Abstract
Heightened delta-beta correlation has been conceptualized as reflecting exaggerated neural regulation and has been implicated in anxiety. Behavioral inhibition (BI) is a temperament characterized by wariness to novelty and is a robust predictor of anxiety, but delta-beta correlation has not been investigated in relation to childhood BI. We examined the relation between BI and between-subjects (i.e., across participants) and within-subjects (i.e., across data epochs) measures of baseline EEG delta-beta correlation in 118 children. Using a between-subjects measure, children scoring high on BI had higher delta-beta correlation relative to low BI children at frontal and central, and marginally higher in parietal, brain regions. Using a within-subjects measure, continuous BI scores were positively correlated with central and parietal delta-beta correlation. Delta-beta correlation may be a neural correlate of BI in childhood that displays differences in region specificity, correlation strength, and variability of correlation values when comparing between- and within-subjects measures.
Keywords: behavioral inhibition, delta-beta correlation, EEG, temperament, child
Behavioral inhibition (BI) is an early emerging temperamental style characterized by fear and wariness in response to novelty (Garcia-Coll & Kagan, 1984). Studying the neurobiological correlates of BI may provide greater precision into our understanding of the biological processes that may underlie this phenotype (Kagan, Reznick, & Snidman, 1987, 1988). Some researchers have conceptualized elevated delta-beta correlation as marking exaggerated regulation on a neural level, possibly underlying the rigid and overcontrolled nature of anxiety-related phenotypes (Phelps, Brooker, & Buss, 2016). Indeed, heightened delta-beta correlation has been implicated in social anxiety in adult samples (Harrewijn, Schmidt, Westenberg, Tang, & van der Molen, 2017; Harrewijn et al., 2018; Miskovic et al., 2010; 2011b). Given that BI is one of the most robust prospective predictors of social anxiety (Clauss & Blackford, 2012), we hypothesized that delta-beta correlation would be particularly relevant to the phenotype of BI early in development. Importantly, we extend the literature by examining both a between-subjects (i.e., across participants) and within-subjects (i.e., across data epochs) measure of delta-beta correlation (Schutter & Knyazev. 2012) in order to bring greater clarity as to how these two methods may be similarly or differentially related to behavioral phenotypes such as BI in childhood.
Delta-Beta Corrlation
Electroencephalogram (EEG) recordings allow for the relatively non-invasive acquisition of neural electrical activity across different frequency bands, each with different proposed functions and behavioral correlates (Engel, Fries, & Singer, 2001; Knyazev & Slobodskaya, 2003; Schürmann & Başar, 2001). Slow wave brain oscillations (e.g., delta) have been linked to subcortical brain areas responsible for motivation, emotion, and reward processing, whereas fast wave brain oscillations (e.g., beta) are thought to reflect intra-cortical connections that may be linked to attentional control, cognitive processing, and regulation (Engel, Fries, & Singer, 2001; Knyazev & Slobodskaya, 2003). Accordingly, there is speculation that the correlation of fast-wave and slow-wave frequencies may reflect interactions between cortical and subcortical circuitry, which can be captured by examining the strength of the correlation between delta and beta frequency bands (Knyazev, 2007; Knyazev & Slobodskaya, 2003; Schutter & Knyazev, 2012).
Over the last two decades, there has been increasing interest in trying to better understand the functional significance and behavioral correlates of delta-beta correlation (also referred to as coupling). Studies with adults have reported a higher delta-beta correlation in relation to heightened trait social anxiety (Miskovic et al., 2010; 2011b), higher state anxiety (Knyazev, 2011), higher scores on the behavioral inhibition system (van Peer, Roelofs, & Spinhoven, 2008), attentional bias to threat (Putman, 2011), higher baseline endogenous salivary cortisol levels (Schutter & Van Honk, 2005), as well as following the administration of exogenous cortisol (van Peer, Roelofs, & Spinhoven, 2008). Heightened salivary cortisol levels have been implicated in behavioral inhibition (Kagan et al., 1987) and anxiety (Schiefelbein & Susman, 2006) and may be a neuroendocrine correlate of fear (Schulkin et al., 2005). Based on these behavioral correlates of delta-beta correlation, greater synchrony between delta and beta oscillations is thought to reflect exaggerated regulation on a neural level, possibly underlying the rigid and overcontrolled nature of anxiety-related phenotypes. Thus, it has been inferred that delta-beta correlation may be a proxy for an individual’s emotion regulatory capacities.
Extending this work, recent studies with younger samples have begun to investigate the relation between anxiety-related behavioral phenotypes and delta-beta correlation to see if the patterns revealed in adults may be present earlier in development. The results from these studies with infants and children mirror findings with adult samples, such that six-month-old infants with high cortisol reactivity exhibited heightened resting state delta-beta correlation across frontal, central, and parietal regions (Brooker, Phelps, Davidson & Goldsmith, 2016). Among samples of toddlers and preschoolers, heightened resting state delta-beta brain correlation in the frontal and central brain regions is evident among two-year-olds with high levels of dysregulated fear (i.e., high fear in low threat contexts; Phelps, Brooker, & Buss, 2016) and heightened resting state frontal delta-beta correlation among three-year-olds with high levels of observed social fear (Najjar & Brooker, 2017). Most recently, higher resting state frontal brain delta-beta correlation was noted among school-aged children with high basal salivary cortisol levels and among children with high parent-report social anxiety (but not non-social anxiety) relative to children low on these measures (Poole & Schmidt, 2019). Collectively, this body of work suggests that heightened resting state delta-beta correlation may be a neural correlate of fear and social anxiety early in development.
Between-Subjects and Within-Subjects Measures of Delta-Beta Correlation
Traditionally, the magnitude of delta-beta correlation has been computed using a between-subjects approach by comparing the strength of the correlation between delta and beta power bands in two distinct groups. Although in some instances this is guided by broadly agreed upon categories, such as clinical diagnostic status (e.g., Harrewijn et al., 2018; Miscovic et al., 2011), oftentimes researchers are required to classify individuals into less-validated groups using methods such as a median split (e.g., Miscovic & Schmidt, 2009; Putman, 2011; Putman et al., 2012) or a mean split (e.g., Brooker et al., 2016; Phelps, Brooker & Buss, 2016) in order to employ between-subjects measures of delta-beta correlation. This can lead to difficulty interpreting and generalizing findings given the arbitrary nature of creating such groups. Although the between-subjects measure of delta-beta correlation is informative and has been widely used as outlined above, there has been some concern as to whether a between-subjects measure can be extrapolated to the individual level and interpreted as a process occurring within an individual. This has led to the suggestion of using a within-subjects measure of delta-beta correlation (Schutter & Knyazev, 2012).
