Abstract
Objective:
To determine the effectiveness of silicone and pressure garments (alone and in combination) in children receiving scar management post-burn.
Design:
Multicentre, parallel-group, randomized controlled trial.
Setting:
Hospital outpatient clinics, colocated research centre, or the participant’s home.
Participants:
Children (0–18 years) referred for burn scar management.
Interventions:
Participants were randomized to (1) topical silicone gel only, (2) pressure garment therapy only, or (3) combined topical silicone gel and pressure garment therapy.
Main measures:
Primary outcomes included scar thickness and itch intensity at the primary end-point of six months post-burn injury. The outcome assessor and data analyst were blinded for scar thickness.
Results:
Participants (N = 153; silicone n = 51, pressure n = 49, combined n = 53) had a median (inter-quartile range) age of 4.9 (1.6, 10.2) years and percent total body surface area burn of 1% (0.5%, 3%) and were 65% male. At six months post-burn injury, intention-to-treat analysis identified thinner scars in the silicone (n = 51 scar sites) compared to the combined group (n = 48 scar sites; mean difference (95% confidence interval) = –0.04 cm (–0.07, –0.00), P = 0.05). No other between-group differences were identified for scar thickness or itch intensity at six months post-burn.
Conclusion:
No difference was identified in the effectiveness of silicone and pressure interventions alone. No benefit to a combined silicone and pressure intervention was identified for the prevention and management of abnormal scarring in children at six months post-burn injury, compared to the silicone or pressure interventions alone.
Keywords: Children, burn, cicatrix, scar management, silicone, pressure garment
Introduction
Managing abnormal post-burn scarring remains challenging for rehabilitation teams despite a global reduction in burn injury incidence, mortality, and severity over the last 30 years.1–3 Potential risk factors for abnormal burn scarring include, but are not limited to, burn depth, total body surface area burned (TBSA), length of wound healing, skin type, anatomical burn site, and wound healing type.4–6 In those who develop abnormal scarring, the peak severity of scarring is six months post-burn.7,8 Preventing abnormal scarring post-burn is the first priority of scar management,9 with non-invasive interventions initiated to prevent or reduce scar severity and improve overall scar appearance and health-related quality of life.10 Non-invasive scar interventions may include silicone products, pressure garment therapy, exercise, massage, and ultrasound.10 While these interventions have been used in routine practice for over 40 years, their effectiveness remains unclear,11 particularly in children who have not been the focus of previous trials. The current study focuses on silicone and pressure garment therapy as these are commonly used interventions in burn centres worldwide.10
Silicone products are postulated to reduce tension at the scar border (influencing the mechanotransduction pathway) and provide an occlusive barrier to the skin, thus mimicking a mature stratum corneum and preventing trans-epidermal water loss.12–15 Pressure garments are hypothesized to induce hypoxia in the scar microvasculature and impact the mechanotransduction pathway by providing support to the extra-cellular matrix, stimulating apoptosis.15,16 The combined application of these two interventions has been hypothesized to improve scar outcomes more than either intervention alone.13,17
Systematic reviews10,11,18,19 evaluating the effectiveness of silicone and pressure garment therapy have reported conflicting evidence of effectiveness. Previous investigations are limited by a focus on scar characteristics rather than patient-centred outcomes such as treatment burden and health-related quality of life. Furthermore, benefits have rarely been reported in light of harms as recommended20 despite skin maceration and breakdown, rash, pruritus, and changes to bony growth being potential harms of silicone and pressure garment therapy.11,18,21 Due to the potential influence of wound healing type on scar development,4–6 this should also be accounted for in investigations.
This study aimed to determine the effectiveness of topical silicone gel and pressure garment therapy alone and in combination for the prevention and management of abnormal post-burn scarring in children.
Method
A multi-centre, three-arm, parallel-group, randomized controlled trial was conducted between August 2016 and November 2018. The study had ethics approval from the Children’s Health Queensland Human Research Ethics Committee: HREC/15/QRCH/249 (SSA/16/QRCH/217, University of Queensland Human Research Ethics Committee: Approval number 2016000558). The study was registered on the Australian and New Zealand Clinical Trial Registry (ANZCTR): ACTRN12616001100482. The study is reported according to the CONSORT guidelines.22
Participants were recruited from the outpatient clinic at a large tertiary-level metropolitan children’s hospital burns centre. Follow-up data collection was completed at the burns centre, a research centre colocated with the burns centre, four regional hospitals located up to 620 km from the primary setting, and participant’s homes. One author (not involved in data collection) completed individual patient randomization using computer-generated random numbers and nested permuted blocks of 12 to ensure balance between groups. Participants were stratified by surgical intervention received (skin grafting or spontaneous skin healing in the acute phase, post-acute scar reconstructive surgery) and then randomly allocated to a treatment group in a 1:1:1 ratio. Concealment of treatment allocation was via sealed, opaque, identical, and serially numbered envelopes prepared in advance by an independent party not involved in the trial. The allocation list was stored securely in a research facility separate to the recruitment location. An independent party opened envelopes after baseline measurements were completed.
