swaab, 2002). Conversely, the structural aspects of OT-expressing neurons, such as number and size, among other factors, remain the same with age (Ishunina & Swaab, 2002). However, OT trafficking and release from central neurons is decreased with age (Sannino, Chini, & Grinevich, 2017). Therefore, physiological changes with age may explain our findings of higher OT in young women and higher AVP in older adults, although further research is needed, especially among the adolescent and young adult groups with regard to puberty, reproduction, and interactions between reproductive hormones such as estrogen with OT and AVP across the lifespan.
In addition to biological processes, however, human aging is typically accompanied by profound interpersonal and relational changes (e.g., loss of partners or reductions in social networks). This combined with findings of protective effects of social relationships on age-related cognitive outcomes (Ellwardt, Aartsen, Deeg, & Steverink, 2013), supports the need for a thorough future examination of OT and AVP as key “social hormones” across adulthood and into old age. Also, determination of modulatory dynamics between OT and AVP in interaction with a network of other neurochemicals, including testosterone, estrogen, cortisol, dopamine, and serotonin, which show sex differences and age-related change (Ebner et al., 2014), will advance understanding of the role OT and AVP, jointly, play in aging.
Plasma OT and AVP Levels were Highly Negatively Associated
We observed a strong negative association between plasma OT and AVP levels in the total sample and among all sex-by-age subgroups. This finding contributes to a recent trend in the literature of these peptides as interdependent, in line with the proposition that OT and AVP interactively form an evolved, dynamic, integrated pathway and that presence of both peptides is required for a variety of behaviors and regulatory functions (C. Sue Carter, 2017a).
Overall, our findings are in line with clinical work suggesting a link between lower OT while higher AVP levels have been associated with several psychiatric disorders, including major depressive disorders (de Winter et al., 2003). In support of a coordinated, reversed relationship between these two neuropeptides is also evidence that in the amygdala AVP can directly excite a subpopulation of neurons, whereas OT can indirectly inhibit these same neurons (Raggenbass, 2008). For example, OT and AVP show coordinated effects on social approach behavior and social stress (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Both social stress and social anxiety engage the amygdala–cingulate circuit and the hypothalamus–pituitary–adrenal (HPA) axis and both these systems are enhanced by AVP while OT reduces amygdala and HPA axis reactivity to social stressors.
Plasma OT and AVP Levels Were Functionally Associated with Attachment and Cognition
Largely in line with Weisman et al. (2013), our data showed that higher endogenous plasma OT levels were associated with lower self-reported attachment anxiety in young men, while there was no association in young women (or older adults). Convergent with Weisman et al., there was no indication of an association between OT (or AVP) and attachment avoidance, although there was a relationship with attachment anxiety. Previous studies have shown that OT increased positive emotions in those securely attached and negative emotions in those anxiously attached (Bartz et al., 2010). Furthermore, higher peripheral OT levels were associated with increased expressions of secure attachment (Alaerts et al., 2019). Our findings further supported a relationship between OT and attachment anxiety in young men. Regarding attachment avoidance our data, in line with Weisman et al., suggested no relationship with OT. However, it is possible that the assessment of attachment avoidance via the self-report measure used in the present study (and in Weisman et al.) is not sufficiently sensitive.
The observed association between high plasma OT levels and low attachment anxiety in young men supports an anxiolytic effect of OT in males, in line with theories suggesting that OT reduces social anxiety and promote social approach in both humans and other animals (De Dreu, 2014). For example, higher OT levels were associated with higher levels of trust and trustworthiness (Zak, Kurzban, & Matzner, 2005) and social bonding (Schneiderman, Zagoory-Sharon, Leckman, & Feldman, 2012). Further, elevation of OT levels via intranasal spray, combined with social support, lowered physiological stress responses and anxiety during a stress test (Heinrichs et al., 2004). Corresponding to the OT findings, higher endogenous plasma AVP levels were associated with greater self-reported attachment anxiety in young men, although this association was not detected in young women (or in older adults). Aligning with these results is evidence of involvement of AVP in human stress-related diseases, such as anxiety and mood disorders (Dinan, O’brien, Lavelle, & Scott, 2004).
