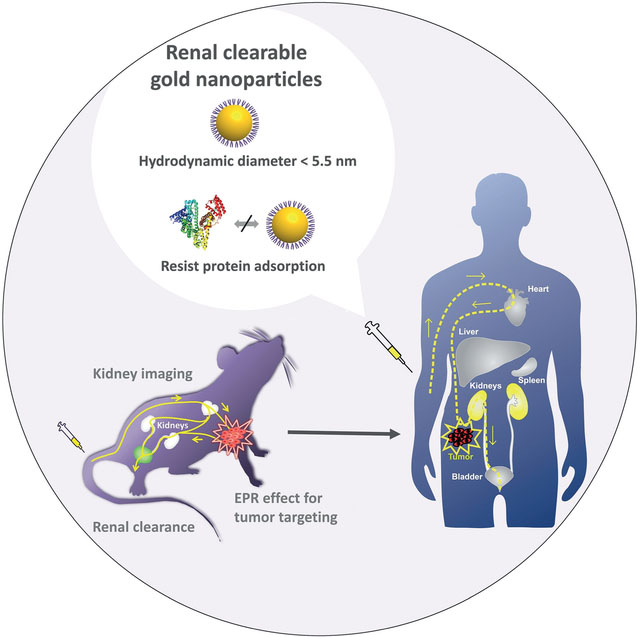
Abstract
With more and more engineered nanoparticles (NPs) being translated to the clinic, the United States Food and Drug Administration (FDA) has recently issued the latest draft guidance on nanomaterial-containing drug products with an emphasis on understanding their in vivo transport and nano–bio interactions. Following these guidelines, NPs can be designed to target and treat diseases more efficiently than small molecules, have minimum accumulation in normal tissues, and induce minimum toxicity. In this Minireview, we integrate this guidance with our ten-year studies on developing renal clearable luminescent gold NPs. These gold NPs resist serum protein adsorption, escape liver uptake, target cancerous tissues, and report kidney dysfunction at early stages. At the same time, off-target gold NPs can be eliminated by the kidneys with minimum accumulation in the body. Additionally, we identify challenges to the translation of renal clearable gold NPs from the bench to the clinic.
Keywords: clinical translation, gold, medical imaging, nanoparticles, renal clearance
Graphical Abstract
1. Introduction
In December 2017, the U.S. Food and Drug Administration (FDA) issued the document entitled “Draft Guidance for Industry—Drug Products, Including Biological Products, that Contain Nanomaterials”.[1] This is the latest guidance document on the application of nanotechnology in human drug products, in which nanomaterials are present in the finished dosage form and can function as active ingredients, carriers for active ingredients, or inactive ingredients. In this guidance, FDA-regulated products that involve nanotechnology refer to materials with at least one dimension in the nanoscale range (approximately 1–100 nm), or materials that exhibit properties that are attributed to their dimensions, even if the dimensions fall outside the nanoscale range (Page 1). This document, combined with other guidances on drugs, provides both general principles and specific considerations for quality control and nonclinical and clinical studies of nanomedicines.
Engineered nanoparticles (NPs), owing to their high surface energy, are known to favor serum protein adsorption, which could lead to changes in their organ distribution, excretion pathways, and biocompatibility.[2–5] For example, in this guidance the FDA pointed out that “elimination of the nanomaterial-protein complex occurs mainly through phagocytosis by macrophages of the mononuclear phagocyte system, predominantly in the liver and spleen”, while “small hydrophilic nanomaterials may be eliminated by the kidney” (Page 5). Different from organic NPs that can degrade in vivo, inorganic NPs (such as quantum dots and gold NPs) are often nonbiodegradable and nonspecifically accumulate in the liver and spleen for months to years.[6–9] Such long-term retention in the body raises the safety concern, as the FDA commented that “Components that are nonbiodegradable can accumulate and persist longer than biodegradable components and can consequently produce effects related to chronic exposure to these components” (Page 15). Thus, this guidance emphasizes the importance of understanding the nano–bio interactions while considering the impact of serum protein binding in the native physiological environment so that potential accumulation and resulted health hazards of NPs can be minimized.
In addition to altering the organ distribution of NPs, the highly dynamic process of protein adsorption/desorption on NPs makes it challenging to understand and precisely control in vivo transport of engineered NPs. Significant efforts have been dedicated to the investigation of the effect of protein coronas on nano—bio interactions.[10–12] In the past ten years, we took a different path to address this issue by developing luminescent gold nanoparticles (AuNPs) that are highly resistant to serum protein binding (Figure 1 and Table S1).[9,13–15] In addition, these AuNPs have overall hydrodynamic diameters below the kidney filtration threshold (about 5.5 nm);[16–17] therefore, they can be eliminated out of the body through the urinary system with high efficiency comparable to those of clinically used small-molecule imaging agents,[9] renal clearable quantum dots[16] and C’ dots (Cornell dots).[18–19] We further applied these renal clearable luminescent AuNPs to explore some fundamental physiological questions on the nanoscale, including how physicochemical properties of renal clearable NPs affect their pharmacokinetics and renal clearance, whether they can still target tumors through the enhanced permeability and retention (EPR) effect, how to use these NPs to address the challenges in kidney disease diagnosis that no other probes have not been able to overcome so far, and whether they are biocompatible enough for the future clinical translation. In the following sections, we will revisit our efforts from a viewpoint of in vivo transport and nano–bio interactions in tissues/organs and integrate the results with the latest FDA guidance on nanomedicines. We hope that the knowledge we have gained from these renal clearable AuNPs will also help accelerate the clinical translations of many other engineered NPs, in particular non-biodegradable NPs.
Figure 1.
Key findings in the development of renal clearable luminescent gold nanoparticles towards clinical translation. #Effects of physicochemical properties on pharmacokinetics and renal clearance. §Interaction of renal clearable gold nanoparticles with tumor microenvironment. ‡Interaction of renal clearable gold nanoparticles with the kidney. The references are shown in Table S1 in the Supporting Information. Milestones in the development of renal clearable nanomedicines are also marked, such as the development of renal clearable quantum dots by Choi and co-workers (Ref. [16]), report of C’ dots (Cornell dots) and first-in-human trial of C’ dots by Wiesner, Bradbury, and co-workers(Refs. [18,19]).
2. Synthesis of Renal Clearable Luminescent Metal Nanoparticles
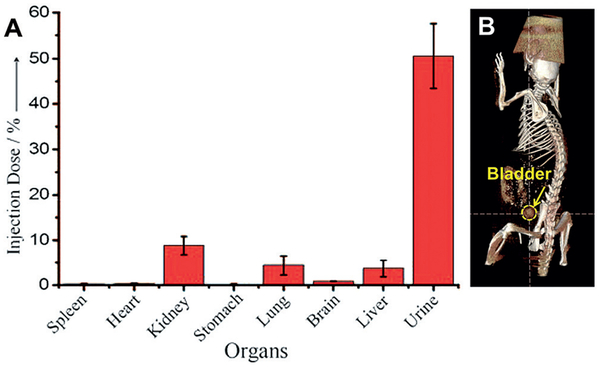
The synthesis of highly luminescent glutathione-coated gold nanoparticles (GS-AuNPs) with core sizes of 1–3 nm was first described in Zheng’s PhD dissertation in 2005[20] and reported in 2010.[21] Efficient renal clearance of these luminescent GS-AuNPs was discovered in 2009, when we explored the application of these NPs in bioimaging and reported in 2011 (Figure 2).[22] At 24 h post intravenous injection (p.i.), more than 50% of injected dose (%ID) of GS-AuNPs were excreted in urine; and the liver retained only 3.7%ID,[22] in contrast to conventional gold nanomaterials, 15–90% of which were found in the liver.[23–29]
Figure 2.
Renal clearance of luminescent gold nanoparticles with core size of 2 nm and glutathione-coated surface (GS-AuNPs). A) Biodistribution of GS-AuNPs in mice at 24 h after intravenous injection (n =3). The percentage of the injected dose was calculated based on the gold concentration measured by ICP-MS. B) Accumulation of AuNPs in the bladder can be detected by X-ray computed tomography (CT) imaging of a live mouse at 30 min after intravenous injection of GS-AuNPs. Reproduced with permission from Ref. [22]. Copyright 2011, John Wiley and Sons.
The renal clearable luminescent GS-AuNPs are created using a gentle reduction method, which involves a dissociation of AuI-glutathione polymers at room temperature in aqueous solution.[15,20–21] Glutathione serves as both stabilizer and weak reducing agent, which is different from previous method for the synthesis of non-luminescent GS-AuNPs reduced by strong reductants such as NaBH4.[30–31] This gentle reduction method leaves 40–50% of gold atoms in the AuI state.[21] Based on the S–AuI and AuI–AuI interactions, ligand-to-metal charge transfer (LMCT) and ligand-to-metal-metal charge transfer (LMMCT) contribute to intrinsic luminescence of GS-AuNPs with a large Stokes shift (> 200 nm), microsecond lifetime, and quantum yield of 3.5% in aqueous solution.[32–33]
This gentle reduction method can be applied to create many other luminescent metal NPs with core sizes of 1–3 nm and having different emission wavelength, core density, and surface chemistry by using different metal salts or thiol ligands while optimizing the reaction conditions (such as pH, time, temperature, concentration, and metal-to-thiol ratio). For example, thermal reduction of HAuCl4 in the presence of glutathione generated near-infrared (NIR)-emitting GS-AuNPs with a core size of 2.5 nm.[22,34–35] By tuning the HAuCl4-to-glutathione ratio in thermal reduction, we synthesized the same sized GS-AuNPs with dual emission at 600 and 810 nm, and discovered the emission was independent on particle size.[36] The glutathione-coated 700 nm-emitting silver NPs with a core size of 2.6 nm can be obtained by replacing HAuCl4 with AgNO3 while tuning metal-to-thiol ratios.[37] Instead of using glutathione, using thiolated PEG1000 or glycine–cysteine (Gly–Cys) can produce NIR-emitting PEG1000-AuNPs[38] or 650 nm-emitting Gly–Cys-AuNPs[39] with a core size of 2.3 nm. Mixing cysteamine with glutathione led to growth of 575 nm-emitting AuNPs with a core size of 2.7 nm and surface coated by both glutathione and cysteamine (GC-AuNPs).[40]
In general, these luminescent metal NPs have low affinity for serum proteins owing to the glutathione coating. Glutathione is a natural tri-peptide (γ-l-glutamyl–l-cysteinyl–glycine) having low affinities for proteins[22] and present in abundance in the cytoplasm.[22,41–43] These glutathione-coated metal NPs not only have high stability in the native physiological environment but also resist protein adsorption and maintain ultra-small hydrodynamic diameters (< 5.5 nm, below the kidney filtration threshold) in vivo.[22,40] Therefore, they can pass through the glomerular filtration barrier and be excreted in urine. In addition, low affinity for serum proteins enables these NPs to retain their original hydrodynamic diameters and surface chemistry in the body. This allows us to correlate physicochemical properties of the NPs with their pharmacokinetics, renal clearance, and interactions with tissues and organs as discussed in the following section.
3. Effects of Physicochemical Properties on Pharmacokinetics and Renal Clearance
In the guidance on drug products containing nanomaterials, the FDA proposed a risk-based framework to evaluate potential risks associated with nanomedicines, which can guide researchers to reduce uncertainties during product development. A list of potential risk factors is suggested on page 6. For instance, the FDA recommends “understanding the mechanism by which the physicochemical properties of the material impact its biological effects (e.g., effect of particle size on pharmacokinetic parameters)”. In this section, we summarize our previous results and discuss how the physicochemical properties of ultra-small metal NPs affect the pharmacokinetics (PK) and renal clearance.