Inclusion criteria included children managed in the acute phase post-burn (up to 16 years of age) or who received burn-scar reconstructive surgery (up to 18 years of age) at the burns centre. Children included were those who received skin grafting, children with spontaneously healing wounds that did not heal by day 17 post-burn with a % total body surface area burned (%TBSA) ⩽ 40%, and children who received reconstruction surgery for a pre-existing burn scar. A parent or guardian who was able to provide informed consent was required. Children with a cognitive impairment impeding communication were enrolled though were not required to complete self-report measures.
Exclusion criteria included children with comorbidities potentially influencing primary outcomes (i.e. dermatological or neurological disorders); referred to local health services before scar management commenced; and isolated facial, ear, or genital burns. The latter criteria were added to the study protocol early after commencement of the trial (after initial registration with ANZCTR but prior to publication of the study protocol) with the rationale documented in Supplemental Appendix 1.
All participants received standard care for the acute burn injury as determined by the burns multidisciplinary team. Baseline assessment was completed prior to scar intervention randomization at approximately 95% wound re-epithelialisation (refer to Supplemental Appendix 1 for additional information). Due to the nature of the interventions, blinding of participants and health professionals was not possible. However, blinded ultrasound assessment of scar thickness was completed and only the measurements of this blinded assessor were used in data analysis. A blinded data analyst completed analysis of the primary outcomes.
Participants were randomized to (1) medical use topical silicone gel only (Strataderm®; Stratpharma, Basel, Switzerland); (2) pressure garment only (Therapeutic Support Laboratory®, TSL; Abbotsford, VIC, Australia)); or (3) combined topical silicone gel (Strataderm®) and pressure garment therapy (TSL®). The interventions investigated during this study were considered components of standard care at the participating burns centre prior to the trial; therefore, known potential adverse effects (e.g. irritation from topical silicone gel or friction caused by pressure garments) had a standardized management protocol. Detailed intervention information is provided in Supplemental Appendix 2.
Primary outcomes included scar thickness and itch intensity. Primary outcomes were measured on two scar sites at baseline, one week post-scar management commencement, and three and six months post-burn or burn scar reconstruction surgery. Scar thickness was measured using the GE Healthcare Ultrasound (intra-observer reproducibility, intraclass correlation coefficient (ICC) = 0.95, smallest detectable change (SDC) = 0.06 cm, standard error of measurement (SEM) = 0.02 cm in children with burn scars).23 The primary approach for scar itch intensity was caregiver proxy report on the itch item of the Patient and Observer Scar Assessment Scale (POSAS).24 The secondary approach included child self-report (five years and older) on a numeric rating scale (NRS) and caregiver observation on the Toronto Paediatric Itch Scale.25
Secondary outcomes were measured on one scar site at baseline (excluding adherence, treatment satisfaction, and adverse effects) and three and six months post-burn or burn scar reconstruction surgery. Secondary outcomes included scar severity (patient and observer reports on the POSAS24 and DSMII ColorMeter® (measured on two scar sites; Cortex Technology, Hadsund, Denmark)), health-related quality of life (generic: Child Health Utility-9D;26 disease specific: Brisbane Burn Scar Impact Profile (BBSIPca0-8,27 BBSIPca8-18 31,28 BBSIP8-18))29, adherence (questionnaire designed for the study purposes), and treatment satisfaction (caregiver and treating occupational therapist perspective using an 11-point NRS). Adverse effects (type and number of effects),20 intervention fidelity30 (paper-based checklist designed for the study purposes), intervention burden (single items addressing intervention burden from the BBSIP, all versions), and interface pressure (Pliance X®; Novel Electronics, Munich, Germany) were also included. Full details for outcome measures are provided in the study protocol31 and Supplemental Appendix 3.
A sample size estimate was based on scar thickness for a single scar site at six months post-burn. To detect a statistically significant between-group difference of 0.76 mm in scar thickness17 with 80% power, an alpha value of 0.017 (due to three pairwise comparisons), and a pooled standard deviation of 1.0 mm, 36 participants were required in each of the three groups. Assuming 20% attrition, recruitment of 135 participants (45 per group) was needed at baseline.
Descriptive statistics were used to summarize sociodemographic, clinical characteristics, and intervention data. Between-group differences in the outcomes were investigated using mixed-effects regression models. Fixed effects entered in the model were time and group, as well as a time-by-group interaction. Participants were entered as random effects, to account for the possible non-independence of outcomes within children when outcomes were measured at each site. Effectiveness was determined using the results of ‘intention-to-treat’ (ITT) analyses at six months post-burn injury or burn scar reconstruction. As pre-specified, a per-protocol approach was also undertaken to investigate the sensitivity of results. Another sensitivity analysis was conducted by inputting missing outcome data using 10 created data sets.32 A pre-specified analysis by surgical group (spontaneous wound healing, skin grafting, scar reconstruction surgery) was completed, as was a post hoc analysis by scar location (upper limb, lower limb, torso). Significance was set at less than 0.05.
