Abstract
PURPOSE
To assess the efficacy of pembrolizumab in patients with advanced relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS).
PATIENTS AND METHODS
CITN-10 is a single-arm, multicenter phase II trial of 24 patients with advanced MF or SS. Patients were treated with pembrolizumab 2 mg/kg every 3 weeks for up to 24 months. The primary end point was overall response rate by consensus global response criteria.
RESULTS
Patients had advanced-stage disease (23 of 24 with stage IIB to IV MF/SS) and were heavily pretreated with a median of four prior systemic therapies. The overall response rate was 38% with two complete responses and seven partial responses. Of the nine responding patients, six had 90% or more improvement in skin disease by modified Severity Weighted Assessment Tool, and eight had ongoing responses at last follow-up. The median duration of response was not reached, with a median response follow-up time of 58 weeks. Immune-related adverse events led to treatment discontinuation in four patients. A transient worsening of erythroderma and pruritus occurred in 53% of patients with SS. This cutaneous flare reaction did not result in treatment discontinuation for any patient. The flare reaction correlated with high PD-1 expression on Sézary cells but did not associate with subsequent clinical responses or lack of response. Treatment responses did not correlate with expression of PD-L1, total mutation burden, or an interferon-γ gene expression signature.
CONCLUSION
Pembrolizumab demonstrated significant antitumor activity with durable responses and a favorable safety profile in patients with advanced MF/SS.
INTRODUCTION
Mycosis fungoides (MF) and Sézary syndrome (SS) are the most common subtypes of cutaneous T-cell lymphomas (CTCLs). Patients with advanced-stage MF/SS experience progressively worsening disease and decreased overall survival (OS).1 Most current systemic therapies are associated with a response rate of less than 50%, and these responses are generally short-lived.2-6 Therapies with more durable responses in these patients with advanced disease are needed.
Antibodies that target immune checkpoint molecules can induce deep and durable responses in a wide variety of malignancies. Immune checkpoint antibodies against programmed cell death protein 1 (PD-1) act by targeting inhibitory PD-1 molecules expressed by exhausted T cells. By interfering with these inhibitory signaling pathways, these drugs can reinvigorate antitumor T cells and lead to long-lasting clinical responses.
Although immune checkpoint antibodies act through targeting of exhausted T cells, PD-1 also is expressed by malignant T cells in certain subtypes of T-cell lymphomas, including MF and SS.7-11 The function of PD-1 in these malignant T cells is unclear. Despite its frequent expression, monoallelic and biallelic copy number loss of PD-1 has been reported in CTCLs.12-14 Genomic alterations also have been observed in MF/SS that involve the PD-1 ligands PD-L115 and PD-L2.16 Furthermore, PD-L1 often is expressed in the skin microenvironment, including the malignant T cells.7,17 Thus, components of the PD-1/PD-L1 axis seem to be both commonly expressed and frequently altered in MF/SS.
Treatment with the anti-PD-1 antibody nivolumab was associated with a 15% overall response rate (ORR) in the MF type of CTCL (two of 13 patients) reported as a subset of a multicohort study.16 Pembrolizumab is an immunoglobulin G4, anti-PD-1 monoclonal antibody that has not been studied in patients with CTCL. Here, we report the results of a Cancer Immunotherapy Trials Network multicenter phase II trial (CITN-10) of pembrolizumab in the treatment of patients with refractory or relapsed MF and SS.
PATIENTS AND METHODS
Study Design and Patient Population
CITN-10 is a multicenter, phase II, single-arm trial that investigated the efficacy of pembrolizumab in 24 patients with relapsed or refractory MF and SS. The trial enrolled across eight centers in the United States. Eligibility criteria included a diagnosis of MF or SS (clinical stage IB to IV) that had relapsed, was refractory, or had progressed after at least one standard systemic therapy. Other eligibility criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status of 0 or 1, measurable disease on the basis of the modified Severity Weighted Assessment Tool (mSWAT),18 and adequate organ function as assessed by laboratory testing. Exclusion criteria included CNS disease; active autoimmune disease that required systemic treatment within the past 3 months; treatment with radiation, phototherapy, histone deacetylase inhibitor, retinoids, interferons, therapeutic doses of systemic corticosteroids, or denileukin diftitox within 2 weeks; treatment with cytotoxic agents, investigational therapies, or tumor-targeting monoclonal antibodies within 4 weeks; treatment with alemutuzumab within 8 weeks; treatment with any T-cell stimulatory or checkpoint antibody within 15 weeks; or any history of prior anti-PD-1, anti-PD-L1, or anti-PD-L2 therapy.
Treatments and Assessments
Patients received pembrolizumab 2 mg/kg as an intravenous infusion every 3 weeks. Treatment continued up to 24 months or until either withdrawal of consent, an unacceptable adverse event (AE), disease progression, or investigator decision. Treatment could be held for up to 12 weeks for persistent treatment-related toxicity. Safety was monitored throughout the study and for 30 days after treatment discontinuation. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Immune-related AEs (irAEs) were defined as AEs of unknown etiology associated with drug exposure and consistent with an immune phenomenon that may be predicted on the basis of the nature of pembrolizumab, its mechanism of action, and reported experience with immunotherapies that have a similar mechanism of action.
Responses were assessed according to consensus global response criteria18 that included composite assessment of the skin by mSWAT,3 lymph nodes and viscera by computed tomography or positron emission tomography imaging, and blood tumor burden assessed by flow cytometry. Global responses were first assessed at week 12, then every 6 weeks (two cycles) until week 30, and then every 12 weeks (four cycles) thereafter. Patients with progressive disease could be continued in the trial until confirmation of progression performed 4 weeks later. Patients with confirmed progression at repeat assessment could be continued in the trial at the discretion of the investigator if clinically stable, with repeat skin assessments every 3 weeks and global assessment every 12 weeks, and if neither the skin, lymph node, nor blood compartment continued to progress by 25% or more above the new baseline at the time progression.
