Abstract
Few studies have documented relationships between endovascular therapy, duplex ultrasonography (DUS), post thrombotic syndrome (PTS), and quality of life (QOL). The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial randomized 692 patients with acute proximal deep vein thrombosis (DVT) to receive anticoagulation or anticoagulation plus pharmacomechanical catheter directed thrombolysis (PCDT). Compression DUS was obtained at baseline, 1-month and 12-months. Reflux DUS was obtained at 12-months in a subset of 126 patients. Clinical outcomes were collected over 24 months. At 1-month, patients who received PCDT had less residual thrombus compared to Control patients evidenced by non-compressible common femoral vein (CFV) (21% vs. 35%, p < 0.0001), femoral vein (51% vs. 70%, p < 0.0001) and popliteal vein (61% vs. 74%, p < 0.0001). At 12 months in the ultrasound substudy, valvular reflux prevalence was similar between groups (85% vs. 91%, p=0.35). CFV non-compressibility at 1 month was associated with higher rates of any PTS (61% vs. 46%, p<0.001), a higher incidence of moderate-or-severe PTS (30% vs. 19%, p=0.003), and worse QOL (difference 8.2 VEINES-QOL points; p=0.004) at 24 months. Valvular reflux at 12 months was associated with moderate-or-severe PTS at 24 months (30% vs. 0%, p=0.01).. In summary, PCDT results in less residual thrombus but does not reduce venous valvular reflux. CFV non-compressibility at 1 month is associated with more PTS, more severe PTS, and worse QOL at 24 months. Valvular reflux may predispose to moderate-or-severe PTS.
Keywords: Deep vein thrombosis, thrombolytic therapy, ultrasound, post-thrombotic syndrome
Introduction
Post thrombotic syndrome (PTS) describes a spectrum of adverse clinical signs and symptoms that may develop after deep vein thrombosis (DVT). Clinical features range from minor limb discomfort to severe leg pain, intractable edema, irreversible skin changes, and ulceration1. These sequelae often result in reduced quality of life (QOL) and financial burden2, 3. Unfortunately, PTS is common, developing in approximately 40% of patients after a first episode of symptomatic DVT3, 4. Once it develops, treatment of PTS is often ineffective. Little is known about how to prevent PTS once a DVT has occurred1, 5, 6.
The natural history of DVT7, the use of duplex ultrasonography (DUS) for the diagnosis of DVT,8 and longitudinal post-DVT DUS characteristics have all been described9, 10. Venous thrombus burden diminishes in most patients following DVT and often a lumen is re-established, a process known as recanalization. Proximal thrombus location and greater thrombus burden are associated with lower recanalization rates than thrombi that are confined to the distal venous segments11. More rapid thrombus resolution may result in improved valvular function12, 13. In tandem, after DVT, the prevalence of venous reflux increases over time10. Venous hypertension, vein wall inflammation and valvular reflux are considered central to the pathophysiology of PTS14
The Acute Venous Thrombosis: Thrombus Removal with Adjunctive Catheter-Directed Thrombolysis (ATTRACT) trial (ClinicalTrials.gov ) randomized patients with acute proximal DVT to receive anticoagulation or anticoagulation plus pharmacomechanical catheter-directed thrombolysis (PCDT)15. In ATTRACT, patient outcomes were collected over 2 years, including the occurrence and severity of PTS and health-related QOL. The main study outcomes have been reported elsewhere16–18. We performed the current analyses to describe the extent of residual thrombus and valvular reflux during the 12 months after proximal DVT, to determine if PCDT reduced residual thrombus and valvular reflux, and to determine if residual thrombus and reflux in the first 12 months resulted in increased PTS and reduced QOL at 24 months.
Methods
This was a phase 3, multicenter, randomized, open-label, assessor-blinded, controlled clinical trial16. The trial was approved by the institutional review boards at all participating centers, and all participants provided written informed consent.
Patients with acute symptomatic proximal DVT involving the femoral, common femoral, and/or iliac veins (with or without other involved ipsilateral veins) were enrolled at 56 centers in the United States. Participants were excluded if they had symptoms for more than 14 days. Complete inclusion and exclusion criteria have been previously published15.
Patients were randomly assigned in a 1:1 ratio to receive anticoagulation (control) or anticoagulation and PCDT (intervention). Randomization was stratified according to clinical center and thrombus extent (i.e. iliofemoral DVT or femoral-popliteal DVT). All patients in both treatment groups were provided sized-to-fit 30–40 mmHg, knee-high, graduated elastic compression stockings and were instructed to wear them during the daytime throughout follow-up. Clinical follow-up was performed through 24 months post-randomization.
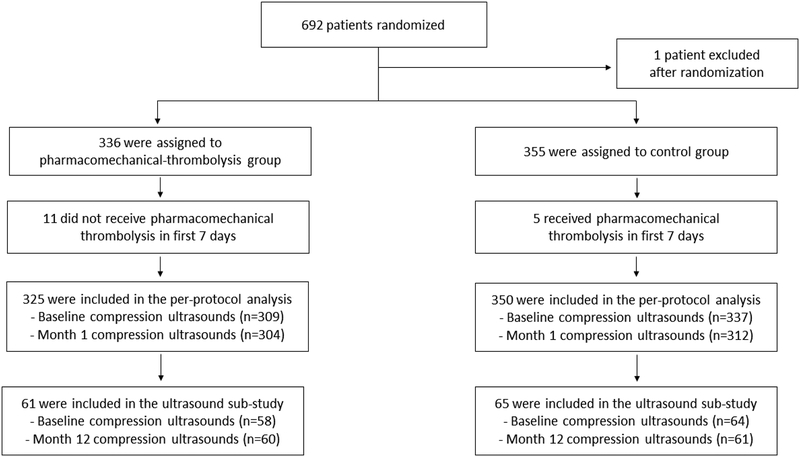
A study flow diagram is presented in Figure 1. In the overall trial, 692 patients were randomized (355 to Control, 337 to PCDT). One patient was found to not have qualifying proximal DVT immediately after randomization to PCDT and was excluded from all analyses. Five control arm patients crossed over to receive PCDT during the first 7 days post-randomization, and 11 patients randomized to the PCDT arm did not have the procedure within 7 days, leaving 350 patients in the control arm and 325 patients in the PCDT arm for the per-protocol data set that was used in these analyses.