Possible explanations for the sex-differential association between plasma AVP concentrations and attachment anxiety come from animal work. AVP receptors in the amygdala are locally expressed in higher levels in males compared to females (Wang & De Vries, 1995). Strong effects of AVP on amygdala functioning may lead to higher baseline levels of plasma AVP in men relative to women, which may be responsible for pronounced effects of plasma AVP on amygdala activation in men (Neumann & Landgraf, 2012). Male-specific effects of AVP are also consistent with animal work supporting synergistic mechanisms that involve testosterone and AVP in male subjects. It is possible that stronger brain-periphery AVP communication in males is a function of amplifying effects of male gonadal hormones on AVP activation of the amygdala.
Higher endogenous plasma OT levels and lower endogenous plasma AVP levels were associated with faster sensorimotor processing speed in the total sample, and particularly pronounced in young men and for AVP in older men, advancing knowledge on the role of these neuropeptides in (non-social) cognition. While the mechanisms underlying this relationship are currently still unclear, there is some discussion in the literature of OT administration stimulating adult neurogenesis in a rodent model (Jafarzadeh, Javeri, Khaleghi, & Taha, 2014).
In our study, endogenous AVP levels were associated with sensorimotor processing (assessed via the Digit Symbol) in the total sample, but AVP levels were only associated with short-term verbal learning memory (assessed via the RAVLT) in the young women. It is possible that the Digit Symbol was overall more sensitive in determining interindividual differences than the RAVLT, as can be seen in the relatively larger standard deviation for Digit Symbol than the RAVLT. The findings of a relationship between neuropeptide concentrations with sensorimotor processing speed but no indication of a relationship with short-term verbal learning memory are in accord with evidence of domain-specificity in the link between inflammatory processes and cognition (Lin et al., 2018), as well as work from an intervention study in which antioxidant supplementation (resveratrol) was associated with improvement on processing speed but not memory (Anton et al., 2018). Thus, it is possible that variable effects of neuropeptide levels are at work for individual cognitive domains. Supporting this hypothesis is evidence that memory-enhancing effects of AVP appear to take place during encoding rather than at recall (Beckwith, Petros, Bergloff, Swenson, & Paulson, 1995) and that AVP enhanced attention and arousal instead of directly affecting memory (Gais, Sommer, Fischer, Perras, & Born, 2002). Also, AVP in young and older participants elevated event-related potentials in the brain, which were associated with attentional mechanisms, but did not improve age-related cognitive impairments (Dodt, Theine, Uthgenannt, Born, & Fehm, 1994). Similarly, repeated intranasal administration of AVP to healthy older adults did not improve general long-term memory, although certain aspects of word recall were enhanced (Perras, Droste, Born, Fehm, & Pietrowsky, 1997).
Limitations
Some methodological limitations need to be considered when interpreting our results. First, the sample size was small, especially when considering sex-by-age subgroups. Results from power simulations showed that our sample would have had to be n > 300 to allow detection of a significant age effect with a power of 0.80 given the small size for the age effect for OT as suggested by our data. Our simulations furthermore showed that the available sample size of 101 participants for the present analysis was sufficient to detect a medium (see significant effect for AVP) or a large age effect. Our power simulations also suggested that a sample size of n > 3000 would have been needed to detect a small sex-by-age interaction as suggested by our data. However, our sample size was sufficiently powered to detect the medium effect size for sex differences for OT in our data. A future investigation with a larger sample size is warranted to confirm and possibly extend the reported findings.
Also, our sample consisted of generally healthy adults without known pathology, allowing us to meet the eligibility criteria of the larger project which included neuroimaging and intranasal OT administration (Ebner et al., 2016). Our participants were community-living, physically, cognitively, and emotionally highly functioning individuals, and the range of values in relationship attachment and cognitive performance was restricted. Future studies need to target a broader, more representative sample, as well as patients with a range of relevant disorders (e.g., social anxiety, dementia), allowing for greater variability in attachment scores and cognitive performance measures and for broader generalizability of the findings. Compared to research in animal models, a tight fit between the human behavioral repertoire and specific neuroendocrine processes is challenging to detect and more research will be needed to tease apart specific conditions, subgroups, cultural backgrounds, socioeconomic contexts, or variability in personal experience that may be related to variability within the general sample and their specific associations with modulations in neuroendocrine processes.