Descriptive data were summarized using SPSS (SPSS, Chicago, IL, USA) and regression models were constructed using Stata (StataCorp, College Station, TX, USA).
Results
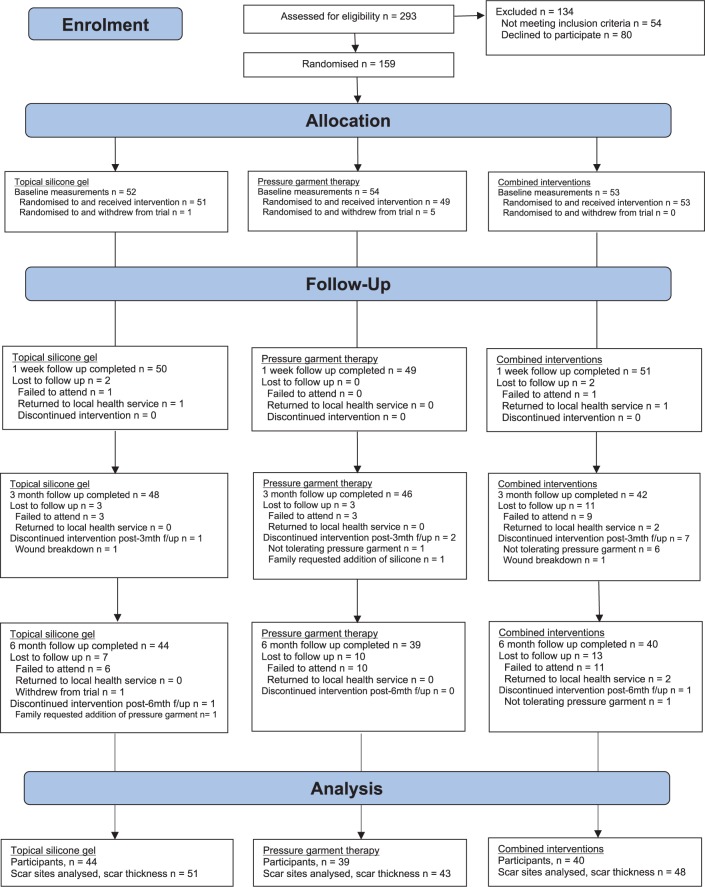
Participants (n = 159) were recruited until the sample size was reached and attrition was accounted for. Six participants withdrew from the trial immediately after randomization (silicone n = 1; pressure n = 5; combined n = 0). Figure 1 details the number of participants recruited and available for scar assessment at each time point (including reasons for missing data).
Figure 1.
CONSORT flowchart.
Participants allocated to an intervention (n = 170 scar sites from 153 children) had a median (interquartile range (IQR)) age of 4.9 (1.6, 10.2) years, a median (IQR) %TBSA burned of 1.0% (0.5, 3.0), and were predominantly male (n = 99, 65%). Burns were mainly located on the limbs (n = 133, 87%; see Table 1). Table 2 displays baseline results for select outcome measures. Supplemental Table 1 details baseline characteristics of participants who declined or withdrew from the trial.
Table 1.
Social and clinical demographics of the included participants.