End Points
The primary end point was ORR by consensus global response criteria.18 Responses were defined as a confirmed partial response or complete response, with the confirmation assessment no less than 4 weeks after criteria for response were first met. Secondary end points included duration of response (DOR; time from first response to progression), progression-free survival (PFS; time from enrollment to disease progression or death as a result of any cause), and OS (time from enrollment to death).
Immunohistochemistry
Skin biopsy specimens were fixed in formalin and embedded in paraffin. Immunohistochemistry (IHC) for PD-1 (clone NAT105; Cell Marque, Rocklin, CA), PD-L1 (clone 22C3; Merck Research Laboratories, Rahway, NJ), PD-L2 (clone 3G2; Merck Research Laboratories), CD3 (clone CD3-12; Abd Serotec, Kidlington, UK), CD4 (clone 4B12; Leica Biosystems, Wetzlar, Germany), CD8 (clone CD8/144B; Dako, Glostrup, South Denmark), FoxP3 (clone 236A/E7; Abcam, Cambridge, UK), and CD163 (clone 10D6; Thermo Fisher Scientific, Waltham, MA) was performed as previously described.19 IHC was graded according to the percentage positive of the total mononuclear cell infiltrate.
Whole-Exome Sequencing
For patients with circulating Sézary cells at baseline, Sézary cells from peripheral blood mononuclear cells (PBMCs) underwent fluorescence-activated cell sorting on the basis of CD4 and CD26 expression. For all others, skin biopsy specimens were microdissected to enrich for tumor involvement. Sections from paraffin-embedded blocks were used to identify tumor-involved areas, and unstained slides were made from adjacent sections and briefly stained with hematoxylin to identify the corresponding areas before microdissection. Germline DNA was obtained by CD4 depletion of PBMCs using the Dynabeads CD4 Positive Isolation Kit (Thermo Fisher Scientific), and in cases with circulating Sézary cells, the samples were subsequently further purified by fluorescence-activated cell sorting to collect CD4−/CD14+ monocytes and CD4−/CD56+ natural killer cells. Twenty-five to 50 ng of genomic DNA was sheared with an LE220 Focused-ultrasonicator (Covaris, Woburn, MA) and used to prepare a sequencing library using the KAPA Hyper Prep Kit (Kapa Biosystems, Wilmington, MA) according to the manufacturer’s instructions. Exome enrichment was performed with the SureSelectXT Human All Exon V6 + COSMIC bait set (Agilent Technologies, Santa Clara, CA) according to manufacturer’s instructions. Libraries were sequenced on an Illumina HiSeq 2500 sequencer in two by 125-base pairs paired-end mode with mean target coverage of approximately 200 times. Reads were aligned to the University of California, Santa Cruz, hg19 reference genome with the Burrows-Wheeler MEM aligner,20 and duplicate reads were removed. Indel realignment and base quality recalibration were performed using the Genome Analysis Toolkit version 3.5 (Broad Institute, Cambridge, MA), and single nucleotide variants were called using a consensus of VarScan 2,21 Strelka2,22 and MuTect23 variant callers with comparison with paired germline DNA. Copy number analysis was performed using CNVkit.24
Gene Expression Profiling
NanoString (NanoString Technologies, Seattle, WA) gene expression analysis of an 18-gene T-cell–inflamed interferon-γ–related signature was performed on skin biopsy specimens as previously described.25
Mass Cytometry
CyTOF (Fluidigm, South San Francisco, CA) analysis was performed on Ficoll gradient–purified, cryopreserved PBMCs collected before pembrolizumab treatment from 22 patients as previously described.26,27 Forty parameters were collected, including a panel of 36 antibodies (Data Supplement). Samples were analyzed by viSNE using all antibody parameters, except PD-1, using Cytobank (www.cytobank.org) to identify Sézary cells. PD-1 expression was then measured on the gated Sézary cells.
Statistical Analyses
The study was designed according to a Simon’s optimal two-stage design. Nine patients were enrolled in stage 1. One or more responses were required in stage 1 to proceed with enrollment of an additional 15 patients in stage 2. The study was powered to detect an increase in the response rate of 5% to 25% at the one-sided 0.1 significance level with 90% power. The null hypothesis was rejected if three or more responses were observed in 24 patients. The Kaplan-Meier method was used to analyze DOR, PFS, and OS. Mann-Whitney U test was used for correlative studies. All analyses were two-sided with a significance level of 0.05. All analyses were completed using SAS 9.4 (SAS Institute, Cary, NC) or Prism 8 (GraphPad Software, San Diego, CA) software.
RESULTS
Baseline Patient Characteristics
Of the 24 enrolled patients, nine had MF, and 15 had SS (Table 1). Patients included those with tumor stage disease and erythrodermic presentation, and 96% had stage IIB or higher. Most patients were heavily pretreated with a median of four lines of prior systemic therapy. Four patients had a reported histologic feature of large-cell transformation, an adverse prognostic factor. All enrolled patients received at least one dose of pembrolizumab. Seven patients discontinued treatment because of disease progression. Three patients discontinued because of irAEs, and four patients discontinued because of unrelated AEs. Four patients continued with pembrolizumab treatment at their last follow-up, including three who completed 2 years of therapy with ongoing response.
TABLE 1.