The scope of the present study could have been extended by assessing responses to social-cognitive tasks, that had previously been shown responsive to OT enhancement (via intranasal administration) such as tests of facial expression identification (Leppanen et al., 2017; Shahrestani et al., 2013) and face recognition (Heinrichs et al., 2004; Rimmele et al., 2009), during the visit at which baseline plasma OT and AVP levels were assessed. In fact, as mentioned above, participants in the present study came to a second visit in which they self-administered a single dose of either 24 IUs OT or placebo via nasal spray after which they engaged in social-cognitive tasks (see published in Ebner et al., 2015, 2016; Horta et al., 2019). However, this data cannot be applied to analyses in the present context as we would have to consider interactions between endogenous OT levels (plasma OT levels as measured at baseline) with exogenous OT administration and the current sample is too small to allow testing these interaction effects. Also, baseline OT assessments did not take place on the same day as the OT intervention. Moving forward in this direction with a sufficiently large sample size and including plasma neuropeptide concentrations at multiple time points before and after the exogenous OT intervention will be a fruitful extension to enhance understanding of the OT system in young and older men and women.
We measured plasma OT and AVP levels once in our sample based on evidence of high interindividual stability, at least over a period of 6 months (Weisman et al., 2013). Also, we measured endogenous neuropeptide levels in plasma. The link between central and peripheral neuropeptide levels is not yet fully understood (Crockford, Deschner, Ziegler, & Wittig, 2014). However, there is evidence of a positive correlation between cerebrospinal fluid and plasma OT concentrations. This evidence validates central and peripheral markers of OT and suggests that measurements of peripheral levels of some hormones may well reflect central levels (Carson et al. 2014). Social voles have a different distribution of neuropeptide receptors than solitary voles, with no difference in amount of neuropeptide (Perkeybile et al., 2019). For this reason, comparing neuropeptide concentrations may be less informative than measuring neuropeptide receptors expression patterns as genetic sequence difference can alter the expression and geography of receptors in the brain. In particular, future longitudinal and behavioral research is needed to further understand the (epi)genetic, brain, and immunological relationships between endogenous neuropeptide levels and behavioral correlates of attachment and cognition in humans, as well as the consequences of early experience, which have been shown based on animal research to have epigenetic effects on OT systems (Perkeybile et al., 2019). Future research efforts in this direction may lead to the development of more specific biomarkers and might ultimately guide the creation of more peptide-based pharmacological or behavioral interventions.
Conclusion
Our findings provide support for sex- and age-differential plasma OT and AVP concentrations in humans. Highly negatively intercorrelated neuropeptide levels were detected across all sex-by-age subgroups, and benefits for attachment anxiety and sensorimotor processing were suggested for higher plasma OT levels and lower AVP levels, with these effects particularly pronounced in young men. Taken together, the integrated approach adopted in this study identified variations in endogenous neuropeptide levels in humans, supporting a possible sex- and age-specific role for these systems as “difference makers” in attachment and cognition. Moving forward, this line of work has potential to clarify the complex relationship between biological and psychosocial factors that contribute to sex differences and individual differences in aging trajectories.
Highlights.
Integrated assessment of OT and AVP plasma levels in young and older men and women
Evidence of sex and age differences in OT and AVP plasma concentrations in humans
Strong negative association between OT and AVP levels across sex and age groups
Sex- and age-moderated functional link of OT and AVP with attachment and cognition
Support of sex-/age-specific role of OT and AVP as difference makers in behavior
Acknowledgments
The authors are grateful to the research teams from the Social-Cognitive and Affective Development lab and the Institute on Aging at University of Florida for assistance in study implementation, data collection, and data management, Song Lai and Tammy Nicholson for brain imaging, and Marilyn Horta for constructive suggestions for the manuscript.