| Topical silicone gel onlya,b | Pressure garment onlya,b | Combineda,b,c | |
|---|---|---|---|
| No. of participants | 51 | 49 | 53 |
| Age, median (IQR) | 3.54 (1.52, 8.78) | 8.86 (1.82, 10.87) | 4.86 (1.75, 10.05) |
| Male Gender | 31 (61%) | 26 (53%) | 42 (79%) |
| Skin type | |||
| Type I – Always burn | 4 (8%) | 6 (12%) | 6 (11%) |
| Type II – Tan with difficulty | 8 (16%) | 9 (18%) | 7 (13%) |
| Type III – Tan about average | 22 (43%) | 16 (33%) | 17 (32%) |
| Type IV – Tan more than average | 7 (14%) | 12 (25%) | 13 (25%) |
| Type V – Brown skin | 9 (17%) | 5 (10%) | 7 (13%) |
| Type VI – Black skin | 1 (2 %) | 0 (0%) | 1 (2%) |
| Missing | 0 (0%) | 1 (2%) | 2 (4%) |
| %TBSA of burn, median (IQR) | 1.25 (0.5, 3.0) | 1.00 (0.5, 2.0) | 1.50 (0.5, 3.0) |
| Missing | 3 | 6 | 3 |
| Burn depth | |||
| Full thickness | 8 (16%) | 9 (18%) | 8 (15%) |
| Deep partial | 30 (59%) | 24 (49%) | 28 (53%) |
| Superficial partial | 10 (19%) | 11 (23%) | 14 (26%) |
| Missing | 3 (6%) | 5 (10%) | 3 (6%) |
| Mechanism of injury | |||
| Scald | 16 (31%) | 12 (25%) | 18 (34%) |
| Contact | 19 (37%) | 24 (49%) | 21 (40%) |
| Flame | 1 (2%) | 3 (6%) | 3 (6%) |
| Friction | 9 (18%) | 5 (10%) | 6 (11%) |
| Electrical | 0 (0%) | 1 (2%) | 2 (3%) |
| Chemical | 3 (6%) | 1 (2%) | 0 (0%) |
| Other | 1 (2%) | 0 (0%) | 0 (0%) |
| Missing | 2 (4%) | 3 (6%) | 3 (6%) |
| Scar location | |||
| Torso | 10 (20%) | 6 (12%) | 4 (8%) |
| Upper limb | 23 (45%) | 20 (41%) | 27 (51%) |
| Lower limb | 18 (35%) | 23 (47%) | 22 (41%) |
| Surgical group | |||
| Spontaneous | 34 (67%) | 31 (63%) | 33 (63%) |
| Grafted | 14 (27%) | 13 (27%) | 15 (28%) |
| Reconstruction | 3 (6%) | 5 (10%) | 5 (9%) |
| Days to re-epithelialisation, median (IQR) | 26 (21, 34) | 24 (20, 29) | 25 (21.5, 31.5) |
| Wound infection | 8 (16%) | 8 (16%) | 12 (23%) |
| Missing | 0 (0%) | 0 (0%) | 1 (2%) |
| Additional interventions | |||
| Ranging exercises | 2 (4%) | 2 (4%) | 6 (11%) |
| Massage | 4 (8%) | 1 (2%) | 2 (4%) |
| Number of itch medications | |||
| 0 | 33 (65%) | 34 (69%) | 37 (70%) |
| 1 | 15 (29%) | 13 (27%) | 10 (19%) |
| 2 | 3 (6%) | 1 (2%) | 6 (11%) |
| 3 | 0 (0%) | 1 (2%) | 0 (0%) |
| Type of itch medication | |||
| Gabapentin | 4 (8%) | 5 (10%) | 6 (11%) |
| Cetirizine | 14 (28%) | 11 (22%) | 14 (26%) |
| Promethazine | 2 (4%) | 1 (2%) | 2 (4%) |
| Loratadine | 1 (2%) | 1 (2%) | 0 (0%) |
| Interface pressure (mmHg) | |||
| Stationary | N/A | 35.10 (32.49) | 25.22 (23.04) |
| Dynamic | N/A | 33.67 (31.15) | 32.02 (26.45) |
| Caregiver education | |||
| Completed post school qualifications | 33 (64%) | 22 (45%) | 27 (51%) |
| Completed senior high school | 8 (16%) | 15 (31%) | 16 (30%) |
| Completed junior high school | 8 (16%) | 10 (20%) | 6 (11%) |
| Did not go to school | 1 (2%) | 0 (0%) | 1 (2%) |
| Missing | 1 (2%) | 2 (4%) | 3 (6%) |
IQR: interquartile range; TBSA: total body surface area; NA: not applicable.
Number (percentage) except where indicated.
Missing data were stated, otherwise there was no missing data.
Combined = topical silicone gel + pressure garment therapy.
Table 2.
Baseline scores of select outcomes.
| Topical silicone gel |
Pressure garment therapy |
Combineda |
|
|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | |
| Scar thickness (cm) | 0.17 (0.08), 60 | 0.16 (0.08), 50 | 0.16 (0.08), 60 |
| Scar itch intensity (NRS) | |||
| Caregiver report | 4.51 (2.62), 59 | 4.94 (2.68), 54 | 4.16 (2.83), 63 |
| Child self-report | 3.10 (3.16), 21 | 3.59 (2.86), 27 | 3.25 (2.52), 24 |
| Scar severity (POSAS) | |||
| Caregiver overall | 7.25 (2.39), 48 | 6.90 (2.43), 49 | 6.61 (2.32), 51 |
| Observer overall | 4.17 (1.12), 60 | 4.15 (1.18), 53 | 4.02 (1.17), 62 |
| Scar colour (Colorimeter) | |||
| Erythema a* parameter | 34.83 (6.64), 58 | 33.23 (8.97), 52 | 31.42 (8.69), 61 |
| Pigmentation L* parameter | −15.40 (10.22), 51 | −15.90 (8.74), 47 | −12.84 (12.73), 58 |
| Impact of burn scar (BBSIP) | |||
| Caregivers <8 years | 2.43 (0.83), 36 | 2.02 (0.53), 23 | 2.16 (0.89), 32 |
| Caregivers ≥8 years | 2.00 (0.61), 14 | 2.34 (0.82), 26 | 2.15 (0.94), 20 |
| Child self-report 8–18 years | 1.90 (0.91), 12 | 2.02 (0.84), 25 | 1.95 (0.92), 19 |
SD: standard deviation; NRS: numeric rating scale; POSAS: Patient and Observer Scar Assessment Scale; Erythema a* parameter and Pigmentation L* paramter: DMSII ColorMeter; BBSIP: Brisbane Burn Scar Impact Profile.