A within-subjects measure of delta-beta correlation is computed by examining the correlation of delta and beta power extracted from multiple epochs from each participant’s data collection period, resulting in each individual having his or her own delta-beta correlation value, as opposed to only having a group level value. This is thought to have both theoretical and methodological advantages. Within-subjects delta-beta correlation scores are based on each individual’s time series of delta and beta power, and unlike the between-subjects approach, can reflect the extent to which these bands correlate over time for each individual. This method results in correlation scores for each individual, allowing us to investigate delta-beta correlation as an individual differences variable and examine its continuous association with other variables of interest (e.g., traits). While each level of analysis may provide unique insight into functioning, in practice, reporting both between-subjects and within-subjects measures of delta-beta correlation is rarely employed. As such, it remains relatively unclear if a between-subjects and within-subjects measure of delta-beta correlation are assessing similar constructs.
In one study, adults scoring high on social anxiety differed in frontal delta-beta correlation compared to individuals low on social anxiety during anticipation and recovery of a speech task using a between-subjects measure (Harrewijn et al., 2016). Although the relation was not significant using a within-subjects measure of delta-beta correlation, results were in the same direction with a small effect size, suggesting that the two approaches may yield similar, but not identical results. A similar pattern of results using between- and within-subjects measures of delta-beta correlation was reported among a sample of children using resting delta-beta correlation in relation to social anxiety (Poole & Schmidt, 2019).
The Current Study
Although previous work has examined the affective and behavioral correlate, of delta-beta correlation, there are at least three gaps in the literature. First, to our knowledge, published work on delta-beta correlation in samples of infants, toddlers, and children has largely been restricted to the use of between-subject measures, and so it remains less clear if a within-subjects measure of delta-beta correlation may be sensitive to distinct socioemotional profiles in early development. Second, the behavioral correlates of delta-beta correlation have been examined in relatively small samples of adults or young children (i.e., toddlerhood and preschool years), and so comparably little is known about the correlates of delta-beta correlation during early childhood. This is a particularly important developmental period to study as children enter novel classroom settings and are expected to engage in more complex social interactions, undergo cognitive development responsible for the understanding of social evaluation (Crozier & Burnham, 1990; Lagattuta, & Thompson, 2007), and place a greater importance on peer acceptance (Werner & Crick, 2004). Further, during the early school age years children’s neural regulatory networks also undergo further maturation, which may be linked to anxious-related tendencies (Piaget, 1970).
Finally, no studies have specifically examined the temperamental trait of behavioral inhibition (BI) in relation to delta-beta correlation.1 As mentioned, BI is an early emerging and relatively stable temperament characterized by fear and wariness in response to novelty (Garcia-Coll & Kagan, 1984), and is one of the most robust predictors of prospective social anxiety (Clauss, & Blackford, 2012). Given that previous work has suggested that delta-beta correlation is particularly relevant to social anxiety (Harrewijn, Schmidt, Westenberg, Tang, & van der Molen, 2017; Harrewijn et al., 2018; Miskovic et al., 2010; 2011b; Poole & Schmidt, 2019), it is plausible that delta-beta correlation may be an early neural correlate among behaviorally inhibited children. By studying delta-beta correlation in relation to BI in childhood, we may further our understanding into the putative neural processes underlying this phenotype.
In light of these gaps, we wished to extend the current literature by examining both between-subjects and within-subjects measures of resting state delta-beta correlation in relation to BI in early childhood using a relatively large sample. We chose to focus on baseline EEG measures because resting state delta-beta coupling demonstrates stability in childhood and is thought to reflect an individual’s trait-like dispositional regulatory style (Knyazev et al., 2019), which is in line with the conceptualization of BI. Consistent with previous work, we hypothesized that children who scored high on parent-report of BI would exhibit significantly higher delta-beta correlation using a between-subjects measure relative to children low on parent-report of BI (Najjar & Brooker, 2017; Phelps, Brooker, & Buss, 2016). Similar to previous studies of preschoolers, we predicted that these patterns of findings would be significant across front;., cen.al, and parietal regions (Brooker, Phelps, Davidson & Goldsmith, 2016).
Given the lack of research reporting relations between childhood temperament and a within-subjects measure of delta-beta correlation, we did not make specific predictions for this metric. However, some existing work has found that between-subjects and within-subjects measures of delta-beta correlation produced statistically similar results (Knyazev, 2011), while other work has found similar patterns of results, but the within-subjects measure did not reach conventional levels of statistical significance (i.e., Harrewijn et al., 2016; Poole & Schmidt, 2019). Accordingly, similar to our hypotheses with a between-subjects measure, we hypothesized that children scoring higher on BI would have a pattern of higher within-subjects delta-beta correlation across the same neural regions.
Method
Sample
Participants included 118 typically developing children (Mage = 6.07 years, SD = 0.76 years; 50% girls) and their primary caregivers (94% biological mother) participating in a larger study of socioemotional functioning in BI. The majority of children were White (90.3%), followed by African American (3.5%), Asian (2.7%), Hispanic (2.7%), and Native American (0.8%). Families were recruited from a university database of families interested in participating in research studies, community outreach, and word-of-mouth.
Sufficient sample size was confirmed by conducting a power analysis in G*Power (Faul, Erdfelder, Lang, & Buchner, 2007). Using effect sizes from three previous studies that compared between-subjects delta-beta correlation in groups of children (i.e., Miskovic et al., 2011a, q = 1.38, N = 16; Phelps, Brooker, & Buss, 2016, q = 0.58, N = 30; Poole & Schmidt, 2019, q = 0.64, N = 42) a power analyses suggested that in order to detect a significant difference in between-subjects delta-beta correlation, we would require a minimum sample size of 40 participants (i.e., 20 participants per group) to detect the mean effect size from previous studies (Power = 0.80, α = 0.05). In addition, a power analysis using effect sizes from three previous studies that compared resting state within-subjects delta-beta correlation among adults (i.e., Harrewijn et al. 2016, d = 0.20, N = 56; Knyazev et al. 2011, d =1.96, N = 39; Poppelaars et al., 2018, d = 0.20, N = 52) revealed that in order to detect a significant difference in within-subjects delta-beta correlation, we would require a minimum sample size of 54 participants to detect the mean effect size from previous studies (Power = 0.80, α = 0.05).
Procedure
Parents provided written consent and children provided verbal assent prior to participation. Children were introduced to our EEG system and had four minutes of baseline EEG collected while they were seated and relaxed. As part of the larger study, children then completed a computer task examining their responses to peer feedback and affect-biased attention (not reported here; see Morales, Vallorani, & Pérez-Edgar, 2019). While the children completed these tasks, parents completed questionnaires related to their child’s temperament. Families received monetary compensation and children also received a small toy and a certificate for participation. The Pennsylvania State University’s Institutional Review Board approved this study.
Electroencephalogram (EEG) Data Collection and Reduction
EEG activity was continuously recorded during a four-minute baseline period (alternating between one-minute eyes open and one-minute eyes closed) while the child was seated using a 128-channel geodesic sensor net (Electrical Geodesics Inc., Eugene, Oregon). Data from each channel was digitized at a 250 Hz sampling rate. EEG channels were collected with reference to Cz and, re-referenced offline to the average of the left and right mastoids. Vertical eye movements were recorded from electrodes placed approximately 1 cm above and below each eye. Horizontal eye movements were monitored with electrodes placed approximately 1 cm at the outer canthi of each eye. Impedances were kept below 50 kΩ.