PK describes a series of processes including absorption, distribution, metabolism, and excretion (ADME) that happen after NPs are introduced into the body.[44] To study the blood PK of NPs, we used ICP-MS to monitor the blood concentrations of the metal NPs at different time points after intravenous injection within one or two days. We then fitted the blood concentration–time curves to obtain PK parameters, such as distribution half-life (t1/2α), elimination half-life (t1/2β), the volume of distribution (Vd), clearance (CL), and the area under the curve (AUC) (calculation method is shown in the Supporting Information). Generally, the PK profile of renal clearable metal NPs follows a two-compartment model with short distribution half-lives (t1/2α < 6 min) and relatively long elimination half-lives (t1/2β = 1–12 h; the values of PK parameters may vary with the PK modeling methods).
The PK parameters can be used to quantitatively describe in vivo transport behavior of renal clearable NPs. For example, the volume of distribution (Vd) reflects the propensity of renal clearable NPs to extravasate from blood vessels and enter the extravascular space. The total plasma volume of mice is 1.56 mL,[45] and the extracellular fluid volume of mice is 6 mL.[46] For renal clearable NPs that tend to be confined in the blood vessel (weak extravasation), the Vd will approach1.56 mL. For NPs that can quickly cross the blood vessel and distribute to the extracellular space (strong extravasation), the Vd will approach 6 mL. Since the renal clearable metal NPs are almost exclusively excreted through the renal excretion and liver uptake of the NPs is very low, the elimination half-life (t1/2β) reflects the efficiency of the body in eliminating NPs through the urinary system, and clearance (CL) is determined by both renal clearance and extravasation. The distribution half-life (t1/2α) reflects how fast the NPs can distribute to the whole body. The area under the curve of blood PK (AUC) indicates the total amount of NPs in blood that the body is exposed to during a given period of time.
3.1. Particle Size Effect
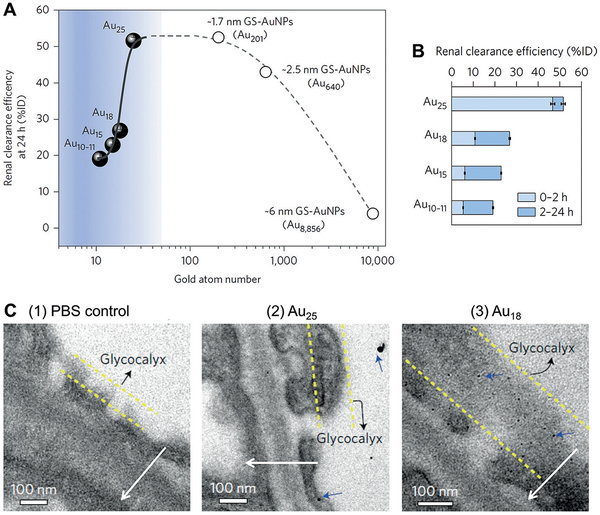
Using a series of GS-AuNPs with different core sizes (2.5 nm and 1.7 nm NPs and atomic precisely sub-nanometer clusters), we found that the glomerular filtration barrier can behave as an atomically precise bandpass filter to significantly slow down the renal clearance of smaller particles in the sub-nanometer size regime while maintaining the renal clearance at a comparable level in the range of 1–3 nm (core size; Figure 3).[47] The 2.5 nm and 1.7 nm GS-AuNPs and 1.2 nm Au25SG18 (SG = glutathione) clusters had a comparable level of renal clearance efficiency (43–53%ID) at 24 h p.i. (Figure 3A). In contrast, from Au25 to Au18, a seven-Au-atom decrease in cluster size led to a four-fold decrease in renal clearance efficiency from 46.71 to 10.79% ID at 2 h p.i. (Figure 3B). Further decreasing the cluster size to Au15 and Au10–11 resulted in a decline of the 2-h renal clearance efficiency to 6.03 and 5.29% ID, respectively. Consistent with the change of renal clearance efficiency, PK studies revealed that CL decreased exponentially from 2.04 to 0.71, 0.50, and 0.41 mLh−1 as the number of gold atoms decreased from 25 to 18, 15, and 11, respectively, and the t1/2β of gold nanoclusters increased exponentially from 2.06 to 4.47, 5.31, and 5.65 h (Table S2).
Figure 3.
Particle size effect on the renal clearance of glutathione-coated gold nanoparticles (GS-AuNPs) from 6 nm to sub-nanometer regime. A) Renal clearance efficiencies of Au10–11, Au15, Au18, and Au25, and GS-AuNPs with core sizes of 1.7 nm (Au201), 2.5 nm (Au640), and 6 nm (Au8856) at 24 h after intravenous injection vs. number of gold atoms. Percentage of injected dose, %ID. B) Renal clearance efficiencies of Au10–11, Au15, Au18, and Au25 at 0–2 h and 2–24 h after intravenous injection (n =3 for Au10–11, Au15, and Au25; n= 6 for Au18). C) Electron microscopy (EM) images of the ultrastructure of the glomerular filtration membrane from mice at 10 min after intravenous injection of phosphate buffered saline (PBS control), Au25 or Au18. Although gold clusters were too small to be directly revealed with EM, they can be visualized with EM after silver staining that increases the particle core size and contrast. (1) No silver nanoparticles were observed in the control glomeruli after silver staining and (2) only a few large silver-enhanced Au25 were found in the glomeruli of the mice injected with Au25. (3) In contrast, a large number of monodispersed silver-enhanced Au18 (blue arrows) were retained on the glycocalyx of the endothelium and podocytes. Arrows point the direction of filtration from vascular lumen to Bowman’s space. Reproduced with permission from Ref. [47]. Copyright 2017, Nature Publishing Group.
The reason of reduced renal clearance of gold nanoclusters with core size below Au25 is because these sub-nanometer gold nanoclusters can be trapped in the glycocalyx layer of endothelial cells and podocytes in a glomerular filtration membrane (Figure 3C). Since glycocalyx lines the whole-body blood vessels, binding of gold nanoclusters to glycocalyx led to a decrease in extravasation from blood vessels. Therefore, the Vd decreased linearly from 6.07 to 4.55, 3.84, and 3.30 mL with decreasing number of gold atoms from 25 to 18, 15, and 11, respectively.
3.2. Particle Density Effect
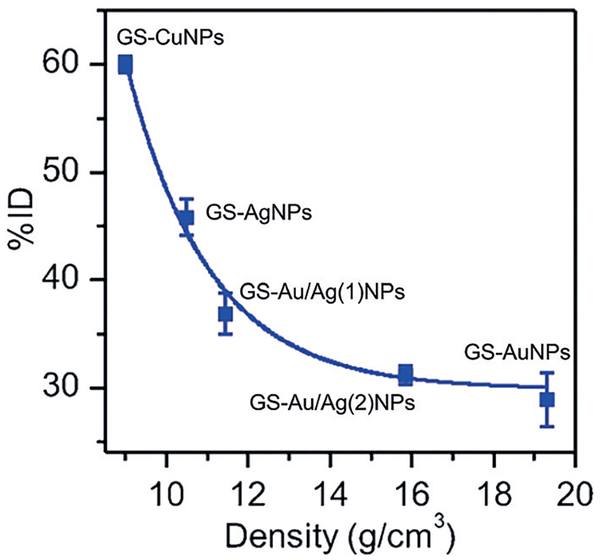
To understand the effect of particle density on renal clearance and PK, we synthesized four types of ultra-small noble-metal NPs: GS-AuNPs with high density core (19.3 gcm−3), GS-AgNPs with low density core(10.5 gcm−3), and two Au–Ag hybrid NPs (GS-Au/Ag(1)NPs and GS-Au/Ag(2)NPs with core density of 11.4 and 15.9 gcm−3, respectively).[37] These NPs had the same core size of 2.5 nm and a glutathione-coated surface. At 2 h p.i., GS-AgNPs showed a high renal clearance of 45.87%ID, followed by 36.90%ID for GS-Au/Ag(1)NPs, 31.22%ID for GS-Au/Ag(2)NPs, and 29.19%ID for GS-AuNPs. The GSCuNPs with a lower core density (8.9 gcm−3) than GS-AgNPs exhibited an even higher clearance efficiency at 2 h p.i. (60%ID).[48] These results indicated the 2-h renal clearance efficiency of these NPs exponentially decreased with an increase in the particle density (Figure 4), which is consistent with PK studies of these metal NPs (Table S3). With a decrease in the core density from 19.3 gcm−3 (GS-AuNPs) to 10.5 gcm−3 (GS-AgNPs), CL increased from 0.54 to2.25 mLh−1, indicating low-density NPs can be eliminated more rapidly through the urinary system than high-density NPs. Reducing particle density not only enhanced the renal clearance of NPs but also greatly expedited the distribution of NPs in the body. Vd increased from 2.98 mL to 8.97 mL as particle density decreased from GS-AuNPs to GS-AgNPs. We hypothesize that the particle-density-dependent renal clearance fundamentally originates from the margination of engineered NPs in the laminar blood flow; the high-density AuNPs tend to marginate toward the blood vessel walls more quickly and be transported in the laminar blood flow more slowly than low-density AgNPs.[37,49–51] The GS-AgNPs travel faster than GS-AuNPs in laminar blood flow, resulting in more rapid kidney clearance. This might also explain why the low-density NPs, such as silica NPs (C’ dots), can be eliminated more rapidly than AuNPs through the urinary system.
Figure 4.
Particle density effect on the renal clearance of glutathione (GS)-coated metal nanoparticles (NPs). The amount of NPs excreted into urine at 2 h after intravenous injection (percentage of injected dose, %ID) exponentially decreases with an increase in the particle density (d), which can be expressed as: renal clearance=2.5×103exp-(−d/2.1)+29.9, R2 =0.98 (n =3). Reproduced with permission from Ref. [37]. Copyright 2016, John Wiley and Sons.
3.3. Zwitterionic Ligand versus PEGylation
PEGylation is the most common and successful surface chemistry to reduce nonspecific accumulation and prolong blood circulation of NPs.[23,52–56] To compare PEGylation and zwitterionization, we synthesized PEG1000-AuNPs with the same core size (2.5 nm) and 810 nm emission as GS-AuNPs.[38] PEG-AuNPs also exhibited a two-compartment PK. The CL of PEG-AuNPs was only third of that of GS-AuNPs (0.70 mLh−1 vs. 2.16 mLh−1; Table S4), suggesting relatively slow renal clearance of PEG-AuNPs. The slow renal clearance of PEG-AuNPs originated from their slow transport in the body. The distribution half-life of PEG-AuNPs was tenfold higher than that of GS-AuNPs (56.1 min vs. 5.41 min), and the elimination half-life of PEG-AuNPs was two-fold longer than that of GS-AuNPs (1.86 h vs. 0.94 h). On the other hand, Vd of PEG-AuNPs (1.88 mL) was close to the total plasma volume of mice (1.56 mL), suggesting PEG-AuNPs tend to be confined in the blood vessel. These results indicated that the speed with which the NPs can be transported to the kidney also affects the renal clearance kinetics.
3.4. Length of Zwitterionic Ligands
To understand the ligand-length effect on renal clearance of AuNPs, we synthesized two types of luminescent AuNPs coated with cysteine and glycine–cysteine, respectively (Cys-AuNPs and Gly–Cys-AuNPs).[39] They had the same core size (2.3 nm). Due to instability in the presence of proteins, Cys-AuNPs were accumulated in liver and spleen and had reduced renal clearance efficiency (21.5%ID at 24 h p.i.). Surprisingly, a single amino acid increase in length from cysteine to glycine–cysteine can significantly improve the stability of NPs in physiological conditions and enhance their renal clearance. The Gly–Cys-AuNPs showed a renal clearance efficiency of 41.6%ID at 24 h p.i, which was 1.93-fold of that of Cys-AuNPs (21.5%ID at 24 h p.i.) and comparable to that of GS-AuNPs (50%ID at 24 h p.i.). The accumulation of Gly–Cys-AuNPs in liver and spleen was only 1.19%IDg−1 (percentage of injected dose per gram of tissue) and 3.44%IDg−1, respectively. Both types of AuNPs exhibited two-compartment PK with rapid elimination and short retention in blood. The distribution half-lives were 2.46 min and 3.18 min for Gly-Cys-AuNPs and Cys-AuNPs, respectively, indicating a fast distribution of particles in the body. The elimination half-lives of Cys-AuNPs (0.74 h) was shorter than that of Gly–Cys-AuNPs (1.05 h; Table S5), suggesting quick clearance of Cys-AuNPs from the blood owing to both renal excretion and liver uptake.