Figure 1:
Consort diagram
Clinical outcomes
PTS was assessed at all scheduled follow-up visits by clinician examiners who were blinded to treatment allocation. The occurrence of PTS was counted when, in the index leg, a patient had a Villalta Scale score19 of 5 or greater or a venous stasis ulcer at one or more of the 6, 12, 18 or 24 month scheduled follow-up visits after randomization. PTS was also counted if a patient underwent an unplanned endovascular procedure during follow-up due to severe, progressive venous-related limb symptoms (unless a Villalta score within the previous 4 weeks was lower than 5). The severity of PTS was assessed using the continuous Villalta score and was also categorized as moderate-or-severe PTS if the Villalta score was ≥10 on any occasion. PTS severity was also measured by the venous clinical severity score (VCSS, score ranges from 0 to 27)20. For both scales, higher scores indicate more severe PTS.
In the study, venous disease-specific QOL was assessed at baseline and through 24 months post-randomization using the patient-reported Venous Insufficiency Epidemiological and Economic Study Quality of Life (VEINES-QOL) measure where lower scores indicated reduced QOL21.
Ultrasound assessments
Prior to randomization, all patients were required to have baseline venous compression DUS that assessed the compressibility of the common femoral vein (CFV), femoral vein (FV), and popliteal vein (PV) in the index leg. To minimize barriers to enrollment, these exams could be done at external facilities. The exams had to be done within 7 days prior to randomization.
At the 30-day post-randomization visit, all patients were required to undergo bilateral venous DUS to evaluate residual thrombus extent. On these exams, the compressibility of the veins (CFV, FV, PV) was recorded as being either fully compressible (defined as complete apposition of the vein walls during application of external ultrasound probe pressure), or non-compressible (any other state). In addition, the extent of proximal vein thrombus was drawn on a standardized figure, and the residual antero-posterior diameter of the externally compressed vein was assessed (as a measure of residual thrombus) for the CFV at the inguinal ligament level and for the PV at the mid-popliteal fossa level.
Ultrasound Substudy:
An ultrasound substudy, with enrollment of 142 consecutive patients in 5 designated clinical centers, was planned as part of the original study design. To estimate the sample size, we assumed a 50% risk of deep venous reflux in the Control Arm and hypothesized that PCDT would provide a 50% relative risk reduction for this outcome. Including 10% inflation for losses due to withdrawal, death, or inadequate exams, a sample of 142 patients overall was determined to provide 80% power to detect a difference of this size (α error = 0.05; two-sided).
During the first 12 months of follow-up, 13 patients were lost to follow-up, and an additional 3 patients could not have their ultrasound examinations included in the analysis due to data transmission errors. Hence, a total of 126 patients (61 PCDT Arm, 65 Control Arm) had analyzable data from the 12-month ultrasound exams. Of note, beyond 7 days post-randomization, just one patient in the Control Arm subsequently underwent a venous endovascular procedure in the index limb.
These patients underwent a detailed venous DUS of the index leg 12 months post-randomization with evaluation of thrombus extent and valvular reflux. The protocol for performing DUS was standardized across all sites by the ultrasound core laboratory. Briefly, patients were placed in a supine position with the leg externally rotated at the hip and slightly flexed at the knee. Veins were serially visualized from the CFV to the tibio-peroneal trunk. In each segment B-mode gray scale imaging was performed with and without compression maneuvers, as well as color and spectral Doppler imaging.
Reflux DUS was performed in the standing position22. Using an automated cuff inflator/deflator with appropriately sized cuffs, the presence of reflux was assessed in the CFV, FV, profunda femoral vein, PV, great saphenous vein, and small saphenous vein. While insonating the vein, the cuff was rapidly inflated and then rapidly deflated. Spectral Doppler waveforms as well as valve closure time following augmentation were recorded. Color Doppler was used to display presence or absence of flow reversal within the vein. Reversed flow >0.5 seconds was considered positive for reflux in any given deep or superficial vein segment. For purposes of this analysis, the outcome ”any reflux” was defined as the presence of reflux in any of the veins evaluated; “deep reflux” was defined as the presence of reflux in the CFV, FV, profunda femoral vein, or PV; and “superficial reflux” was defined as the presence of reflux in the great saphenous vein or small saphenous vein.
The ultrasound studies were performed in vascular laboratories that were accredited by the American College of Radiology or the Intersocietal Accreditation Commission – Vascular Testing Division. The exams were performed by registered vascular technologists who had completed an ultrasound protocol training session.
An independent core-laboratory (VasCore, the Vascular Ultrasound Core Laboratory, Massachusetts General Hospital, Boston, MA) credentialed the sonographers, led the ultrasound protocol training, provided ongoing quality oversight for the DUS exams, and adjudicated the ultrasound substudy 12-month compression and reflux DUS exams. Baseline and 1-month compression DUS exams were not routinely reviewed by the core laboratory.
A modified Venous Segmental Disease Score (VSDS) was calculated, as not all segments were available to report the original VSDS23. Obstruction was scored in the CFV (2 points), FV (1 point), profunda femoral vein (1 point), PV (2 points), and great saphenous vein (1 point); the points were summed to calculate the VSDS obstruction score (total possible = 7 points). If any flow was present on color and/or spectral Doppler, a score of ‘0’ was assigned to the segment. If there was no flow present on color and spectral Doppler, the total possible points for that segment were assigned. Reflux was scored in the great saphenous vein (1 point), small saphenous vein (0.5 point), CFV (1 point), FV (1 point), profunda femoral vein (1 point), and PV (2 points); the points were summed to calculate the VSDS reflux score (total possible = 6.5 points). If flow reversal was present for >0.5 seconds, the full possible score was counted for that segment. Missing segments were scored as ‘0’, for both the obstruction and reflux scores.
Statistical Analysis
Because the focus of these analyses was on disease mechanisms, the analysis population consisted of those patients who were randomized, had DVT at enrollment, and received the assigned treatment. Only the index leg was included in the analysis. Descriptive statistics were used to summarize demographic and clinical characteristics using mean (standard deviation) or median (25th, 75th) for continuous variables, and frequency (percentage) for categorical variables. Evaluation of differences in venous sonographic outcomes (non-compressible vs compressible vein segments) between the control and PCDT groups used multivariable logistic regression to adjust for baseline compressibility status. Differences in residual diameter between the control and PCDT groups were evaluated using multivariable linear regression to adjust for baseline compressibility status.
