Abstract
Lipid droplets (LDs) are fat storage organelles integral to energy homeostasis and a wide range of cellular processes. LDs physically and functionally interact with many partner organelles, including the ER, mitochondria, lysosomes, and peroxisomes. Recent findings suggest that the dynamics of LD inter-organelle contacts is in part controlled by LD intracellular motility. LDs can be transported directly by motor proteins along either actin filaments or microtubules, via Kinesin-1, Cytoplasmic Dynein, and type V Myosins. LDs can also be propelled indirectly, by hitchhiking on other organelles, cytoplasmic flows, and potentially actin polymerization. Although the anchors that attach motors to LDs remain elusive, other regulators of LD motility have been identified, ranging from modification of the tracks to motor co-factors to members of the perilipin family of LD proteins. Manipulating these regulatory pathways provides a tool to probe whether altered motility affects organelle contacts and has revealed that LD motility can promote interactions with numerous partners, with profound consequences for metabolism. LD motility can cause dramatic redistribution of LDs between a clustered and a dispersed state, resulting in altered organelle contacts and LD turnover. We propose that LD motility can thus promote switches in the metabolic state of a cell. Finally, LD motility is also important for LD allocation during cell division. In a number of animal embryos, uneven allocation results in a large difference in LD content in distinct daughter cells, suggesting cell-type specific LD needs.
Keywords: lipid droplet, cytoskeleton, contact, molecular motor, cell division, metabolism
Advances in microscopy, genome editing, and computational biology have dramatically increased our understanding of how cells organize themselves. A major paradigm shift has been the recognition that cellular organelles frequently interact with each other via specific contact sites. These contacts are dynamic and highly regulated, and they are key for understanding how organelle functions are integrated into the cell as a whole. As the reviews in this special issue demonstrate, this new perspective is transforming our understanding of lipid droplets (LDs), the organelles specialized for intracellular fat storage.
LDs play crucial roles in energy homeostasis, diverse aspects of lipid metabolism, and the handling of specific proteins (Walther and Farese, 2012; Hashemi and Goodman, 2015; Welte, 2015b; Kimmel and Sztalryd, 2016; Welte and Gould, 2017). They sequester harmful lipids and proteins and provide fatty acids for energy production, membrane lipids, and signaling molecules. LDs have thus critical functions in diverse cells, including in adipose tissue, liver and skin, many reproductive tissues, as well as the immune and nervous systems (Saka and Valdivia, 2012; Walther and Farese, 2012; Krahmer et al., 2013; Arrese et al., 2014; Gluchowski et al., 2017; Pennetta and Welte, 2018). After originating from the ER, LDs physically and functionally interact with many cellular organelles, including other LDs, the ER, mitochondria, peroxisomes, and lysosomes; eventually, they are broken down by cytoplasmic lipases and via autophagy.
In many cells, LDs show extensive intracellular motility (Welte, 2009; Valm et al., 2017). Such motility has the potential to bring LDs into the vicinity of other organelles and thus is expected to contribute to the formation, dynamics, and dissolution of inter-organellar contacts. Such roles for motility are certainly well established for other intracellular organelles: for example, in mitochondria, a complex of Milton and Miro1/2 mediates the recruitment of both Dynein and Kinesin for transport along microtubules as well as of Myosin 19 for transport along actin filaments (Oeding et al., 2018). This motility plays a crucial role in bringing different mitochondria together for fusion (Cagalinec et al., 2013), an adaptive stress response that improves mitochondrial metabolism and survival. Lysosomes are highly motile as well (reviewed in Pu et al., 2016), displaying Kinesin-1, Dynein-Dynactin, and Myosin IIa driven transport; such active transport is, for example, crucial for lysosomal fusion with autophagosomes during autophagy (Nakamura and Yoshimori, 2017). Below, we review what is known about LD motility and its contribution to inter-organelle contacts, with a particular emphasis on recent papers. For a summary of the older literature on LD motility, we refer the reader to three comprehensive reviews (Welte, 2009, 2015a; Welte and Gould, 2017).
LD motility is widespread
Like other intracellular structures, LDs can display a variety of states of motion: free diffusion, confined Brownian motion (e.g., around a tether point), and directed motion along linear tracks (Maucort et al., 2014; Norregaard et al., 2017). A given LD can repeatedly switch between such states, though the exact pattern varies dramatically between different types of cells. For example, in early Drosophila embryos, essentially all LDs constantly move back-and-forth along linear paths, with only occasional brief pauses and no obvious diffusive behavior (Gross et al., 2000, 2002). In contrast, in the hepatocyte cell line HuH-7, LDs typically oscillate in a confined area, but some switch to rapid unidirectional movement in a transient fashion (Targett-Adams et al., 2003). Because linear motion of LDs is by far the best understood, both molecularly and functionally, the following discussion will focus on this type of motility.
Directed LD motion has been observed in a wide variety of systems: from yeasts and fungal hyphae, to insect and vertebrate embryos, to a plethora of cultured mammalian cells (for reviews, see Welte, 2009, 2015a; Welte and Gould, 2017). Recently, advances in imaging technologies have uncovered many additional instances of LD motility; these discoveries expand both the experimental approaches to monitor LD motion and the systems in which its mechanisms and functions can be studied. Particularly exciting are label-free methods which have been used to follow LDs in real time: methods based on Raman scattering for plant seedlings, mouse oocytes, and insect embryos (Dou et al., 2012; Bradley et al., 2016; Waschatko et al., 2016); confocal reflection microscopy for Drosophila ovaries (Gaspar and Szabad, 2009); and near-infrared spectroscopy for fish embryos (Ishigaki et al., 2016). In addition, new fluorescent dyes have been developed for in-vivo imaging of LDs (e.g., Collot et al., 2018; Zheng et al., 2019). These methods are well suited for live imaging, making them complementary with older, fixed-imaging techniques. Concordantly, advances in computational analysis allow for monitoring many organelles at the same time through demixing of multi-spectral imaging. They reveal, e.g., with which types of organelles LDs make contact (Valm et al., 2017). Finally, intra-vital imaging has, for the first time, been able to monitor the motion of individual LDs in mammary gland cells during lactation (Masedunskas et al., 2017).
Molecular mechanisms of LD motility
To evaluate to what extent LD motility contributes to inter-organellar contacts, it is essential to experimentally modulate this motility. Thus, a good understanding of its molecular basis is crucial.
Directed motion of LDs is typically brought about by cytoskeletal motors, such as Kinesins, Dyneins, and Myosins (Welte, 2009; Welte and Gould, 2017). In the simplest case, LDs are directly pulled by motors, either unidirectionally along actin filaments or microtubules or – if opposing motors work together – back-and-forth along microtubules, displaying so-called bidirectional transport (Fig. 1 A, B) (Welte, 2004). Motors can also bring about LD motion indirectly, such as in the filamentous fungus Ustilago where LDs “hitchhike” on motor-driven early endosomes (Fig. 1C) (Guimaraes et al., 2015). In a second indirect mechanism, cytoskeletal motors can power bulk motion of the entire cytoplasm that then drags LDs along – together with many other organelles (Fig. 1D). Two such instances occur during Drosophila oogenesis: nurse cells, the fifteen sister cells of the oocyte, undergo actin and Myosin-II dependent contraction, resulting in LDs, as well as other cytoplasmic content, being squeezed through cytoplasmic bridges into the oocyte (Wheatley et al., 1995). At the same time, the oocyte undergoes Kinesin-1/microtubule-driven cytoplasmic streaming that mixes the incoming nurse cell contents with the existing ooplasm (Palacios and St Johnston, 2002).
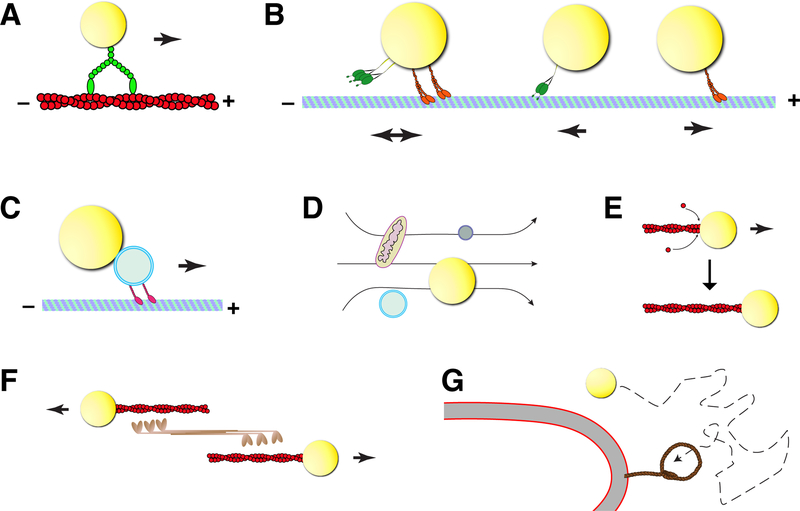
Figure 1:
Mechanisms of LD motility.