3.5. Surface-Charge Effect
To understand the surface-charge effect on renal clearance and PK of AuNPs, we compared two same-sized, negatively charged zwitterionic AuNPs, GS-AuNPs (core size of 2.1 nm, zeta potential of −48.0 mV at pH 7.4)[40,57] and glutathione/cysteamine-coated AuNPs (GC-AuNPs, core size of 2.3 nm, zeta potential of −22.1 mV at pH 7.4). At 1 h post-intravenous injection, liver uptake of the two types of NPs was less than 7%IDg−1 but GC-AuNPs showed much lower renal clearance efficiency (10%ID) than GS-AuNPs (28%ID), indicating that the renal clearance was slowed down with decreasing zeta potential in the negative charge range. PK studies suggested that GC-AuNPs and GS-AuNPs had comparable Vd values (2.81 vs. 2.98 mL; Table S6). The distribution half-life of GC-AuNPs was slightly longer than that of GS-AuNPs (6.5 vs. 3.9 min). However, GC-AuNPs and GS-AuNPs were significantly different in CL (0.50 vs.2.11 mLh−1) and t1/2β (3.92 vs. 0.98 h). These PK parameters suggested that renal clearance of GC-AuNPs was slowed down in the kidneys, rather than NPs being transported more slowly to the kidney. The mechanism of charge-dependent renal clearance of AuNPs needs to be further investigated.
4. Interactions with Tissues and Organs
Nanomedicines may have different chemical, physical, or biological properties compared to their larger-scale counterparts. Therefore, FDA emphasized the importance of understanding the interaction of nanomaterials with biological systems, for example, the impact of intrinsic (e.g., disease, age, sex) and extrinsic factors (e.g., co-administered drugs) on exposure and response (Page 5). In the case of renal clearable AuNPs, their interaction with normal tissues and organs are relatively weak as indicated by the low body accumulation. At 14 days p.i., less than 5%ID of GS-AuNPs was found in major organs (heart, liver, spleen, lungs, and kidneys).[58] The total amount of sub-nanometer glutathione-coated gold nanoclusters in major organs was 1.5%ID at one month after the injection.[47] These results indicated that almost all the injected renal clearable AuNPs can be eliminated from the body. However, the interactions of renal clearable AuNPs with tissues and organs could become stronger in diseased tissues and organs, such as tumor and diseased kidney. In this section, we first discuss dose-dependent transport of GS-AuNPs in the bloodstream because it affects the exposure of all tissues and organs to the NPs, followed by discussion of the interaction of NPs with background tissues (such as skin) since it determines the contrast of noninvasive in vivo fluorescence imaging. Then we discuss the interaction of NPs with normal kidney, obstructed kidney, and tumor microenvironment.
4.1. Dose-Dependent Transport in the Bloodstream
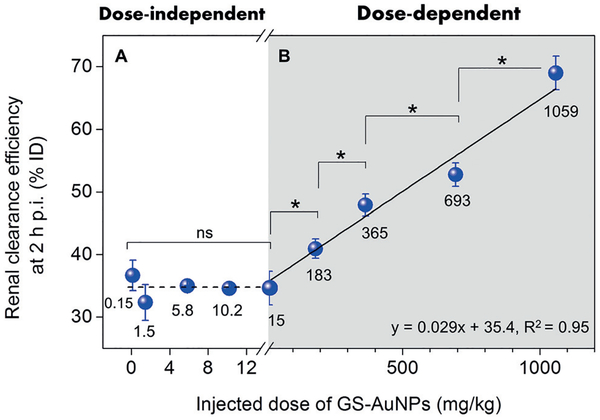
Dose dependency in PK and clearance has been investigated in small molecule-based imaging and therapeutic agents and is the key to maximizing the potential and minimizing the toxicity in clinic.[59–61] However, it is less understood than how dose affects the in vivo transport of nanoparticles. To explore the dose effect on PK and renal clearance of AuNPs, we intravenously injected 200 mL phosphate-buffered saline containing GS-AuNPs into CD-1 mice at nine different doses (0.15–1059 mgkg−1).[58] We discovered that the renal clearance efficiency at 2 h p.i. was independent of injected dose at a low-dose range from 0.15 to 15 mgkg−1 and remained constant at about 35%ID, but it linearly increased from 34.6 to 41.0, 47.9, 52.7, and 69.0%ID as dose increase from 15 to 183, 365, 693, and 1059 mgkg−1 (Figure 5). Consistent with these results, PK studies revealed that when the dose increased from 15, 183 to 1059 mgkg−1, the clearance (CL) of GS-AuNPs was increased from 1.31 to 3.11 to 3.87 mLh−1, corresponding to a decline of elimination half-live (t1/2β) from 3.25 to 0.71 to 0.31 h (Table S7).
Figure 5.
Dose effect on renal clearance of glutathione-coated gold nanoparticles (GS-AuNPs) with core size of 2.5 nm after intravenous injection. A) The renal clearance efficiency at 2 h post injection was independent of injected dose at a low-dose range from 0.15 to 15 mgkg−1, B) but it linearly increased from 34.6 to 69.0 percentage of injected dose (%ID) as dose increased from 15 to 1059 mgkg−1 (n =3 for each group; *P<0.05; ns, no significant difference, P> 0.05). Reproduced with permission from Ref. [58]. Copyright 2017, John Wiley and Sons.
This accelerated renal clearance at high doses was attributed to dose-dependent transport of GS-AuNPs in bloodstream as suggested by PK parameters. The volume of distribution (Vd) at 15 mgkg−1 is 6.13 mL, comparable to the extracellular fluid volume of mice (ca. 6 mL). This data suggests that at low doses GS-AuNPs can easily marginate toward the blood vessel wall and extravasate into the extravascular space, thereby dramatically reducing the blood concentration and slowing down the renal clearance. With an increase of dose to 1059 mgkg−1, the Vd decreased to 1.73 mL, as small as the total plasma volume of mice (ca. 1.56 mL). This data indicated that at high doses, the extravasation of NPs from blood vessels is low and more GS-AuNPs are confined in the blood vessels; as a result, more NPs were directly transported to the kidneys and excreted in urine.
4.2. Low Affinity to and Rapid Clearance from Background Tissues such as Skin
Both NPs and small molecules can be engineered to exhibit NIR emission, which improves the light penetration into tissues and is favorable for noninvasive in vivo fluorescence imaging. However, different physicochemical properties of the two types of materials could lead to distinct interactions with tissues. For instance, NIR organic dyes with large hydrophobic π-conjugated systems tend to accumulate in the skin lipid membranes, owing to high lipophilicity.[62–64] As shown in Figure 6A, in the case of noninvasive in vivo fluorescence imaging, rapid accumulation of organic fluorophore IRDye 800CW in the skin obscured real fluorescence signals from the kidneys, the organs located under the skin.[65] As a result, it is necessary to surgically remove the skin (invasively) when using renal clearable dyes as contrast agents for kidney imaging.[66] In contrast, luminescent inorganic nanoparticles, such as GS-AuNPs, can exhibit NIR emissions while having a hydrophilic surface.
Figure 6.
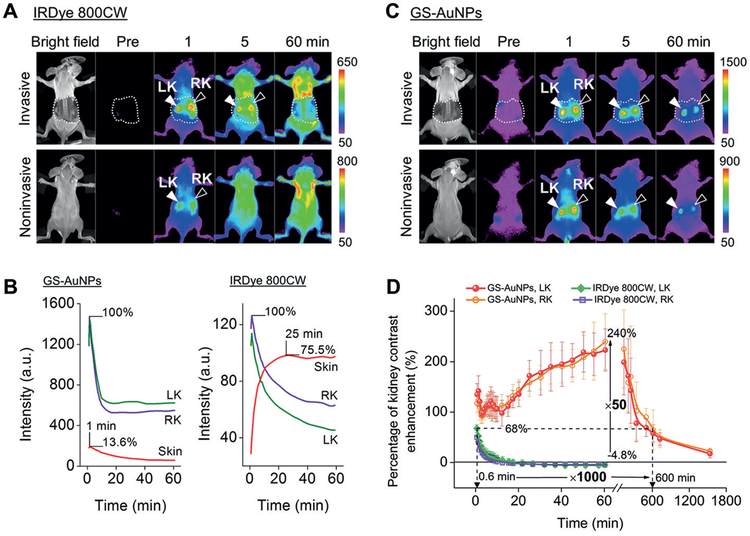
Comparison of renal-clearable NIR-emitting IRDye 800CW and renal-clearable NIR-emitting GS-AuNPs in in vivo fluorescence kidney imaging. A) Whole-body images of mice were obtained invasively (skin above the kidney was surgically removed) and noninvasively (hair-removed skin was intact) before and after intravenous injection of IRDye 800CW (Ex/Em filters:710/790 nm) or C) GS-AuNPs (Ex/Em filters: 710/830 nm). LK=left kidney, RK =right kidney. B) Time-fluorescence intensity curves of the kidneys and skin obtained from invasive imaging after iv injection of GS-AuNPs and IRDye 800CW. D) Comparison of time-dependent kidney contrast enhancements in percentage after GS-AuNPs and IRDye 800CW injection, respectively (n = 3). Reproduced with permission from Ref. [65]. Copyright 2015, John Wiley and Sons.
To compare renal clearable NIR-emitting GS-AuNPs with renal clearable NIR fluorophore IRDye 800CW in the interaction with background tissues such as skin, we used in vivo fluorescence imaging to monitor the skin signal of nude mice during 0–24 h after intravenous injection of the fluorescent agents.[35] The skin intensity of mice receiving IRDye 800CW reached its maximum at 50 min p.i. The clearance of IRDye 800CW from the skin exhibited a mono-exponential decay with a half-life of 2.3 h. In contrast, GS-AuNPs showed a rapid distribution to and rapid clearance from the skin. The skin intensity reached the maximum within 10 min. More than 90% of the maximum signal was eliminated from the skin with a half-life of 43.4 min, and only 10% of the maximum signal remained after 5 h. These results indicated that most of GS-AuNPs were cleared from the skin at a rate three-fold faster than that of IRDye 800CW. Moreover, the skin accumulation of GS-AuNPs was much lower than that of organic dyes. The maximum intensity of fluorescence from the skin after GS-AuNPs injection was only 13.6% of the maximum intensity of the signal from the kidney, whereas the skin-to-kidney ratio of IRDye 800CW fluorescence was 75.5% (Figure 6B).[65] The low affinity to and rapid clearance of NIR-emitting GS-AuNPs from background tissues leads to rapid tumor detection[35] and high-contrast noninvasive fluorescence imaging of the kidney and its function (Figure 6C),[65,67] which cannot be achieved with organic dyes. NIR-emitting GS-AuNPs can increase the percentage of kidney-contrast enhancement and imaging-time window by approximately 50- and 1000-fold over that of IRDye 800CW (Figure 6D). In addition to the rapid removal from the background tissues for imaging, another type of background removal, systemic removal of the particles from the body, is also highly efficient. Only less than 5%ID of GS-AuNPs was retained in major organs at 14 days p.i., indicating almost all the injected GS-AuNPs can be removed from the body.[58]