Baseline Characteristics and Responses
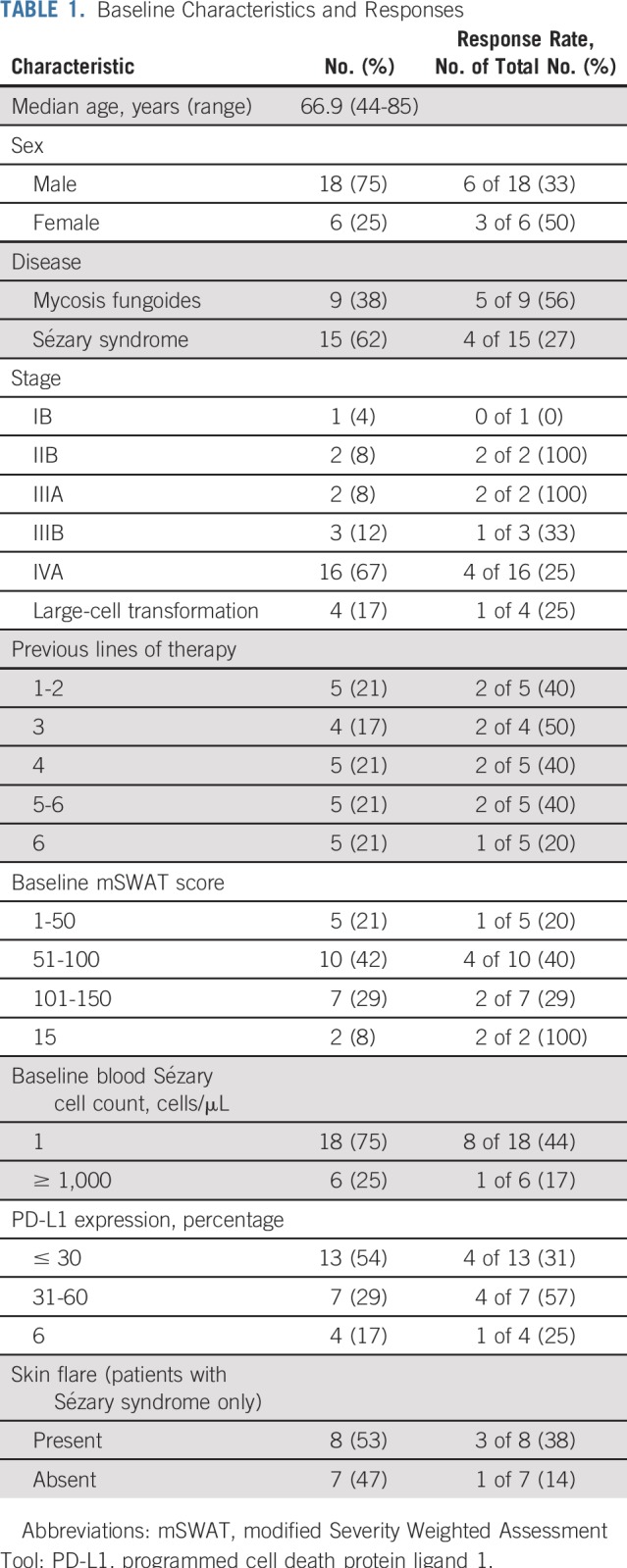
Clinical Activity
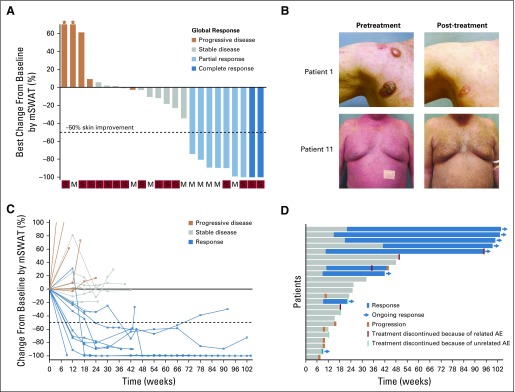
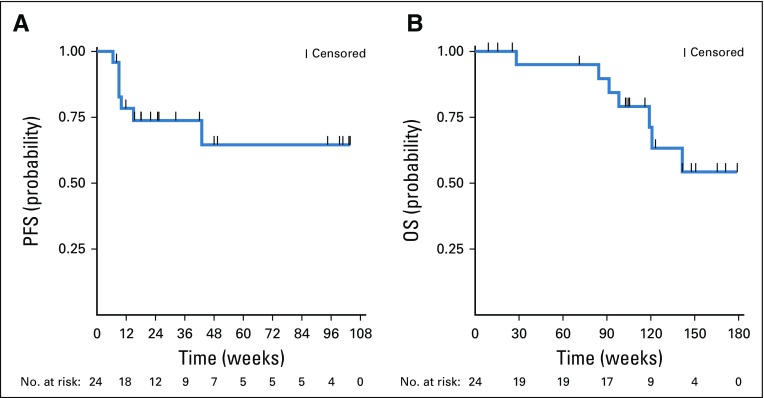
The ORR was 38% (nine of 24 patients) with two complete responses (Fig 1A). Responses occurred in patients with MF and SS, including those with erythrodermic and tumor stage presentations (Table 1; Fig 1B). Patients with four or more lines of prior therapy had a similar response rate as those with fewer than four prior treatments (33% v 44%; P = .68 by Fisher’s exact test). The median DOR was not reached, with a median time of response follow-up of 58 weeks (Figs 1C and 1D). Only one responding patient subsequently lost his response. This patient had discontinued treatment with pembrolizumab because of pneumonitis 8 weeks before subsequent disease progression. At 1 year, the PFS and OS rates were 65% and 95%, respectively (Figs 2A and 2B).