Combined = topical silicone gel + pressure garment therapy.
ITT analysis of scar thickness identified thinner scars in the silicone and pressure alone groups compared to the combined group, with the difference significant between the silicone and combined intervention groups. There were no significant differences when comparing scar thickness of the silicone and pressure alone groups (Table 3). Standardized mean differences (effect sizes) are reported in Supplemental Table 3. Sensitivity analyses (ITT analysis with missing outcome data imputed, Table 3 and per protocol analysis, Supplemental Table 2) had similar findings. Assessment of scar thickness after stratification by surgical group (Supplemental Table 4) identified thicker scars in the combined intervention group compared to the silicone alone group for participants who received skin grating. Stratification by scar location (Supplemental Table 5) identified thicker scars in the combined intervention group compared to both interventions alone for participants with scars located on the upper limbs. The mean (SD) of burn scar thickness measurements for the primary and blinded investigators were within 0.05 cm of each other which is equal to the inter-rater SEM of the GE Healthcare Ultrasound device in this population.23
Table 3.
Mixed-effects regression results for primary outcomes at six months post-burn injury.
| Topical silicone gel |
Pressure garment therapy |
Combineda |
Topical silicone gel vs. pressure garment |
Topical silicone gel vs. combined |
Combined vs. pressure garment therapy |
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD), n | Mean (SD), n | Mean (SD), n | Mean difference (CI) | P | Mean difference (CI) | P | Mean difference (CI) | P | |
| Scar thickness (cm) | |||||||||
| Complete case | 0.18 (0.10), 51 | 0.18 (0.09), 43 | 0.22 (0.14), 48 | −0.00 (−0.04, 0.04) | 0.93 | −0.04 (−0.07, −0.00) | 0.05 | 0.04 (−0.00, 0.07) | 0.07 |
| Multiple imputation | – | – | – | −0.00 (−0.04, 0.04) | 0.92 | −0.03 (−0.07, 0.01) | 0.11 | 0.03 (−0.01, 0.07) | 0.18 |
| Scar itch intensity (NRS) | |||||||||
| Caregiver report | 1.90 (1.72), 50 | 1.83 (1.69), 41 | 1.65 (1.45), 48 | 0.03 (−0.91, 0.98) | 0.94 | 0.20 (−0.71, 1.12) | 0.67 | −0.17 (−1.12, 0.79) | 0.73 |
| Child self-report | 1.71 (2.59), 17 | 1.04 (1.61), 23 | 0.83 (1.29), 18 | 0.69 (−0.76, 2.15) | 0.35 | 0.80 (−0.74, 2.35) | 0.31 | −0.11 (−1.54, 1.32) | 0.88 |
SD: standard deviation; CI: confidence interval; NRS: numeric rating scale.
Combined = topical silicone gel + pressure garment therapy.
Caregiver-reported burn scar itch intensity was similar between the three groups (Table 3, Supplemental Table 6). Sensitivity analysis confirmed this finding (Supplemental Table 2). Stratification by surgical group and scar location or assessment of child self-report and caregiver observation for children below the age of five years (Supplemental Table 7) did not alter these results. Standardized mean differences (effect sizes) are reported in Supplemental Table 3.
No statistically significant differences were identified at six months post-burn injury for scar severity (Supplemental Table 8), scar colour (Supplemental Table 9), health-related quality of life (Supplemental Table 10), intervention fidelity (Supplemental Table 11), interface pressure (Supplemental Table 12), or intervention burden (Supplemental Table 13). No serious adverse effects were identified (Supplemental Table 14), though a statistically significant difference in the rate of adverse effects was observed in the silicone compared to the pressure group. Caregiver and occupational therapist treatment satisfaction is reported in Supplemental Table 15.
Caregiver-reported adherence to silicone alone at least once per day was better than when combined with pressure garment therapy (91.43% vs. 82.76%). Caregiver-reported adherence to pressure alone (as a percentage compared to the recommended 23 hours per day) was better than when used in combination with silicone, mean (SD) = 77.51% (31.99) vs. 58.48% (42.89). A per protocol analysis of adherence is reported in Supplemental Table 16.
Discussion
Together the findings indicate there was no benefit to using a combination of topical silicone gel and pressure garment therapy at six months post-burn, the typical peak of abnormal burn scar characteristics,7,8 compared to silicone or pressure therapy alone. Greater scar thickness was observed in the combined group when compared to the silicone group. In addition, superior caregiver-reported treatment satisfaction and adherence were reported when interventions were used individually rather than in combination. No difference was observed between the silicone and pressure garment alone groups for any outcome. However, the results should be interpreted in light of more adverse effects in the pressure alone group compared to the silicone alone group.