4.3. Interaction with Healthy and Diseased Kidney.
4.3.1. High Specificity to the Kidney
Renal clearable AuNPs show high specificity to the kidney because the kidney receives 22% of blood from cardiac output and is the major excretory organ for these NPs.[68–69] For example, the kidney accumulation of NIR-emitting GS-AuNPs was 21.39%IDg−1 at 5 min p.i., 5–9-fold greater than liver- and spleen-uptake (4.04 and 2.37%IDg−1, respectively).[70] After being excreted in urine, the kidney accumulation of GS-AuNPs decreased to 7.54% IDg−1 at 1 h p.i., resulting in a 64.7% decrease compared to that at 5 min p.i.[70]
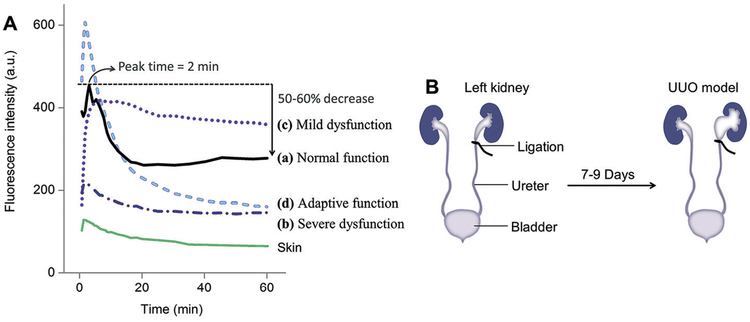
This kidney clearance kinetics of NIR-emitting GS-AuNPs can be noninvasively monitored at high temporal resolution with in vivo fluorescence imaging with minimum interference from background tissues (Figure 6C).[65,67] The kidney time–intensity curve of GS-AuNPs exhibited a rapid ascending segment with a peak value at 2 min, followed by a descending segment with a 50–60% decrease of peak value at 1 h p.i. (Figure 7A, pattern a, “Normal function”), consistent with a 64.7% decrease from 5 min to 1 h p.i. in kidney accumulation of NPs measured by ICP-MS.
Figure 7.
Noninvasive staging of kidney dysfunction with renal-clearable NIR-emitting GS-AuNPs. A) After intravenous injection of GS-AuNPs, the patterns of time-fluorescence intensity curve of the kidney obtained by noninvasive in vivo imaging can be correlated with the degrees of renal damage caused by ureteral obstruction. B) Unilateral ureteral obstruction (UUO) in mice was achieved by complete ligation of the left ureter while the right ureter was kept intact. Reproduced with permission from Ref. [67]. Copyright 2016, John Wiley and Sons.
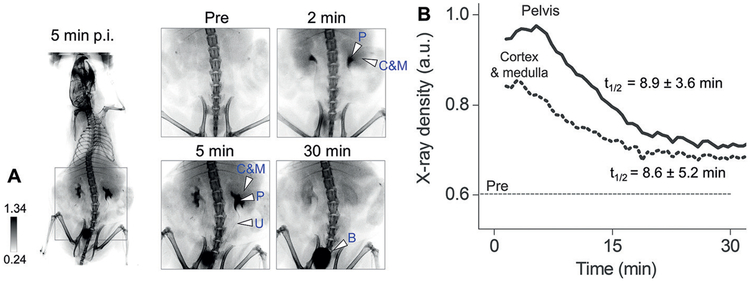
The clearance kinetics of GS-AuNPs in different compartments of the kidney can be noninvasively quantified with X-ray imaging.[71] Owing to high X-ray attenuation of gold atoms, GS-AuNPs were clearly observed in the renal cortex, medulla, and pelvis of the kidneys at 2 min p.i. and then were cleared through the pelvis into the ureter and bladder during 2–30 min p.i. (Figure 8A). We derived the time-dependent X-ray density curves from the two kidneys. The decay half-lives of the curves were 8.6 ± 5.2 min and 8.9 ± 3.6 min for cortico-medullary area and pelvic area, respectively, which corresponded to the clearance of NPs from renal cortex and medulla to pelvis and from pelvis to ureter (Figure 8B). The decay half-lives showed no significant differences, suggesting that GS-AuNPs were transported through different compartments of the kidney at a comparable rate in normal mice.
Figure 8.
In vivo X-ray imaging of transport of renal clearable gold nanoparticles in the normal kidneys. A) Representative noninvasive X-ray images of CD-1 mice before and after intravenous injection of NIR-emitting GS-AuNPs (core size of 2.5 nm; injection dose of 1000 mgkg−1 body weight) that include a whole-body image taken at 5 min p.i. and zoomed-in images of the urinary system taken before injection (Pre) and at 2, 5, and 30 min after injection (“C & M”, cortex and medulla; “P”, pelvis; “B”, bladder; “U”, ureter). B) Representative X-ray density vs. time curves derived from cortex, medulla, and pelvis in kidneys obtained from noninvasive X-ray images of mice after injection of GS-AuNPs. Reproduced with permission from Ref. [71]. Copyright 2017, John Wiley and Sons.
4.3.2. Interaction with Obstructed Kidney and Unobstructed Contralateral Kidney in the Same Animal
The transport of GS-AuNPs in the kidney is highly sensitive to the kidney function changes at early stages that cannot be even identified with conventional renal function biomarkers such as blood urea nitrogen (BUN) and serum creatinine. Using noninvasive fluorescence in vivo imaging, we studied the NP transport in the kidney at two different dysfunction stages in a unilateral ureteral obstruction (UUO) mouse model (Figure 7B).[67] At a mild stage, NPs can still be carried to the obstructed kidney at a level comparable to the normal status, but the clearance of NPs from the obstructed kidney was clearly slowed down (Figure 7A, pattern c). When the kidney injury developed to a relatively severe stage, blood perfusion was dramatically reduced, leading to low accumulation of NPs (Figure 7A, pattern b). When the obstructed kidney was severely damaged, the unobstructed contralateral kidney in the same animal exhibited both enhanced accumulation and clearance of GS-AuNPs relative to normal status, implying the adaptive function of the kidney (Figure 7A, pattern d).
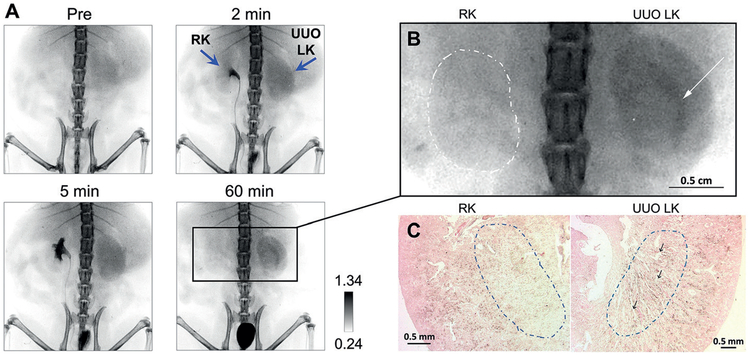
With noninvasive X-ray imaging, we found the elimination of GS-AuNPs from the renal cortex and medulla into the pelvis was significantly slowed down in the obstructed kidney, while transport of GS-AuNPs from bloodstream to glomeruli in the renal cortex was not significantly delayed in the obstructed kidney (Figure 9A).[71] GS-AuNPs were found to accumulate at the interface of medulla and pelvis owing to the dilatation and atrophy of renal tubules in the medulla induced by ureter ligation (Figure 9B). The pathological change of the kidneys also alters the interaction of NPs with kidneys at the cellular level. In unobstructed contralateral kidneys, the kidney tissue remained normal after exposure to large amount of GS-AuNPs, and the GS-AuNPs were mainly accumulated in the cortex at 1 h p.i. For UUO mice on day 1 post operation, glomeruli and proximal tubules in the cortex remained normal and contained particles, extensive tubular dilatation and atrophy were found on the boundary of the pelvis and medulla, where the transport of GS-AuNPs was interrupted, and the NPs were retained in the tubular lumen (1 h p.i.) (Figure 9C). These results indicated that the interrupted transport of GS-AuNPs within the damaged medulla caused the unique distribution of GS-AuNPs in the UUO kidney. On day 8 post operation when tubular dilatation and atrophy were more pronounced, some AuNPs appeared inside the normal tubular epithelial cells at the junction of the cortex and medulla, right below the medullary area where extensive tubular impairment was observed. These results indicated that the tubular cells started to internalize GS-AuNPs that could not be eliminated.
Figure 9.
Altered in vivo transport of renal clearable gold nanoparticles in the unilateral ureteral obstruction (UUO) mouse model. A) Representative X-ray images of UUO mouse at one day after ureteral ligation before and after intravenous injection of NIR-emitting GS-AuNPs with core size of 2.5 nm (injection dose of 1000 mgkg−1 body weight). RK =right kidney, LK=left kidney. B) Magnified X-ray image at 60 min p.i. of GS-AuNPs. Arrow indicates the area where NPs were selectively retained in obstructed kidney (UUO LK), and dashed line indicates contralateral normal kidney (RK). C) H&E and silver-stained sections of kidneys shown in (B), which were harvested right after 60-min dynamic X-ray imaging. Dashed circles indicate medullary area; arrows indicate the dilated tubules in which a large amount of GS-AuNPs were accumulated in the obstructed kidney. Reproduced with permission from Ref. [71]. Copyright 2017, John Wiley and Sons.
4.4. Interactions with Tumor Microenvironment
4.4.1. EPR Effect for Passive Targeting of Solid Tumors
NPs can be accumulate selectively in tumor tissues over normal tissues at higher concentrations for much longer periods of time through the enhanced permeability and retention (EPR) effect, which originates from the dense, leaky microvasculature and impaired lymphatic drainage in solid tumors.[56] For a long time, the EPR effect has been considered as an excretion pathway-dependent phenomenon; only non-renal clearable NPs can target the tumor through the EPR effect because they can be engineered to have a long blood-circulation time, and renal clearable NPs have negligible EPR effect owing to their rapid body clearance.[72] In 2013, through a comprehensive comparison of the passive tumor targeting of renal-clearable NIR-emitting GS-AuNPs and a renal clearable organic dye, IRDye 800CW, we found that the EPR effect can still be retained in renal clearable GS-AuNPs, which exhibited a much longer tumor retention time and faster normal tissue clearance than IRDye 800CW.[35] Through the EPR effect, renal clearable GS-AuNPs can target different types of cancers, including breast,[35,37–38,47] prostate,[57] and brain cancers[73] (Figure 10 and Table S8).
Figure 10.
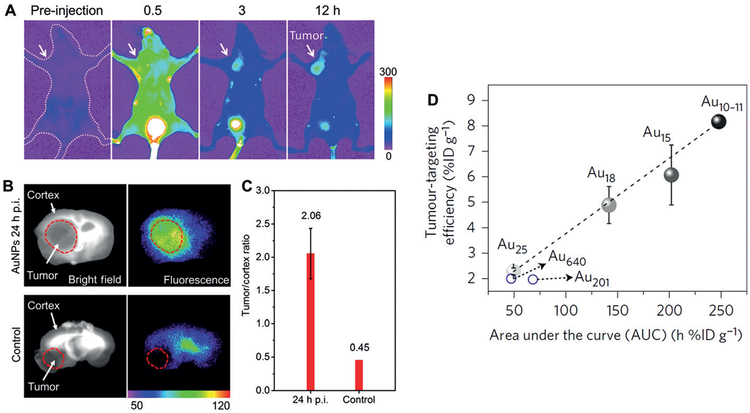
Tumor targeting of renal clearable NIR-emitting GS-AuNPs and gluathione-coated sub-nanometer gold clusters through the enhanced permeability and retention (EPR) effect. A) Representative in vivo NIR fluorescence images of mice bearing MCF-7 breast cancer xenografts at 0.5, 3, and 12 h after intravenous injection of GS-AuNPs (Ex/Em filters: 470/830 nm). Reproduced with permission from Ref. [35]. Copyright 2013, American Chemical Society. B) Bright-field and fluorescence images of glioma-bearing brain slices with (“AuNPs 24 h p.i.”) or without (“Control”) intravenous injection of GS-AuNPs. C) Tumor/cortex fluorescence ratios showed a 4.6-fold increase from “Control” to “AuNPs 24 h p.i.”. Reproduced with permission from Ref. [73]. Copyright 2017, Tsinghua University Press and Springer-Verlag Berlin Heidelberg. D) The linear relationship between tumor-targeting efficiencies of gold clusters in xenograft model of MCF-7 at 24 h p.i. and area under the pharmacokinetics curve (AUC) (n = 3). Reproduced with permission from Ref. [47]. Copyright 2017, Nature Publishing Group.