This work was supported by the Department of Psychology at University of Florida, the McKnight Brain Research Foundation, and the University of Florida Center for Cognitive Aging and Memory, a University of Florida Clinical and Translational Science pilot award (NIH/NCATS; UL1 TR000064), and a Scientific Research Network on Decision Neuroscience and Aging pilot award (NIH/NIA, R24 AG039350) to NCE. While working on this manuscript, NCE was in part supported by the NIH-funded Claude D. Pepper Older Americans Independence Center (NIH/NIA, P30AG028740) and the Cognitive Aging and Memory Clinical Translational Research Program at University of Florida. Peptide assay development was sponsored by NICHD (P01 HD075750) to CSC.
Footnotes
None of the authors has a conflict of interest.
The pattern of results was comparable when one young male with outlying values for OT and one older male with outlying values for AVP were removed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaerts K, Bernaerts S, Vanaudenaerde B, Daniels N, & Wenderoth N (2019). Amygdala–Hippocampal Connectivity Is Associated With Endogenous Levels of Oxytocin and Can Be Altered by Exogenously Administered Oxytocin in Adults With Autism. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(7), 655–663. 10.1016/j.bpsc.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Ansseau M, Legros J-J, Mormont C, Cerfontaine J-L, Papart P, Geenen V, … Franck G (1987). Intranasal oxytocin in obsessive-compulsive disorder. Psychoneuroendocrinology, 12(3), 231–236. Retrieved from http://www.sciencedirect.com.lp.hscl.ufl.edu/science/article/pii/0306453087900096 [DOI] [PubMed] [Google Scholar]
- Anton SD, Ebner NC, Dzierzewski JM, Zlatar ZZ, Gurka MJ, Dotson VM, … Manini TM (2018). Effects of 90 Days of Resveratrol Supplementation on Cognitive Function in Elders: A Pilot Study. The Journal of Alternative and Complementary Medicine, 24(7), 725–732. 10.1089/acm.2017.0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza JA, Grewal NS, Ropacki S, Perez P, Gonzalez A, & Zak PJ (2013). Effects of a 10-day oxytocin trial in older adults on health and well-being. Experimental and Clinical Psychopharmacology, 21(2), 85–92. 10.1037/a0031581 [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, Bolger N, Kolevzon A, Ludwig N, & Lydon JE (2010). Effects of oxytocin on recollections of maternal care and closeness. Proceedings of the National Academy of Sciences. 10.1073/pnas.1012669107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith BE, Petros TV, Bergloff PJ, Swenson RR, & Paulson R (1995). Failure of posttrial administration of vasopressin analogue (DDAVP) to influence memory in healthy, young, male volunteers. Peptides, 16(8), 1327–1328. [DOI] [PubMed] [Google Scholar]
- Beckwith BE, Till RE, & Schneider V (1984). Vasopressin analog (DDAVP) improves memory in human males. Peptides, 5(4), 819–822. 10.1016/0196-9781(84)90028-7 [DOI] [PubMed] [Google Scholar]
- Brandt J, Spencer M, & Folstein M (1988). The telephone interview for cognitive status. Neuropsychiatry, Neuropsychology, & Behavioral Neurology, 1(2), 111–117. [Google Scholar]
- Brandtzaeg OK, Johnsen E, Roberg-Larsen H, Seip KF, MacLean EL, Gesquiere LR, Leknes S, Wilson SR (2016). Proteomics tools reveal startlingly high amounts of oxytocin in plasma and serum. Scientific Reports, 6(1), 31693 10.1038/srep31693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A, Ruffman T, Murray JE, & Glue P (2014). Oxytocin improves emotion recognition for older males. Neurobiology of Aging, 35(10), 2246–2248. 10.1016/j.neurobiolaging.2014.04.021 [DOI] [PubMed] [Google Scholar]
- Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, … Parker KJ (2014). Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Molecular Psychiatry. 10.1038/mp.2014.132 [DOI] [PubMed] [Google Scholar]