FIG 1.
(A) Best change from baseline in skin by modified Severity Weighted Assessment Tool (mSWAT) scoring. Bar color reflects global response according to global response criteria. (B) Representative images showing response in tumor stage (top) and erythrodermic disease (bottom). (C) Longitudinal change from baseline skin mSWAT score. (D) Time to response and response duration. AE, adverse event; M, mycosis fungoides; S, Sézary syndrome.
FIG 2.
Kaplan-Meier curves of (A) progression-free survival (PFS) and (B) overall survival (OS).
Six patients experienced progressive disease by global response assessment as their best response. Skin was the primary site of progression in four patients, whereas two patients had progression only noted by lymph node criteria. One patient with stage IB developed new tumor-type (T3) disease in the skin on treatment, thus meeting criteria for progression.
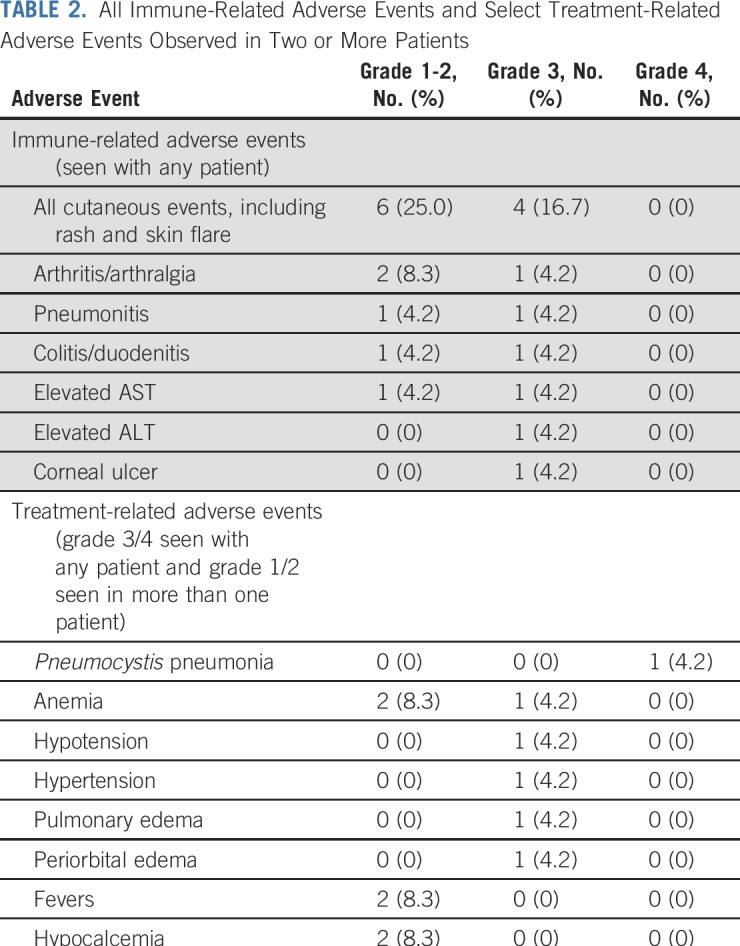
Safety
There were no grade 4 or 5 irAEs. A total of 11 irAEs of any grade were observed among nine patients. Four patients discontinued treatment because of an irAE, all grade 3 (pneumonitis, duodenitis, AST/ALT increase, and corneal ulcer; Table 2). One additional patient discontinued treatment after completion of the trial because of grade 2 colitis that developed 24 months into treatment. Of these irAEs, corneal ulcer has not been reported as a previous irAE with pembrolizumab. This AE occurred in a patient with a history of epithelial basement membrane corneal dystrophy, which may be related to this unique toxicity, and was associated with concomitant development of an inflammatory arthritis.
TABLE 2.
All Immune-Related Adverse Events and Select Treatment-Related Adverse Events Observed in Two or More Patients
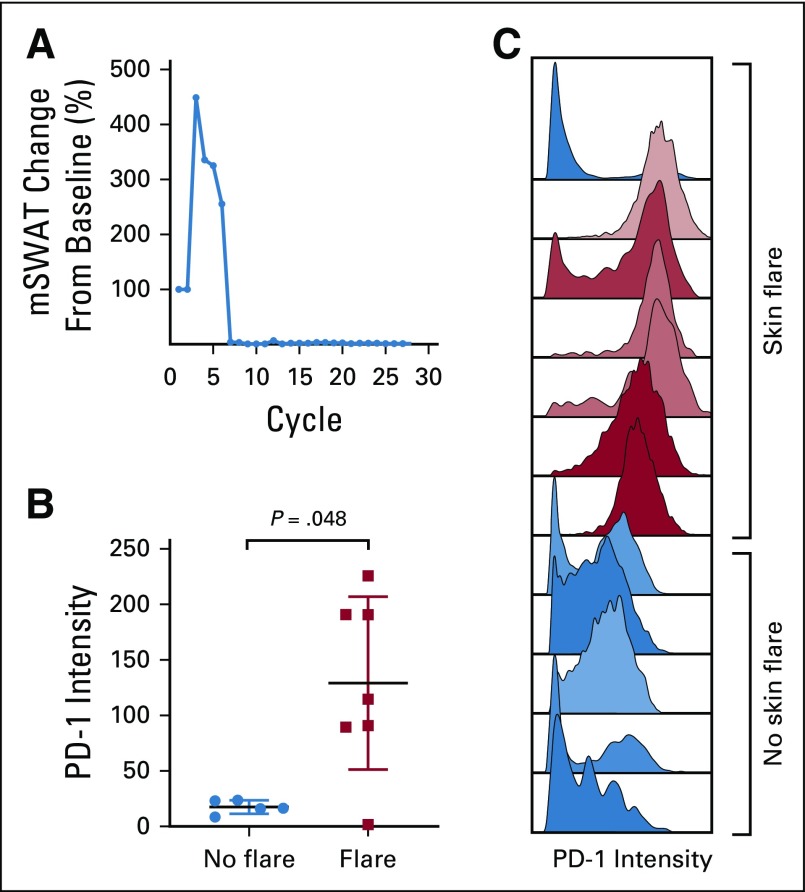
Eight (53%) of 15 patients with SS experienced marked worsening of erythema and pruritus and, in some, peripheral edema early in their treatment course (Fig 3A). Six of the eight affected patients experienced symptoms with their first cycle of treatment. In most, the flare remitted within 12 weeks with only supportive management and topical corticosteroids, and no patient required discontinuation of pembrolizumab therapy. One patient experienced a prolonged reaction that eventually subsided with systemic corticosteroids. This transient skin toxicity did not occur in patients with MF and did not associate with other irAEs. Of patients with a skin flare reaction, 38% (three of eight patients) later achieved a partial or complete response. Only 14% of patients with SS (one of seven patients) who did not experience a flare reaction went on to achieve a response.