All data preparation and processing were conducted offline using Brain Vision Analyzer (Brain Products GmbH, Germany). Data were filtered with a high-pass frequency of 0.1 Hz, a low-pass frequency of 40 Hz, and a 60 Hz notch filter. Data were visually inspected first, to remove electrodes with high impedance or noisy signal. Ocular artifacts from eye blinks and horizontal eye movements were corrected using the method developed by Gratton (Gratton et al., 1983). Data were then segmented into 1 sec epochs using 50% overlap and baseline corrected. Before artifact rejection, we selected the electrodes of interest in frontal (F3, Fz, F4), central (C3, Cz, C4) and parietal sites (P3, Pz, P4). Epochs exceeding ± 120 μV, a voltage step of more than 75 μV between sample points, or a maximum voltage difference of less than .50 μV within any 100-ms interval were marked as artifacts and automatically removed. Data were also visually inspected for any remaining artifacts. EEG power was then computed using a Fast-Fourier Transformation with full spectrum and a Hamming window length of 50%.
Between-Subjects and Within-Subjects Measures.
For the between-subjects measure of delta-beta correlation, regional absolute EEG power (in μV2) was derived in the delta (0 to 4 Hz) and beta (14 to 20 Hz) frequency bands. A natural log (ln) transformation was performed on all EEG power data to reduce skewness. In order to derive a within-subjects measure of delta-beta correlation, we also extracted delta (0 to 4 Hz) and beta (14 to 20 Hz) power for each 20 second, and then calculated the correlation between log-transformed delta power and log-transformed beta power for each individual participant across the epochs. The average number of epochs included in the within-subjects correlation was 21.24 (Range: 9 to 26).
Parent-Report of Child Behavioral Inhibition
Parents completed the 30-item Behavioural Inhibition Questionnaire (BIQ) which encompasses children’s wariness and inhibition in response to both social and non-social novel stimuli (Bishop, Spence, & McDonald, 2003). Parents rate how characteristic each item is for their child on a scale from 1 = hardly ever to 7 = almost always. Example items comprising this subscale include: “My child is shy when first meeting new children” and “My child is nervous or uncomfortable in new situations”. Parent-report on the BIQ has been correlated with laboratory observations of BI (Dyson, Klein, Olino, Dougherty, & Durbin, 2011). The BIQ demonstrated excellent internal consistency in our sample (α = .95). A total of 108 parents completed the BIQ, and total scores in our sample ranged from 36 to 168.
Missing Data
Of the 108 children with parent-reports of BI, there were missing baseline EEG data due to excessive noise (n = 2), child refusal (n = 10), and equipment failure (n = 2), resulting in 94 children with baseline EEG data. Of these children, there was one extreme value (3 SD ± mean) detected and removed for frontal beta power and two extreme values removed for frontal delta power, resulting in 92 children who had data for frontal delta-beta correlation measures. No extreme values were detected for delta and beta power in central or parietal regions, resulting in 94 children who had data for central and parietal delta-beta correlations. Children with complete EEG data versus those missing EEG data did not significantly differ on child age, gender, or BIQ scores (ps > .46). Pairwise deletion was used for all analyses.
Data Analyses
First, to test the magnitude of cross-frequency correlation using a between-subjects approach, we used partial correlations (controlling for child age and gender) between delta and beta spectral power in total frontal (average of F3, Fz, F4), central (average of C3, Cz, C4), and parietal (average of P3, Pz, P4) region for children with high parent-reported BI (upper 50%, n = 47; M = 110.76; SD = 18.14) versus low parent-reported BI (lower 50%, n = 45; M = 65.78, SD = 14.69). To test for differences in the strength of delta-beta correlation between groups, we used Fisher’s r-to-z transformations. Effect sizes for significant between-group differences were calculated as the difference between Fisher’s Z scores (q = Z1 − Z2). Results employing an extreme group design using a cut point of 119 on the total BIQ to denote BI (n = 13) and non-BI (n = 81), as used in previous studies (Suarez, Morales, Metcalf, & Pérez- Edgar, 2019), can be found in our Supplementary Materials.
Second, we examined the relation between the within-subjects measure of delta-beta correlation and continuous scores on the BIQ using a series of partial correlations (controlling for child age and gender), separately for frontal, central, and parietal regions. To supplement these analyses, we additionally examined mean differences on the within-subjects measure of delta-beta correlation for children with high BI and low BI using an ANCOVA (controlling for child age and gender), for comparability to our between-subjects measure above. All statistical analyses were performed using SPSS Version 24.0. For all main results, we present both the uncorrected p-value, in addition to the corrected p-value using the false discovery rate (FDR) for multiple comparisons (Benjamini & Hochberg, 1995).
Results
Descriptive Statistics
Table 1 presents descriptive statistics for main study variables. All EEG delta and beta power values were log transformed to reduce skewness. Parent-report of child BI was normally distributed. Child age was negatively correlated with delta and beta absolute power across frontal, central, and parietal regions (ps < .001), and girls had significantly lower delta and beta power across frontal, central, and parietal regions (ps < .001). Therefore, child age and gender were controlled for in all analyses. There were no gender differences on child age (p = .477) or parent-reported BI (p = .949), and age was not correlated with parent-reported BI (p = .509).
Table 1.
Descriptive statistics for main study variables
| Mean | SD | Range | |
|---|---|---|---|
| BIQ Scores | 88.27 | 27.94 | 36.00 – 168.00 |
| Between-Subjects Delta-Beta Correlation | |||
| Delta Power | |||
| Frontal | 2.84 | 0.48 | 1.39 – 4.00 |
| Central | 2.31 | 0.65 | 0.81 – 3.77 |
| Parietal | 2.11 | 0.64 | 0.52 – 3.65 |
| Beta Power | |||
| Frontal | −0.68 | 0.57 | −1.84 – 1.11 |
| Central | −0.97 | 0.67 | −2.35 – 0.91 |
| Parietal | −1.02 | 0.72 | −2.72 – 0.94 |
| Within-Subjects Delta-Beta Correlation | |||
| Frontal Coupling | 0.25 | 0.27 | −0.46 – 0.79 |
| Central Coupling | 0.27 | 0.29 | −0.36 – 0.86 |
| Parietal Coupling | 0.38 | 0.29 | −0.52 – 0.83 |
Note: EEG power values are natural log transformed. The between-subjects values reflect mean power values, while the within-subjects values reflect the mean within-subject correlation coefficient. BIQ = behavioral inhibition questionnaire; SD = standard deviation
Between-Subjects Measure of Delta-Beta Correlation
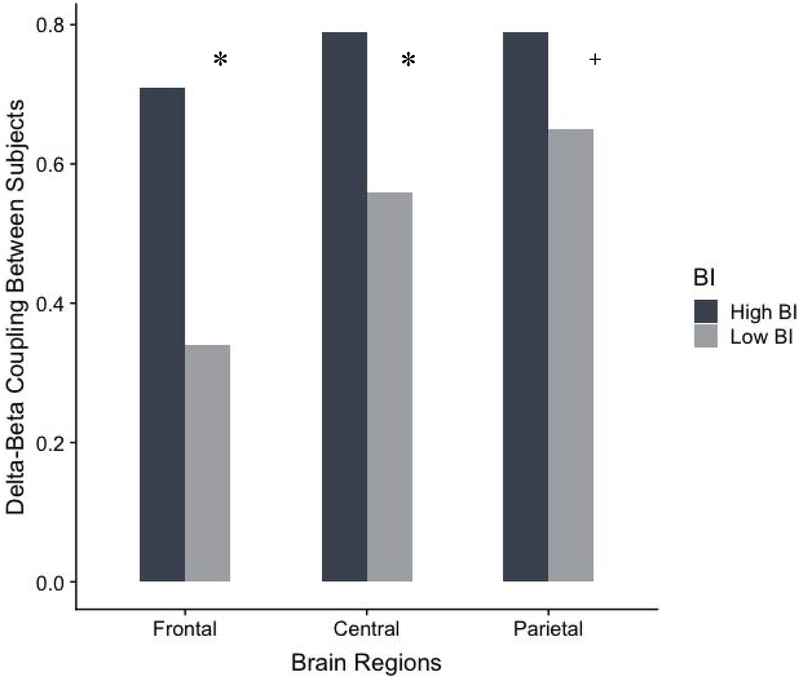
Figures 1 and 2 illustrates between-subjects delta-beta correlations for frontal, central, and parietal regions among children with high versus low parent-reported BI, controlling for child age and gender.