Despite potential differences in mechanism of action for silicone products and pressure garments,14–16 the goal of both interventions, alone and in combination, remains the same. The goal of interventions is to reduce keratinocyte, fibroblast, mast cell, and histamine production, thus increasing collagenase activity and subsequent collagen breakdown.12,14,15 Results of this study suggest that it is plausible that both interventions may be equally effective at achieving this goal on their own, particularly in the thinner skin of the paediatric population.
The finding of greater scar thickness in the combined group compared to the silicone group and no between-group differences for scar severity is in contrast to previous findings in adults.17,33,34 Reduced thickness and increased pliability in paediatric pre-injury skin compared to adults35,36 and a lower mean %TBSA in the study sample than typically reported in adult studies may partly explain these findings. In addition, the use of a preventive approach mirroring clinical practice may have meant the severity of scarring and subsequent impact on health-related quality of life of the children in this trial was lower than in adults. It should also be noted that the minimally clinically important difference for scar thickness has not been determined. The extent of the difference in this study was not considered clinically important as it was below the SDC of the GE Healthcare Ultrasound device.23
Assessment of potential influencing factors identified no differences between the groups in fidelity or interface pressure. Thus, it is reasonable to conclude that the study results were due to the intervention and not because of variation in the type of intervention received.37 Interestingly, the reported rates of adherence were worse when interventions were used in combination.
Adherence can be hampered by increasing intervention complexity and frequency, and reduced convenience.38,39 Therefore, the increased requirements of the combined intervention group may have increased the complexity and frequency of the intervention such that adherence was negatively influenced. There was an increased intervention burden reported by caregivers of children aged less than eight years using pressure alone compared to silicone alone; however, results were not statistically significant.
Also of interest was the finding of a greater number of adverse effects in the pressure alone group compared to the silicone alone group but not in the combined group (with an equivalent pressure dosage) compared to silicone alone. This may suggest that silicone provides a layer of protection to the skin for adverse effects such as skin irritation or wound breakdown. Alternatively, the reduced adherence to pressure in the combined group may have reduced the likelihood of participants experiencing these effects.
Attrition is a well-documented concern in burn scar research with up to 80% attrition reported in paediatric studies over an undisclosed timeframe.40 In a bid to prevent attrition, additional processes were initiated prior to recruitment such as research appointments after business hours to reduce the burden on families travelling during school and work times. A greater rate of attrition was identified in the combined intervention group compared to the pressure and silicone alone groups at six months post-burn injury or burn scar reconstruction surgery indicating a potential intervention effect on attendance at appointments. To determine the influence of attrition, an analysis of burn-scar thickness at six months post-burn using multiple imputations for missing data was completed. As this analysis did not identify a between-group difference in scar thickness, there is further support for the conclusion that there was no benefit to a combined intervention approach for the children involved in this study.
The study results may not generalize to the limited number of participants included with Fitzpatrick Skin Type VI (black skinned persons) and facial scar sites which were not included. The fewer number of participants receiving scar reconstruction surgery for a pre-existing burn scar compared to those with acute burn injuries also suggests that the findings require replication for this group in particular. Furthermore, the scar sites assessed in this study may not be representative of the entire scar. Assessment of the audio recordings for intervention fidelity was unable to be completed due to technical reasons.
Strengths of this trial include the collection of outcomes such as health-related quality of life, adverse effects, intervention satisfaction and burden, intervention fidelity, and interface pressure enabling a comprehensive investigation of intervention effectiveness. Further investigation is required to confirm the results of this study. The cost-effectiveness and effectiveness of the study interventions to prevent long-term surgical procedures and improve outcomes at 12 months or longer post-burn is also yet to be determined and will be reported in future publications.
Clinical messages.
There was no benefit to using combined silicone and pressure therapy for physical or sensory scar outcomes at six months post-burn.
Greater frequency of adverse effects was observed in the pressure compared to the silicone group.
Combined silicone and pressure interventions may reduce intervention adherence and satisfaction.
Supplemental Material
Supplemental material, Supplemental_Material for Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: a randomized controlled trial by Jodie Wiseman, Robert S Ware, Megan Simons, Steven McPhail, Roy Kimble, Anne Dotta and Zephanie Tyack in Clinical Rehabilitation
Acknowledgments
The authors would like to acknowledge all the children, families, and health professionals who participated in this study and extend their gratitude to all the staff at the Pegg Leditschke Children’s Burns Centre at the Queensland Children’s Hospital (formerly Lady Cilento Children’s Hospital), Australia for their support and assistance throughout data collection. The authors would also like to acknowledge the assistance of Rachael Adams, James Brannigan, and Cody Frear (UQ Science and Medical students) for their contribution to medical chart data collection and data cleaning. An Australian Government Research Training Scholarship, Children’s Health Foundation Top Up scholarship, and a grant given to the University of Queensland by Stratpharma funded the study.