FIG 3.
(A) Change in skin modified Severity Weighted Assessment Tool (mSWAT) scoring for patient 17 with transient skin flare reaction. (B) Mass cytometry analysis of programmed cell death protein 1 (PD-1) expression of peripheral blood Sézary cells at baseline for 12 patients by skin flare status. (C) Histogram of PD-1 expression of peripheral blood Sézary cells for 12 patients.
Through mass cytometry profiling of PBMCs, we found that the skin flare reaction was associated with high expression of PD-1 on circulating Sézary cells before therapy (Figs 3B and 3C). PD-1 expression on Sézary cells was an average of sevenfold higher in patients who developed the flare reaction compared with those who did not.
Exploratory Biomarker Studies
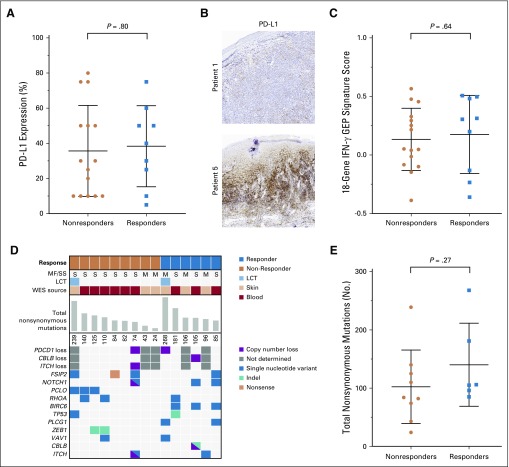
Pretreatment and on-treatment formalin-fixed skin biopsy specimens were analyzed by IHC for expression of PD-1, PD-L1, PD-L2, CD3, CD8, CD3, FOXP3, and CD163. There was a wide range of PD-L1 expression among patients (5% to 80%), but no significant correlation was found between clinical outcome and either pretreatment expression, the change in expression of PD-L1, or any other marker studied (Figs 4A and 4B). Gene expression of these skin biopsy specimens also was profiled by NanoString. An 18-gene T-cell–inflamed interferon-γ–related signature has been described that predicts responses to pembrolizumab in solid malignancies.25 The score of this 18-gene gene expression profiling signature did not differ between responding and nonresponding patients (Fig 4C). We were unable to identify any cell populations that were associated with clinical responses through mass cytometry with viSNE analysis. Of note, clinical responses occurred even in patients with markedly aberrant immune profiles (Data Supplement). Thus, the immune dysfunction associated with advanced SS does not seem to preclude responses to immunotherapies.
FIG 4.
(A) Programmed cell death protein ligand 1 (PD-L1) expression in baseline skin biopsy specimens by immunohistochemistry by response status. (B) Representative images of biopsy specimens with low (top) and high (bottom) PD-L1 intensity. (C) T-cell–inflamed interferon-γ (ΙFN-γ)–related gene expression profiling (GEP) signature scores in baseline skin biopsy specimens by response status. (D) Summary of whole-exome sequencing (WES) results, with selected genomic events displayed. (E) Tumor mutation burden determined by WES by response status. LCT, large-cell transformation; MF, mycosis fungoides; SS, Sézary syndrome.
Whole-exome sequencing did not reveal any translocations of PD-L1 or PD-L2, but monoallelic loss of PD-1 was seen in two of 10 evaluable patients. We detected focal deletions and mutations in two genes putatively involved in PD-1–mediated repression: CBLB28,29 and ITCH30 (Data Supplement). One of the patients with a complete response had both a focal chromosome 3 deletion that targeted CBLB and a second focal deletion of exon 15 of CBLB that resulted in a frameshift mutation. The total mutational burden was not significantly different between responding and nonresponding patients (Figs 4D and 4E).
DISCUSSION
Pembrolizumab demonstrated significant clinical activity in this trial of heavily pretreated patients with MF and SS. The measure of clinical response in this study represents the composite global response of all compartments (consensus criteria) and is comparable to CTCL studies using similar criteria. Thus, the ORR of 38% in this trial compares favorably with recently approved agents for this patient population, including mogamulizumab5 (ORR, 28%) and brentuximab vedotin31 (ORR, 65% in CD30+ MF), which also used the consensus response criteria in their pivotal trials. The response rate slightly favored patients with MF (five of nine patients; 56%) over those with SS (four of 15 patients; 27%; P = .2 by Fisher’s exact test), but deep and durable responses occurred in both diseases. No difference was found between patients with MF and SS with regard to their potential biomarkers (Data Supplement) that could explain the difference in response rates. Ongoing studies of immune checkpoint treatment may help to answer whether pembrolizumab is truly more efficacious in patients with MF.