The association of compressibility status of vein segments (non-compressible vs compressible) with late clinical and anatomical outcomes used t-tests for continuous outcomes and chi square tests for categorical outcomes in the overall cohort. To examine if change in CFV compressibility from baseline to 1 month influenced late clinical and anatomical outcomes, these analyses were also done on the following mutually exclusive subgroups: those who had a compressible CFV at baseline and those who had a non-compressible CFV at baseline, using the same analysis methods.
In the ultrasound substudy, the association of compressibility status of vein segments (non-compressible vs compressible) and presence of any or deep reflux with late clinical and anatomical outcomes used Wilcoxon tests for continuous outcomes and chi square tests for categorical outcomes.
A two-sided P value of 0.01 or lower was considered statistically significant for all secondary efficacy outcome analyses in the ATTRACT Trial (including all analyses presented here) to reduce the likelihood of false positive findings that simply represented random chance in the context of extensive multiple testing. All analyses were conducted in SAS v9.4 (SAS Institute, Cary NC).
Results
Baseline thrombus distribution, as evident from the proportion of non-compressible venous segments, was similar in the PCDT and control arms (Table 1). The baseline characteristics of the ultrasound substudy patients were similar to the characteristics of the overall ATTRACT trial population (Table 1).
Table 1:
Baseline characteristics for overall trial participants and ultrasound sub-study participants
| Overall Trial | Ultrasound Substudy | |||||
|---|---|---|---|---|---|---|
| Total1 (N=675) | Control (N=350) | Total1 (N=126) | Control (N=65) | |||
| Age (years), median (SD) | 52.0 (42.0, 62.0) | 53.0 (43.0, 62.0) | 52.0 (41.0, 59.0) | 52.0 (42.0, 62.0) | ||
| Male, n (%) | 416 (62%) | 219 (63%) | 76 (60%) | 43 (66%) | ||
| White, n (%) | 528 (78%) | 271 (77%) | 107 (85%) | 53 (82%) | ||
| Hispanic or Latino, n (%) | 41 (6%) | 26 (7%) | 9 (7%) | 6 (9%) | ||
| BMI, median (SD) | 30.6 (26.8, 35.7) | 30.5 (26.2, 35.1) | 31.4 (27.5, 35.6) | 31.1 (26.4, 36.4) | ||
| eGFR, median (SD) | 84.2 (72.0, 98.0) | 88.0 (74.0, 102.0) | 93.5 (70.0, 108.0) | 102.0 (92.0, 116.0) | ||
| DVT Left Leg, n (%) | 416 (62%) | 213 (61%) | 78 (62%) | 38 (59%) | ||
| Non-compressible CFV, n (%) | 364/644 (57%) | 185/336 (55%) | 67/122 (55%) | 36/64 (56%) | ||
| Non-compressible FV, n (%) | 598/644 (93%) | 312/336 (93%) | 116/122 (95%) | 63/64 (98%) | ||
| Non-compressible PV, n (%) | 554/643 (86%) | 287/335 (86%) | 108/121 (89%) | 60/63 (95%) | ||
Treatment groups are per-protocol patients
BMI=Body Mass Index, eGFR=Estimated Glomerular Filtration Rate, DVT=Deep Vein Thrombus, CFV=Common Femoral Vein, FV=Femoral Vein, PCDT=Pharmacomechanical Catheter-Directed Thrombolysis, PV=Popliteal Vein, SD=Standard Deviation
Effect of PCDT Treatment Upon DUS Outcomes
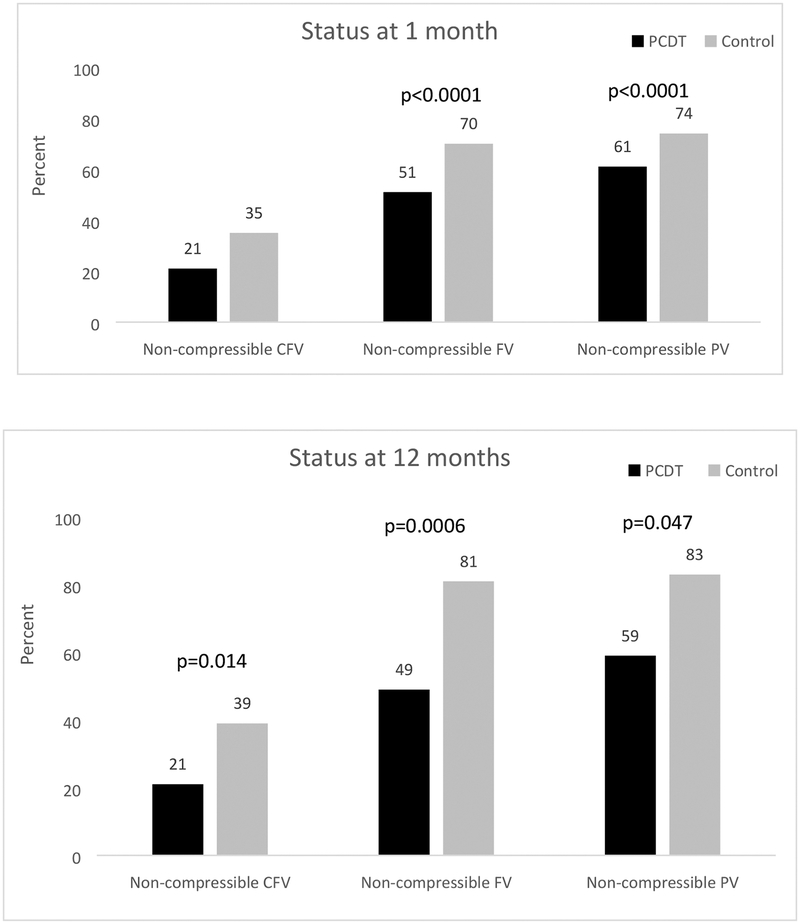
At 1 month, in the overall trial, patients in the PCDT arm had less residual thrombus as evidenced by: a lower proportion of non-compressible venous segments (Table 2) - CFV (21% PCDT vs. 35% Control, p<0.0001), FV (51% PCDT vs. 70% Control, p<0.0001), and PV (61% PCDT vs. 74% Control, p<0.0001) (Figure 2); and smaller residual diameters of the CFV and PV (Table 2).