Figure 1.
Group differences for the between-subjects measure of delta-beta correlation for children with high BI and low BI. Notes: *p < .05; +p < .10
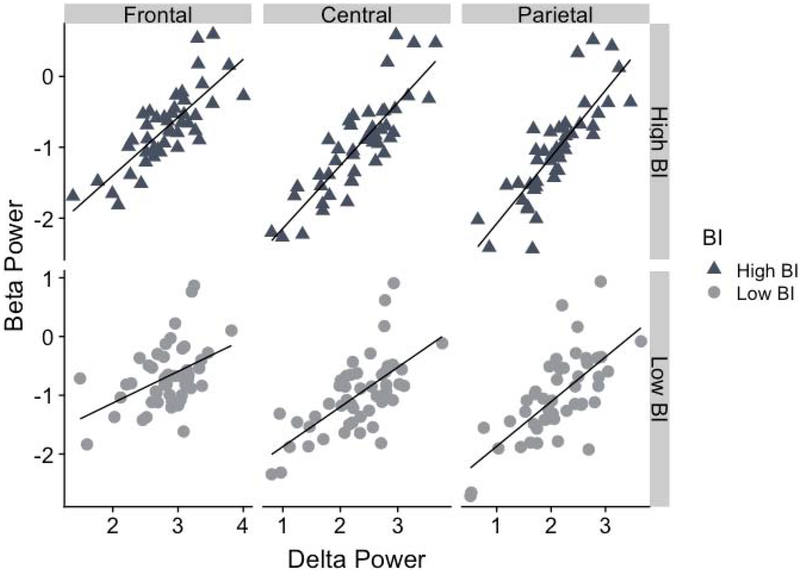
Figure 2.
Scatterplots depicting the between-subjects correlation of delta and beta power for children with high BI and low BI for the frontal, central, and parietal regions.
There was a large effect size for the frontal region, such that delta-beta correlation was significantly stronger among high BI children (r = .71, p < .001) compared to the low BI children (r = .34, p = .022; z = 2.38, uncorrected p = .007, FDR-corrected p = .033, q = 0.53). There was a medium sized effect when examining the central region, such that delta-beta correlation was significantly stronger among high BI children (r = .79, p < .001) compared to the low BI children (r = .56, p < .001; z = 1.96, uncorrected p = .022, FDR-corrected p = .039, q = 0.44). Finally, there was a marginal small effect for the parietal region, such that delta-beta correlation was higher among high BI children (r = .79, p < .001) compared to the low BI children (r = .65, p < .001; z = 1.32, uncorrected p = .086, FDR-corrected p = 0.110, q = 0.30).
We also examined whether there were group differences in absolute delta and beta power for each region between children rated as high in BI versus low BI; no significant differences were noted (ps > .495). This suggests that the two BI groups differed specifically in the synchrony of delta and beta oscillations.
Within-Subjects Measure of Delta-Beta Correlation
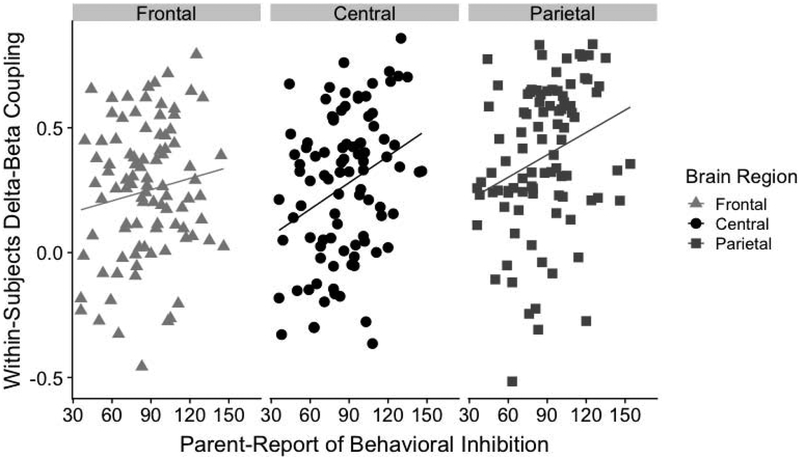
Figure 3 presents the scatterplots for the correlations between continuous scores of parent-report BI and within-subjects delta-beta correlation for frontal, central, and parietal regions. Before examining correlations between the two measures, we examined Cook’s distance in order to assess influential observations (defined as 3 SD above the mean Cook’s distance). This resulted in one case being removed for the frontal region, one case removed from the central region, and two cases removed for the parietal region. Results revealed significant positive relations between the within-subjects measure of delta-beta correlation and continuous scores of BI for parietal (r = .33, uncorrected p = .002, FDR-corrected p = .009) and central regions (r = .33, uncorrected p = .002, FDR-corrected p = .009), but not for the frontal region (r = .13, uncorrected p = .205, FDR-corrected p = .231).
Figure 3.
Scatterplots depicting the relation between continuous scores of behavioral inhibition and the within-subjects measure of delta-beta correlation for the frontal, central, and parietal regions.