Responses were remarkably durable. The only patient with loss of response had discontinued treatment 8 weeks before his disease progression. In later follow-up, a second patient who completed the trial with an ongoing partial response later lost his response after discontinuing pembrolizumab 12 weeks earlier. This may indicate that ongoing treatment is needed to maintain responses in this patient population, which would contrast with experience in other malignancies, such as melanoma, where it seems that treatment can safely be discontinued without jeopardizing clinical response.32
The safety and tolerability of pembrolizumab was similar to other studies of PD-1 inhibitors. All irAEs resolved upon discontinuation of treatment and administration of appropriate supportive therapy. A unique toxicity of acute worsening of erythema and pruritus was reported in patients with SS. This flaring of disease symptoms was not easily distinguishable from true disease progression. Patients with the flare reaction were managed with supportive care, including short-term increased application of topical corticosteroids until symptoms eventually subsided. Clinicians treating patients with SS with PD-1 or other immune checkpoint inhibitors should be aware of this potential skin flare reaction. We would advise that patients with SS with acute worsening of disease symptoms be cautiously continued on therapy to allow for resolution of a potential flare. High expression of PD-1 on the surface of circulating Sézary cells may represent a simple and effective predictor of this reaction that should be validated in future studies. Although we used mass cytometry in this study to discover the association of PD-1 expression with the flare reaction, more generalizable assays, such as flow cytometry, may be more practical in assessing PD-1 expression in patients with SS who receive pembrolizumab.
There is theoretical concern that blockade of PD-1 signaling may accelerate growth of T-cell malignancies because PD-1 acts to inhibit proliferation of T cells. Two studies have supported this concern. In a murine model of T-cell lymphoma, PD-1 was found to be a haploinsufficient tumor repressor and that inhibition of PD-1 could accelerate tumor growth.14 In addition, a trial of anti-PD-1 antibody therapy in adult T-cell leukemia/lymphoma found explosive progression of disease in the first three patients treated.33 Only one patient in our trial experienced unequivocal worsening of their disease as manifested by development of new tumor disease. We cannot conclude from this single patient that there is a true potential to cause progression in patients. Future studies should continue to address this possibility. One concern with the use of pembrolizumab in T-cell lymphomas has been that lymphomas with monoallelic deletion of PD-1 may experience additional acceleration in growth if PD-1 signaling is completely blocked by antibody treatment. We identified two patients with monoallelic deletion of PD-1 in our cohort. These patients had a partial response or stable disease as their best response, and there was no indication of worsening of their disease after treatment. We did discover focal deletions of two genes believed to be involved in PD-1 signaling: CBLB and ITCH. In particular, CBLB has been shown to be required for PD-1–mediated inhibition of T cells.28,29 Of note, the single patient with biallelic focal deletions of CBLB achieved a complete response with pembrolizumab therapy and remains on treatment more than 42 months later. Additional trials may help to determine whether deletion of PD-1 or alterations in genes associated with PD-1 signaling correlate with response to PD-1 targeting therapy.
In conclusion, pembrolizumab monotherapy has promising activity in relapsed and refractory MF and SS, with remarkably durable responses. Additional studies that combine PD-1/PD-L1 checkpoint antibodies with other therapies currently are under way and may reveal effective strategies to boost the response rate. More studies are needed to identify potential biomarkers of response and to better assess whether there may exist a small subset of patients who may develop acceleration of their disease in response to treatment.
Footnotes
Presented in part at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, December 3-6, 2016; 60th Annual Meeting of the American Society of Hematology, San Diego, CA, December 1-4, 2018; 3rd World Congress of Cutaneous Lymphomas, New York, NY, October 26-28, 2016; and EORTC Cutaneous Lymphoma Group Meeting, St Gallen, Switzerland, September 27-29, 2018.
Supported by National Cancer Institute grant 5UM1CA154967 (M.A.C.). Additional funding support is from Merck, which provided pembrolizumab and partial funding; from the Haas Family Foundation; and from federal funds from the National Cancer Institute under contract HHSN261200800001E.
Listen to the podcast by Dr Amengual at http://ascopubs.org/journal/jco/podcasts
Clinical trials information: NCT02243579.