Table 2.
Effect of PCDT on Venous Sonographic Outcomes at 1 month and 12 months
| Overall Trial | Status at 1 Month | Control | p-value2 |
| CFV diameter (mm), mean (SD) | 3.4 (7.3) | 0.0002 | |
| PV diameter (mm), mean (SD) | 6.4 (7.2) | <0.0001 | |
| Ultrasound Substudy | Status at 12 Months | Control | p-value2 |
| CFV diameter (mm), mean (SD) | 3.2 (4.7) | 0.003 | |
| PV diameter (mm), mean (SD) | 7.2 (5.1) | 0.004 | |
| Obstruction score3, mean (SD) | 0.14 (0.4) | 0.49 | |
| Reflux score3, mean (SD) | 3.0 (1.7) | 0.39 | |
| Any reflux present, n (%) | 57/63 (91%) | 0.35 | |
| Deep reflux present, n (%) | 54/63 (86%) | 0.71 | |
| Superficial reflux present, n (%) | 23/63 (37%) | 0.44 |
Treatment groups are per-protocol patients
Comparisons adjusted for baseline compressibility status for CFV, PV and FV at 1 month and 1 year
Scores are derived from the modified Venous Segmental Disease Scale (VSDS)
CFV=Common Femoral Vein,, PCDT=Pharmacomechanical Catheter-Directed Thrombolysis, PV=Popliteal Vein, SD=Standard Deviation
Figure 2.
Effect of PCDT on Venous Compressibility at 1 month and 12 months
CFV=Common Femoral Vein, FV=Femoral Vein, PCDT=Pharmacomechanical Catheter-Directed Thrombolysis, PV=Popliteal Vein
At 12 months, in the ultrasound substudy participants, patients in the PCDT arm also had less residual thrombus, which reached statistical significance for the proportion with FV non-compressibility (Table 2 and Figure 2). The VSDS obstruction score at 12 months did not differ between groups. The distribution of completely obstructed venous segments at 12 months can be found in Supplemental Table S1.
At 12 months, in the ultrasound substudy, the proportions of patients with reflux in any vein (85% PCDT vs. 91% Control, p=0.35) and any deep vein (83% PCDT vs. 86% Control, p=0.71) were similar in both groups. The anatomical distribution of refluxing segments can be found in Supplemental Table S2. VSDS reflux score at 12 months was similar in both groups, as was the occurrence of reflux in any superficial vein (43% PCDT vs. 37% Control, p=0.44) (Table 2).
Relationship of 1-month DUS findings to 12-month and 24-month outcomes
In the overall trial, CFV non-compressibility at 1 month was associated with a higher rate of PTS (61% vs. 46%, p<0.001), a higher rate of moderate-or-severe PTS (30% vs. 19%, p=0.003), and lower QOL scores (difference 8.2 VEINES-QOL scale units, p=0.004) at 24 months (Table 3). In contrast, the presence of either femoral vein or popliteal vein non-compressibility at 1 month was not associated with a lower rate of PTS (52% vs. 44%, p=0.07), moderate-or-severe PTS (23% vs. 19%, p=0.23), or better QOL (p=0.26). In the analysis that included only those patients with a non-compressible CFV at baseline, restoration of CFV compressibility at 1-month was associated with better clinical outcomes – this reached statistical significance for 24-month PTS (62% vs. 46%, p=.004) and QOL (difference 7.3 VEINES-QOL scale points, p=0.01), but not moderate-or-severe PTS (29% vs. 21%, p=0.07) (Supplemental Table S3). In the analysis that included only those patients with a compressible CFV at baseline, there continued to be no relationship between 1-month compressibility of the femoral and popliteal veins and late clinical outcomes (Supplemental Table S3).
Table 3.
Association of venous non-compressibility at 1 month with late clinical, anatomical, and physiological outcomes
| Clinical Outcome | Status of CFV at 1 Month | Status of FV and PV at 1 Month | |||||
|---|---|---|---|---|---|---|---|
| Compressible | p-value | Both compressible | p-value | ||||
| Overall Trial | Any PTS, n (%) | 205/447 (46%) | <0.001 | 70/160 (44%) | 0.07 | ||
| Moderate-or-Severe PTS, n (%) | 85/447 (19%) | 0.003 | 30/160 (19%) | 0.23 | |||
| Villalta score at 24 montds, mean (SD) | 4.0 (4.6) | 0.10 | 3.9 (4.4) | 0.24 | |||
| VCSS score at 24 montds, mean (SD) | 2.1 (2.7) | 0.21 | 1.7 (2.4) | 0.02 | |||
| VEINES-QOL score at 24 montds, mean (SD) | 81.4 (20) | 0.004 | 79.3 (24) | 0.26 | |||
| Anatomical/Physiological Outcome | Status of CFV at 1 Month | Status of FV and PV at 1 Month | |||||
| Compressible | p-value | Both compressible | p-value | ||||
| Ultrasound Substudy | Non-compressible CFV at 12 months, n (%) | 1/83 (1%) | <0.001 | 5/28 (18%) | 0.86 | ||
| Non-compressible FV at 12 months, n (%) | 46/84 (55%) | 0.35 | 2/28 (7%) | <0.001 | |||
| Non-compressible PV at 12 months, n (%) | 53/84 (63%) | 0.18 | 0/28 (0%) | <0.001 | |||
| Obstruction score at 12 months, mean (SD) | 0.1 (0.4) | 0.15 | 0.04 (0.2) | 0.33 | |||
| CFV diameter (mm), mean (SD) | 0.05 (0.4) | <0.001 | 0.6 (1.5) | 0.57 | |||
| PV diameter (mm), mean (SD) | 2.8 (2.4) | 0.07 | 0 (0.0) | <0.001 | |||
| Reflux score at 12 months, mean (SD) | 2.8 (1.7) | 0.57 | 1.9 (1.7) | 0.002 | |||
| Any reflux present at 12 months, n (%) | 74/84 (88%) | 0.76 | 19/27 (70%) | 0.002 | |||
| Deep reflux present at 12 months, n (%) | 71/84 (85%) | 0.87 | 19/27 (70%) | 0.02 | |||
CFV=Common Femoral Vein, FV=Femoral Vein, PTS=Post-thrombotic syndrome, PV=Popliteal Vein, SD=Standard Deviation, VCSS=Venous Clinical Severity Score, VEINES-QOL=Venous Insufficiency Epidemiological and Economic Study Quality of Life
In the ultrasound substudy, CFV non-compressibility at 1 month did not predict the presence of valvular reflux in the deep veins or any veins at 12 months (Table 3). In contrast, FV or PV non-compressibility at 1 month appeared to be associated with more valvular reflux at 12 months which reached statistical significance for any reflux (93% vs. 70%, p=0.002) and the VSDS Reflux Score (3.2 [1.6] vs. 1.9 [1.7], p = 0.002) but not for deep reflux (88% vs. 70%, p = 0.02).