The tumor-targeting efficiency of renal clearable AuNPs can be further enhanced by tuning the pharmacokinetics of NPs. High blood concentration, long elimination half-life, and large AUC are favorable for improving the tumor targeting efficiency because extravasation of NPs from tumor vasculature to extracellular tumor microenvironment is an accumulative process. Renal clearable AuNPs with large AUC often have high tumor targeting efficiencies. For example, at 12 h p.i., PEG-AuNPs showed a high tumor-targeting efficiency in MCF-7 breast cancer model (8.3%IDg−1), which was 3.6-fold higher than that of GS-AuNPs (2.3%IDg−1).[38] The reason is that PEG-AuNPs had three-fold larger AUC than GS-AuNPs (142.8%IDhg−1 vs. 47.2%IDhg−1, 0–24 h p.i.). GC-AuNPs targeted PC-3 (human prostate cancer) xenograft tumors at an efficiency of 4.36% IDg−1, which was about two-fold higher than that of GS-AuNPs (2.72%IDg−1), owing to larger AUC (201.6 vs. 47.5%IDhg−1).[57] More interestingly, for sub-nanometer gold nanoclusters, a linear relationship was found between the AUC and the tumor-targeting efficiency in xenograft MCF-7 model: with an increase in AUC from 49.31 to 141.55, 201.81, and 247.58%IDhg−1, the 24-h tumor accumulation linearly increased from 2.28 to 4.89, 6.07, and 8.17%IDg−1 for Au25, Au18, Au15, and Au10–11, respectively (Figure 10D).[47] This result further indicates the tumor tageting of these gold clusters originates from the EPR effect.
On the other hand, it should be noted that passive tumor targeting of ultrasmall GS-AuNPs and gold clusters is different from the traditional EPR effect-enabled tumor accumulation of larger sized NPs (e.g., NPs with hydrodynamic diameters of 10–100 nm). While these large NPs are able to extravasate from tumor blood vessels with pore size of 60–400 nm,[74] they cannot penetrate the pores of blood vessels in normal tissues (6–7 nm).[69] In contrast, extravasation of ultrasmall GS-AuNPs and gold clusters also happens in normal tissues, which is indicated by the observation that their Vd of 3–6 mL in mice is larger than the total plasma volume of mice (1.56 mL). In addition, a previous study revealed that, compared with large NPs, small NPs can more easily intravasate from tumor extracellular space back into the circulation, leading to shorter tumor retention than large NPs.[75] Therefore, self-assembly of sub-5 nm ultrafine iron oxide NPs (core size of 3.5 nm) into large NPs in the tumor interstitial space has been demonstrated to prevent the NPs from reentering into the circulation so as to enhance their passive tumor tageting.[76] Regarding renal clearable AuNPs, although they had a much longer tumor retention time than IRDye 800CW, a small molecule, the difference between ultra-small GS-AuNPs and conventional large NPs in extravastion and intravasation in tumors still needs further investigation.
4.4.1. Tumor Vasculature and Acidity Effects
In addition to dense, leaky microvasculature that enables the EPR effect, acidic extracellular pH values (pHe = 6.5–7.2) is also a general feature of solid tumors.[77–80] Design of pH-responsive to further enhance the tumor accumulation of NPs or to improve the imaging contrast of tumors.[81–84] On the other hand, different types of solid tumors could have different extracellular pH values and distinct microvasculature, such as different microvascular densities (MVDs), different vascular sizes, and different vascular endothelial areas. Therefore, tumor vasculature and acidity effects are both involved in the tumor targeting of NPs. But it is unknown that how these two factors affect the tumor permeability and retention of NPs, particular for ultras-mall NPs with hydrodynamic diameters much smaller than the pore size of the tumor vasculature (60–400 nm).[74]
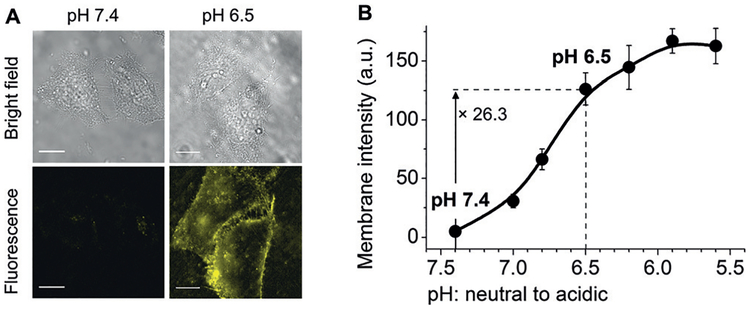
To understand the tumor vasculature and acidity effects on the tumor targeting of NPs, we compared the accumulation of renal clearable glutathione/cysteamine-coated AuNPs (GC-AuNPs) with acidity targeting to the GS-AuNPs without acidity targeting in two prostate cancer models PC-3 (pHe = 6.9, high MVD, small vascular endothelial area) and LNCaP tumors (pHe=6.5, low MVD, large vascular endothelial area).[57] The luminescent renal clearable GC-AuNPs were designed to target the tumor acidic microenvironment by showing acidic pH-induced adsorption onto the live-cell membrane while having little interaction with cells at neural pH (Figure 11).[40,57] Through biodistribution studies at 1, 24, and 72 h after intravenous injection, we found that for the same type of AuNPs, differences in the tumor microvasculature had little impact on the tumor permeability of NPs in early targeting stages (at 1 h p.i.), but a large vascular endothelium and lower vasculature densities can increase the retention of NPs in tumors (at 24 and 72 h p.i.). On the other hand, an acidic tumor microenvironment only temporarily enhanced the accumulation of the GC-AuNPs (with acidity targeting) in LNCaP tumor (with a more acidic pHe values) at 24 h p.i., and the tumor vasculature dictated the tumor targeting of renal-clearable AuNPs over the entire targeting process from 1 to 72 h p.i. through the EPR effect. Thus, to enhance the tumor targeting of renal-clearable NPs, tuning pharmacokinetics of NPs to enhance the EPR effect should always be taken into account first. Other strategies, such as targeting tumor acidity, can also further enhance the tumor accumulation of NPs but the two strategies need to be synchronized in time. For example, at 24 h p.i., because of EPR effect and tumor acidity effect, the GC-AuNPs exhibited a tumor targeting efficiency of 9.48%IDg−1 in LNCaP tumor, which was comparable to targeting efficiencies of renal clearable NPs with long blood-circulation time (8.3%IDg−1 for PEG-AuNPs and 8.17%IDg−1 for Au10–11 in MCF-7 tumor).
Figure 11.
Renal-clearable luminescent gold nanoparticles coated with both glutathione and cysteamine (GC-AuNPs) were designed for targeting tumor acidity. A) Bright-field and fluorescence images of live HeLa cells incubated with GC-AuNPs at pH 7.4 and 6.5 in PBS at 25 °C for 10 min (scale bar =20 μm). B) Luminescence intensity of cell membranes after incubation with GC-AuNPs at different pH values in phosphate buffered solution at 25° C for 10 min. Reproduced with permission from Ref. [57]. Copyright 2017, John Wiley and Sons.
5. Safety Assessment of Renal Clearable Gold Nanoparticles
In addition to the latest FDA guidance on nanomedicines, many other FDA guidance documents for human drugs have set up a series of standards that can guide us to design the preclinical studies of nanomedicines in a rational way; so that key challenges in clinical translation can be addressed in a timely fashion. For example, regarding medical imaging agents, FDA recommends conducting necessary nonclinical safety assessments before Phase I clinical trial in “Final Guidance: Developing Medical Imaging Drug and Biological Products, Part 1: Conducting Safety Assessments”.[85] These nonclinical safety assessments include but are not limited to assessing toxicity to major organs and organ systems that the imaging agent is intended to visualize and conducting expanded acute single dose toxicity study according to the guidance “Single Dose Acute Toxicity Testing for Pharmaceuticals”.[86] In this guidance on toxicity testing, to evaluate acute toxicity of agents, it is required to use at least two mammalian species, including a reasonable nonrodent species. Moreover, animals need to be observed for 14 days after injection of the agents and gross necropsies should be performed on all animals. These nonclinical safety assessments not only provide preliminary information about target organs of toxicity but also identify the no-observed-adverse-effect-level (NOAEL) of agents in animal species, which will help with the selection of starting doses of Phase I human studies.[85]
5.1. Safety Assessment in Mice and Non-human Primates
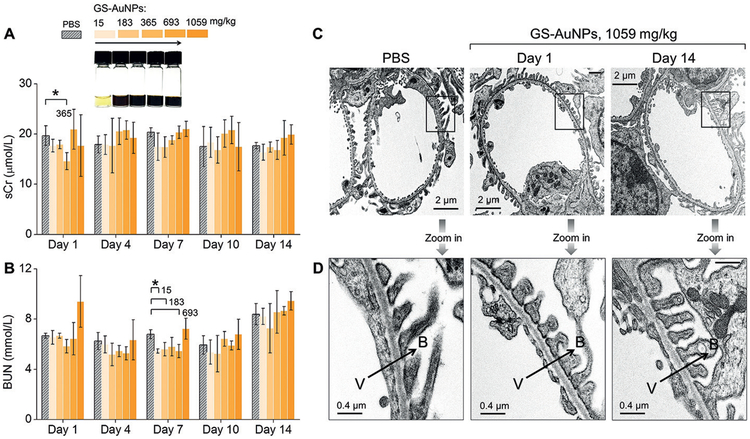
To assess the biocompatibility of renal clearable AuNPs, we chose NIR-emitting GS-AuNPs with core size of 2.5 nm because of their great potential in cancer imaging and kidney functional imaging.[35,65,67] We chose two animal species, CD-1 mice and cynomolgus monkeys, which are often used in toxicity assessments of nanoparticles.[58] To identify the no-observed-adverse-effect-level (NOAEL) in CD-1 mice, we conducted a 14-day acute single toxicity study at nine doses from 15 to 1059 mgkg−1 through intravenous administration. Because kidneys are the major organ of excretion of GS-AuNPs, we first investigated kidney function and kidney structure of the mice after being exposure to GS-AuNPs. We monitored the two renal function markers, blood urea nitrogen (BUN) and serum creatinine (sCr), for 14 days after intravenously injecting 15–1059 mgkg−1 GS-AuNPs into the mice. No significant changes in BUN and sCr levels were found from day 1 to day 14, compared to control mice injected with phosphate buffer saline (Figure 12A). No structural changes were observed on the renal glomeruli and tubules even at the dose of 1059 mgkg−1, according to TEM (Figure 12B) histological studies at day 14 after NP injection. Histological analysis of liver, spleen, heart and lungs at day 1 and day 14 revealed no abnormalities after injection of 1059 mgkg−1 NPs. These results suggested the NOAEL of GS-AuNPs is larger than 1000 mgkg−1 in CD-1 mice, 2–25-fold higher than the maximal tolerated doses (MTDs) of the highly biocompatible silica NPs in CD-1 mice (30–450 mgkg−1).[87]
Figure 12.