(A, B) LDs are transported as direct cargo by molecular motors along cytoskeletal filaments: transport along actin filaments is driven by plus-end directed Myosins (A); transport along microtubules can be unidirectional towards either the plus-or minus end (powered by Kinesin-1 or Cytoplasmic Dynein, respectively) or bidirectional, using both types of motors (B). (C) LDs hitchhike on other organelles that are transported by motors along microtubules. (D) Molecular motors promote bulk flow of the cytoplasm that drags many organelles, including LDs, along. (E) Polymerization of actin filaments may push LDs through the cytoplasm. (F) Myosin II may move LDs indirectly, by sliding actin filaments with attached LDs against each other. (G). LD moving by Brownian motion until it is captured by a tether on a target organelle.
Some cases of LD motility may not be due to molecular motors, but nevertheless depend on the cytoskeleton. For example, in U2OS cells, localized polymerization of actin is necessary to drive clustered LDs away from each other (Pfisterer et al., 2017). Conceivably, LDs are pushed directly by growing actin filaments (Fig. 1E); alternatively – or in addition – actin filaments polymerized from LDs might be pushed against each other by Myosin II, a motor that slides filaments (Fig. 1F) (Pfisterer et al., 2017). Actin-based mechanisms also promote the dissociation of LDs from peroxisomes in seedlings of Arabidopsis (Cui et al., 2016).
Unequivocal proof that actin-and microtubule-based mechanisms are responsible for LD motion exists only for a moderate number of systems. However, the presence of either actin or tubulin in numerous LD proteomes is consistent with the idea that these cytoskeletal elements drive LD motility in many cells. In addition to the cases summarized previously (Welte, 2009, 2015a; Welte and Gould, 2017), there are recent examples ranging from plants and fungi to mammalian systems (Brocard et al., 2017; Yu et al., 2017; Zhi et al., 2017; Speziali et al., 2018, Pfisterer, 2017 #394). These data have to be interpreted cautiously as actin and tubulin in LD proteomes might simply represent biochemical contamination, given that they are abundant cytoplasmic proteins. However, actin, tubulin, and a motor subunit (KIF16B) were also detected in LD proteomes using much more stringent proximity-labelling strategies (Bersuker et al., 2018). As such stringent techniques become more wide-spread, the true extent of LD-cytoskeletal associations will become clearer. Of course, the mere presence of cytoskeletal elements does not prove by itself that they are responsible for LD motion; after all, LDs can act as storage sites for diverse proteins (Welte and Gould, 2017). Thus, functional tests are required, such as those detailed in the next section.
Finally, simple Brownian motion might lead to an inhomogeneous LD distribution in the cell if diffusing LDs get captured at specific sites, e.g., when they form contacts with partner organelles (Fig. 1G). For example, newly formed LDs in Drosophila cultured cells will change their spatial distribution over the course of several hours and eventually congregate into clusters (Guo et al., 2008). These clusters seem to result from slow aggregation of randomly moving LDs, not concerted transport to a specific region. This pattern is consistent with the idea that diffusing LDs happen to collide and then remain attached, potentially through a tethering mechanism (Fig. 1G).
Strategies for modulating LD motility: targeting tracks and motors
Given that most LD motility depends on actin filaments or microtubules, there is a simple general strategy to probe whether LD motility contributes to organelle contacts: alter the actin or microtubule tracks, pharmacologically or genetically, and determine effects on LD contacts. For example, when COS-7 cells are treated with Nocodazole to disassemble microtubules, the contact landscape of LDs changes dramatically: fewer LDs are engaged in contacts with mitochondria or peroxisomes, ternary contacts between LDs, peroxisomes, and the Golgi decrease, and simultaneous contacts between LDs, ER, and lysosomes increase (Valm et al., 2017). These observations implicate microtubule-driven motility of LDs and/or those other organelles in controlling inter-organellar contacts. In a hepatocyte cell line, microtubules are stabilized in response to Septin-9 upregulation, which then promotes LD relocalization to the perinuclear region (Akil et al., 2016), with the potential of novel inter-organelle contacts. The actin-disrupting drug Latrunculin prevents the migration of LDs towards the animal pole in zebrafish embryos (Dutta and Kumar Sinha, 2015) as well as proper allocation of LDs into the spores of fission yeast (Yang et al., 2017). In the fungus Metarhizium acridum deletion of the beta tubulin gene impairs LD trafficking (Zhang et al., 2017). Finally, post-translational modification of microtubule tracks can have a profound impact on LD distribution and organelle contacts: in Vero cells, nutrient deprivation results in the assembly of a network of microtubules enriched in detyrosinated tubulin; transport along these microtubules promotes LD dispersion which in turn increases LD-mitochondrial contacts (Herms et al., 2015). In contrast, in hepatic cells, microtubule acetylation impairs LD motility (Groebner et al., 2019); it was proposed that Cytoplasmic Dynein binds more strongly to these modified tracks, thus stalling LD motion. Intriguingly, cells with increased microtubule acetylation displayed reduced LD-LD interactions, suggesting that LD motion promotes contacts between LDs.
A downside of abolishing LD motility via altering cytoskeletal tracks is that many other cytoskeleton-dependent processes may also be disrupted, including the trafficking, biogenesis, and turnover of various organelles. Such pleiotropic effects make it challenging to confidently attribute changes in LD inter-organellar contacts to altered LD motion. A more targeted approach is to specifically abolish the motors powering LD transport. In those cases where the microtubule motors responsible for LD motion have been identified, they are almost invariably the plus-end motor Kinesin-1 and the minus-end motor Cytoplasmic Dynein (Welte, 2015a; Welte and Gould, 2017). They can be found on their own or in combination. Thus, inhibiting these two motors is typically the first choice for manipulating LD motility. Kinesin-1 even powers the indirect transport of LDs by cytoplasmic flow in Drosophila oocytes (Serbus 2005; Palacios 2002). The only exception we are aware of is in Ustilago, where LDs are dragged along by early endosomes propelled via Kinesin-3/Cytoplasmic Dynein (Guimaraes et al., 2015). Intriguingly, the Kinesin-3 family member KIF16B was recovered as a high-confidence LD protein in two mammalian cell lines (Bersuker et al., 2018), though whether it promotes motility in those cases has not yet been tested. Actin-based transport of LDs involves motors from three different myosin families: in budding yeast, LDs are transported from the mother to the daughter cell via a type V Myosin (Knoblach and Rachubinski, 2015), a motor that carries cargoes along its tracks analogous to Kinesin-1 (Fig. 1A). Declustering of LDs in U2OS cells (Pfisterer et al., 2017) and transport of LDs to the cortex of zebrafish embryos (Dutta and Kumar Sinha, 2015) depends on a different Myosin, non-muscle Myosin II. This myosin does not directly transport cargo, but rather slides actin filaments against each other. Thus, in these instances, LDs might be pushed by moving actin filaments (Fig. 1F). Finally, the single-headed Myosin I is also implicated in LD motility; its pharmacological inhibition results in local accumulation of LDs at the cleavage furrow in zebrafish embryos (Gupta et al., 2017). However, the underlying mechanism is not yet clear.
The identification of LD motors has led to an unexpected side-benefit for motor studies, namely new opportunities to study the activity of these motors in vivo (reviewed in Welte, 2015a; Welte and Gould, 2017). Although in principle any organelle could be used for such investigations, LDs are particularly suited because of their unique biophysical properties. On the one hand, the size and shape of LDs make them ideal for motion tracking, as the position of the LD center can be pinpointed within a few nanometers even by standard light microscopy (Gross et al., 2000). On the other hand, LDs can be manipulated in vivo with optical tweezers, allowing the forces driving LD motion to be determined at the single LD level, including their variation over time (Shubeita et al., 2008). Most recently, this combination of properties was used to uncover that Cytoplasmic Dynein has an intrinsic ability to upregulate force production if it encounters an opposing force (Reddy et al., 2016; Chapman et al., 2019).
Strategies for modulating LD motility: focus on LD-specific interventions
Kinesin-1, Cytoplasmic Dynein, and Myosins are not restricted to LDs, but typically perform multiple independent functions in cells, including the trafficking of other organelles. Thus, to manipulate just LD motility, it is necessary to target LD-specific co-factors of the motor machinery.