Relationship of 12-month DUS findings to 24-month clinical outcomes
In the ultrasound substudy, compressibility of the CFV at 12 months appeared to be associated with favorable clinical outcomes at 24 months – these relationships approached statistical significance for any PTS (83% vs. 57%, p=0.02) but were less compelling for moderate-or-severe PTS (39% vs. 22%, p=0.09) and QOL (difference 7.5 VEINES-QOL scale points, p=0.08) (Table 4). Compressibility of both the femoral vein and popliteal vein (compared to non-compressibility of one or both) at 12 months was not associated with lower rates of PTS (56% vs. 64%, p=0.40) or with less moderate-or-severe PTS (21% vs. 27%, p=0.41) at 24 months.
Table 4.
Association of 12-month venous sonographic outcomes with 24-month clinical outcomes in Ultrasound Sub-study
| Clinical Outcome | Status of CFV at 12 Months | Status of FV and PV at 12 Months | ||||
|---|---|---|---|---|---|---|
| Compressible | p-value | Both compressible | p-value | |||
| Any PTS, n (%) | 56/99 (57%) | 0.02 | 22/39 (56%) | 0.40 | ||
| Moderate-or-Severe PTS, n (%) | 22/99 (22%) | 0.09 | 8/39 (21%) | 0.41 | ||
| Villalta score at 24 montds, mean (SD) | 4.2 (4.3) | 0.06 | 4.1 (4.3) | 0.45 | ||
| VCSS score at 24 montds, mean (SD) | 1.9 (2.5) | 0.11 | 1.5 (2.2) | 0.05 | ||
| VEINES-QOL score at 24 montds, mean (SD) | 79.7 (22) | 0.08 | 78.8 (24) | 0.37 | ||
| Clinical Outcome | Any Reflux at 12 Months | Deep Reflux at 12 Months | ||||
| No | p-value | No | p-value | |||
| Any PTS, n (%) | 8/15 (53%) | 0.47 | 11/19 (58%) | 0.70 | ||
| Moderate-or-Severe PTS, n (%) | 0/15 (0%) | 0.01 | 1/19 (5%) | 0.02 | ||
| Villalta score at 24 months, mean (SD) | 2.7 (2.7) | 0.10 | 3.2 (3.1) | 0.23 | ||
| VCSS score at 24 months, mean (SD) | 1.3 (1.9) | 0.12 | 1.4 (1.8) | 0.24 | ||
| VEINES-QOL score at 24 months, mean (SD) | 82.0 (20) | 0.39 | 79.9 (22) | 0.53 | ||
CFV=Common Femoral Vein, FV=Femoral Vein, PTS=Post-thrombotic syndrome, PV=Popliteal Vein, SD=Standard Deviation, VCSS=Venous Clinical Severity score, VEINES-QOL=Venous Insufficiency Epidemiological and Economic Study Quality of Life
While reflux at 12 months was not associated with more any PTS (63% vs. 53%, p=0.47), it was associated with more moderate-or-severe PTS (30% vs. 0%, p=0.01) at 24 months. Similarly, there was no association of deep vein reflux with any PTS (63% vs. 58%, p=0.70), but deep vein reflux appeared to be associated with moderate-or-severe PTS (30% vs. 5%, p=0.02). Superficial vein reflux was associated with any PTS (78% vs. 51%, p=0.003), and also appeared to be associated with moderate-or-severe PTS (37% vs. 19%, p=0.03) (Table 5).
Table 5.
Association of 12-month superficial vein reflux with 24-month clinical outcomes in Ultrasound Sub-study
| Clinical Outcome | Any Superficial Reflux at 12 Months | ||
|---|---|---|---|
| No | p-value | ||
| Any PTS, n (%) | 38/74 (51%) | 0.003 | |
| Moderate-or-Severe PTS, n (%) | 14/74 (19%) | 0.03 | |
| Villalta score at 24 months, mean (SD) | 3.8 (4.3) | 0.005 | |
| VCSS score at 24 months, mean (SD) | 1.7 (2.1) | 0.02 | |
| VEINES-QOL score at 24 months, mean (SD) | 80.9 (20) | 0.06 | |
PTS=Post-thrombotic syndrome, SD=Standard Deviation, VCSS=Venous Clinical Severity score, VEINES-QOL=Venous Insufficiency Epidemiological and Economic Study Quality of Life
Of patients who had a compressible CFV and no valvular reflux at 12 months follow-up, 6/13 (46%) developed PTS but none (0%) developed moderate-or-severe PTS. Of patients who had both a non-compressible CFV and valvular reflux at 12 months follow-up, PTS developed in 16/20 (80%) and moderate-or-severe PTS developed in 9/20 (45%).
Discussion
In this large, randomized study of anticoagulation alone versus anticoagulation and PCDT for acute proximal DVT, our findings collectively suggest that: 1) the use of PCDT is associated with lower thrombus burden at 1-month and likely also at 12 months; 2) a thrombus-free CFV at 1 month is associated with improved 24-month clinical outcomes, including PTS, moderate-or-severe PTS, and QOL, but the same is not true for a thrombus-free femoral-popliteal venous segment; 3) in patients presenting with CFV thrombus, successful restoration of full CFV compressibility during the first month is associated with reduced PTS and improved QOL, and possibly also with reduced moderate-or-severe PTS; 4) the use of PCDT is not associated with less venous valvular reflux (deep or superficial) at 12 months; and 5) venous valvular reflux appears to play a role in progression to moderate-or-severe PTS.