- Carstensen LL (2006). The influence of a sense of time on human development. Science, 312(5782), 1913–1915. 10.1126/science.1127488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2017a). The oxytocin-vasopressin pathway in the context of love and fear. Frontiers in Endocrinology, 8(DEC), 356 10.3389/fendo.2017.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2017b). The Role of Oxytocin and Vasopressin in Attachment. Psychodynamic Psychiatry, 45(4), 499–517. 10.1521/pdps.2017.45.4.499 [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, & Schwertz D (2007). Oxytocin: behavioral associations and potential as a salivary biomarker. Annals of the New York Academy of Sciences, 1098(1), 312–322. 10.1196/annals.1384.006 [DOI] [PubMed] [Google Scholar]
- Conrad CD, & Bimonte-Nelson HA (2010). Impact of the hypothalamic-pituitary-adrenal/gonadal axes on trajectory of age-related cognitive decline. Progress in Brain Research, 182, 31–76. Retrieved from http://www.sciencedirect.com/science/article/pii/S0079612310820023 [DOI] [PubMed] [Google Scholar]
- Crockford C, Deschner T, Ziegler TE, & Wittig RM (2014). Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Frontiers in Behavioral Neuroscience, 8(March), 68 10.3389/fnbeh.2014.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, & Herpertz SC (2010). Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology, 35(1), 83–93. 10.1016/j.psyneuen.2009.06.016 [DOI] [PubMed] [Google Scholar]
- De Dreu CKW (2014). Oxytocinergic circuitry motivates group loyalty In Mikulincer M & Shaver RP (Eds.), Mechanisms of Social Connection: From Brain to Group. (pp. 391–407). Washington, DC, US: American Psychological Association; Retrieved from http://portal.idc.ac.il/en/symposium/hspsp/2012/documents/cdedreu12.pdf [Google Scholar]
- de Vries GJ (2008). Sex differences in vasopressin and oxytocin innervation of the brain Progress in Brain Research. Elsevier; 10.1016/S0079-6123(08)00402-0 [DOI] [PubMed] [Google Scholar]
- de Winter RFP, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, & Goekoop JG (2003). Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 28(1), 140–7. 10.1038/sj.npp.1300002 [DOI] [PubMed] [Google Scholar]
- Dinan TG, O’brien S, Lavelle E, & Scott LV (2004). Further neuroendocrine evidence of enhanced vasopressin V 3 receptor responses in melancholic depression. Psychological Medicine, 34(1), 169–172. [DOI] [PubMed] [Google Scholar]
- Dodt C, Theine K-J, Uthgenannt D, Born J, & Fehm HL (1994). Basal secretory activity of the hypothalamo-pituitary-adrenocortical axis is enhanced in healthy elderly. An assessment during undisturbed night-time sleep. European Journal of Endocrinology, 131(5), 443–450. 10.1530/eje.0.1310443 [DOI] [PubMed] [Google Scholar]
- Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, & Cohen RA (2016). Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology, 69, 50–59. 10.1016/j.psyneuen.2016.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Horta M, Lin T, Feifel D, Fischer H, & Cohen RA (2015). Oxytocin modulates meta-mood as a function of age and sex. Frontiers in Aging Neuroscience, 7(SEP), 175 10.3389/fnagi.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Kamin H, Diaz V, Cohen RA, & MacDonald K (2014). Hormones as “difference makers” in cognitive and socioemotional aging processes. Frontiers in Psychology, 5(OCT), 1595 10.3389/fpsyg.2014.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner NC, Maura GM, Macdonald K, Westberg L, & Fischer H (2013). Oxytocin and socioemotional aging: Current knowledge and future trends. Frontiers in Human Neuroscience, 7, 487 10.3389/fnhum.2013.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, … Amri E-Z (2008). Oxytocin Controls Differentiation of Human Mesenchymal Stem Cells and Reverses Osteoporosis. STEM CELLS, 26(9), 2399–2407. 10.1634/stemcells.2008-0127 [DOI] [PubMed] [Google Scholar]