A powerful approach would be to specifically abolish motor recruitment to LDs. Although it has been known for almost two decades that specific motors move LDs (Gross et al., 2000), their mode of attachment to LDs remains unclear. This is in contrast to many other motor cargoes, where motor adapters are well characterized (Akhmanova and Hammer, 2010). Physical interactions between microtubule motors and various LD associated proteins have indeed been uncovered, such as with the perilipin Plin3 (Gu et al., 2019), the nesprin Klar (Gaspar et al., 2014), and the GTPase Arf1 (Rai et al., 2017). However, with the data currently available, it is hard to determine whether those interactions recruit the motors to the LDs or if they control the activity of motors recruited by other means. For example, in hepatic cells, a Dynein subunit co-immunoprecipitates with PLIN3 and partially colocalizes with LDs by immunostaining, but it is not yet known if that colocalization is PLIN3-dependent (Gu et al., 2019). In Drosophila embryos lacking Klar, LDs still move bidirectionally, with much reduced forces and velocities (Welte et al., 1998); thus Klar is at best one of several anchors that recruit motors to LDs (for alternative possibilities, see Welte, 2015a). Arf1 levels on LDs, Kinesin-1 levels on LDs, and LD motility all change together in response to metabolic signals (Rai et al., 2017). However, it remains possible that a common upstream signal promotes independent recruitment of Kinesin-1 and Arf1 to LDs, and Arf1 then modulates the Kinesin-1 activity on LDs. In addition, since Arf1 can affect the movement of multiple organelles (Kaczmarek et al., 2017), it cannot be a specific LD motor anchor. A yet unexplored possibility is that motors might be anchored to lipids in the phospholipid monolayer of the LDs, rather than to LD proteins. A precedent exists in the Golgi and endosome localization of Kinesin-1, which is recruited via a protein complex that recognizes specific membrane lipids (Mahajan et al., 2019).
Even though motor anchors on LDs remain elusive, the motor co-factors already identified allow modulation of LD motility. Inactivation of Arf1 via Brefeldin-A in hepatic cells disrupts the intracellular distribution of LDs similar to Kinesin-1 knockdown (Rai et al., 2017). In the absence of the LD-specific isoform of Klar, most LDs in Drosophila embryos are mislocalized to the yolk cell (Guo et al., 2005), which presumably results in globally altered LD-organelle contacts. Similarly, lack of the Kinesin-1 cofactor Halo causes global redistribution of LDs in these embryos (Arora et al., 2016), but apparently not of other organelles (Gross et al., 2003). And several perilipins are known to localize to LDs and alter their motility and intracellular distribution, such as ectopically expressed PLIN1 in fibroblasts (Marcinkiewicz et al., 2006; Orlicky et al., 2013), PLIN2 in Huh-7 and NIH3T3 cells (Boulant et al., 2008; Cruz et al., 2019), and LSD-2/PLIN2 in Drosophila embryos (Welte et al., 2005). For PLIN1 and LSD-2, the phosphorylation state of the protein correlates with distinct intracellular distributions (Welte et al., 2005; Marcinkiewicz et al., 2006); this is also the case for PLIN6 in fish, a protein that localizes to carotenoid droplets, proposed to be homologous to LDs (Granneman et al., 2017). For ectopically expressed PLIN1, it was shown that the relevant kinase is Protein Kinase A and that phosphorylation of specific sites is responsible for modulating LDs’ intracellular distribution (Marcinkiewicz et al., 2006). More broadly, various experimental treatments have been reported to alter LD motility in cultured cells, such as exposure to certain herbal extracts (Haselgrubler et al., 2019) or modulation of the extracellular pH (Nardi et al., 2019a).
Reciprocal relationship between LD motility and organelle contacts
Just as LD motion can promote or break interactions with other organelles, so too can organelle contacts positively and negatively modulate LD motility. As discussed above, LDs in Ustilago tightly interact with early endosomes, which allows them to utilize the endosomal motor machinery for transport (Guimaraes et al., 2015). In turn, organelle tethers are implicated in impeding LD motion. A prime example are members of the VPS13 protein family that tether mitochondria and the ER to each other (Kumar et al., 2018; Yeshaw et al., 2019).They are also present on LDs (Bersuker et al., 2018; Kumar et al., 2018; Yeshaw et al., 2019) and may help establish contacts between the ER and LDs. Live imaging of cells expressing a VPS13A GFP fusion revealed that transient active motion of LDs correlates with loss of VPS13A from LDs; when VPS13A was regained, LD motion ceased. This correlation suggests that tethering of LDs to the ER restricts their motion (Yeshaw et al., 2019). Consistent with this model, overexpression of VPS13A reduces LD motility (Yeshaw et al., 2019). A related phenomenon may occur during cell division in budding yeast. Here, some LDs remain in contact with the nuclear envelope in the mother cell, while the rest are transported along actin filaments to the daughter (Knoblach and Rachubinski, 2015). Nuclear-envelope anchoring may prevent some LDs from productively engaging with Myosin motors, thus retaining them in the mother. In an analogous manner, mitochondria bound to LDs tend to display much reduced motility (Benador et al., 2019); it will be interesting to determine whether mitochondria-LD contacts in turn impair LD motion.
Reciprocal relationship between LDs and the cytoskeleton
Not only do cytoskeletal elements control LD motility, but LDs can modulate actin and microtubule organization. In prostate cancer cells, LDs regulate a non-centrosomal microtubule organizing center which is important for proliferation (Nardi et al., 2018; Nardi et al., 2019b). LDs in U2OS cells carry the actin nucleator Formin-like 1 (FMNL1) and can nucleate actin filaments in vitro. This activity is likely important in vivo, as FMLN1 promotes the recruitment of non-muscle Myosin to LDs, which in turn helps LDs to dissociate from each other (Pfisterer et al., 2017). This declustering role may be widespread as LD proteomes from numerous systems include regulators of actin polymerization. Furthermore, the LD-associated protein ORP2 physically interacts with actin regulators and affects the formation of actin filaments in a nutrient dependent manner (Olkkonen et al., 2019), though the detailed mechanisms remain to be worked out. In a potentially analogous case, the LD-associated protein Utrophin forms an atypical cytoskeletal filament when complexed with beta-2Syntrophin. These filamentous structures appear to be independent from other cytoskeletal filaments and regulate LD size in adipocytes (Krautbauer et al., 2019).
Functional consequences of LD motility
Contacts between LDs and other organelles play crucial roles for inter-organelle metabolite exchange. For example, fatty acids from triglyceride breakdown can be shuttled to mitochondria for beta-oxidation (Rambold 2015). Reciprocally, mitochondria bound to LDs can provide the substrates necessary for the synthesis of neutral lipids as demonstrated in mammalian brown adipose tissue (Benador et al., 2018). As LD motility alters the spatial position of LDs relative to the rest of the cell and, thus, presumably to other organelles, it is expected to have a profound effect on establishing and breaking LD-organelle contacts and therefore, on the flux of lipids through the cell.
Indeed, such functional effects of LD motion have already been demonstrated in several cases. LD motility along microtubules can control the extent of contacts between LDs and mitochondria, and thus, how well fatty acids from LDs are used as fuel (Herms et al., 2015). In the liver, activation of Kinesin-1 on LDs promotes lipoprotein secretion; it has been hypothesized that active motion of LDs increases their contacts with the ER, leading to the transfer of lipids needed for the assembly of lipoproteins in the ER lumen (Rai et al., 2017).
In additional cases, a role of LD motility for lipid transfer between organelles has been inferred, though not yet conclusively demonstrated. A number of intracellular pathogens, both bacteria and protozoa, deploy a specialized strategy for taking advantage of LD-stored nutrients, while remaining protected from attack inside phagosome-derived organelles, called parasitophorous vacuoles. Infection somehow promotes the translocation of LDs to the parasitophorous vacuole, establishment of intimate LD-vacuole contacts, and even uptake of the LDs into the vacuole (Cocchiaro et al., 2008; Toledo et al., 2016). Whether active LD motion is required for this process has not yet been established but seems likely. In mammary gland cells, LDs originate all over the cell and move along linear paths towards the apical surface, in an intermittent manner (Masedunskas et al., 2017). Once apical, they are released by an unconventional secretion mechanism, so that the LDs that end up in milk are wrapped by remnants of the plasma membrane. This pattern of LD motility is presumably functionally important: LDs grow in part by LD-LD fusion. It seems likely that fusion is enhanced by confining LDs to linear paths. In addition, active transport to the apical side is expected to promote contact with the plasma membrane for secretion. Once the mechanism driving LD motility in these cells is better described, these conjectures become testable.