Venous obstruction and valvular reflux contribute to venous hypertension, which has been considered a central component of the pathophysiology of PTS. The “open vein hypothesis” has posited that preservation of late venous patency and valvular competence may prevent PTS, and that early thrombus removal may assist this process24–26. In a series of ultrasound studies in anticoagulated DVT patients, Meissner et al. found that venous segments showing delayed thrombus clearance were more likely to develop valvular reflux, and that reflux developed more often if there was DVT propagation or re-thrombosis27. In a randomized trial evaluating compression therapy, Prandoni et al. found that PTS developed more frequently in proximal DVT patients with residual venous thrombus or popliteal valvular reflux at 6-month follow-up (n = 180, 47% vs. 23%, p < 0.01)28. In a prospective analysis of 93 patients who presented with iliofemoral DVT and underwent CDT, combining various DUS-derived measures including thrombus burden, venous obstruction, and venous valvular reflux was useful in PTS prediction29.
However, the relationships among thrombus burden, the presence and sites of valvular reflux, and PTS have not been consistent24. Previous studies have demonstrated substantial rates of both residual thrombus and valvular reflux in patients with and without PTS. While the degree of initial venous occlusion may correlate with later reflux29, the effect of treatment has remained uncertain.
Role of Residual Thrombus Burden and Venous Obstruction
In ATTRACT, PCDT resulted in reduced thrombus burden at 1 month and at 12 months in the lower extremity proximal veins. This finding is very similar to what was observed in a previous multicenter randomized trial that evaluated CDT in 189 patients with proximal DVT (the CaVenT study). In that study, iliofemoral venous obstruction at 2 years (assessed by a combination of DUS and air plethysmography) was less prevalent in patients who underwent CDT (25% vs. 40%)30, to a similar degree as in ATTRACT. Furthermore, in CaVenT, iliofemoral venous obstruction was present in more patients who developed PTS (Villalta score ≥ 5) as compared with those without PTS (44% vs. 23%).
Of note, the proportion of patients with residual thrombus during follow-up was higher than one might expect given the extensive nature of the endovascular procedures in both studies. It is unclear if this is the result of venographically occult thrombus that remained after PCDT, versus later development of asymptomatic recurrent thrombosis, and if additional focus on minimizing recurrence in the early weeks after PCDT (e.g., with improved antiplatelet and anticoagulation strategies, improved PCDT technique up front, or additional imaging surveillance) may prove beneficial in increasing the effectiveness of PCDT in PTS prevention.
Role of Valvular Reflux
In the Control arm of the ultrasound substudy of the ATTRACT trial, 86% of patients had deep venous valvular reflux after 12 months follow-up. Although alternate thresholds have been applied to define deep vein reflux, we deliberately chose an inclusive threshold of 0.5 seconds to avoid missing instances of reflux. In any case, this finding is consistent with previous studies that have reported high rates of valvular reflux in patients who experienced a proximal DVT and were treated with either anticoagulation alone (including the randomized CaVenT study, 83% at 2 years)30 or with systemic thrombolysis (Laiho et al, 81%)31.
However, in ATTRACT, PCDT did not reduce the occurrence of valvular reflux. This finding differs substantially from what has been observed in previous retrospective studies32 and smaller randomized trials30, 33. In a randomized trial comparing pulse-spray CDT to anticoagulation alone for patients with iliofemoral DVT, results at 6 months were available in 35 patients and reflux was present in fewer patients in the pulse-spray CDT arm (11% vs. 41%)33. In the CaVenT trial (n=189), femoral-popliteal reflux at 2 years was present in fewer patients who underwent CDT (66.7% vs. 83.2%, p = 0.009)30. In that study, patients who developed PTS had more venous reflux at 2 years as compared with those who did not develop PTS (89.8% vs. 61.9%).
The reasons for the differences in the effect of catheter intervention upon valvular reflux between ATTRACT and CaVenT are unknown. In both studies the presence of reflux was adjudicated by an independent core-laboratory, but the thrombus removal method used (CDT in CaVenT, PCDT in ATTRACT) differed to an extent. One possibility is that the use of mechanical thrombectomy devices for PCDT may promote valve injury, which could be either macroscopic or related to aggravation of inflammation or other biological mechanisms. Another possibility is that a longer period of thrombolysis (e.g., the 48 hours in CaVenT versus the 20 hours in ATTRACT) could provide more complete thrombus clearance or improved inflow, contributing to restoration of normal vein function. Of course, it is also possible that the observed differences in valvular reflux between the studies are unrelated to the procedure type but stem more from differences in the conduct of the ultrasound assessments, differences in study size or location (Norway versus United States), or from the overall level of methodological rigor applied. Unfortunately, ATTRACT does not provide insight into how to minimize the development of valvular reflux.
Study Limitations
This study is not without limitations. For budgetary reasons, it was only possible to perform detailed ultrasound examinations at 12 months in 142 patients, which reduced our ability to assess inter-relationships with the 12-month ultrasound assessments. Baseline and 1-month compression ultrasound assessments were not routinely centrally interpreted. Information on the baseline presence of reflux was not available since the study patients were only identified after the diagnosis of acute DVT; patients with known established ipsilateral PTS were excluded, but historical information on the presence of varicose veins or chronic venous insufficiency prior to the onset of the index DVT was not recorded. Our analysis involved substantial multiple testing. Finally, while most of the design and outcomes were pre-specified, the sonographers did not use the defined VSDS venous obstruction criteria, which reduced the utility of that assessment.
Conclusion
In conclusion, residual thrombus and valvular reflux are present with high frequency after proximal DVT. PCDT leads to reduced late residual thrombus burden but does not prevent venous valvular reflux from developing. While less thrombus burden in the CFV is associated with less PTS, less moderate-or-severe PTS, and better QOL, that is not the case for thrombus burden in the FV and PV. Valvular reflux may have a role in progression to moderate-or-severe PTS. Thus, to reduce PTS, additional study of the open vein hypothesis in the iliofemoral venous segment may be helpful, but new insights into alternative mechanisms (biochemical, genetic, inflammatory, and/or microvascular) will likely be needed to fully elucidate the pathophysiology of PTS and to identify new targets for therapy.