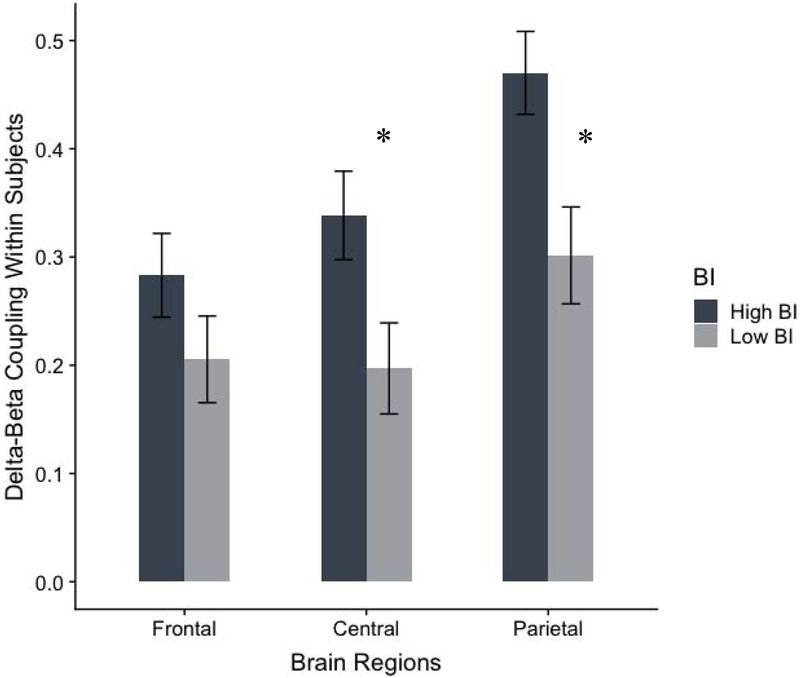
Figure 4 illustrates mean-level differences for the within-subjects measure of delta-beta correlation for frontal, central, and parietal regions among children with high versus low parent-report BI, controlling for child age and gender. Results from the ANCOVA mirror those using partial correlations as reported above, such that there was a higher within-subjects delta-beta correlation at the central region for children rated as high BI (M = 0.33, SE = 0.40) versus low BI (M = 0.21, SE = 0.40; F(1,90) = 4.60, uncorrected p = 0.035, FDR-corrected p = .053, d = 0.45), and at the parietal region for children rated as high BI (M = 0.46; SE = 0.04) versus low BI (M = 0.31, SE = 0.40; F(1,90, = 6.80, uncorrected p = 0.011, FDR-corrected p = 0.024, d = 0.55). Results for the frontal region for children rated as high BI (M = 0.28; SE = 0.04) versus low BI (M = 0.21, SE = 0.40) did not reach statistical significance (F(1,90) = 1.23, uncorrected p = 0.270, FDR-corrected p = 0.270, d = 0.24).
Figure 4.
Group differences for the within-subjects measure of delta-beta correlation for children with high BI and low BI. Notes: *p < .05. Error bars reflect standard error of the mean.
Discussion
We sought to examine resting state delta-beta correlation in relation to the temperamental trait of behavioral inhibition in a sample of typically-developing children. We compared both a between-subjects and a within-subjects approach for deriving estimates of delta-beta correlation. Using a between-subjects measure of delta-beta correlation, we found that children scoring high on parent-report of BI had higher delta-beta correlation relative to children low on BI at frontal, central, and (marginally) parietal brain regions. Using a within-subjects measure of delta-beta correlation, we found that parent-report BI was related to a higher delta-beta correlation at central and parietal regions.
Our findings converge with previous studies finding a heightened between-subjects delta-beta correlation in relation to dysregulated fear (Phelps, Brooker, & Buss, 2016), social fear (Najjar & Brooker, 2017), and social anxiety (Poole & Schmidt, 2019) in children. Adult samples have also noted higher between-subjects delta-beta correlation in relation to social anxiety (Miskovic et al., 2010; 2011b), state anxiety (Knyazev, 2011), the behavioral inhibition system (van Peer, Roelofs, & Spinhoven, 2008), and attentional bias to threat (Putman, 2011). Although speculative, a higher delta-beta correlation has previously been conceptualized as reflecting the efforts of regulatory networks to downregulate arousal in the subcortical networks (Knyazev, 2007; Knyazev & Slobodskaya, 2003; Schutter & Knyazev, 2012). This may suggest that children rated as high on BI are showing signs of over-control on a neural level, perhaps reflecting their effort to downregulate tonically high levels of arousal (Blackford et al., 2014; Jarcho et al., 2013; see also Henderson, Pine, & Fox, 2015, for a review).
In terms of brain region specificity, our effect sizes were largest for the frontal region when examining a between-subjects measure of delta-beta correlation in relation to BI, although we also found medium sized effects for the central region, and small (marginal) effects for the parietal region. These findings are consistent with work in toddlers and preschoolers that has likewise found differences in delta-beta correlation across anterior and posterior sites of the brain in relation to salivary cortisol (Brooker et al., 2016) and observed fear (Najjar & Brooker, 2017), but diverge with studies of older children and adults that have found specificity of between-subjects delta-beta correlation to frontal networks in relation to anxiety-related phenotypes (e.g., Miskovic et al., 2011a; Poole & Schmidt, 2019). This may suggest that the frontal region of the brain is particularly important for trait-level propensities towards avoidance and fear later in life, but that these patterns are broader and evident across both posterior to anterior brain regions during early childhood. An alternate methodological explanation is that these differences in effects across regions could be caused by volume conduction, where the effect originates from the frontal region and is carried over slightly to central and posterior locations, enhancing the detected but smaller effects in these regions (Olejniczak, 2006).
An important methodological contribution of our study is the inclusion of a within-subjects measure of delta-beta correlation in addition to the between-subjects measure typically seen in the literature. This is an important extension because there has been some concern as to whether a between-subjects measure delta-beta correlation can accurately be interpreted as an individual differences factor (Schutter & Knyazev, 2012). Our findings provide partial support that a within-subjects measure of resting state delta-beta correlation may yield similar results as a between-subjects measure of delta-beta correlation, at least in relation to our sample variation in BI during early childhood.
Despite the generally consistent patterns across the between-subjects and within-subjects measures of delta-beta correlation, there are some differences that should be noted. The relation differed based on brain region such that the within-subjects measure was only related to BI for the central and parietal regions, and not the frontal region. It remains unclear why a within-subjects measure versus a between-subjects measure may be more sensitive to detecting significant findings in the posterior brain regions. An additional study among adults found that the parietal (but not frontal) region of the brain showed heightened delta-beta correlation in relation to higher levels of attentional control (Morillas-Romero et al., 2015). The parietal region of the brain plays an influential role in attentional and executive functions (Balle et al., 2013; Posner, Rueda, & Kanske, 2007) and thus heightened delta-beta correlation at this region may be capturing the distinct attentional processes among behaviorally inhibited children (Henderson, Pine, & Fox, 2015). Further, it may be the case that early in development a within-subjects measure of delta-beta correlation may be observed most strongly in posterior brain regions during childhood, which has recently been demonstrated (Knyazev et al., 2019).