Modulation of lipid exchange by LD motility may also be important in cancer. LDs often accumulate in cancer cells and profoundly affect their metabolism (Koizume and Miyagi, 2016). Intriguingly, in a number of cancer types, the velocity with which LDs move positively correlates with cancer severity (Nardi et al., 2019a). The mechanistic basis for this correlation remains to be worked out but might involve exchange of lipids between LDs and other organelles.
LDs do not only store lipids, but also specific proteins (Welte, 2007; Welte and Gould, 2017). Thus, LD motility might also promote delivery of proteins to their correct cellular destination. The strongest case for such a role comes from the life cycle of the Hepatitis C virus. Upon Hepatitis C infection, newly synthesized viral core protein accumulates on LDs and promotes LD relocalization to the perinuclear region (Boulant et al., 2008). This motion is hypothesized to promote contacts between sites of viral RNA replication and the core-loaded LDs to promote assembly of mature viral particles. In early Drosophila embryos, LDs store large amounts of specific histones (Cermelli et al., 2006); these histones promote proper chromatin assembly (Li et al., 2012; Johnson et al., 2018) and can help fight bacterial infections (Anand et al., 2012). LDs in these embryos are highly motile, which might enhance LD contacts with bacteria and thus killing activity as well as affecting how quickly histones reach the nucleus.
There may even be a role for LD motility in cytokinesis. In both mammalian cells (Atilla-Gokcumen et al., 2014) and budding yeast (Yang et al., 2016), interfering with triglyceride synthesis causes cytokinesis defects, possibly because toxic diglycerides accumulate (Yang et al., 2016). Intriguingly, during mammalian cytokinesis, neutral lipids – and thus presumably LDs – accumulate at the cytokinetic furrow and persist in the midbody, the structure that links the two daughter cells (Atilla-Gokcumen et al., 2014). Although these LDs might be generated on site, it is conceivable that they are transported from elsewhere in the cell to act as a local sink for newly produced toxic lipids. Alternatively, LDs might accumulate there via capture by actin filaments/microtubules after diffusion, as midbodies are particularly rich in cytoskeletal elements.
LD motility and global cellular LD distribution
A common pattern associated with LD motility is that it seems to switch LDs between two distinct distributions within the cell: clustered and dispersed (Fig. 2). The dramatic spatial rearrangement of LDs between these two states has the potential to radically alter the landscape of inter-organellar LD contacts (Fig. 2, insets). In at least one case, such a remodeling of the LD “interactome” has been directly demonstrated and linked to profound changes in the physiology of the cell (Herms et al., 2015). Namely, in starved Vero cells, LD dispersal promotes contacts with mitochondria, transfer of fatty acids from LDs to mitochondria, and thus both mitochondrial respiration and LD consumption (Herms et al., 2015).
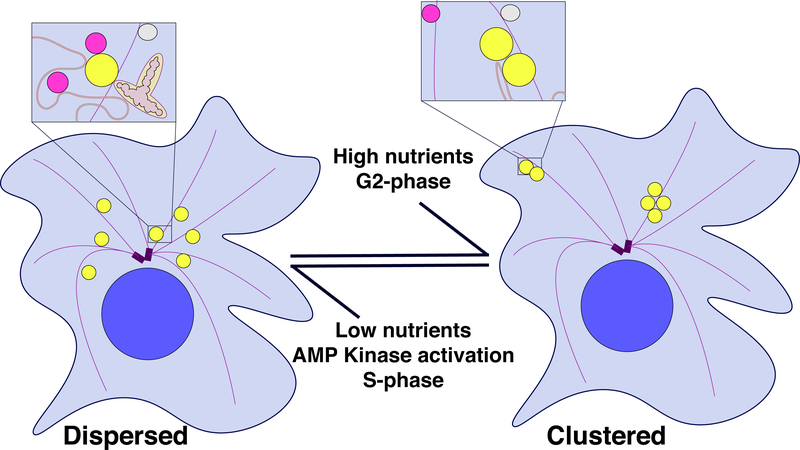
Figure 2:
Alternative LD distributions may control organelle contacts and cellular metabolism.
The main cartoon depicts the two distributions LDs adopt in cells; insets highlight consequences for organelle contacts. The black arrows indicate the internal and external factors that can lead to switching between the two LD distributions. In the clustered distribution, LDs are stored away from interacting organelles and are thus preserved for later use. In the dispersed distribution, LDs have higher chance of contacting a partner organelle, allowing for LD utilization. For simplicity, the whole cell view displays a reduced number of cellular structures. The blown-up panels emphasize those structures implicated in affecting LD function through contacts: LDs (yellow), lysosomes (magenta), unidentified “other” vesicle (grey), mitochondria (oblong, two-tone grey), microtubule (purple filaments) emanating from centrosome (purple), nuclei (large blue circle), ER (red lines with a darkened interior lumen), and cytoplasm (light blue).
Switching between a dispersed and a clustered state has been observed under diverse circumstances. For example, it can occur in response to the cell’s nutritional status (Herms et al., 2015; Nguyen et al., 2017) or due to cell cycle progression (Cruz et al., 2019). Similar LD repositioning also happens developmentally, such as in canine oocytes (Ariu et al., 2016) as well as in newly fertilized mouse embryos (Bradley et al., 2016); in the latter case, nutritional changes in the culture medium alter LD distribution further. Early Drosophila embryos also undergo precisely timed LD repositioning, with LDs first spreading broadly through the peripheral cytoplasm, then accumulating near the central yolk, and finally returning to a dispersed configuration (Welte, 2015b). LD redistribution from dispersed to clustered can also result from infection with diverse intracellular pathogens (Boulant et al., 2008; Cocchiaro et al., 2008; Toledo et al., 2016). Finally, ectopic expression of Plin1 in fibroblasts or HEK293 cells causes perinuclear clustering of LDs; subsequent activation of Protein Kinase A disperses the LDs (Marcinkiewicz et al., 2006; Orlicky et al., 2013).
We propose that toggling between LD clustering and dispersal may allow cells to achieve distinct metabolic states, by quickly and globally altering the contacts LDs engage in. Details will depend on the particular cell type, especially the distribution of potential partner organelles. For example, lysosomes degrade LDs through multiple types of autophagy and show a polarized cellular distribution, often with a perinuclear pool that actively engages in autophagy (Cabukusta and Neefjes, 2018). In cells with that configuration, co-accumulation of LDs around the nucleus would then promote LD turnover via autophagy. Indeed, interfering with the perinuclear accumulation of lysosomes (via interfering with their Dynein-dependent motility) can impair LD turnover (Tapia et al., 2019).
A possible correlate of this idea of a motility-driven metabolic switch are recent observations on the intracellular positioning of LDs in human muscle. Muscle contains two distinct types of mitochondria, intermyofibular and subsarcolemmal, each with a corresponding pool of LDs. In highly trained skiers, LDs positioned in the intermyofibrillar region, but not LDs positioned in the subsarcolemmal region, were consumed during 1 hour of exhaustive exercise (Koh et al., 2017). Another study obtained similar results, showing that trained individuals store the bulk of their LDs in the intermyofibrillar region while individuals with type 2 diabetes had a higher portion in the subsarcolemmal region. Upon exercise training, the type 2 diabetic patients shifted to an ‘athlete like’ intermyofibrillar distribution of their LDs (Daemen et al., 2018). In obese human males, exercise increases intracellular interactions between LDs and mitochondria (Shepherd et al., 2017). As of yet, it is unclear whether the observed differential LD positioning in human muscle is due to regulated LD motility or other mechanisms, but the similarities to intracellular positioning in cultured cells are intriguing and warrant further studies.
The mechanisms that bring about the switch between clustered and dispersed states has been elucidated in two cases. In Drosophila embryos, LD repositioning is mediated by precise temporal regulation of the synthesis and destruction of the Kinesin co-factor Halo, which upregulates motion away from the nucleus and thus causes LD clustering near the yolk (Arora et al., 2016). In Vero cells, nutritional status is sensed via AMP Kinase; in response to kinase activation, the amount of detyrosinated tubulin increases and the detyrosinated microtubules arrange into a non-centrosomal array (Herms et al., 2015). LDs preferentially interact with these detyrosinated microtubules and disperse on them.