Supplementary Material
Acknowledgements
The authors wish to thank Victoria B. Sova, Sandra M. Croteau, and the entire network of investigators and study staff at the ATTRACT Trial coordinating centers, core laboratories, vascular ultrasound laboratories, and clinical centers (see Appendix).
Sources of funding
The ATTRACT Trial was supported by grants from the National Heart, Lung, and Blood Institute (NHLBI) for the clinical coordinating center (U01-HL088476 to Washington University in St. Louis) and data coordinating center (U01-HL088118 to McMaster University, Hamilton, ON); the Washington University Center for Translational Therapies in Thrombosis, which is supported by a grant from the NHLBI (U54-HL112303); the Washington University Institute of Clinical and Translational Sciences, which is supported by a grant from the National Center for the Advancement of Translational Sciences (UL1-TR00044810); Boston Scientific; Covidien (now Medtronic); Genentech; the Society of Interventional Radiology Foundation; the Canada Research Chairs Program (Tier 1 support to Dr. Susan Kahn); the CanVECTOR Network (funded by Canadian Institutes of Health Research CDT-142654, to Dr. Kahn); the Heart and Stroke Foundation of Canada (Investigator Award to Dr. Kearon); and a Jack Hirsh Professorship in Thrombosis (to Dr. Kearon). BSN Medical donated the compression stockings.
Disclosures
Ido Weinberg - None; Suresh Vedantham - Grant support from Cook Medical; Amber Salter - None; Gail Hadley - None; Noor Al-Hammadi - None; Clive Kearon - None; Jim A. Julian - None; Mahmood K. Razavi - Consulting fees from Abbott, Boston Scientific, Medtronic, Veniti, and Volcano/Phillips; Heather L. Gornik - Research support from CVR Global, Flexlife Health/Zin Medical - Equity; Samuel Z. Goldhaber - Grant support from BiO2 Medical; grant support and consulting fees, Boehringer Ingelheim, BMS, Daiichi Sankyo, Janssen, Portola, Bayer, and BTG/Ekos; Anthony J. Comerota - Consulting fees from Medtronic; Andrei L. Kindzelski - None; Robert M. Schainfeld - None; John F. Angle - Consultant for Proteon Therapeutics; grant support from Siemens Medical; Sanjay Misra - Consultant for Medtronic, DSMB Chair for Flexstent and Cordis Johnson & Johnson; Jonathan A. Schor - None; Darren Hurst - None; Michael R. Jaff - Holds equity in Embolitech and Venarum; uncompensated advisor, Boston Scientific, Cordis Corporation; compensated advisor: Medtronic, Sanofi, BTG; consultant, Volcano/Phillips.
References
- 1.Kahn SR, Shapiro S, Wells PS, Rodger MA, Kovacs MJ, Anderson DR, Tagalakis V, Houweling AH, Ducruet T, Holcroft C, Johri M, Solymoss S, Miron MJ, Yeo E, Smith R, Schulman S, Kassis J, Kearon C, Chagnon I, Wong T, Demers C, Hanmiah R, Kaatz S, Selby R, Rathbun S, Desmarais S, Opatrny L, Ortel TL, Ginsberg JS and investigators SOXt. Compression stockings to prevent post-thrombotic syndrome: a randomised placebo-controlled trial. Lancet. 2014;383:880–8. [DOI] [PubMed] [Google Scholar]
- 2.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johri M and Ginsberg JS. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–12. [DOI] [PubMed] [Google Scholar]
- 3.Ashrani AA and Heit JA. Incidence and cost burden of post-thrombotic syndrome. J Thromb Thrombolysis. 2009;28:465–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prandoni P, Lensing AW, Cogo A, Cuppini S, Villalta S, Carta M, Cattelan AM, Polistena P, Bernardi E and Prins MH. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Ten Cate-Hoek AJ, Amin EE, Bouman AC, Meijer K, Tick LW, Middeldorp S, Mostard GJM, Ten Wolde M, van den Heiligenberg SM, van Wissen S, van de Poel MH, Villalta S, Serne EH, Otten HM, Klappe EH, Bistervels IM, Lauw MN, Piersma-Wichers M, Prandoni P, Joore MA, Prins MH, Ten Cate H and investigators ID. Individualised versus standard duration of elastic compression therapy for prevention of post-thrombotic syndrome (IDEAL DVT): a multicentre, randomised, single-blind, allocation-concealed, non-inferiority trial. Lancet Haematol. 2018;5:e25–e33. [DOI] [PubMed] [Google Scholar]
- 6.Mol GC, van de Ree MA, Klok FA, Tegelberg MJ, Sanders FB, Koppen S, de Weerdt O, Koster T, Hovens MM, Kaasjager HA, Brouwer RE, Kragten E, Schaar CG, Spiering W, Arnold WP, Biesma DH and Huisman MV. One versus two years of elastic compression stockings for prevention of post-thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ. 2016;353:i2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kearon C Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. [DOI] [PubMed] [Google Scholar]
- 8.White RH, McGahan JP, Daschbach MM and Hartling RP. Diagnosis of deep-vein thrombosis using duplex ultrasound. Ann Intern Med. 1989;111:297–304. [DOI] [PubMed] [Google Scholar]
- 9.Franzeck UK, Schalch I, Jager KA, Schneider E, Grimm J and Bollinger A. Prospective 12-year follow-up study of clinical and hemodynamic sequelae after deep vein thrombosis in low-risk patients (Zurich study). Circulation. 1996;93:74–9. [DOI] [PubMed] [Google Scholar]
- 10.Markel A, Manzo RA, Bergelin RO and Strandness DE Jr Valvular reflux after deep vein thrombosis: incidence and time of occurrence. J Vasc Surg. 1992;15:377–82; discussion 383–4. [PubMed] [Google Scholar]
- 11.Tick LW, Doggen CJ, Rosendaal FR, Faber WR, Bousema MT, Mackaay AJ, P VANB MH. Predictors of the post-thrombotic syndrome with non-invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J Thromb Haemost. 2010;8:2685–92. [DOI] [PubMed] [Google Scholar]