Another observation is that although the direction of findings between BI and delta-beta correlation was similar when using a within-subjects and between-subjects approach, the correlation coefficients were numerically higher when using a between-subjects approach relative to a within-subjects approach. This is most likely due to the decreased statistical power in within-subjects analyses given the lower number of epochs used to calculate the correlations as well as increased variability. The exact functional significance of this variation is not clear. It is possible that between-subjects measures may overestimate the correlation between delta and beta given the group-based approach by averaging over individual variation. This may also be related to the fact that the between-subjects approach on average resulted in positive correlations, whereas inspection of the values from the within-subjects correlations reveal a range from negative to positive correlations, providing further support that the two approaches may reflect somewhat different measures. It is also possible that trait- and state-level variations in delta-beta coupling may relate to BI in fundamentally different ways. That is, individuals’ variation in relation to their average delta-beta coupling may reflect a distinct neural process and need not map onto how sample-level differences in coupling relate to BI. With larger samples, future studies could clarify this question using analytical methods that partition trait- and state-level variation in delta-beta coupling and can compare their unique influence on BI as well as how they interact.
A comparison of both a between-subjects and within-subjects measure of delta-beta correlation within the same study has rarely been reported, but our findings are somewhat consistent with available studies. For example, studies have found differences in frontal brain delta-beta correlation at rest in children (Poole & Schmidt, 2019) and in response to social stress in adults (Harrewijn et al., 2016) depending on individual differences of social anxiety. These data are consistent with the direction of findings when using a within-subjects measure, despite failing to reach statistical significance. In addition, a study by Knyazev et al. (2011) found similar results when examining a between-subjects and within-subjects measure of delta-beta correlation among adults in relation to state anxiety. Similarly, in our study we found higher resting state delta-beta correlation in children high on BI using both a between-subjects approach and within-subjects approach.
Although the exact reason for differences in direction of the results is not clear, it may be due to methodological differences in computing delta-beta correlation (i.e., between-subjects versus within-subjects measure), our use of a community sample while some others have used selected samples (Harrewijn et al., 2018), the younger age in our sample while others have used adult samples (Harrewijn et al., 2018; Morillas-Romero et al., 2015; Poppelaars et al., 2018; Putman, Arias-Garcia, Pantazi, & van Schie, 2012), or the specific subtype of anxiety (e.g., social, general, or fear). It is also important to note that we used a resting state measure of EEG whereas some others have relied on EEG collected in response to a social stressor (Harrewijn et al., 2018; Poppelaars et al., 2018). Previous work has noted that EEG markers at baseline and during anticipation of a stressor are not always correlated with the same traits or outcomes (e.g., Pérez-Edgar, Kujawa, Nelson, Cole, & Zapp, 2013).
Although we find distinct patterns of delta-beta correlation (across the between-subjects and within-subjects measures) among children scoring higher on BI, our study design is cross-sectional. Thus, it remains unclear whether a higher delta-beta correlation predisposes a child to an overcontrolled temperamental style, or if conversely, these patterns of brain activity develop as a result of the temperament. There is some experimental evidence to suggest that a between-subjects measure of frontal brain delta-beta correlation is sensitive to intervention effects. Specifically, a reduction in frontal delta-beta correlation was found in a sample of adults diagnosed with social anxiety disorder (SAD) after they completed a cognitive behavioral therapy intervention, which paralleled decreases in social anxiety symptoms (Miskovic, Moscovitch, et al., 2011). Additional intergenerational studies have found that heightened delta-beta correlation may be a marker of prospective anxiety risk (before the onset of symptoms), such that children of socially anxious parents show a higher frontal brain resting state delta-beta correlation in late childhood (Miskovic et al., 2011a), and recent work found that heightened frontal delta-beta correlation in response to social stress may be an endophenotype of SAD (Harrewijn et al., 2018). However, until longitudinal studies systematically examine whether delta-beta correlation reflects a susceptibility factor for prospective anxiety, these findings remain speculative.
Limitations
The results of the current study should be interpreted in the context of the following limitations. First, as mentioned, the study design was cross-sectional and thus the findings should be interpreted as correlational in nature. Second, our measure of BI was restricted to parent-report. Although parent-report is considered a valid measure of child temperament (Garstein, Bridget, & Low, 2012), is correlated with observational measures (Smith et al., 2012), and has associated biological profiles (Fu, Taber-Thomas, & Pérez-Edgar, 2017), future work should aim to incorporate observational indices of BI. Third, some of our findings were non-significant when correcting for multiple comparisons, and accordingly should be interpreted with appropriate caution. Fourth, our sample was fairly homogenous in terms of ethnicity and therefore should be replicated in additional samples to ensure generalizability. This is particularly important as the correlates of BI may differ based on culture (e.g., Chen, Chen, Li, & Wang, 2009). Fifth, each participant did not have the exact. same number of useable EEG epochs, and differences in epochs between participants can negatively impact the signal-to-noise ratio (Cohen, Elger, & Fell, 2009). Finally, the exact functional significance of delta-beta correlation is not known (Canolty & Knight, 2010), and there is debate as to whether delta power actually reflects subcortical regions (Blaeser, Connors, & Nurmikko, 2017; Harrewijn, Schmidt, Westenberg, Tang, & van der Molen, 2017).
Conclusion
Our preliminary findings suggest that both a between-subjects and within-subjects measure of delta-beta correlation may distinguish children with high versus low levels of BI. Although both approaches of computing delta-beta correlation were related to this temperamental style, there were slight differences in the strength of the correlation and the brain regions where this pattern was found across measures. We recommend that future studies continue to examine a within-subjects measure of delta-beta correlation in order to replicate our findings, and more importantly, use a longitudinal study design in order to examine the stability of a delta-beta correlation and its validity to predict or moderate prospective psychopathology among children at temperamental risk, as has been done with other within-subject measures of resting brain activity such as frontal EEG asymmetry (Coan & Allen, 2004; Peltola et al., 2014). This is an important future direction that will help increase our understanding of the functional significance and predictive utility of delta-beta correlation in early childhood.
Supplementary Material
Highlights.
We examined the relation between behavioral inhibition (BI) and between-subjects and within-subjects measures of resting delta-beta correlation in typically-developing children.
High BI children had a higher between-subjects delta-beta correlation relative to low BI children at frontal and central, and marginally higher in parietal, brain regions.
Continuous scores of BI were positively correlated with central and parietal within subjects delta-beta correlation.
Delta-beta correlation may be a neural correlate of BI in childhood.
Acknowledgments
This research was supported by a Canadian Institute of Health Research (CIHR) Doctoral Research Award and a CIHR Michael Smith Foreign Study Supplement awarded to KLP, an NIMH Diversity Supplement (R01 MH109692–02S1) to BA, and the McCourtney Early Career Professorship and the McCourtney Professorship in Child Studies awarded to KPE. The authors wish to thank the children and their primary caregivers for their participation in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
It should be noted that among samples of adults, the behavioral inhibition system (BIS) — which assesses one’s sensitivity to impending punishment (Carver & White, 1994; Gray, 1981)—has been examined in relation to delta-beta correlation (Putman, 2011; van Peer, Roelofs, & Spinhoven, 2008). Behavioral inhibition (BI), on the other hand, is conceptualized as a temperamental trait assessed in early childhood and reflects wariness in response to novel stimuli (Garcia-Coll & Kagan, 1984). Although there is conceptual similarity between the behavioral inhibition system (BIS) and the temperament behavioral inhibition (BI), the two constructs are not synonymous (See Barker, Buzzell, & Fox, 2019, for a review).