LD motility controls LD allocation during cell division
In addition to the two LD distribution states discussed in the previous section, there is another case where LD distribution is globally controlled, namely during cell division. As detailed below, there are numerous cases in which the LDs of the mother cell are unequally distributed to the daughters. This is particularly dramatic in many animal embryos, where different progeny derived from the same fertilized egg can end up with vastly different LD inheritance. Presumably, this differential inheritance amongst the daughters is determined by the different lipid needs and by different potential LD interactomes in their respective lineages. For example, some cells might rely heavily on triglycerides for energy metabolism and thus would favor interactions with mitochondria, while others predominantly require fatty acids substrates for lineage-specific signaling molecule production. Embryonic sister cells with differing LD inheritances have yet to be studied, but we propose that they potentially display related differences in LD interactomes and LD utilization. In the following, we summarize known instances of such uneven allocation and document what is known about the underlying LD motility events.
The behavior of LDs during cell division has not been extensively studied, but early indications are that LDs are actively allocated between daughter cells. For example, in NIH 3T3 cells, LDs associate tightly with spindle poles during mitosis, which presumably promotes even allocation amongst the daughters (Cruz et al., 2019). In the budding yeast S. cerevisiae, cytokinesis is asymmetric with the larger daughter (‘mother’ cell) retaining the majority of the cytoplasmic contents and the smaller daughter (bud) receiving a lesser share. LDs, in contrast, are actively transported by Myosin along actin filaments into the forming bud (Wolinski et al., 2011; Knoblach and Rachubinski, 2015). This LD-bud deposition method is similar to the mechanism that deposits mitochondria into the bud (Vevea et al., 2014). These similarities suggest that LDs and mitochondria are especially valuable, warranting an active transport mechanism into the nascent bud, unlike the randomly allocated bulk of cytoplasmic contents.
Interestingly, the allocation of LDs between different cells is not always balanced. In the fission yeast S. pombe, growth media low in nutrients induces spore formation, a type of specialized division in which a diploid precursor cell (ascus) generates four stress-resistant haploid spores. The spores are loaded with LDs from the ascus cytoplasm, using actomyosin machinery and providing the spore with energy until nutrient conditions improve (Yang et al., 2017). As a result of this allocation, the remaining ascus cytoplasm is depleted of LDs, while the spores are enriched for them. An analogous situation occurs during Drosophila oogenesis where sixteen sister cells share a common cytoplasm as a result of incomplete cytokinesis. One of the sisters, the oocyte, undergoes meiosis and eventually becomes haploid; the remaining fifteen nurse cells generate tens of thousands of LDs that are transported to the oocyte in an actomyosin dependent manner and provide a major energy source for the future embryo. It would be interesting to know if other types of asymmetric cell divisions (for example, those of stem cells) also are characterized by uneven allocation of LDs to daughter cells.
During animal embryogenesis, LD allocation from a fertilized egg to an ever-growing number of embryonic cells can be particularly dramatic, often involving large-scale LD redistribution (see Welte, 2009). In the eggs of the Japanese rice fish (Medaka), LDs are initially everywhere. As development proceeds, they accumulate via microtubule-dependent motion at the vegetal pole. In Drosophila oocytes, LDs are homogenously distributed, but by 90 minutes into embryogenesis, they are enriched near the plasma membrane and depleted from the center of the embryo (Welte, 2015a). In mouse oocytes, LDs are inhomogeneously distributed into aggregates at meiosis II, but after fertilization disperse all over the zygote (Bradley et al., 2016).
Many of these LD rearrangements result in uneven allocation of LDs between the cells of the developing embryo. In Medaka, LDs are depleted at the animal pole, i.e., the precursor of the embryo proper (blastodisc), and instead the yolk sac contains the majority of LDs. A similar pattern was observed in zebrafish, where the yolk region stains much more heavily with the LD dye Nile Red than the blastodisc; however, LDs are continually being transported into the blastodisc using actomyosin machinery (Dutta and Kumar Sinha, 2015; Gupta et al., 2017). In Xenopus oocytes, triglycerides (and thus presumably LDs) are also highly enriched at the vegetal versus the animal pole (Lee et al., 2006; Papan et al., 2007; Shrestha et al., 2014); as a result, at gastrula stages, LDs are highly enriched in the endoderm and depleted from the ectoderm (Sehy et al., 2001; Papan et al., 2007). In Drosophila, at the cellular blastoderm stage, the epithelial cells inherit the majority of LDs, while the central yolk cell contains relatively few (Welte et al., 1998). At the moment, the organismal consequences of such uneven allocation across the embryo remain unknown. However, it would not be surprising if it profoundly affects embryo physiology and development.
Conclusion
Since the topic of intracellular LD motility was first reviewed a decade ago (Welte, 2009), many more cases of such motility have been discovered, driven by the widespread use of live imaging and improvements in imaging technology. In a few cases, the basic mechanisms underlying motility are fairly well understood, providing a framework for probing its biological role. As the examples covered in this review show, there is already good evidence that LD motility contributes to establishing and breaking the contacts LDs make with other organelles. Most likely, this is just the tip of the iceberg, as the relationship between LD motility and contacts has so far been examined only in a limited number of cell types and for a few types of organelles. New ways to manipulate LD motility will provide ever sharper tools to distinguish the effect of LD motility from other cellular processes. Further, the ongoing search for specific regulators of that motility, including LD-specific motor anchors, will help to improve such tools and is thus important beyond simply understanding the mechanisms of motility. Eventually, the insights from studies of single cells should help make it possible to attack the challenging problem of why animal embryos invest considerable energy to rearrange their LD content and allocate it differentially between cells.
Acknowledgements
This work was supported by National Institutes of Health grants to MDK (F31 HD100127) and MAW (RO1 GM102155). We thank two anonymous reviewers, Roxan Stephenson, Asmita Dutta, Pakinee Phromsiri, and Jonathon Thomalla for helpful comments on the manuscript.
References
- Akhmanova A, and Hammer JA 3rd., (2010). Linking molecular motors to membrane cargo. Curr Opin Cell Biol 22, 479–487. doi: 10.1016/j.ceb.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil A, Peng J, Omrane M, Gondeau C, Desterke C, Marin M, Tronchere H, Taveneau C, Sar S, Briolotti P, et al. (2016). Septin 9 induces lipid droplets growth by a phosphatidylinositol-5-phosphate and microtubule-dependent mechanism hijacked by HCV. Nat Commun 7, 12203. doi: 10.1038/ncomms12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand P, Cermelli S, Li Z, Kassan A, Bosch M, Sigua R, Huang L, Ouellette AJ, Pol A, Welte MA, and Gross SP. (2012). A novel role for lipid droplets in the organismal antibacterial response. Elife 1, e00003. doi: 10.7554/eLife.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariu F, Strina A, Murrone O, Falchi L, Bebbere D, Ledda S, Zedda MT, Pau S, and Bogliolo L. (2016). Lipid droplet distribution of immature canine oocytes in relation to their size and the reproductive stage. Anim Sci J 87, 147–150. doi: 10.1111/asj.12432 [DOI] [PubMed] [Google Scholar]