Evaluation of renal function and structure of kidney filtration barrier of CD-1 mice after intravenous injection of renal clearable NIR-emitting GS-AuNPs with core size of 2.5 nm at doses from 15 to 183, 365, 693, and 1059 mgkg−1. A) Levels of serum creatinine (sCr) and B) blood urea nitrogen (BUN) were monitored within 14 days after NP injection. Mice receiving PBS served as control (n =4 for each group, *P< 0.05). Representative transmission electron microscopy (TEM) images of C) glomeruli and D) glomerular filtration membranes were taken from mice injected with 1059 mgkg−1 GS-AuNPs at Day 1 and Day 14. V= vascular lumen, B= Bowman’s space, arrows point the direction of filtration. Reproduced with permission from Ref. [58]. Copyright 2017, John Wiley and Sons.
We then conducted a preliminary 90-day safety assessment of GS-AuNPs in cynomolgus monkeys.[58] The injection dose of 250 mgkg−1 was selected based on the NOAEL of GS-AuNPs in CD-1 mice (> 1000 mgkg−1). The mouse dose was divided by 4.0 to obtain the monkey dose, according to the FDA guidance “Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers”.[88] Three monkeys received a single injection of GS-AuNPs at 250 mgkg−1 body weight through intravenous drip. No abnormal behaviors were observed after receiving GS-AuNPs. Serum biochemistry analysis, urinalysis, complete blood count, and blood clotting tests were conducted every week. The results indicated that GS-AuNPs were well-tolerated by monkeys and did not induce adverse effects within 90 days following the single injection at 250 mgkg−1. Therefore, the NOAEL of GS-AuNPs should be larger than 250 mgkg−1 in cynomolgus monkeys.
5.2. Pharmacokinetics and Renal Clearance in Mice versus Non-human Primates
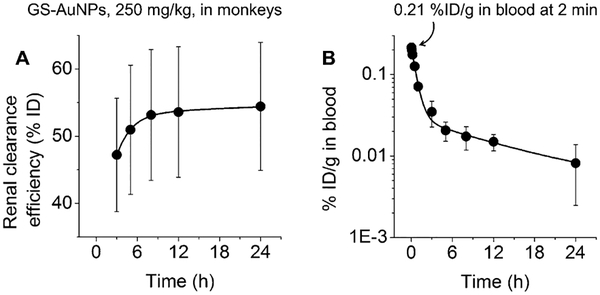
To study the excretion pathway of GS-AuNPs in monkeys, we measured the amount of gold in urine and feces with ICP AES and ICP-MS after intravenous injection of 250 mgkg−1 GS-AuNPs (Figure 13A).[58] At 24 h p.i., only 1.1%ID was detected in feces, whereas urine contained 48.6–65.4%ID. This result indicated that the efficient renal clearance observed in mice can also be translated to non-human primates.
Figure 13.
A) Renal clearance and B) blood pharmacokinetics of intravenously injected NIR-emitting GS-AuNPs with core size of 2.5 nm (250 mgkg−1) in cynomolgus monkeys (n= 3). Reproduced with permission from Ref. [58]. Copyright 2017, John Wiley and Sons.
The PK profile of renal clearable GS-AuNPs in monkeys also followed a two-compartment model, consistent with the results in mice (Figure 13B). However, the initial blood concentration of gold at 2 min p.i. in monkeys was only 0.21 ± 0.03%IDg−1, which was 60–150-fold lower than the values in mice (12.7, 20.3, and 31.2%IDg−1 at 2 min, for injection doses at 15, 183, and 1059 mgkg−1). This difference in PK between small and large animals is consistent with a 1200-fold decrease in the blood or plasma concentration of renal clearable silica NPs (C’ dots) in human (ca. 0.01%IDg−1)[18] compared to that in mice (ca. 12%IDg−1) at 4 h p.i.[89] This significant decrease in initial blood concentration from mouse to monkey and human is caused by a significant increase of blood volume from small animals to large animals and humans; the average blood volume of mouse, monkey, and human is estimated to be about 2, 200, and 5000 mL, respectively (Table S9).
6. Integration of Nanoparticles with Clinical Imaging Modalities
In the FDA guidance for medical imaging drugs,[85] the imaging agents are classified into two general categories, contrast agents for X-ray imaging and computed tomography (CT), magnetic resonance imaging (MRI) and ultrasonography, and diagnostic radiopharmaceuticals for nuclear imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). Contrast agents need to be injected at high doses to provide enough contrast between the region of interest and surrounding area, whereas diagnostic radiopharmaceuticals can be detected at ultra-low doses because of high sensitivity of nuclear imaging. For example, the clinically used doses of 18F agents for PET and 99mTc agents for SPECT are about 1 × 10−11–5 × 10−10 mmolkg−1, which are generally eight to eleven orders of magnitude lower than the doses of contrast agents for MRI, X-ray imaging, and CT (0.05–5 mmolkg−1) (Tables S10 and S11). Considering that the toxicity and health hazards of NPs are strongly dependent on their dosages, the development of radioactive isotope-labeled or -doped nanomedicines as diagnostic radiopharmaceuticals might address the safety concerns.
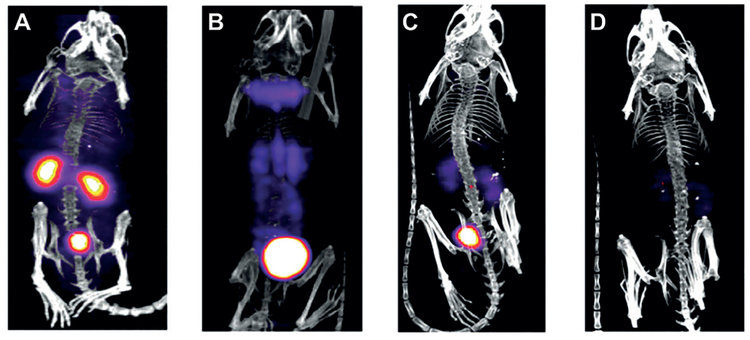
Renal clearable AuNPs can serve as contrast agents for X-ray imaging[22,71] owing to the high X-ray attenuation of gold atoms.[90–93] The minimal dose for imaging the transport of GS-AuNPs in the kidneys of mice is 1000 mgkg−1,[71] corresponding to 5.5 × 10−3 mmolkg−1 (10–1000-fold lower than the clinical doses of iodine agents for X-ray imaging and CT, 0.05–5 mmolkg−1; Table S11). To further reduce the injection dose of AuNPs for imaging, we explored the development of renal clearable AuNP-based diagnostic radiopharmaceuticals for nuclear imaging. Using thermal reduction of HAuCl4 and 198Au3+ in the presence of glutathione, we synthesized NIR-emitting GS-[198Au]AuNPs for SPECT with radiochemical purity of nearly 100%.[70] The radioactive GS-[198Au]AuNPs maintained the same core size (2.6 nm) and hydrodynamic diameter (3.0 nm) as nonradioactive GS-AuNPs (core size of 2.5 nm and hydrodynamic diameter of 3.3 nm).
To further investigate the potential application of GS-[198Au]AuNPs in SPECT imaging, we monitored kidney clearance kinetics of these NPs with SPECT (Figure 14). The mouse receiving 37 MBq of the NPs was imaged at 10 min and 1, 4, and 24 h after intravenous injection. At 10 min p.i., the kidneys and bladder were clearly seen on SPECT images (Figure 10A). From 10 min to 1 h, the kidney signal dramatically decreased, while bladder signal increased, indicating the renal clearance of GS-[198Au]AuNPs (Figure 10B,C). At 24 h p.i., weak signals were observed in the kidneys and bladder, indicating that the majority of GS- [198Au]AuNPs was cleared out from the body within 24 h p.i. (Figure 10D). For SPECT imaging of the kidney clearance kinetics of NPs, the dose of radioactive GS-[198Au]AuNPs (37 MBq) is estimated to be less than 8.1 × 10−7 mmolkg−1, which is four orders lower than the dose of GS-AuNPs for X-ray imaging (5.5 × 10−3 mmolkg−1; Table S11).
Figure 14.
Representative single-photon emission computed tomography (SPECT) images of balb/c mice intravenously injected with 37 MBq of GS-[198Au]AuNPs at A) 10 min, B) 1 h, C) 4 h, and D) 24 h p.i. Reproduced with permission from Ref. [70]. Copyright 2012, John Wiley and Sons.
7. Summary and Outlook
7.1. Comparison of Renal Clearable Gold Nanoparticles with Small Molecule-Based Contrast Agents in Disease Detection
Owing to unique nano–bio interactions, renal clearable luminescent AuNPs show strengths in disease detection that cannot be provided by small-molecule agents: 1) The EPR effect enables the passive tumor targeting of NPs.[35,38] 2) NIR-emitting AuNPs can interact less with background tissues than conventional NIR dyes, enabling 50- and 1000-fold increases in kidney-contrast enhancement and imaging time windows over IRDye 800CW,[65] respectively. The high-contrast fluorescence imaging of the kidneys allows for noninvasive staging of kidney dysfunction that cannot be achieved with conventional renal function biomarkers.[67] The low affinity for and rapid clearance from background tissues of the NPs also contributes to rapid cancer detection with NIR-emitting GS-AuNPs.[35] 3) The transport of renal clearable AuNPs in the kidney is slower than that of small molecules (e.g., diatrizoate meglumine, a clinically used iodinated agent), leading to a dramatic enhancement in the contrast of the kidneys and ureters under X-ray.[71]
In addition, renal clearable luminescent AuNPs provide a platform to readily integrate different types of signals for multiple imaging modalities, which is challenging for small molecules. For example, the renal clearable NIR-emitting gold nanoparticles can potentially serve as a probe that allows the kidney and its function as well as injuries to be imaged by multiple high-resolution imaging techniques at the size scales from centimeters down to nanometers (CT, planar X-ray, and fluorescence imaging for small-animal imaging, fluorescence microscopy for tissue and cell imaging, and electron microscopy for imaging of subcellular structures at nanometer resolution).
7.2. Comparison of Renal Clearable Gold Nanoparticles with Conventional Large Gold Nanoparticles in Disease Detection and Treatment
Gold nanomaterials have been extensively investigated in disease diagnosis and therapy. They can serve as contrast agents for X-ray imaging,[22,71,90–93] agents for cancer photothermal therapy,[54,94] radiation sensitizers for cancer radiation therapy,[95–97] and drug delivery systems for cancer chemo-therapy.[98–101] While gold nanoparticles are considered biocompatible because they are relatively inert in biological environments,[102] the FDA has not approved any gold-based nanomedicines.[103–105] One of the major challenges in clinical translation of nanomaterials is the potential toxicity, which is caused by their long-term body accumulation owing to the large core size or serum-protein adsorption.
The development of ultra-small renal clearable AuNPs could potentially address the safety concern, and renal clearable AuNPs have been demonstrated to retain some of the functionalities of conventional AuNPs, for example, as contrast agents for planar X-ray imaging and CT and EPR effect for passive targeting of solid tumors. Regarding the radiosensitization of renal clearable AuNPs,[106] we found that surface chemistry played a key role in determining radiosensitization of the ultra-small AuNPs. The 2-h incubation with GS-AuNPs can reduce survival rates of MCF-7 cells under irradiation of clinically used megavoltage photon beam (6 MV) at a low dosage of 2.25 Gy. In contrast, PEG-AuNPs can render MCF-7 cells more resistant to irradiation. This difference was independent of cell uptake efficiency of NPs and might be related to the differences in X-ray-induced photoelectron generation and/or interactions between AuNPs and X-ray-triggered reactive oxygen species (ROS). The applications of renal clearable AuNPs in photothermal therapy and drug delivery are currently under investigation.