Contributor Information
Kristie L. Poole, Department of Psychology, Neuroscience & Behaviour, McMaster University;
Berenice Anaya, Department of Psychology, The Pennsylvania State University;.
Koraly E. Pérez-Edgar, Department of Psychology, The Pennsylvania State University
References
- Balle M, Bornas X, Tortella-Feliu M, Llabrés J, Morillas-Romero A, Aguayo-Siquier B, & Gelabert JM (2013). Resting parietal EEG asymmetry and cardiac vagal tone predict attentional control. Biological Psychology, 93, 257–261. doi: 10.1016/j.biopsycho.2013.02.012 [DOI] [PubMed] [Google Scholar]
- Barker TV, Buzzell GA, & Fox NA (2019). Approach, avoidance, and the detection of conflict in the development of behavioral inhibition. New Ideas in Psychology, 53, 2–12. doi: 10.1016/j.newideapsych.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bishop G, Spence SH, & McDonald C (2003). Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Development, 74, 1899–1917. 10.1046/j.1467-8624.2003.00645.x [DOI] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, & VanDerKlok RM (2014). Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biological Psychology, 99, 15–25. 10.1016/j.biopsycho.2014.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaeser AS, Connors BW, & Nurmikko AV (2017). Spontaneous dynamics of neural networks in deep layers of prefrontal cortex. Journal of Neurophysiology, 117, 1581–1594. 10.1152/jn.00295.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Phelps RA, Davidson RJ, & Goldsmith HH (2016). Context differences in delta beta coupling are associated with neuroendocrine reactivity in infants. Developmental Psychobiology, 58, 406–418. doi: 10.1002/dev.21381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, & Knight RT (2010). The functional role of cross-frequency coupling. Trends in Cognitive Sciences, 14, 506–515. doi: 10.1016/j.tics.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, & White TL (1994). Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology, 67, 319–333. doi: 10.1037/0022-3514.67.2.319 [DOI] [Google Scholar]
- Chen X, Chen H, Li D, & Wang L (2009). Early childhood behavioral inhibition and social and school adjustment in Chinese children: A 5- year longitudinal study. Child Development, 80, 1692–1704. 10.1111/j.1467-8624.2009.01362.x [DOI] [PubMed] [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 1066–1075. doi: 10.1016/j.jaac.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, & Allen JJ (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–50. doi: 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Cohen MX, Elger CE, & Fell J (2009). Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. Journal of Cognitive Neuroscience, 21, 390–402. 10.1162/jocn.2008.21020 [DOI] [PubMed] [Google Scholar]
- Crozier W, & Burnham M (1990). Age related differences in children’s understanding of shyness. British Journal of Developmental Psychology, 8, 179–185. https://doi-org.libaccess.lib.mcmaster.ca/10.1111/j.2044-835X.1990.tb00832.x [Google Scholar]
- Dyson MW, Klein DN, Olino TM, Dougherty LR, & Durbin CE (2011). Social and non-social behavioral inhibition in preschool-age children: differential associations with parent-reports of temperament and anxiety. Child Psychiatry and Human Development, 42, 390–405. doi: 10.1007/s10578-011-0225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Fries P, & Singer W (2001). Dynamic predictions: Oscillations and synchrony in top–down processing. Nature Reviews Neuroscience, 2, 704–716. doi: 10.1038/35094565 [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fu X, Taber-Thomas BC, & Pérez-Edgar K (2017). Frontolimbic functioning during threat-related attention: relations to early behavioral inhibition and anxiety in children. Biological Psychology, 122, 98–109. 10.1016/j.biopsycho.2015.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, & Low C (2012) Asking questions about temperament: Self-and other-report measures across the lifespan In Zentner M & Shiner RL (Eds.), Handbook of Temperament (pp. 183–208). New York: Guilford Press. [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Gray JA (1981). A critique of Eysenck’s Theory of Personality In Eysenck HJ,, editor. A Model for Personality (pp. 246–276). Springer Berlin; Heidelberg. [Google Scholar]
- Harrewijn A, Schmidt LA, Westenberg PM, Tang A, & van der Molen MJ (2017). Electrocortical measures of information processing biases in social anxiety disorder: A review. Biological Psychology, 129, 324–348. 10.1016/j.biopsycho.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Harrewijn A, Van der Molen MJW, & Westenberg PM (2016). Putative EEG measures of social anxiety: Comparing frontal alpha asymmetry and delta-beta cross-frequency correlation. Cognitive, Affective, & Behavioral Neuroscience, 16, 1086–1098. DOI: 10.3758/s13415-016-0455-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, & Fox NA (2015). Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology, 40, 1–18. DOI: 10.1038/npp.2014.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, & Ernst M (2013). The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology, 92, 306–314. 10.1016/j.biopsycho.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1987). The physiology and psychology of behavioral inhibition in children. Child Development, 58, 1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, & Snidman N (1988). Biological bases of childhood shyness. Science, 240, 167–171. doi 10.1126/science.3353713 [DOI] [PubMed] [Google Scholar]
- Knyazev GG (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience & Biobehavioral Reviews, 31, 377–395. doi: 10.1016/j.neubiorev.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskaya HR, & Wilson GD (2004). Personality and brain oscillations: Developmental aspects In: Shohov SP, (Ed.). Advances in Psychology Research. (pp. 3–34). New York: Nova Science Publishers. [Google Scholar]
- Knyazev GG (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neuroscience & Biobehavioral Reviews, 36, 677–695. doi: 10.1016/j.neubiorev.2011.10.002 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, & Slobodskaya HR (2003). Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. International Journal of Psychophysiology, 48, 247–261. 10.1016/S0167-8760(03)00C72-2 [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Schutter DJ, & van Honk J (2006). Anxious apprehension increases coupling of delta and beta oscillations. International Journal of Psychophysiology, 61, 283–287. doi: 10.1016/j.ijpsycho.2005.12.003 [DOI] [PubMed] [Google Scholar]
- Knyazev G, Savostyanov AN, Bocharov AV, Tamozhnikov SS, Kozlova EA, Leto IV, & Slobodskaya HR (2019). Cross-frequency coupling in developmental perspective. Frontiers in Human Neuroscience, 13, 158 10.3389/fnhum.2019.00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev GG (2011). Cross-frequency coupling of brain oscillations: an impact of state anxiety. International Journal of Psychophysiology, 80, 236–245. doi: 10.1016/j.ijpsycho.2011.03.013 [DOI] [PubMed] [Google Scholar]