- Arora GK, Tran SL, Rizzo N, Jain A, and Welte MA. (2016). Temporal control of bidirectional lipid-droplet motion in Drosophila depends on the ratio of kinesin-1 and its co-factor Halo. J Cell Sci 129, 1416–1428. doi: 10.1242/jcs.183426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese EL, Saudale FZ, and Soulages JL. (2014). Lipid Droplets as Signaling Platforms Linking Metabolic and Cellular Functions. Lipid Insights 7, 7–16. doi: 10.4137/LPI.S11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilla-Gokcumen GE, Muro E, Relat-Goberna J, Sasse S, Bedigian A, Coughlin ML, Garcia-Manyes S, and Eggert US. (2014). Dividing cells regulate their lipid composition and localization. Cell 156, 428–439. doi: 10.1016/j.cell.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Liesa M, and Shirihai OS. (2019). Mitochondria Bound to Lipid Droplets: Where Mitochondrial Dynamics Regulate Lipid Storage and Utilization. Cell Metab 29, 827–835. doi: 10.1016/j.cmet.2019.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, Acin-Perez R, Shum M, Oliveira MF, Cinti S, et al. (2018). Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab 27, 869–885 e866. doi: 10.1016/j.cmet.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersuker K, Peterson CWH, To M, Sahl SJ, Savikhin V, Grossman EA, Nomura DK, and Olzmann JA. (2018). A Proximity Labeling Strategy Provides Insights into the Composition and Dynamics of Lipid Droplet Proteomes. Dev Cell 44, 97–112 e117. doi: 10.1016/j.devcel.2017.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, and McLauchlan J. (2008). Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule-and dynein-dependent manner. Traffic 9, 1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x [DOI] [PubMed] [Google Scholar]
- Bradley J, Pope I, Masia F, Sanusi R, Langbein W, Swann K, and Borri P. (2016). Quantitative imaging of lipids in live mouse oocytes and early embryos using CARS microscopy. Development 143, 2238–2247. doi: 10.1242/dev.129908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocard L, Immel F, Coulon D, Esnay N, Tuphile K, Pascal S, Claverol S, Fouillen L, Bessoule JJ, and Brehelin C. (2017). Proteomic Analysis of Lipid Droplets from Arabidopsis Aging Leaves Brings New Insight into Their Biogenesis and Functions. Front Plant Sci 8, 894. doi: 10.3389/fpls.2017.00894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabukusta B, and Neefjes J. (2018). Mechanisms of lysosomal positioning and movement. Traffic 19, 761–769. doi: 10.1111/tra.12587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagalinec M, Safiulina D, Liiv M, Liiv J, Choubey V, Wareski P, Veksler V, and Kaasik A. (2013). Principles of the mitochondrial fusion and fission cycle in neurons. J Cell Sci 126, 2187–2197. doi: 10.1242/jcs.118844 [DOI] [PubMed] [Google Scholar]
- Cermelli S, Guo Y, Gross SP, and Welte MA. (2006). The lipid-droplet proteome reveals that droplets are a protein-storage depot. Curr Biol 16, 1783–1795. doi: 10.1016/j.cub.2006.07.062 [DOI] [PubMed] [Google Scholar]
- Chapman DE, Reddy BJN, Huy B, Bovyn MJ, Cruz SJS, Al-Shammari ZM, Han H, Wang W, Smith DS, and Gross SP. (2019). Regulation of in vivo dynein force production by CDK5 and 14–3-3epsilon and KIAA0528. Nat Commun 10, 228. doi: 10.1038/s41467-018-08110-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchiaro JL, Kumar Y, Fischer ER, Hackstadt T, and Valdivia RH. (2008). Cytoplasmic lipid droplets are translocated into the lumen of the Chlamydia trachomatis parasitophorous vacuole. Proc Natl Acad Sci U S A 105, 9379–9384. doi: 10.1073/pnas.0712241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collot M, Fam TK, Ashokkumar P, Faklaris O, Galli T, Danglot L, and Klymchenko AS. (2018). Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J Am Chem Soc 140, 5401–5411. doi: 10.1021/jacs.7b12817 [DOI] [PubMed] [Google Scholar]
- Cruz ALS, Carrossini N, Teixeira LK, Ribeiro-Pinto LF, Bozza PT, and Viola JPB. (2019). Cell Cycle Progression Regulates Biogenesis and Cellular Localization of Lipid Droplets. Mol Cell Biol 39. doi: 10.1128/MCB.00374-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Hayashi Y, Otomo M, Mano S, Oikawa K, Hayashi M, and Nishimura M. (2016). Sucrose Production Mediated by Lipid Metabolism Suppresses the Physical Interaction of Peroxisomes and Oil Bodies during Germination of Arabidopsis thaliana. J Biol Chem 291, 19734–19745. doi: 10.1074/jbc.M116.748814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daemen S, Gemmink A, Brouwers B, Meex RCR, Huntjens PR, Schaart G, Moonen-Kornips E, Jorgensen J, Hoeks J, Schrauwen P, and Hesselink MKC. (2018). Distinct lipid droplet characteristics and distribution unmask the apparent contradiction of the athlete’s paradox. Mol Metab 17, 71–81. doi: 10.1016/j.molmet.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang D, Jung Y, Cheng JX, and Umulis DM. (2012). Label-free imaging of lipid-droplet intracellular motion in early Drosophila embryos using femtosecond-stimulated Raman loss microscopy. Biophys J 102, 1666–1675. doi: 10.1016/j.bpj.2012.01.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, and Kumar Sinha D. (2015). Turnover of the actomyosin complex in zebrafish embryos directs geometric remodelling and the recruitment of lipid droplets. Sci Rep 5, 13915. doi: 10.1038/srep13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar I, and Szabad J. (2009). In vivo analysis of MT-based vesicle transport by confocal reflection microscopy. Cell Motil Cytoskeleton 66, 68–79. doi: 10.1002/cm.20334 [DOI] [PubMed] [Google Scholar]
- Gaspar I, Yu YV, Cotton SL, Kim DH, Ephrussi A, and Welte MA. (2014). Klar ensures thermal robustness of oskar localization by restraining RNP motility. J Cell Biol 206, 199–215. doi: 10.1083/jcb.201310010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluchowski NL, Becuwe M, Walther TC, and Farese RV Jr., (2017). Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. doi: 10.1038/nrgastro.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Kimler VA, Zhang H, Ye X, Luo X, Postlethwait JH, and Thummel R. (2017). Lipid droplet biology and evolution illuminated by the characterization of a novel perilipin in teleost fish. Elife 6. doi: 10.7554/eLife.21771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groebner JL, Giron-Bravo MT, Rothberg ML, Adhikari R, Tuma DJ, and Tuma PL. (2019). Alcohol-induced microtubule acetylation leads to the accumulation of large, immobile lipid droplets. Am J Physiol Gastrointest Liver Physiol. doi: 10.1152/ajpgi.00026.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP, Guo Y, Martinez JE, and Welte MA. (2003). A determinant for directionality of organelle transport in Drosophila embryos. Curr Biol 13, 1660–1668 [DOI] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, and Wieschaus EF. (2000). Dynein-mediated cargo transport in vivo. A switch controls travel distance. J Cell Biol 148, 945–956. doi: 10.1083/jcb.148.5.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SP, Welte MA, Block SM, and Wieschaus EF. (2002). Coordination of opposite-polarity microtubule motors. J Cell Biol 156, 715–724. doi: 10.1083/jcb.200109047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Yang Y, Cao X, Zhao Y, Gao X, Sun C, Zhang F, Yuan Y, Xu Y, Zhang J, et al. (2019). Plin3 protects against alcoholic liver injury by facilitating lipid export from the endoplasmic reticulum. J Cell Biochem 120, 16075–16087. doi: 10.1002/jcb.28889 [DOI] [PubMed] [Google Scholar]