The development of renal clearable luminescent AuNPs also offers new opportunities for disease detection and treatment: 1) The high specificity for the kidney provides a foundation for developing nanomedicines for diagnosis and treatment of kidney diseases. For example, the development of renal clearable luminescent AuNPs makes it possible to deliver drugs to renal tubules from the lumen side, which is often not accessible to non-renal clearable NPs. 2) Noninvasive fluorescence kidney functional imaging provides a low-cost, highly sensitive tool for kidney function assessment in preclinical studies. 3) GS-AuNP-conjugated insulin can retain bioactivity in lowering the blood glucose of normal and diabetic mice at a comparable level as unlabeled insulin, suggesting the potential of renal clearable AuNPs in protein labeling and delivery.[107] 4) To study biodistribution of nanomaterials, it may be necessary to label nanomaterials with radioactive isotopes or fluorescent dyes. The FDA recommends collecting data to demonstrate that the label does not substantially affect the biodistribution of the nanomaterials. The intrinsic emission of AuNPs can simplify the data interpretation of fluorescence imaging and circumvent potential interference from organic dyes conjugated on the particles.5) The intrinsic emission of AuNPs also makes it possible to design renal clearable fluorescent probes that can report on local chemical environment conditions, such as pH, in vivo.[36,108]
7.3. Future Work Towards Clinical Translation
The FDA requires that all drug products containing nanoparticles must be manufactured in accordance with current good manufacturing practices (CGMPs). To establish robust control strategies and implement effective process validation protocols, it is essential to understand the potential risks to product safety, identity, strength, quality, and purity characteristics during the manufacturing process (Page 11).
In contrast to small molecules, individual engineered nanoparticles are often heterogeneous in size, shape, and surface chemistry, resulting in an additional barrier for their success in the clinical translation since the slight differences in these parameters could lead to dramatic differences in body clearance, biodistribution, and disease targeting. Therefore, the FDA suggests identifying critical quality attributes (CQAs) of nanomaterials that determine their function and potential impact on product performance. These attributes include but are not limited to “chemical composition, average particle size, particle size distribution, general shape and morphology, and stability, both physical and chemical”. The FDA recommends examining and controlling CQAs during product development and manufacturing process (Page 7). In our previous research, we have identified the CQAs of ultrasmall metal NPs that determine their PK and renal clearance, such as particle size, core density, and surface ligands. We also found some CQAs that determine photophysical properties of NPs. For example, the emission maximum of GS-AuNPs was determined by surface coverage of glutathione on NPs,[36] and the quantum yield was related to the valence state of gold atoms.[21] These CQAs can be used to control the quality of products during the manufacturing process. Nevertheless, with a decade’s effort, renal clearable AuNPs have shown great potential in disease diagnosis and treatment and many challenges in their clinical translation have been addressed. Although there are still some critical issues ahead, what we have achieved lays down a solid foundation for their future success in the clinic. Moreover, the fundamental understanding we gained of the in vivo transport and nano–bio interactions of these renal clearable gold nanoparticles can potentially be generalized to other engineered NPs, including non-biodegradable NPs as well as polymeric NPs that are either renal clearable[109] or can degrade into small fragments with size in the renal clearable size range (hydrodynamic diameter of less than 5.5 nm), so that they can be designed in a more rational way to meet the FDA requirements for their successful clinical translation in the future.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the financial support from the National Institutes of Health [NIH, R01DK103363 (JZ), R01DK115986 (JZ) and R43DK116368 (MY)], the Cancer Prevention and Research Institute of Texas [CPRIT 120588 (JZ), 140544 (JZ) and 160866 (JZ)], Welch Research Foundation [AT-1974-20180324 (JZ)], and Cecil H. and Ida Green Professorship (JZ) from the University of Texas at Dallas. We are grateful to all the collaborators for their stimulating discussions and invaluable contributions to these studies.
Biographies

Mengxiao Yu is a research assistant professor at the University of Texas at Dallas and is the Chief Scientist and Chief Executive Officer of ClearNano, Inc. She received her BS in chemistry from Beijing Normal University in China in 2004 and her PhD in chemistry from Fudan University in 2009. After that, she joined Dr. Jie Zheng’s group at UT Dallas as a postdoctoral fellow and was promoted to research scientist in 2014 and research assistant professor in 2015. Her current research focuses on the development of nanoprobes and technologies for early detection of kidney disease such as drug-induced nephrotoxicity.

Jing Xu received her BS in pharmaceutics from China Pharmaceutical University in 2011. She joined Dr. Jie Zheng’s lab at the University of Texas at Dallas in 2013 and received PhD in chemistry in 2018. Her research focuses on the nano–bio interactions of renal clearable gold nanoparticles with the kidneys in vitro and in vivo.

Jie Zheng is a full professor and holds Cecil H. and Ida Green Professorship in Systems Biology at the University of Texas at Dallas. He received his BS degree from Inner Mongolian University in China in 1994 and PhD degree from Georgia Institute of Technology in 2005. After three-years of postdoctoral research at Harvard University, he joined the chemistry department at UT Dallas as an assistant professor in 2008 and was promoted to full professor in 2018. His research focuses on luminescent metal nanostructures, fundamental understanding of in vivo nanoparticle transport, and physiology on the nanoscale, and developing nanotechnologies for early detection and treatment of diseases such as cancer and kidney disease. He is the founder of ClearNano, Inc.
Footnotes
Conflict of interest
Dr. Jie Zheng and Dr. Mengxiao Yu have financial interests in ClearNano, Inc., which is dedicated to developing and commercializing technologies for early diagnosis of kidney disease.
Contributor Information
Mengxiao Yu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA); ClearNano, Inc., Venture Development Center, The University of Texas at Dallas, 17217 Waterview Parkway, Suite 1.202, Dallas, TX 75252 (USA).
Jing Xu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA).
Jie Zheng, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA); ClearNano, Inc., Venture Development Center, The University of Texas at Dallas, 17217 Waterview Parkway, Suite 1.202, Dallas, TX 75252 (USA).
References
- [1]. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM588857.pdf.
- [2].Lynch I, Dawson KA, Nano Today 2008, 3, 40–47. [Google Scholar]
- [3].Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA,McNeil SE, Adv. Drug Delivery Rev 2009, 61, 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walkey CD, Chan WC, Chem. Soc. Rev 2012, 41, 2780–2799. [DOI] [PubMed] [Google Scholar]
- [5].Vinluan RD III, Zheng J, Nanomedicine 2015, 10, 2781–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang L, Li Y-F, Zhou L, Liu Y, Meng L, Zhang K, Wu X,Zhang L, Li B, Chen C, Anal. Bioanal. Chem 2010, 396, 1105–1114. [DOI] [PubMed] [Google Scholar]
- [7].Goel R, Shah N, Visaria R, Paciotti GF, Bischof JC, Nanomedicine 2009, 4, 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ye L, Yong K-T, Liu L, Roy I, Hu R, Zhu J, Cai H, Law W-C, Liu J, Wang K, Liu J, Liu Y, Hu Y, Zhang X, Swihart MT, Prasad PN, Nat. Nanotechnol 2012, 7, 453. [DOI] [PubMed] [Google Scholar]
- [9].Yu M, Zheng J, ACS Nano 2015, 9, 6655–6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA, Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Åberg C, Mahon E, Dawson KA, Nat. Nanotechnol 2013, 8, 137. [DOI] [PubMed] [Google Scholar]
- [12].Walkey CD, Olsen JB, Song F, Liu R, Guo H, Olsen DWH, Cohen Y, Emili A, Chan WC, ACS Nano 2014, 8, 2439–2455. [DOI] [PubMed] [Google Scholar]
- [13].Zhou C, Yang S, Liu J, Yu M, Zheng J, Exp. Biol. Med 2013, 238, 1199–1209. [DOI] [PubMed] [Google Scholar]
- [14].Liu J, Yu M, Zhou C, Zheng J, Mater. Today 2013, 16, 477–486. [Google Scholar]
- [15].Xu J, Peng C, Yu M, Zheng J, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2017, 9, e1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Soo Choi H, Liu W, Misra P, Tanaka E, Zimmer JP, Itty Ipe B, Bawendi MG, Frangioni JV, Nat. Biotechnol 2007, 25, 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du B, Yu M, Zheng J, Nat. Rev. Mater 2018, 10.1038/s41578-018-0038-0033. [DOI] [Google Scholar]
- [18].Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD,Mohan P, Ye Y, Humm J, Gçnen M, Kalaigian H, Schçder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS, Sci. Transl. Med 2014, 6, 260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burns AA, Vider J, Ow H, Herz E, Penate-Medina O, Baumgart M, Larson SM, Wiesner U, Bradbury M, Nano Lett. 2009, 9, 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zheng J, “Fluorescent noble metal nanoclusters”, Georgia Institute of Technology Theses and Dissertations, 2005. [Google Scholar]
- [21].Zhou C, Sun C, Yu M, Qin Y, Wang J, Kim M, Zheng J, J. Phys. Chem. C 2010, 114, 7727–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhou C, Long M, Qin Y, Sun X, Zheng J, Angew. Chem. Int. Ed 2011, 50, 3168–3172; Angew. Chem. 2011, 123, 3226 – 3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang Y, Liu Y, Luehmann H, Xia X, Brown P, Jarreau C, Welch M, Xia Y, ACS Nano 2012, 6, 5880–5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y, Liu Y, Luehmann H, Xia X, Wan D, Cutler C, Xia Y, Nano Lett. 2013, 13, 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wang Y, Black KCL, Luehmann H, Li W, Zhang Y, Cai X,Wan D, Liu S-Y, Li M, Kim P, Li Z-Y, Wang LV, Liu Y, Xia Y, ACS Nano 2013, 7, 2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao Y, Sultan D, Detering L, Cho S, Sun G, Pierce R, Wooley KL, Liu Y, Angew. Chem. Int. Ed 2014, 53, 156–159; Angew. Chem. 2014, 126, 160 – 163. [DOI] [PubMed] [Google Scholar]
- [27].Melancon MP, Lu W, Yang Z, Zhang R, Cheng Z, Elliot AM, Stafford J, Olson T, Zhang JZ, Li C, Mol. Cancer Ther 2008, 7, 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cheng K, Kothapalli S-R, Liu H, Koh AL, Jokerst JV, Jiang H, Yang M, Li J, Levi J, Wu JC, Gambhir SS, Cheng Z,J. Am. Chem. Soc 2014, 136, 3560–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sun X, Huang X, Yan X, Wang Y, Guo J, Jacobson O, Liu D,Szajek LP, Zhu W, Niu G, Kiesewetter DO, Sun S, Chen X, ACS Nano 2014, 8, 8438–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Negishi Y, Takasugi Y, Sato S, Yao H, Kimura K, Tsukuda T,J. Am. Chem. Soc 2004, 126, 6518–6519. [DOI] [PubMed] [Google Scholar]