AUTHOR CONTRIBUTIONS
Conception and design: Holbrook E. Kohrt, Michael S. Khodadoust, Steven P. Fling, Richard Shine, Elad Sharon, Martin A. Cheever, Youn H. Kim
Financial support: Elad Sharon, Martin A. Cheever, Youn H. Kim
Administrative support: Elad Sharon, Martin A. Cheever, Youn H. Kim
Provision of study material or patients: Michael S. Khodadoust, Alain H. Rook, Pierluigi Porcu, Francine Foss, Alison J. Moskowitz, Andrei Shustov, Satish Shanbhag, Lubomir Sokol, Steven P. Fling, Nirasha Ramchurren, Steven M. Horwitz, Martin A. Cheever, Youn H. Kim
Collection and assembly of data: Michael S. Khodadoust, Alain H. Rook, Pierluigi Porcu, Francine Foss, Alison J. Moskowitz, Andrei Shustov, Satish Shanbhag, Lubomir Sokol, Steven P. Fling, Nirasha Ramchurren, Asa Davis, Richard Shine, Shufeng Li, Sophia Fong, Jinah Kim, Yi Yang, Wendy M. Blumenshein, Jennifer H. Yearley, Biswajit Das, Rajesh Patidar, Vivekananda Datta, Erin Cantu, Justine N. McCutcheon, Chris Karlovich, P. Mickey Williams, Priyanka B. Subrahmanyam, Holden T. Maecker, Steven M. Horwitz, Elad Sharon, Martin A. Cheever, Youn H. Kim
Data analysis and interpretation: Michael S. Khodadoust, Alain H. Rook, Pierluigi Porcu, Francine Foss, Alison J. Moskowitz, Andrei Shustov, Satish Shanbhag, Lubomir Sokol, Steven P. Fling, Nirasha Ramchurren, Shufeng Li, Sophia Fong, Jinah Kim, Wendy M. Blumenshein, Jennifer H. Yearley, Biswajit Das, Rajesh Patidar, Vivekananda Datta, Erin Cantu, Justine N. McCutcheon, Chris Karlovich, P. Mickey Williams, Priyanka B. Subrahmanyam, Holden T. Maecker, Steven M. Horwitz, Elad Sharon, Martin A. Cheever, Youn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jco/site/ifc.
Michael S. Khodadoust
Consulting or Advisory Role: Kyowa Hakko Kirin, Seattle Genetics
Alain H. Rook
Honoraria: Trillium Therapeutics
Consulting or Advisory Role: miRagen Therapeutics
Speakers’ Bureau: Mallinckrodt
Patents, Royalties, Other Intellectual Property: Patent pending for the treatment of cutaneous T-cell lymphoma with topical resiquimod
Pierluigi Porcu
Honoraria: Celgene, Innate Pharma, Kiowa Hakko Kirin, Viracta Therapeutics
Consulting or Advisory Role: Innate Pharma, Viracta Therapeutics
Research Funding: Infinity Pharmaceuticals (Inst), Celgene (Inst), Millennium Pharmaceuticals (Inst), Seattle Genetics (Inst)
Francine Foss
Consulting or Advisory Role: Celgene, Seattle Genetics, Spectrum Pharmaceuticals, Eisai, Verastem, miRagen Therapeutics, Mallinckrodt
Speakers’ Bureau: Seattle Genetics
Research Funding: miRagen Therapeutics (Inst), Kura (Inst), Kyowa Hakko Kirin (Inst), Daiichi Sankyo (Inst), Eisai (Inst), Astex Pharmaceuticals (Inst)
Alison J. Moskowitz
Honoraria: Seattle Genetics
Consulting or Advisory Role: Seattle Genetics, ERYTECH Pharma, Bristol-Myers Squibb, Cell Medica, ADC Therapeutics, Takeda Pharmaceuticals, miRagen Therapeutics, Kyowa Hakko Kirin
Research Funding: Incyte (Inst), Seattle Genetics (Inst), Merck (Inst), Bristol-Myers Squibb (Inst)
Andrei Shustov
Honoraria: Kyowa Hakko Kirin
Consulting or Advisory Role: Kyowa Hakko Kirin
Research Funding: Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Spectrum Pharmaceuticals
Satish Shanbhag
Consulting or Advisory Role: Takeda Pharmaceuticals
Research Funding: American Regent
Lubomir Sokol
Consulting or Advisory Role: EUSA Pharma
Steven P. Fling
Research Funding: Merck
Travel, Accommodations, Expenses: Cambridge Healthtech Institute
Robert Pierce
Stock and Other Ownership Interests: OncoSec Medical, Sensei Biosciences, Calithera Biosciences (I), Immunomic Therapeutics
Consulting or Advisory Role: Pulse Biosciences, Calithera Biosciences, AbbVie, Juno Therapeutics (I), Seattle Genetics (I)
Research Funding: Pulse Biosciences (Inst), Juno Therapeutics (Inst), Immunomic Therapeutics (Inst), X4 Pharmaceuticals (Inst), Incyte (Inst), Minerva Therapeutics (Inst)
Travel, Accommodations, Expenses: Pulse Biosciences, Immunomic Therapeutics, Sensei Biotherapeutics
Jennifer H. Yearly
Employment: Merck
Stock and Other Ownership Interests: Merck
Patents, Royalties, Other Intellectual Property: Two patents pending resulting from work conducted as full-time Merck employee
Biswajit Das
Research Funding: Illumina (Inst)
Chris Karlovich
Stock and Other Ownership Interests: Clovis Oncology
Research Funding: Illumina (Inst)
P. Mickey Williams
Research Funding: Illumina (Inst)
Patents, Royalties, Other Intellectual Property: Co-inventor of the diffuse large B-cell lymphoma cell-of-origin patent recently filed by the National Institutes of Health
Holden T. Maecker
Employment: AbbVie (I)
Stock and Other Ownership Interests: AbbVie (I)
Research Funding: Amgen, Ionis
Steven M. Horwitz
Consulting or Advisory Role: Celgene, Millennium Pharmaceuticals, Kyowa Hakko Kirin, Seattle Genetics, ADC Therapeutics, Corvus Pharmaceuticals, Innate Pharma, miRagen Therapeutics, Portola Pharmaceuticals, Verastem, Takeda Pharmaceuticals
Research Funding: Celgene, Seattle Genetics, Takeda Pharmaceuticals, Kyowa Hakko Kirin, Aileron Therapeutics, ADC Therapeutics, Verastem, Forty Seven
Martin A. Cheever
Research Funding: Merck Sharp & Dohme (Inst), Alkermes (Inst), Genentech (Inst), Roche (Inst), Horizon Pharma (Inst), NeoImmuneTech (Inst), Revimmune (Inst)
Youn H. Kim
Consulting or Advisory Role: Seattle Genetics, Eisai, Actelion, Kyowa Hakko Kirin, Millennium Pharmaceuticals, miRagen Therapeutics, Innate Pharma, Medivir
Research Funding: Seattle Genetics (Inst), Millennium (Inst), Eisai (Inst), Merck (Inst), Kyowa Hakko Kirin (Inst), Soligenix (Inst), Innate Pharma (Inst), Neumedicines (Inst), Forty Seven (Inst), Portola Pharmaceuticals (Inst), Horizant (Inst), miRagen Therapeutics (Inst)