- Elabd C, Cousin W, Upadhyayula P, Chen RY, Chooljian MS, Li J, … Conboy IM (2014). Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature Communications, 5, 4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwardt L, Aartsen M, Deeg D, & Steverink N (2013). Does loneliness mediate the relation between social support and cognitive functioning in later life? Social Science & Medicine (1982), 98, 116–24. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24331889 [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Cobb P, & Minassian A (2012). Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophrenia Research, 139, 207–210. 10.1016/j.schres.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, & Zagoory-Sharon O (2011). Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: considering stress and affiliation components of human bonding. Developmental Science, 14(4), 752–61. 10.1111/j.1467-7687.2010.01021.x [DOI] [PubMed] [Google Scholar]
- Fliers E, Swaab DF, Pool CW, & Verwer RWH (1985). The vasopressin and oxytocin neurons in the human supraoptic and paraventricular nucleus changes with aging and in senile dementia. Brain Research, 342(1), 45–53. [DOI] [PubMed] [Google Scholar]
- Francis DD, Young LJ, Meaney MJ, & Insel TR (2002). Naturally Occurring Differences in Maternal Care are Associated with the Expression of Oxytocin and Vasopressin (V1a) Receptors: Gender Differences. Journal of Neuroendocrinology, 14(5), 349–353. 10.1046/j.00071331.2002.00776.x [DOI] [PubMed] [Google Scholar]
- Furman DJ, Chen MC, & Gotlib IH (2011). Variant in oxytocin receptor gene is associated with amygdala volume. Psychoneuroendocrinology, 36(6), 891–897. Retrieved from http://www.sciencedirect.com/science/article/pii/S0306453010003136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gais S, Sommer M, Fischer S, Perras B, & Born J (2002). Post-trial administration of vasopressin in humans does not enhance memory formation (vasopressin and memory consolidation). Peptides, 23(3), 581–583. [DOI] [PubMed] [Google Scholar]
- Grinevich V, Knobloch-Bollmann HS, Eliava M, Busnelli M, & Chini B (2016). Assembling the puzzle: pathways of oxytocin signaling in the brain. Biological Psychiatry, 79(3), 155–164. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Wippich W, Ehlert U, & Hellhammer DH (2004). Selective amnesic effects of oxytocin on human memory. Physiology & Behavior, 83(1), 31–8. 10.1016/j.physbeh.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Hofman MA, & Swaab DF (1994). Alterations in circadian rhythmicity of the vasopressin-producing neurons of the human suprachiasmatic nucleus (SCN) with aging. Brain Research, 651(1–2), 134–142. 10.1016/0006-8993(94)90689-0 [DOI] [PubMed] [Google Scholar]
- Horta M, Ziaei M, Lin T, Porges EC, Fischer H, Feifel D, … Ebner NC (2019). Oxytocin alters patterns of brain activity and amygdalar connectivity by age during dynamic facial emotion identification. Neurobiology of Aging, 78, 42–51. 10.1016/j.neurobiolaging.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Salehi A, Hofman MA, & Swaab DF (1999). Activity of vasopressinergic neurones of the human supraoptic nucleus is age- and sex-dependent. Journal of Neuroendocrinology, 11(4), 251–258. 10.1046/j.1365-2826.1999.00318.x [DOI] [PubMed] [Google Scholar]
- Jafarzadeh N, Javeri A, Khaleghi M, & Taha MF (2014). Oxytocin improves proliferation and neural differentiation of adipose tissue-derived stem cells. Neuroscience Letters, 564, 105–10. 10.1016/j.neulet.2014.02.012 [DOI] [PubMed] [Google Scholar]
- Jennings JR, Nebes RD, & Reynolds CF (1986). Vasopressin peptide (DDAVP) may narrow the focus of attention in normal elderly. Psychiatry Research, 17(1), 31–39. 10.1016/01651781(86)90039-9 [DOI] [PubMed] [Google Scholar]