- Guimaraes SC, Schuster M, Bielska E, Dagdas G, Kilaru S, Meadows BR, Schrader M, and Steinberg G. (2015). Peroxisomes, lipid droplets, and endoplasmic reticulum “hitchhike” on motile early endosomes. J Cell Biol 211, 945–954. doi: 10.1083/jcb.201505086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jangi S, and Welte MA. (2005). Organelle-specific control of intracellular transport: distinctly targeted isoforms of the regulator Klar. Mol Biol Cell 16, 1406–1416. doi: 10.1091/mbc.e04-10-0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Walther TC, Rao M, Stuurman N, Goshima G, Terayama K, Wong JS, Vale RD, Walter P, and Farese RV. (2008). Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature 453, 657–661. doi: 10.1038/nature06928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P, Martin R, Knolker HJ, Nihalani D, and Kumar Sinha D. (2017). Myosin-1 inhibition by PClP affects membrane shape, cortical actin distribution and lipid droplet dynamics in early Zebrafish embryos. PLoS One 12, e0180301. doi: 10.1371/journal.pone.0180301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrubler R, Lanzerstorfer P, Rohrl C, Stubl F, Schurr J, Schwarzinger B, Schwarzinger C, Brameshuber M, Wieser S, Winkler SM, and Weghuber J. (2019). Hypolipidemic effects of herbal extracts by reduction of adipocyte differentiation, intracellular neutral lipid content, lipolysis, fatty acid exchange and lipid droplet motility. Sci Rep 9, 10492. doi: 10.1038/s41598-019-47060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemi HF, and Goodman JM. (2015). The life cycle of lipid droplets. Curr Opin Cell Biol 33, 119–124. doi: 10.1016/j.ceb.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms A, Bosch M, Reddy BJ, Schieber NL, Fajardo A, Ruperez C, Fernandez-Vidal A, Ferguson C, Rentero C, Tebar F, et al. (2015). AMPK activation promotes lipid droplet dispersion on detyrosinated microtubules to increase mitochondrial fatty acid oxidation. Nat Commun 6, 7176. doi: 10.1038/ncomms8176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki M, Kawasaki S, Ishikawa D, and Ozaki Y. (2016). Near-Infrared Spectroscopy and Imaging Studies of Fertilized Fish Eggs: In Vivo Monitoring of Egg Growth at the Molecular Level. Sci Rep 6, 20066. doi: 10.1038/srep20066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MR, Stephenson RA, Ghaemmaghami S, and Welte MA. (2018). Developmentally regulated H2Av buffering via dynamic sequestration to lipid droplets in Drosophila embryos. Elife 7. doi: 10.7554/eLife.36021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek B, Verbavatz JM, and Jackson CL. (2017). GBF1 and Arf1 function in vesicular trafficking, lipid homoeostasis and organelle dynamics. Biol Cell 109, 391–399. doi: 10.1111/boc.201700042 [DOI] [PubMed] [Google Scholar]
- Kimmel AR, and Sztalryd C. (2016). The Perilipins: Major Cytosolic Lipid Droplet-Associated Proteins and Their Roles in Cellular Lipid Storage, Mobilization, and Systemic Homeostasis. Annu Rev Nutr 36, 471–509. doi: 10.1146/annurev-nutr-071813-105410 [DOI] [PubMed] [Google Scholar]
- Knoblach B, and Rachubinski RA. (2015). Transport and retention mechanisms govern lipid droplet inheritance in Saccharomyces cerevisiae. Traffic 16, 298–309. doi: 10.1111/tra.12247 [DOI] [PubMed] [Google Scholar]
- Koh HE, Nielsen J, Saltin B, Holmberg HC, and Ortenblad N. (2017). Pronounced limb and fibre type differences in subcellular lipid droplet content and distribution in elite skiers before and after exhaustive exercise. J Physiol 595, 5781–5795. doi: 10.1113/JP274462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizume S, and Miyagi Y. (2016). Lipid Droplets: A Key Cellular Organelle Associated with Cancer Cell Survival under Normoxia and Hypoxia. Int J Mol Sci 17. doi: 10.3390/ijms17091430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahmer N, Farese RV Jr.,, and Walther TC. (2013). Balancing the fat: lipid droplets and human disease. EMBO Mol Med 5, 973–983. doi: 10.1002/emmm.201100671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautbauer S, Neumeier M, Haberl EM, Pohl R, Feder S, Eisinger K, Rein-Fischboeck L, and Buechler C. (2019). The utrophin-beta 2 syntrophin complex regulates adipocyte lipid droplet size independent of adipogenesis. Mol Cell Biochem 452, 29–39. doi: 10.1007/s11010-018-3409-6 [DOI] [PubMed] [Google Scholar]
- Kumar N, Leonzino M, Hancock-Cerutti W, Horenkamp FA, Li P, Lees JA, Wheeler H, Reinisch KM, and De Camilli P. (2018). VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J Cell Biol 217, 3625–3639. doi: 10.1083/jcb.201807019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Cho JH, Mietchen D, Kim YS, Hong KS, Lee C, Kang D, Park KD, Choi BS, and Cheong C. (2006). Subcellular in vivo 1H MR spectroscopy of Xenopus laevis oocytes. Biophys J 90, 1797–1803. doi: 10.1529/biophysj.105.073502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Thiel K, Thul PJ, Beller M, Kuhnlein RP, and Welte MA. (2012). Lipid droplets control the maternal histone supply of Drosophila embryos. Curr Biol 22, 2104–2113. doi: 10.1016/j.cub.2012.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan D, Tie HC, Chen B, and Lu L. (2019). Dopey1-Mon2 complex binds to dual-lipids and recruits kinesin-1 for membrane trafficking. Nat Commun 10, 3218. doi: 10.1038/s41467-019-11056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz A, Gauthier D, Garcia A, and Brasaemle DL. (2006). The phosphorylation of serine 492 of perilipin a directs lipid droplet fragmentation and dispersion. J Biol Chem 281, 11901–11909. doi: 10.1074/jbc.M600171200 [DOI] [PubMed] [Google Scholar]
- Masedunskas A, Chen Y, Stussman R, Weigert R, and Mather IH. (2017). Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: lipid droplet release is intermittently stimulated by oxytocin. Mol Biol Cell 28, 935–946. doi: 10.1091/mbc.E16-11-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maucort G, Kasula R, Papadopulos A, Nieminen TA, Rubinsztein-Dunlop H, and Meunier FA. (2014). Mapping organelle motion reveals a vesicular conveyor belt spatially replenishing secretory vesicles in stimulated chromaffin cells. PLoS One 9, e87242. doi: 10.1371/journal.pone.0087242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, and Yoshimori T. (2017). New insights into autophagosome-lysosome fusion. J Cell Sci 130, 1209–1216. doi: 10.1242/jcs.196352 [DOI] [PubMed] [Google Scholar]
- Nardi F, Fitchev P, Brooks KM, Franco OE, Cheng K, Hayward SW, Welte MA, and Crawford SE. (2019a). Lipid droplet velocity is a microenvironmental sensor of aggressive tumors regulated by V-ATPase and PEDF. Lab Invest. doi: 10.1038/s41374-019-0296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi F, Fitchev P, Franco OE, Ivanisevic J, Scheibler A, Hayward SW, Brendler CB, Welte MA, and Crawford SE. (2018). PEDF regulates plasticity of a novel lipid-MTOC axis in prostate cancer-associated fibroblasts. J Cell Sci 131. doi: 10.1242/jcs.213579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi F, Franco OE, Fitchev P, Morales A, Vickman RE, Hayward SW, and Crawford SE. (2019b). DGAT1 Inhibitor Suppresses Prostate Tumor Growth and Migration by Regulating Intracellular Lipids and Non-Centrosomal MTOC Protein GM130. Sci Rep 9, 3035. doi: 10.1038/s41598-019-39537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, Nomura DK, and Olzmann JA. (2017). DGAT1-Dependent Lipid Droplet Biogenesis Protects Mitochondrial Function during Starvation-Induced Autophagy. Dev Cell 42, 9–21 e25. doi: 10.1016/j.devcel.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norregaard K, Metzler R, Ritter CM, Berg-Sorensen K, and Oddershede LB. (2017). Manipulation and Motion of Organelles and Single Molecules in Living Cells. Chem Rev 117, 4342–4375. doi: 10.1021/acs.chemrev.6b00638 [DOI] [PubMed] [Google Scholar]
- Oeding SJ, Majstrowicz K, Hu XP, Schwarz V, Freitag A, Honnert U, Nikolaus P, and Bahler M. (2018). Identification of Miro1 and Miro2 as mitochondrial receptors for myosin XIX. J Cell Sci 131. doi: 10.1242/jcs.219469 [DOI] [PubMed] [Google Scholar]
- Olkkonen VM, Koponen A, and Arora A. (2019). OSBP-related protein 2 (ORP2): Unraveling its functions in cellular lipid/carbohydrate metabolism, signaling and F-actin regulation. J Steroid Biochem Mol Biol. doi: 10.1016/j.jsbmb.2019.01.016 [DOI] [PubMed] [Google Scholar]
- Orlicky DJ, Monks J, Stefanski AL, and McManaman JL. (2013). Dynamics and molecular determinants of cytoplasmic lipid droplet clustering and dispersion. PLoS One 8, e66837. doi: 10.1371/journal.pone.0066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios IM, and St Johnston D. (2002). Kinesin light chain-independent function of the Kinesin heavy chain in cytoplasmic streaming and posterior localisation in the Drosophila oocyte. Development 129, 5473–5485. doi: 10.1242/dev.00119 [DOI] [PubMed] [Google Scholar]
- Papan C, Boulat B, Velan SS, Fraser SE, and Jacobs RE. (2007). Two-dimensional and three-dimensional time-lapse microscopic magnetic resonance imaging of Xenopus gastrulation movements using intrinsic tissue-specific contrast. Dev Dyn 236, 494–501. doi: 10.1002/dvdy.21045 [DOI] [PubMed] [Google Scholar]