- Lagattuta KH, & Thompson RA (2007). The development of self-conscious emotions: cognitive processes and social influences In Tracy JL, Robins RW, & Price Tangney J (Eds.), The Self-conscious Emotions: Theory and Research (pp. 91–113). New York Guilford. [Google Scholar]
- Miskovic V, & Schmidt LA (2009). Frontal brain oscillatory coupling among men who vary in salivary testosterone levels. Neuroscience Letters, 464, 239–242. 10.1016/j.neulet.2009.08.059 [DOI] [PubMed] [Google Scholar]
- Miskovic V, Ashbaugh AR, Santesso DL, McCabe RE, Antony MM, & Schmidt LA (2010). Frontal brain oscillations and social anxiety: A cross-frequency spectral analysis during baseline and speech anticipation. Biological Psychology, 83, 125–132. doi: 10.1016/j.biopsycho.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Miskovic V, Campbell MJ, Santesso DL, Van Ameringen M, Mancini CL, & Schmidt LA (2011a). Frontal brain oscillatory coupling in children of parents with social phobia: A pilot study. Journal of Neuropsychiatry and Clinical Neurosciences, 23, 111–114. doi: 10.1176/appi.neuropsych.23.1.111 [DOI] [PubMed] [Google Scholar]
- Miskovic V, Moscovitch DA, Santesso DL, McCabe RE, Antony MM, & Schmidt LA (2011b). Changes in EEG cross-frequency coupling during cognitive behavioral therapy for social anxiety disorder. Psychological Science, 22, 507–516. doi: 10.1177/0956797611400914 [DOI] [PubMed] [Google Scholar]
- Morales S, Vallorani A, & Pérez-Edgar K (2019). Young children’s behavioral and neural responses to peer feedback relate to internalizing problems. Developmental Cognitive Neuroscience, 36, 100610 10.1016/j.dcn.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillas-Romero A, Tortella-Feliu M, Bornas X, & Putman P (2015). Spontaneous EEG theta/beta ratio and delta–beta coupling in relation to attentional network functioning and self-reported attentional control. Cognitive, Affective, & Behavioral Neuroscience, 15, 598–606. 10.3758/s13415-015-0351-x [DOI] [PubMed] [Google Scholar]
- Najjar R, & Brooker RJ (2017). Delta-beta coupling is associated with paternal caregiving behaviors during preschool. International Journal of Psychophysiology, 112, 31–39. doi: 10.1016/j.ijpsycho.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak P (2006). Neurophysiologic basis of EEG. Journal of Clinical Neurophysiology, 23, 186–189. doi: 10.1097/01.wnp.0000220079.61973.6c [DOI] [PubMed] [Google Scholar]
- Peltola MJ, Bakermans-Kranenburg MJ, Alink LRA, Huffmeijer R, Biro S, & van IJzendoorn MH (2014). Resting frontal EEG asymmetry in children: Meta-analyses of the effects of psychosocial risk factors and associations with internalizing and externalizing behavior. Developmental Psychobiology, 56, 1377–1389. 10.1002/dev.21223 [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Kujawa A, Nelson SK, Cole C, & Zapp DJ (2013). The relation between electroencephalogram asymmetry and attention biases to threat at baseline and under stress. Brain and Cognition, 82, 337–343. 10.1016/j.bandc.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps RA, Brooker RJ, & Buss KA (2016). Toddlers’ dysregulated fear predicts delta–beta coupling during preschool. Developmental Cognitive Neuroscience, 17, 28–34. doi: 10.1016/j.dcn.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaget J (1970). Piaget’s theory In Mussen PH (Ed.), Carmichael’s Manual of Child Psychology, (pp. 703–732). New York, NY: Wiley. [Google Scholar]
- Poole KL, & Schmidt LA (2019). Frontal brain delta-beta correlation, salivary cortisol, and social anxiety in children. Journal of Child Psychology and Psychiatry, 60, 646–654. doi: 10.1111/jcpp.13016 [DOI] [PubMed] [Google Scholar]
- Poppelaars ES, Harrewijn A, Westenberg PM, & van der Molen MJ (2018). Frontal delta-beta cross-frequency coupling in high and low social anxiety: An index of stress regulation? Cognitive, Affective, & Behavioral Neuroscience, 18, 1–14. doi: 10.3758/s13415-018-0603-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Rueda MR, & Kanske P (2007). Probing the mechanisms of attention In Cacioppo JT, Tassiary JG, & Berntson GG (Eds.), Handbook of Psychophysiology (pp. 410–431). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Putman P (2011). Resting state EEG delta–beta coherence in relation to anxiety, behavioral inhibition, and selective attentional processing of threatening stimuli. International Journal of Psychophysiology, 80, 63–68. doi: 10.1016/j.ijpsycho.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Putman P, Arias-Garcia E, Pantazi I, & van Schie C (2012). Emotional Stroop interference for threatening words is related to reduced EEG delta–beta coupling and low attentional control. International Journal of Psychophysiology, 84, 194–200. doi: 10.1016/j.ijpsycho.2012.02.006 [DOI] [PubMed] [Google Scholar]
- Putman P, & Roelofs K (2011). Effects of single cortisol administrations on human affect reviewed: Coping with stress through adaptive regulation of automatic cognitive processing. Psychoneuroendocrinology, 36, 439–448. 10.1016/j.psyneuen.2010.12.001 [DOI] [PubMed] [Google Scholar]
- Schiefelbein VL, & Susman EJ (2006). Cortisol levels and longitudinal cortisol change as predictors of anxiety in adolescents. Journal of Early Adolescence, 26, 397–413. 10.1177/0272431606291943 [DOI] [Google Scholar]
- Schulkin J, Morgan MA, & Rosen JB (2005). A neuroendocrine mechanism for sustaining fear. TRENDS Neuroscience, 28, 629–635. DOI: 10.1016/j.tins.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Schürmann M, & Başar E (2001). Functional aspects of alpha oscillations in the EEG. International Journal of Psychophysiology, 39, 151–158. 10.1016/S0167-8760(00)00138-0 [DOI] [PubMed] [Google Scholar]
- Schutter DJ, & Knyazev GG (2012). Cross-frequency coupling of brain oscillations in studying motivation and emotion. Motivation and Emotion, 36, 46–54. doi: 10.1007/s11031-011-9237-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter DJ, & Van Honk EJ (2005). Salivary cortisol levels and the coupling of midfrontal delta-beta oscillations. International Journal of Psychophysiology, 55, 127–129. [DOI] [PubMed] [Google Scholar]
- Smith AK, Rhee SH, Corley RP, Friedman NP, Hewitt JK, & Robinson JL (2012). The magnitude of genetic and environmental influences on parental and observational measures of behavioral inhibition and shyness in toddlerhood. Behavior Genetics, 42, 764–777. doi: 10.1007/s10519-012-9551-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez GL, Morales S, Metcalf K, & Pérez- Edgar KE (2019). Perinatal complications are associated with social anxiety: Indirect effects through temperament. Infant and Child Development, e2130 10.1002/icd.2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Peer JM, Roelofs K, & Spinhoven P (2008). Cortisol administration enhances the coupling of midfrontal delta and beta oscillations. International Journal of Psychophysiology, 67, 144–150. doi: 10.1016/j.ijpsycho.2007.11.001 [DOI] [PubMed] [Google Scholar]
- Werner NE, & Crick NR (2004). Maladaptive peer relationships and the development of relational and physical aggression during middle childhood. Social Development, 13, 495–514. 10.1111/j.1467-9507.2004.00280.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.