Footnotes
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Despite financial support, Stratpharma had no part in the final study design, data collection, or analysis of results. Financial support was provided by Stratpharma for the open access publication of this manuscript. The funders did not have access to the results prior to them being released publicly at the Australian New Zealand Burn Association and the International Society for Burn Injuries conferences. Only the investigators have access to the final trial data set. The investigators have no financial interest in Strataderm or the Stratpharma Company. The principal investigator is a PhD student of The University of Queensland. The investigators have no additional conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an Australian Government Research Training Scholarship and Children’s Health Foundation Top-Up Scholarship (RPCPHD0022017) to the lead author (J.W.) and a research grant provided to The University of Queensland by Stratpharma.
ORCID iD: Jodie Wiseman  https://orcid.org/0000-0002-0605-5710
https://orcid.org/0000-0002-0605-5710
Supplemental material: Supplemental material for this article is available online.
References
- 1. Brusselaers N, Pirayesh A, Hoeksema H, et al. Burn scar assessment: a systematic review of objective scar assessment tools. Burns 2010; 36(8): 1157–1164. [DOI] [PubMed] [Google Scholar]
- 2. Lee KC, Dretzke J, Grover L, et al. A systematic review of objective burn scar measurements. Burns Trauma 2016; 4: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Finnerty CC, Jeschke MG, Branski LK, et al. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet 2016; 388(10052): 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gangemi EN, Gregori D, Berchialla P, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 2008; 10(2): 93–102. [DOI] [PubMed] [Google Scholar]
- 5. Berchialla P, Gangemi E, Foltran F, et al. Predicting severity of pathological scarring due to burn injuries: a clinical decision making tool using Bayesian networks. Int Wound J 2014; 11(3): 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lawrence JW, Mason ST, Schomer K, et al. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res 2012; 33(1): 136–146. [DOI] [PubMed] [Google Scholar]
- 7. Nedelec B, Correa JA, De Oliveira A, et al. Longitudinal burn scar quantification. Burns 2014; 40(8): 1504–1512. [DOI] [PubMed] [Google Scholar]
- 8. Van der Wal M, Vloemans J, Tuinebreijer W, et al. Outcome after burns: an observational study on burn scar maturation and predictors for severe scarring. Wound Repair Regen 2012; 20: 676–687. [DOI] [PubMed] [Google Scholar]
- 9. Monstrey S, Middelkoop E, Vranckx JJ, et al. Updated scar management practical guidelines: non-invasive and invasive measures. J Plast Reconstr Aesthet Surg 2014; 67(8): 1017–1025. [DOI] [PubMed] [Google Scholar]
- 10. Anthonissen M, Daly D, Janssens T, et al. The effects of conservative treatments on burn scars: a systematic review. Burns 2016; 42(3): 508–518. [DOI] [PubMed] [Google Scholar]
- 11. Friedstat J, Hultman S. Hypertrophic burn scar management: what does the evidence show? A systematic review of randomized controlled trials. Ann Plast Surg 2014; 72(6): S198–S201. [DOI] [PubMed] [Google Scholar]
- 12. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesthetic Plast Surg 2008; 32(1): 82–92. [DOI] [PubMed] [Google Scholar]
- 13. Van den Kerckhove E, Stappaerts K, Boeckx W, et al. Silicones in the rehabilitation of burns: a review and overview. Burns 2001; 27(3): 205–214. [DOI] [PubMed] [Google Scholar]
- 14. Tandara AA, Mustoe TA. The role of the epidermis in the control of scarring: evidence for mechanism of action for silicone gel. J Plast Reconstr Aesthet Surg 2008; 61(10): 1219–1225. [DOI] [PubMed] [Google Scholar]
- 15. Yagmur C, Akaishi S, Ogawa R, et al. Mechanical receptor-related mechanisms in scar management: a review and hypothesis. Plast Reconstr Surg 2010; 126(2): 426–434. [DOI] [PubMed] [Google Scholar]
- 16. Li-Tsang CW, Feng B, Huang L, et al. A histological study on the effect of pressure therapy on the activities of myofibroblasts and keratinocytes in hypertrophic scar tissues after burn. Burns 2015; 41(5): 1008–1016. [DOI] [PubMed] [Google Scholar]
- 17. Li-Tsang CW, Zheng YP, Lau JC. A randomized clinical trial to study the effect of silicone gel dressing and pressure therapy on posttraumatic hypertrophic scars. J Burn Care Res 2010; 31(3): 448–457. [DOI] [PubMed] [Google Scholar]
- 18. Anzarut A, Olson J, Singh P, et al. The effectiveness of pressure garment therapy for the prevention of abnormal scarring after burn injury: a meta-analysis. J Plast Reconstr Aesthet Surg 2009; 62(1): 77–84. [DOI] [PubMed] [Google Scholar]
