Abstract
Objective: Yes-associated protein 1 (YAP1) regulates a variety of genes related to cell proliferation, cycle and apoptosis, and plays a role in the pathogenesis of esophageal cancer. It was found that the expression of miR-195 was significantly decreased in esophageal cancer tissues, suggesting its anti-cancer effect. Bioinformatics analysis revealed the targeted relationship between miR-195 and the 3’-UTR of YAP1. This study investigated the role of miR-195 in regulating YAP1 expression and affecting proliferation and apoptosis of esophageal cancer cells. Methods: The tumor tissue and the adjacent tissue of patients with esophageal cancer were collected to detect the expressions of miR-195 and YAP1. Dual luciferase reporter gene assay was adopted to validate the targeted regulation between miR-195 and YAP1. Esophageal cancer EC9706 cells and normal esophageal epithelial HEEC cells were cultured in vitro to measure the expression of miR-195 and YAP1. EC9706 cells were divided into miR-NC group and miR-195 mimic group followed by analysis of cell apoptosis by flow cytometry and cell proliferation by EdU staining. Results: Compared with adjacent tissues, miR-195 was significantly decreased, while YAP1 mRNA and protein were significantly upregulated in esophageal cancer tissues. Dual luciferase reporter gene assay confirmed that there was a targeted relationship between miR-195 and YAP1. Compared with HEEC cells, miR-195 expression was declined, whereas YAP1 was elevated in EC9706 cells. Transfection of miR-195 mimic significantly downregulated YAP1 expression, resulting in increased apoptosis and decreased proliferation of EC9706 cells. Conclusion: Decreased expression of miR-195 plays a regulatory role in increasing YAP1 expression and promoting the pathogenesis of esophageal cancer. Elevation of miR-195 inhibites the expression of YAP1, restrains cell proliferation, and promotes cell apoptosis.
Keywords: miR-195, YAP1, esophageal cancer, proliferation, apoptosis
Introduction
Esophageal cancer (EC) is a common malignant tumor of the digestive tract. Its complicated pathogenesis, difficult prevention, and lack of early diagnosis lead to high morbidity and mortality [1].
Yes-associated protein 1 (YAP1), a major effector and regulatory protein in the classical Hippo-YAP signaling pathway, regulates the expression of several target genes by entering into the nucleus as a transcriptional co-activated form. Its expression was significantly increased in various tumor tissues and cells [2-4]. It was found that increased expression of YAP1 is associated with the development, progression and drug resistance of esophageal cancer [5-7].
MicroRNAs are endogenous, non-coding small-molecule single-stranded RNAs with approximately 22-25 nucleotides in length, which can bind to the 3’-UTR of the target gene to degrade mRNA or inhibit its translation. MicroRNAs that accounted for 1% of human genes regulate the expression of more than 1/3 of human genes [8]. The role of microRNA expression and dysfunction in tumorigenesis has received increasing attention [9,10]. It was demonstrated that the expression of miR-195 in tumor tissues of patients with esophageal cancer is significantly reduced, suggesting that miR-195 may play a role as a tumor suppressor gene in the development of esophageal cancer [11-14]. Bioinformatics analysis found a targeted relationship between miR-195 and the 3’-UTR of YAP1. This study investigated the role of miR-195 in regulating YAP1 expression and affecting proliferation and apoptosis of esophageal cancer cells.
Materials and methods
Main reagents and materials
Normal esophageal epithelial cells HEEC and esophageal cancer cells EC9706 were purchased from Jennio-bio. RPMI 1640, FBS, and Opti-MEM were purchased from Gibco. Lip 2000 was purchased from Invitrogen. ReverTra Ace qPCR RT Kit and SYBR dye were purchased from Toyobo. miR-NC and miR-195 mimic were purchased from Ribobio. EdU flow detection reagent was purchased from Sigma. Rabbit anti-human YAP1 was purchased from Abcam. Rabbit anti-human β-actin antibody was purchased from GeneTex Inc. HRP-conjugated secondary antibody was purchased from Jackson ImmunoResearch. Annexin V/PI apoptosis detection reagent was purchased from Beyotime. RIPA protein extract was purchased from Solarbio. Dual-Luciferase Reporter Assay System and pMIR plasmid were purchased from Promega.