- 12.Meissner MH, Manzo RA, Bergelin RO, Markel A and Strandness DE Jr. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18:596–605; discussion 606–8. [PubMed] [Google Scholar]
- 13.Singh H and Masuda EM. Comparing short-term outcomes of femoral-popliteal and iliofemoral deep venous thrombosis: early lysis and development of reflux. Ann Vasc Surg. 2005;19:74–9. [DOI] [PubMed] [Google Scholar]
- 14.Henke PK and Comerota AJ. An update on etiology, prevention, and therapy of postthrombotic syndrome. J Vasc Surg. 2011;53:500–9. [DOI] [PubMed] [Google Scholar]
- 15.Vedantham S, Goldhaber SZ, Kahn SR, Julian J, Magnuson E, Jaff MR, Murphy TP, Cohen DJ, Comerota AJ, Gornik HL, Razavi MK, Lewis L and Kearon C. Rationale and design of the ATTRACT Study: a multicenter randomized trial to evaluate pharmacomechanical catheter-directed thrombolysis for the prevention of postthrombotic syndrome in patients with proximal deep vein thrombosis. Am Heart J. 2013;165:523–530 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Duncan JR, Nieters P, Derfler MC, Filion M, Gu CS, Kee S, Schneider J, Saad N, Blinder M, Moll S, Sacks D, Lin J, Rundback J, Garcia M, Razdan R, VanderWoude E, Marques V, Kearon C and Investigators AT. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017;377:2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearon C, Gu CS, Julian JA, Goldhaber SZ, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Kahn SR, Kindzelski AL, Slater D, Geary R, Winokur R, Natarajan K, Dietzek A, Leung DA, Kim S and Vedantham S. Pharmacomechanical Catheter-Directed Thrombolysis in Acute Femoral-Popliteal Deep Vein Thrombosis: Analysis from a Stratified Randomized Trial. Thromb Haemost. 2019. [DOI] [PubMed] [Google Scholar]
- 18.Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, Jaff MR, Razavi MK, Kindzelski AL, Bashir R, Patel P, Sharafuddin M, Sichlau MJ, Saad WE, Assi Z, Hofmann LV, Kennedy M, Vedantham S and Investigators AT. Endovascular Thrombus Removal for Acute Iliofemoral Deep Vein Thrombosis: Analysis from a Stratified Multicenter Randomized Trial. Circulation. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–8. [DOI] [PubMed] [Google Scholar]
- 20.Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, Meissner MH, Rutherford RB and American Venous Forum Ad Hoc Outcomes Working G. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387–96. [DOI] [PubMed] [Google Scholar]
- 21.Lamping DL, Schroter S, Kurz X, Kahn SR and Abenhaim L. Evaluation of outcomes in chronic venous disorders of the leg: development of a scientifically rigorous, patient-reported measure of symptoms and quality of life. J Vasc Surg. 2003;37:410–9. [DOI] [PubMed] [Google Scholar]
- 22.van Bemmelen PS, Bedford G, Beach K and Strandness DE. Quantitative segmental evaluation of venous valvular reflux with duplex ultrasound scanning. J Vasc Surg. 1989;10:425–31. [DOI] [PubMed] [Google Scholar]
- 23.Rutherford RB, Padberg FT Jr., Comerota AJ, Kistner RL, Meissner MH and Moneta GL. Venous severity scoring: An adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–12. [DOI] [PubMed] [Google Scholar]
- 24.Roumen-Klappe EM, den Heijer M, Janssen MC, van der Vleuten C, Thien T and Wollersheim H. The post-thrombotic syndrome: incidence and prognostic value of non-invasive venous examinations in a six-year follow-up study. Thromb Haemost. 2005;94:825–30. [DOI] [PubMed] [Google Scholar]
- 25.Grewal NK, Martinez JT, Andrews L and Comerota AJ. Quantity of clot lysed after catheter-directed thrombolysis for iliofemoral deep venous thrombosis correlates with postthrombotic morbidity. J Vasc Surg. 2010;51:1209–14. [DOI] [PubMed] [Google Scholar]
- 26.Comerota AJ, Grewal N, Martinez JT, Chen JT, Disalle R, Andrews L, Sepanski D and Assi Z. Postthrombotic morbidity correlates with residual thrombus following catheter-directed thrombolysis for iliofemoral deep vein thrombosis. J Vasc Surg. 2012;55:768–73. [DOI] [PubMed] [Google Scholar]
- 27.Meissner MH, Caps MT, Zierler BK, Polissar N, Bergelin RO, Manzo RA and Strandness DE, Jr. Determinants of chronic venous disease after acute deep venous thrombosis. J Vasc Surg. 1998;28:826–33. [DOI] [PubMed] [Google Scholar]
- 28.Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost 2005; 3(2):401–402. [DOI] [PubMed] [Google Scholar]
- 29.Yamaki T, Nozaki M, Sakurai H, Takeuchi M, Soejima K and Kono T. High peak reflux velocity in the proximal deep veins is a strong predictor of advanced post-thrombotic sequelae. J Thromb Haemost. 2007;5:305–12. [DOI] [PubMed] [Google Scholar]
- 30.Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM and Klow NE. Residual rates of reflux and obstruction and their correlation to post-thrombotic syndrome in a randomized study on catheter-directed thrombolysis for deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2014;2:123–30. [DOI] [PubMed] [Google Scholar]
- 31.Laiho MK, Oinonen A, Sugano N, Harjola VP, Lehtola AL, Roth WD, Keto PE and Lepantalo M. Preservation of venous valve function after catheter-directed and systemic thrombolysis for deep venous thrombosis. Eur J Vasc Endovasc Surg. 2004;28:391–6. [DOI] [PubMed] [Google Scholar]
- 32.Sillesen H, Just S, Jorgensen M and Baekgaard N. Catheter directed thrombolysis for treatment of ilio-femoral deep venous thrombosis is durable, preserves venous valve function and may prevent chronic venous insufficiency. Eur J Vasc Endovasc Surg. 2005;30:556–62. [DOI] [PubMed] [Google Scholar]
- 33.Elsharawy M and Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.