- Jokinen J, Chatzittofis A, Hellström C, Nordström P, Uvnäs-Moberg K, & Asberg M (2012). Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology, 37(4), 482–90. 10.1016/j.psyneuen.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, … Meyer-Lindenberg A (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience, 25(49), 11489–11493. 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, & Olff M (2016). Intranasal oxytocin normalizes amygdala functional connectivity in posttraumatic stress disorder. Neuropsychopharmacology, 41(8), 2041–2051. 10.1038/npp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen J, Ng KW, Tchanturia K, & Treasure J (2017). Meta-analysis of the effects of intranasal oxytocin on interpretation and expression of emotions. Neuroscience & Biobehavioral Reviews, 78, 125–144. 10.1016/j.neubiorev.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Lin T, Liu GA, Perez E, Rainer RD, Febo M, Cruz-Almeida Y, & Ebner NC (2018). Systemic Inflammation Mediates Age-Related Cognitive Deficits. Frontiers in Aging Neuroscience. Retrieved from https://www.frontiersin.org/article/10.3389/fnagi.2018.00236 [DOI] [PMC free article] [PubMed]
- Lischke A, Berger C, Prehn K, Heinrichs M, Herpertz SC, & Domes G (2012). Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology, 37(4), 475–481. 10.1016/j.psyneuen.2011.07.015 [DOI] [PubMed] [Google Scholar]
- Lopatina OL, Komleva YK, Gorina YV, Higashida H, & Salmina AB (2018). Neurobiological Aspects of Face Recognition: The Role of Oxytocin. Frontiers in Behavioral Neuroscience. Retrieved from https://www.frontiersin.org/article/10.3389/fnbeh.2018.00195 [DOI] [PMC free article] [PubMed]
- MacLean EL, Gesquire LR, Gee N, Levy K, Martin WL, & Carter CS (2017). Validation of salivary oxytocin and vasopressin as biomarkers in domestic dogs. J. Neurosci. Methods 293, 67–76. [DOI] [PubMed] [Google Scholar]
- MacLean EL, Wilson SR, Martin WL, Davis JM, Nazarloo HP, & Carter CS (2019). Challenges for measuring oxytocin: The blind men and the elephant? Psychoneuroendocrinology, 107(May), 225–231. 10.1016/j.psyneuen.2019.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, & Heinrichs M (2011). Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12(9), 524–538. 10.1038/nrn3044 [DOI] [PubMed] [Google Scholar]
- Myers B, Scheimann JR, Franco-Villanueva A, & Herman JP (2017). Ascending mechanisms of stress integration: Implications for brainstem regulation of neuroendocrine and behavioral stress responses. Neuroscience & Biobehavioral Reviews, 74, 366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC (2012). Behavioural Roles of Oxytocin and Vasopressin. In Neuroendocrinology and Behaviour (pp. 49–82). InTech. 10.5772/1666 [DOI] [Google Scholar]
- Neumann ID, & Landgraf R (2012). Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends in Neurosciences, 35(11), 649–659. 10.1016/j.tins.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Neumann ID, & Slattery DA (2016). Oxytocin in General Anxiety and Social Fear: A Translational Approach. Biological Psychiatry, 79(3), 213–221. 10.1016/J.BIOPSYCH.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Carter CS, Wroblewski KL, Puglia MH, Kenkel WM, Lillard TS, … Connelly JJ (2019). Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology, 99, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perras B, Droste C, Born J, Fehm HL, & Pietrowsky R (1997). Verbal memory after three months of intranasal vasopressin in healthy old humans. Psychoneuroendocrinology, 22(6), 387–396. 10.1016/S0306-4530(97)00047-4 [DOI] [PubMed] [Google Scholar]
- Quintana DS, Westlye LT, Alnæs D, Rustan ØG, Kaufmann T, Smerud KT, … Andreassen OA (2016). Low dose intranasal oxytocin delivered with Breath Powered device dampens amygdala response to emotional stimuli: A peripheral effect-controlled within-subjects randomized dose-response fMRI trial. Psychoneuroendocrinology, 69, 180–188. 10.1016/j.psyneuen.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Raggenbass M (2008). Overview of cellular electrophysiological actions of vasopressin. European Journal of Pharmacology, 583(2–3), 243–54. 10.1016/j.ejphar.2007.11.074 [DOI] [PubMed] [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie. Presses Universitaires de France. [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MAS, Vermeiren RRJM, … Rombouts SARB (2011). Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biological Psychiatry, 70(3), 291–297. 