- [31].Negishi Y, Nobusada K, Tsukuda T, J. Am. Chem. Soc 2005, 127, 5261–5270. [DOI] [PubMed] [Google Scholar]
- [32].Zheng J, Zhou C, Yu M, Liu J, Nanoscale 2012, 4, 4073–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huang Y, Fuksman L, Zheng J, Dalton Trans. 2018, 47, 6267–6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tu X, Chen W, Guo X, Nanotechnology 2011, 22, 095701. [DOI] [PubMed] [Google Scholar]
- [35].Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J, J. Am. Chem. Soc 2013, 135, 4978–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu J, Duchesne PN, Yu M, Jiang X, Ning X, Vinluan RD III, Zhang P, Zheng J, Angew. Chem. Int. Ed 2016, 55, 8894–8898; Angew. Chem. 2016, 128, 9040 – 9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Tang S, Peng C, Xu J, Du B, Wang Q, Vinluan RD III, Yu M, Kim MJ, Zheng J, Angew. Chem. Int. Ed 2016, 55, 16039–16043; Angew. Chem. 2016, 128, 16273 – 16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J, Angew. Chem. Int. Ed 2013, 52, 12572–12576; Angew. Chem. 2013, 125, 12804 – 12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ning X, Peng C, Li ES, Xu J, Vinluan III RD, Yu M, Zheng J, APL Mater. 2017, 5, 053406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yu M, Zhou C, Liu J, Hankins JD, Zheng J, J. Am. Chem. Soc 2011, 133, 11014–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA, Science 2006, 312, 1027–1030. [DOI] [PubMed] [Google Scholar]
- [42].Hong R, Han G, Fernμndez JM, B.-j. Kim, N. S. Forbes, V. M. Rotello, J. Am. Chem. Soc 2006, 128, 1078–1079. [DOI] [PubMed] [Google Scholar]
- [43].Verma A, Uzun O, Hu Y, Hu Y, Han H-S, Watson N, Chen S, Irvine DJ, Stellacci F, Nat. Mater 2008, 7, 588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].A. G Louis S. Goodman Goodman and Gilman’s Manual of Pharmacology and Therapeutics, McGraw-Hill, New York, 2007. [Google Scholar]
- [45].Chapman ME, Hu L, Plato CF, Kohan DE, Am. J. Physiol. Renal Physiol 2010, 299, F280–F283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Riches AC, Sharp JG, Thomas DB, Smith SV, J. Physiol 1973, 228, 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Du B, Jiang X, Das A, Zhou Q, Yu M, Jin R, Zheng J, Nat. Nanotechnol 2017, 12, 1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yang S, Sun S, Zhou C, Hao G, Liu J, Ramezani S, Yu M, Sun X, Zheng J, Bioconjugate Chem. 2015, 26, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Decuzzi P, Lee S, Bhushan B, Ferrari M, Ann. Biomed. Eng 2005, 33, 179–190. [DOI] [PubMed] [Google Scholar]
- [50].Randall T, Elliott H, Christopher S, Harihara B, Efstathios K, Nanotechnology 2011, 22, 115101.21387846 [Google Scholar]
- [51].Thompson AJ, Eniola-Adefeso O, Acta Biomater. 2015, 21, 99–108. [DOI] [PubMed] [Google Scholar]
- [52].Iyer AK, Khaled G, Fang J, Maeda H, Drug Discovery Today 2006, 11, 812–818. [DOI] [PubMed] [Google Scholar]
- [53].Della Rocca J, Huxford RC, Comstock-Duggan E, Lin W, Angew. Chem. Int. Ed 2011, 50, 10330–10334; Angew. Chem. 2011, 123, 10514 – 10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chen J, Glaus C, Laforest R, Zhang Q, Yang M, Gidding M,Welch MJ, Xia Y, Small 2010, 6, 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Jokerst JV, Lobovkina T, Zare RN, Gambhir SS, Nanomedicine 2011, 6, 715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Matsumura Y, Maeda H, Cancer Res. 1986, 46, 6387–6392. [PubMed] [Google Scholar]
- [57].Yu M, Zhou C, Liu L, Zhang S, Sun S, Hankins JD, Sun X,Zheng J, Angew. Chem. Int. Ed 2017, 56, 4314–4319; Angew. Chem. 2017, 129, 4378 – 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Xu J, Yu M, Peng C, Carter P, Tian J, Ning X, Zhou Q, Tu Q, Zhang G, Dao A, Jiang X, Kapur P, Hsieh J-T, Zhao X,Liu P, Zheng J, Angew. Chem. Int. Ed 2018, 57, 266–271; Angew. Chem. 2018, 130, 272 – 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gabizon A, Tzemach D, Mak L, Bronstein M, Horowitz AT, J. Drug Targeting 2002, 10, 539–548. [DOI] [PubMed] [Google Scholar]
- [60].Johnson HJ, Swan SK, Heim-Duthoy KL, Nicholls AJ, Tsina I, Tarnowski T, Clin. Pharmacol. Ther 1998, 63, 512–518. [DOI] [PubMed] [Google Scholar]
- [61].Jungbluth GL, Jusko WJ, Antimicrob. Agents Chemother 1989, 33, 839–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Choi HS, Gibbs SL, Lee JH, Kim SH, Ashitate Y, Liu F,Hyun H, Park G, Xie Y, Bae S, Henary M, Frangioni JV, Nat. Biotechnol 2013, 31, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Kumar R, Roy I, Ohulchanskky TY, Vathy LA, Bergey EJ, Sajjad M, Prasad PN, ACS Nano 2010, 4, 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhang X, Tian Y, Zhang H, Kavishwar A, Lynes M, Brownell A-L, Sun H, Tseng Y-H, Moore A, Ran C, Sci. Rep 2015, 5, 13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yu M, Liu J, Ning X, Zheng J, Angew. Chem. Int. Ed 2015, 54, 15434–15438; Angew. Chem. 2015, 127, 15654 – 15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rowe CK, Franco FB, Barbosa JABA, Minnillo BJ,Chow JS, Treves T, Retik AB, Nguyen HT, J. Urol 2012, 188, 1978–1985. [DOI] [PubMed] [Google Scholar]
- [67].Yu M, Zhou J, Du B, Ning X, Authement C, Gandee L, Kapur P, Hsieh J-T, Zheng J, Angew. Chem. Int. Ed 2016, 55, 2787–2791; Angew. Chem. 2016, 128, 2837 – 2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Stanfield C, Principles of human physiology, 12th ed., Pearson Higher Ed, Upper Saddle River, NJ, 2012. [Google Scholar]
- [69].Hall J, Guyton and Hall Textbook of Medical Physiology, 12th ed., Elsevier Inc., Amsterdam, 2010. [Google Scholar]
- [70].Zhou C, Hao G, Thomas P, Liu J, Yu M, Sun S, Öz OK, Sun X, Zheng J, Angew. Chem. Int. Ed 2012, 51, 10118–10122; Angew. Chem. 2012, 124, 10265 – 10269. [DOI] [PubMed] [Google Scholar]
- [71].Xu J, Yu M, Carter P, Hernandez E, Dang A, Kapur P, Hsieh J-T, Zheng J, Angew. Chem. Int. Ed 2017, 56, 13356–13360; Angew. Chem. 2017, 129, 13541 – 13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fang J, Nakamura H, Maeda H, Adv. Drug Delivery Rev 2011, 63, 136–151. [DOI] [PubMed] [Google Scholar]
- [73].Peng C, Gao X, Xu J, Du B, Ning X, Tang S, Bachoo RM,Yu M, Ge W-P, Zheng J, Nano Res. 2017, 10, 1366–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Mei K-C, Bai J, Lorrio S, Wang JT-W, Al-Jamal KT, Biomaterials 2016, 106, 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tang L, Yang X, Yin Q, Cai K, Wang H, Chaudhury I, Yao C, Zhou Q, Kwon M, Hartman JA, Dobrucki IT, Dobrucki LW, Borst LB, Lezmi S, Helferich WG, Ferguson AL, Fan TM, Cheng J, Proc. Natl. Acad. Sci. USA 2014, 111, 15344–15349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Wang L, Huang J, Chen H, Wu H, Xu Y, Li Y, Yi H, Wang YA, Yang L, Mao H, ACS Nano 2017, 11, 4582–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y, Cancer Cell Int. 2013, 13, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Danhier F, Feron O, PrØat V, J. Controlled Release 2010, 148, 135–146. [DOI] [PubMed] [Google Scholar]
- [79].Fukumura D, Jain RK, Microvasc. Res 2007, 74, 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Brown JM, Giaccia AJ, Cancer Res. 1998, 58, 1408–1416. [PubMed] [Google Scholar]
- [81].Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J, Angew. Chem. Int. Ed 2011, 50, 6109–6114; Angew. Chem. 2011, 123, 6233 – 6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yao L, Daniels J, Moshnikova A, Kuznetsov S, Ahmed A, Engelman DM, Reshetnyak YK, Andreev OA, Proc. Natl. Acad. Sci. USA 2013, 110, 465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Du J-Z, Sun T-M, Song W-J, Wu J, Wang J, Angew. Chem. Int. Ed 2010, 49, 3621–3626; Angew. Chem. 2010, 122, 3703 – 3708. [DOI] [PubMed] [Google Scholar]
- [84].Crayton SH, Tsourkas A, ACS Nano 2011, 5, 9592–9601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. www.fda.gov/downloads/Drugs/Guidances/ucm071600.pdf.
- [86]. www.fda.gov/downloads/Drugs/./Guidances/ucm079270.pdf.
- [87].Yu T, Greish K, McGill LD, Ray A, Ghandehari H, ACS Nano 2012, 6, 2289–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88]. www.fda.gov/downloads/Drugs/Guidance/UCM078932.pdf.
- [89].Benezra M, Penate-Medina O, Zanzonico PB, Schaer D, Ow H, Burns A, DeStanchina E, Longo V, Herz E, Iyer S, Wolchok J, Larson SM, Wiesner U, Bradbury MS, J. Clin. Invest 2011, 121, 2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hainfeld J, Slatkin D, Focella T, Smilowitz H, Br. J. Radiol 2006, 79, 248. [DOI] [PubMed] [Google Scholar]
- [91].Au JT, Craig G, Longo V, Zanzonico P, Mason M, Fong Y,Allen PJ, Am. J. Roentgenol 2013, 200, 1347–1351. [DOI] [PubMed] [Google Scholar]
- [92].Cole LE, Ross RD, Tilley JMR, Vargo-Gogola T, Roeder RK, Nanomedicine 2015, 10, 321–341. [DOI] [PubMed] [Google Scholar]
- [93].Xi D, Dong S, Meng X, Lu Q, Meng L, Ye J, RSC Adv. 2012, 2, 12515–12524. [Google Scholar]
- [94].Huang X, El-Sayed MA, J. Adv. Res 2010, 1, 13–28. [Google Scholar]
- [95].Chithrani DB, Jelveh S, Jalali F, van Prooijen M, Allen C,Bristow RG, Hill RP, Jaffray DA, Radiat. Res 2010, 173, 719–728. [DOI] [PubMed] [Google Scholar]
- [96].Zhang X-D, Wu D, Shen X, Chen J, Sun Y-M, Liu P-X, Liang X-J, Biomaterials 2012, 33, 6408–6419. [DOI] [PubMed] [Google Scholar]
- [97].Brun E, Sanche L, Sicard-Roselli C, Colloids Surf. B 2009, 72, 128–134. [DOI] [PubMed] [Google Scholar]
- [98].Han G, Ghosh P, Rotello VM, Nanomedicine 2007, 2, 113–123. [DOI] [PubMed] [Google Scholar]
- [99].Pissuwan D, Niidome T, Cortie MB, J. of Controlled Release 2011, 149, 65–71. [DOI] [PubMed] [Google Scholar]
- [100].Paciotti GF, Myer L, Weinreich D, Goia D, Pavel N, McLaughlin RE, Tamarkin L, Drug Delivery 2004, 11, 169–183. [DOI] [PubMed] [Google Scholar]
- [101].Ghosh P, Han G, De M, Kim CK, Rotello VM, Adv. Drug Delivery Rev 2008, 60, 1307–1315. [DOI] [PubMed] [Google Scholar]
- [102].Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD, Small 2005, 1, 325–327. [DOI] [PubMed] [Google Scholar]
- [103].Zhang L, Gu FX, Chan JM, Wang AZ, Langers RS,Farokhzad OC, Clin. Pharmacol. Ther 2008, 83, 761–769. [DOI] [PubMed] [Google Scholar]
- [104].Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR, Pharm. Res 2016, 33, 2373–2387. [DOI] [PubMed] [Google Scholar]
- [105].Anselmo AC, Mitragotri S, Bioeng. Transl. Med 2016, 1, 10–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Jiang X, Du B, Yu M, Jia X, Zheng J, J. Innov. Opt. Health Sci 2016, 09, 1642003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Vinluan III RD, Yu M, Gannaway M, Sullins J, Xu J, Zheng J, Bioconjugate Chem. 2015, 26, 2435–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Sun S, Ning X, Zhang G, Wang T-CW, Peng C, Zheng J, Angew. Chem. Int. Ed 2016, 55, 2421–2424; Angew. Chem. 2016, 128, 2467 – 2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kang H, Gravier J, Bao K, Wada H, Lee JH, Baek Y, El Fakhri G, Gioux S, Rubin BP, Coll J-L, Choi HS, Adv. Mater 2016, 28, 8162–8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.