Patents, Royalties, Other Intellectual Property: Royalty for chapters in UpToDate
Travel, Accommodations, Expenses: Innate Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Scarisbrick JJ, Prince HM, Vermeer MH, et al. Cutaneous Lymphoma International Consortium study of outcome in advanced stages of mycosis fungoides and Sézary syndrome: Effect of specific prognostic markers on survival and development of a prognostic model. J Clin Oncol. 2015;33:3766–3773. doi: 10.1200/JCO.2015.61.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28:4485–4491. doi: 10.1200/JCO.2010.28.9066. [DOI] [PubMed] [Google Scholar]
- 3.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 4.Duvic M, Hymes K, Heald P, et al. Bexarotene is effective and safe for treatment of refractory advanced-stage cutaneous T-cell lymphoma: Multinational phase II-III trial results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Bagot M, Pinter-Brown L, et al. Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): An international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19:1192–1204. doi: 10.1016/S1470-2045(18)30379-6. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantekure K, Yang Y, Raghunath P, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am J Dermatopathol. 2012;34:126–128. doi: 10.1097/DAD.0b013e31821c35cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samimi S, Benoit B, Evans K, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: Implications for immune suppression. Arch Dermatol. 2010;146:1382–1388. doi: 10.1001/archdermatol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wada DA, Wilcox RA, Harrington SM, et al. Programmed death 1 is expressed in cutaneous infiltrates of mycosis fungoides and Sézary syndrome. Am J Hematol. 2011;86:325–327. doi: 10.1002/ajh.21960. [DOI] [PubMed] [Google Scholar]
- 10.Çetinözman F, Jansen PM, Vermeer MH, et al. Differential expression of programmed death-1 (PD-1) in Sézary syndrome and mycosis fungoides. Arch Dermatol. 2012;148:1379–1385. doi: 10.1001/archdermatol.2012.2089. [DOI] [PubMed] [Google Scholar]
- 11.Querfeld C, Leung S, Myskowski PL, et al. Primary T cells from cutaneous T-cell Lymphoma skin explants display an exhausted immune checkpoint profile. Cancer Immunol Res. 2018;6:900–909. doi: 10.1158/2326-6066.CIR-17-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J, Goh G, Walradt T, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47:1011–1019. doi: 10.1038/ng.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Ni X, Covington KR, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47:1426–1434. doi: 10.1038/ng.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1038/nature24649. Wartewig T, Kurgyis Z, Keppler S, et al: PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature 552:121-125, 2017 [Erratum: Nature 553:238, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ungewickell A, Bhaduri A, Rios E, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47:1056–1060. doi: 10.1038/ng.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basu A, Yearley JH, Annamalai L, et al. Association of PD-L1, PD-L2, and immune response markers in matched renal clear cell carcinoma primary and metastatic tissue specimens. Am J Clin Pathol. 2019;151:217–225. doi: 10.1093/ajcp/aqy141. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koboldt DC, Zhang Q, Larson DE, et al. VarScan 2: Somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Scheffler K, Halpern AL, et al. Strelka2: Fast and accurate calling of germline and somatic variants. Nat Methods. 2018;15:591–594. doi: 10.1038/s41592-018-0051-x. [DOI] [PubMed] [Google Scholar]
- 23.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Talevich E, Shain AH, Botton T, et al. CNVkit: Genome-wide copy number detection and visualization from targeted DNA sequencing. PLOS Comput Biol. 2016;12:e1004873. doi: 10.1371/journal.pcbi.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ayers M, Lunceford J, Nebozhyn M, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subrahmanyam PB, Maecker HT: CyTOF measurement of immunocompetence across major immune cell types. Curr Protoc Cytom 82:9.54.1-9.54.12, 2017 . [DOI] [PMC free article] [PubMed]
- 27.Subrahmanyam PB, Dong Z, Gusenleitner D, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. 2018;6:18. doi: 10.1186/s40425-018-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peer S, Baier G, Gruber T. Cblb-deficient T cells are less susceptible to PD-L1-mediated inhibition. Oncotarget. 2017;8:41841–41853. doi: 10.18632/oncotarget.18360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujiwara M, Anstadt EJ, Clark RB. Cbl-b deficiency mediates resistance to programmed death-ligand 1/programmed death-1 regulation. Front Immunol. 2017;8:42. doi: 10.3389/fimmu.2017.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nurieva R, Thomas S, Nguyen T, et al. T-cell tolerance or function is determined by combinatorial costimulatory signals. EMBO J. 2006;25:2623–2633. doi: 10.1038/sj.emboj.7601146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prince HM, Kim YH, Horwitz SM, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): An international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017;390:555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36:1668–1674. doi: 10.1200/JCO.2017.75.6270. [DOI] [PubMed] [Google Scholar]
- 33.Ratner L, Waldmann TA, Janakiram M, et al. Rapid progression of adult T-cell leukemia-lymphoma after PD-1 inhibitor therapy. N Engl J Med. 2018;378:1947–1948. doi: 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]