10.1016/j.biopsych.2011.02.006 [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, & Klaver P (2009). Oxytocin Makes a Face in Memory Familiar. Journal of Neuroscience, 29(1), 38–42. 10.1523/JNEUROSCI.4260-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, … Montorsi F (2005). Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior, 47(2), 164–169. 10.1016/j.yhbeh.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Sannino S, Chini B, & Grinevich V (2017). Lifespan oxytocin signaling: Maturation, flexibility, and stability in newborn, adolescent, and aged brain. Developmental Neurobiology, 77(2), 158–168. 10.1002/dneu.22450 [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, & Feldman R (2012). Oxytocin during the initial stages of romantic attachment: Relations to couples’ interactive reciprocity. Psychoneuroendocrinology, 37(8), 1277–1285. Retrieved from http://www.sciencedirect.com/science/article/pii/S0306453012000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrestani S, Kemp AH, & Guastella AJ (2013). The impact of a single administration of intranasal oxytocin on the recognition of basic emotions in humans: A meta-analysis. Neuropsychopharmacology, 38(10), 1929–1936. 10.1038/npp.2013.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJW, DiBenedictis BT, & Veenema AH (2019, April 1). Comparing vasopressin and oxytocin fiber and receptor density patterns in the social behavior neural network: Implications for cross-system signaling. Frontiers in Neuroendocrinology, Vol. 53 10.1016/j.yfrne.2019.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZE, Larkin TE, Malley MO, & Albers HE (2014). Oxytocin ( OT ) and arginine-vasopressin ( AVP ) act on OT receptors ( OTRs ) to influence social but not non- social recognition in Syrian hamsters ( Mesocricetus auratus ) Introduction : Experimental Design : Conclusions : Results : References : Hormones and Behavior, 81, 923301. [Google Scholar]
- Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, … Mendez AJ (2011). Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosomatic Medicine, 73(5), 393–400. 10.1097/PSY.0b013e31821df0c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, & Seeman TE (2010). Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychological Science, 21(1), 3–7. 10.1177/0956797609356507 [DOI] [PubMed] [Google Scholar]
- Wang Z, & De Vries GJ (1995). Androgen and estrogen effects on vasopressin messenger RNA expression in the medial amygdaloid nucleus in male and female rats. Journal of Neuroendocrinology, 7(11), 827–831. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981). WAIS-R: Manual: Wechsler adult intelligence scale--revised. New York: Psychological Corporation. [Google Scholar]
- Wei M, Russell DW, Mallinckrodt B, & Vogel DL (2007). The Experiences in Close Relationship Scale (ECR)-short form: reliability, validity, and factor structure. Journal of Personality Assessment, 88(2), 187–204. [DOI] [PubMed] [Google Scholar]
- Weingartner H, Gold P, Ballenger J, Smallberg S, Summers R, Rubinow D, … Goodwin F (1981). Effects of vasopressin on human memory functions. Science, 211(4482), 601–603. 10.1126/science.7455701 [DOI] [PubMed] [Google Scholar]
- Weisman O, Zagoory-Sharon O, Schneiderman I, Gordon I, & Feldman R (2013). Plasma oxytocin distributions in a large cohort of women and men and their gender-specific associations with anxiety. Psychoneuroendocrinology, 38(5), 694–701. [DOI] [PubMed] [Google Scholar]
- Whittington K, Connors B, King K, Assinder S, Hogarth K, & Nicholson H (2007). The effect of oxytocin on cell proliferation in the human prostate is modulated by gonadal steroids: implications for benign prostatic hyperplasia and carcinoma of the prostate. Prostate, 67(10), 1132–1142. 10.1002/pros.20612 [DOI] [PubMed] [Google Scholar]
- Zak P, Kurzban R, & Matzner W (2005). Oxytocin is associated with human trustworthiness. Hormones and Behavior, 48(5), 522–527. 10.1016/j.yhbeh.2005.07.009 [DOI] [PubMed] [Google Scholar]