- 19. Ai JW, Liu JT, Pei SD, et al. The effectiveness of pressure therapy (15-25 mmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Sci Rep 2017; 7: 40185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ioannidis J, Evans S, Gøtzsche P, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med 2004; 141(10): 781–788. [DOI] [PubMed] [Google Scholar]
- 21. Nedelec B, Carter A, Forbes L, et al. Practice guidelines for the application of nonsilicone or silicone gels and gel sheets after burn injury. J Burn Care Res 2015; 36(3): 345–374. [DOI] [PubMed] [Google Scholar]
- 22. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simons M, Kee EG, Kimble R, et al. Ultrasound is a reproducible and valid tool for measuring scar height in children with burn scars: a cross-sectional study of the psychometric properties and utility of the ultrasound and 3D camera. Burns 2017; 43(5): 993–1001. [DOI] [PubMed] [Google Scholar]
- 24. Draaijers LJ, Tempelman FRH, Botman YAM, et al. The Patient and Observer Scar Assessment Scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg 2004; 113(7): 1960–1965; discussion 1966–1967. [DOI] [PubMed] [Google Scholar]
- 25. Everett T, Parker K, Fish J, et al. The construction and implementation of a novel postburn pruritus scale for infants and children aged five years or less: introducing the Toronto Pediatric Itch Scale. J Burn Care Res 2015; 36(1): 44–49. [DOI] [PubMed] [Google Scholar]
- 26. Stevens K. Working with children to develop dimensions for a preference-based, generic, pediatric health-related quality-of-life measure. Qual Health Res 2010; 20(3): 340–351. [DOI] [PubMed] [Google Scholar]
- 27. Tyack Z, Simons M, Kimble R. Brisbane Burn Scar Impact Profile for caregivers of children aged less than 8 years version 1.0. Brisbane, QLD, Australia: The State of Queensland; (Queensland Health), 2013. [Google Scholar]
- 28. Tyack Z, Simons M, Kimble R. Brisbane Burn Scar Impact Profile for caregivers of children 8 years and older version 1.0. Brisbane, QLD, Australia: The State of Queensland; (Queensland Health), 2013. [Google Scholar]
- 29. Tyack Z, Simons M, Kimble R. Brisbane Burn Scar Impact Profile for children 8 to 18 years version 1.0. Brisbane, QLD, Australia: The State of Queensland; (Queensland Health), 2013. [Google Scholar]
- 30. Schoenwald SK, Garland AF, Chapman JE, et al. Toward the effective and efficient measurement of implementation fidelity. Adm Policy Ment Health 2011; 38(1): 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wiseman J, Simons M, Kimble R, et al. Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: study protocol for a randomised controlled trial. Trials 2017; 18(1): 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin DB, Little RJ. Statistical analysis with missing data. Hoboken, NJ: John Wiley & Sons, 2002. [Google Scholar]
- 33. Steinstraesser L, Flak E, Witte B, et al. Pressure garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg 2011; 128(4): 306e–313e. [DOI] [PubMed] [Google Scholar]
- 34. Harte D, Gordon J, Shaw M, et al. The use of pressure and silicone in hypertrophic scar management in burns patients: a pilot randomized controlled trial. J Burn Care Res 2009; 30(4): 632–642. [DOI] [PubMed] [Google Scholar]
- 35. Tan C, Statham B, Marks R, et al. Skin thickness measurement by pulsed ultrasound: its reproducability, validation and variability. Br J Dermatol 1982; 106: 657–667. [DOI] [PubMed] [Google Scholar]
- 36. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermato-endocrinol 2012; 4(3): 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moncher F, Prinz R. Treatment fidelity in outcome studies. Clin Psychol Rev 1991; 11: 247–266. [Google Scholar]
- 38. Szabo MM, Urich MA, Duncan CL, et al. Patient adherence to burn care: a systematic review of the literature. Burns 2016; 42(3): 484–491. [DOI] [PubMed] [Google Scholar]
- 39. Jones LL, Calvert M, Moiemen N, et al. Outcomes important to burns patients during scar management and how they compare to the concepts captured in burn-specific patient reported outcome measures. Burns 2017; 43(8): 1682–1692. [DOI] [PubMed] [Google Scholar]
- 40. McQuaid D, Barton J, Campbell E. Researchers BEWARE! attrition and nonparticipation at large. J Burn Care Rehabil 2003; 24(4): 203–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material for Effectiveness of topical silicone gel and pressure garment therapy for burn scar prevention and management in children: a randomized controlled trial by Jodie Wiseman, Robert S Ware, Megan Simons, Steven McPhail, Roy Kimble, Anne Dotta and Zephanie Tyack in Clinical Rehabilitation