- Pennetta G, and Welte MA. (2018). Emerging Links between Lipid Droplets and Motor Neuron Diseases. Dev Cell 45, 427–432. doi: 10.1016/j.devcel.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer SG, Gateva G, Horvath P, Pirhonen J, Salo VT, Karhinen L, Varjosalo M, Ryhanen SJ, Lappalainen P, and Ikonen E. (2017). Role for formin-like 1-dependent acto-myosin assembly in lipid droplet dynamics and lipid storage. Nat Commun 8, 14858. doi: 10.1038/ncomms14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu J, Guardia CM, Keren-Kaplan T, and Bonifacino JS. (2016). Mechanisms and functions of lysosome positioning. J Cell Sci 129, 4329–4339. doi: 10.1242/jcs.196287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai P, Kumar M, Sharma G, Barak P, Das S, Kamat SS, and Mallik R. (2017). Kinesin-dependent mechanism for controlling triglyceride secretion from the liver. Proc Natl Acad Sci U S A 114, 12958–12963. doi: 10.1073/pnas.1713292114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BJ, Mattson M, Wynne CL, Vadpey O, Durra A, Chapman D, Vallee RB, and Gross SP. (2016). Load-induced enhancement of Dynein force production by LIS1-NudE in vivo and in vitro. Nat Commun 7, 12259. doi: 10.1038/ncomms12259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka HA, and Valdivia R. (2012). Emerging roles for lipid droplets in immunity and host-pathogen interactions. Annu Rev Cell Dev Biol 28, 411–437. doi: 10.1146/annurev-cellbio-092910-153958 [DOI] [PubMed] [Google Scholar]
- Sehy JV, Ackerman JJ, and Neil JJ. (2001). Water and lipid MRI of the Xenopus oocyte. Magn Reson Med 46, 900–906 [DOI] [PubMed] [Google Scholar]
- Shepherd SO, Cocks M, Meikle PJ, Mellett NA, Ranasinghe AM, Barker TA, Wagenmakers AJM, and Shaw CS. (2017). Lipid droplet remodelling and reduced muscle ceramides following sprint interval and moderate-intensity continuous exercise training in obese males. Int J Obes (Lond) 41, 1745–1754. doi: 10.1038/ijo.2017.170 [DOI] [PubMed] [Google Scholar]
- Shrestha B, Sripadi P, Reschke BR, Henderson HD, Powell MJ, Moody SA, and Vertes A. (2014). Subcellular metabolite and lipid analysis of Xenopus laevis eggs by LAESI mass spectrometry. PLoS One 9, e115173. doi: 10.1371/journal.pone.0115173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubeita GT, Tran SL, Xu J, Vershinin M, Cermelli S, Cotton SL, Welte MA, and Gross SP. (2008). Consequences of motor copy number on the intracellular transport of kinesin-1-driven lipid droplets. Cell 135, 1098–1107. doi: 10.1016/j.cell.2008.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speziali G, Liesinger L, Gindlhuber J, Leopold C, Pucher B, Brandi J, Castagna A, Tomin T, Krenn P, Thallinger GG, et al. (2018). Myristic acid induces proteomic and secretomic changes associated with steatosis, cytoskeleton remodeling, endoplasmic reticulum stress, protein turnover and exosome release in HepG2 cells. J Proteomics 181, 118–130. doi: 10.1016/j.jprot.2018.04.008 [DOI] [PubMed] [Google Scholar]
- Tapia D, Jimenez T, Zamora C, Espinoza J, Rizzo R, Gonzalez-Cardenas A, Fuentes D, Hernandez S, Cavieres VA, Soza A, et al. (2019). KDEL receptor regulates secretion by lysosome relocation-and autophagy-dependent modulation of lipid-droplet turnover. Nat Commun 10, 735. doi: 10.1038/s41467-019-08501-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett-Adams P, Chambers D, Gledhill S, Hope RG, Coy JF, Girod A, and McLauchlan J. (2003). Live cell analysis and targeting of the lipid droplet-binding adipocyte differentiation-related protein. J Biol Chem 278, 15998–16007. doi: 10.1074/jbc.M211289200 [DOI] [PubMed] [Google Scholar]
- Toledo DA, D’Avila H, and Melo RC. (2016). Host Lipid Bodies as Platforms for Intracellular Survival of Protozoan Parasites. Front Immunol 7, 174. doi: 10.3389/fimmu.2016.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valm AM, Cohen S, Legant WR, Melunis J, Hershberg U, Wait E, Cohen AR, Davidson MW, Betzig E, and Lippincott-Schwartz J. (2017). Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 546, 162–167. doi: 10.1038/nature22369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vevea JD, Swayne TC, Boldogh IR, and Pon LA. (2014). Inheritance of the fittest mitochondria in yeast. Trends Cell Biol 24, 53–60. doi: 10.1016/j.tcb.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther TC, and Farese RV Jr., (2012). Lipid droplets and cellular lipid metabolism. Annu Rev Biochem 81, 687–714. doi: 10.1146/annurev-biochem-061009-102430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschatko G, Billecke N, Schwendy S, Jaurich H, Bonn M, Vilgis TA, and Parekh SH. (2016). Label-free in situ imaging of oil body dynamics and chemistry in germination. J R Soc Interface 13. doi: 10.1098/rsif.2016.0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA. (2004). Bidirectional transport along microtubules. Curr Biol 14, R525–537. doi: 10.1016/j.cub.2004.06.045 [DOI] [PubMed] [Google Scholar]
- Welte MA. (2007). Proteins under new management: lipid droplets deliver. Trends Cell Biol 17, 363–369. doi: 10.1016/j.tcb.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Welte MA. (2009). Fat on the move: intracellular motion of lipid droplets. Biochem Soc Trans 37, 991–996. doi: 10.1042/BST0370991 [DOI] [PubMed] [Google Scholar]
- Welte MA. (2015a). As the fat flies: The dynamic lipid droplets of Drosophila embryos. Biochim Biophys Acta 1851, 1156–1185. doi: 10.1016/j.bbalip.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA. (2015b). Expanding roles for lipid droplets. Curr Biol 25, R470–481. doi: 10.1016/j.cub.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA, Cermelli S, Griner J, Viera A, Guo Y, Kim DH, Gindhart JG, and Gross SP. (2005). Regulation of lipid-droplet transport by the perilipin homolog LSD2. Curr Biol 15, 1266–1275. doi: 10.1016/j.cub.2005.06.062 [DOI] [PubMed] [Google Scholar]
- Welte MA, and Gould AP. (2017). Lipid droplet functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol Lipids 1862, 1260–1272. doi: 10.1016/j.bbalip.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA, Gross SP, Postner M, Block SM, and Wieschaus EF. (1998). Developmental regulation of vesicle transport in Drosophila embryos: forces and kinetics. Cell 92, 547–557. doi: 10.1016/s0092-8674(00)80947-2 [DOI] [PubMed] [Google Scholar]
- Wheatley S, Kulkarni S, and Karess R. (1995). Drosophila nonmuscle myosin II is required for rapid cytoplasmic transport during oogenesis and for axial nuclear migration in early embryos. Development 121, 1937–1946 [DOI] [PubMed] [Google Scholar]
- Wolinski H, Kolb D, Hermann S, Koning RI, and Kohlwein SD. (2011). A role for seipin in lipid droplet dynamics and inheritance in yeast. J Cell Sci 124, 3894–3904. doi: 10.1242/jcs.091454 [DOI] [PubMed] [Google Scholar]
- Yang HJ, Osakada H, Kojidani T, Haraguchi T, and Hiraoka Y. (2017). Lipid droplet dynamics during Schizosaccharomyces pombe sporulation and their role in spore survival. Biol Open 6, 217–222. doi: 10.1242/bio.022384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PL, Hsu TH, Wang CW, and Chen RH. (2016). Lipid droplets maintain lipid homeostasis during anaphase for efficient cell separation in budding yeast. Mol Biol Cell 27, 2368–2380. doi: 10.1091/mbc.E16-02-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshaw WM, van der Zwaag M, Pinto F, Lahaye LL, Faber AI, Gomez-Sanchez R, Dolga AM, Poland C, Monaco AP, van ISC, et al. (2019). Human VPS13A is associated with multiple organelles and influences mitochondrial morphology and lipid droplet motility. Elife 8. doi: 10.7554/eLife.43561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Li T, Wu N, Jiang L, Ji X, and Huang H. (2017). The Role of Lipid Droplets in Mortierella alpina Aging Revealed by Integrative Subcellular and Whole-Cell Proteome Analysis. Sci Rep 7, 43896. doi: 10.1038/srep43896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jin K, and Xia Y. (2017). Contributions of beta-tubulin to cellular morphology, sporulation and virulence in the insect-fungal pathogen, Metarhizium acridum. Fungal Genet Biol 103, 16–24. doi: 10.1016/j.fgb.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhu W, Ni F, Ai H, Gong S, Zhou X, Sessler JL, and Yang C. (2019). Simultaneous dual-colour tracking lipid droplets and lysosomes dynamics using a fluorescent probe. Chem Sci 10, 2342–2348. doi: 10.1039/c8sc04462g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi Y, Taylor MC, Campbell PM, Warden AC, Shrestha P, El Tahchy A, Rolland V, Vanhercke T, Petrie JR, White RG, et al. (2017). Comparative Lipidomics and Proteomics of Lipid Droplets in the Mesocarp and Seed Tissues of Chinese Tallow (Triadica sebifera). Front Plant Sci 8, 1339. doi: 10.3389/fpls.2017.01339 [DOI] [PMC free article] [PubMed] [Google Scholar]