Clinical information
Forty esophageal cancer patients underwent resection in our hospital from September 2017 to January 2018 were enrolled with mean age of 56.9 (45-71) years old. The esophageal cancer tissues were resected and pathologically confirmed during the operation. A paracancerous tissue of 2 cm or more from the esophageal cancer tissue was collected as the control. All samples had informed consent by the patient and the experimental procedures were approved by the hospital ethics committee.
Cell culture
HEEC and EC9706 cells were cultured in RPMI 1640 medium containing 10% FBS and 1% penicillin-streptomycin at 37°C with 5% CO2. The cells were passaged at 1:4. The cells in log phase were taken for experiments.
Dual luciferase reporter gene assay
The PCR product of the YAP1 3’-UTR full-length fragment or mutant fragment was double-digested and then ligated into the pMIR vector. After sequencing, the plasmid was designated as pMIR-YAP1-WT and pMIR-YAP1-MUT.
The HEK293T cells were transfected with pMIR-YAP1-WT (or pMIR-YAP1-MUT) together with miR-195 mimic (or miR-NC) using Lipofectamine 2000. After incubation for 48 h, the cells were lysed by 100 µL Passive Lysis Buffer and centrifuged at 1000 rpm for 5 min. Next, the lysis was mixed with 50 μL substrate and stopped by 50 µL Stop&Glo. At last, the luciferase activity was detected by Dual-Glo Luciferase Assay System kit according to the manual.
Cell transfection and grouping
EC9706 cells were divided into miR-NC group and miR-195 mimic group. A total of 10 μL of Lip2000, 50 nmoL miR-NC, and 50 nmoL miR-195 mimic were diluted in 100 μL serum-free Opti-MEM medium, and incubated for 5 min at room temperature, respectively. The mixture was added to the cell culture medium for 72 hours. Finally, the cells were collected for detection.
Flow cytometry detection of cell proliferation
EdU solution at 10 μM was added into the cells in logarithmic phase. After incubation for 2 h, cells were seeded for 48 h and digested by trypsin. After fixing in 4% paraformaldehyde for 15-30 min, cells were neutralized by 2 mg/mL glycine for 5 min, incubated in 100 μL TritonX-100 at room temperature, followed by addition of 500 μL Apollo fluid at room temperature under dark for 30 min. Then, cell proliferation was tested on FC500 MCL flow cytometry.
Cell apoptosis detection
The cells were digested by enzyme and collected. After resuspension in 100 μl binding buffer, 5 μl Annexin V-FITC and 5 μl PI were added to the cells and incubated at room temperature for 15 min under darkness. Then cell apoptosis was measured by flow cytometry.
qRT-PCR
Total RNA was extracted using Trizol. ReverTra Ace qPCR RT Kit was used to reverse transcribe RNA to cDNA for PCR reaction. qPCR reaction system contained 2 μg total RNA, 1 μL dNTP, 4 μL RT Buffer, 1 μL RT primer, 2 μL RT Enzyme, 1 μL RNase inhibitor, and ddH2O. qPCR reaction conditions were 40 cycles of 95°C for 15 s, 60°C for 30 s, and 74°C for 30 s on the Bio-Rad CFX96 Real-Time PCR Detection System.
Western blot
Cells and tissues were lysed by 100 μL RIPA. 50 μg proteins were separated by 12% SDS-PAGE gel and 4% concentrated gel. Then the protein was transferred to PVDF membrane at 300 mA for 90 min. Next, the membrane was blocked with 5% skim milk at room temperature for 60 minutes and incubated with primary antibody (YAP1 and β-actin at 1:1000 and 1:8000, respectively) at 4°C overnight. After that, the membrane was incubated with HRP-conjugated secondary antibody (1:15000) at room temperature for 60 min and finally detected by ECL chemiluminescence.
Statistical analysis
All data analyses were performed on SPSS 18.0 software. Measurement data were expressed as mean ± standard deviation and compared by t-test or one-way ANOVA. Bonferroni method was adopted for post hoc test. P < 0.05 was considered significant.
Results
miR-195 and YAP1 mRNA expressions changed in EC tissue
qRT-PCR showed that miR-195 expression was significantly decreased in tumor tissues of patients with esophageal cancer compared with adjacent tissues (Figure 1A). qRT-PCR demonstrated that YAP1 mRNA level was significantly upregulated in tumor tissues of patients with esophageal cancer compared with adjacent tissues (Figure 1B). Western blot analysis revealed that YAP1 protein expression was significantly elevated in tumor tissues of patients with esophageal cancer compared with adjacent tissues (Figure 1C, 1D).
Figure 1.
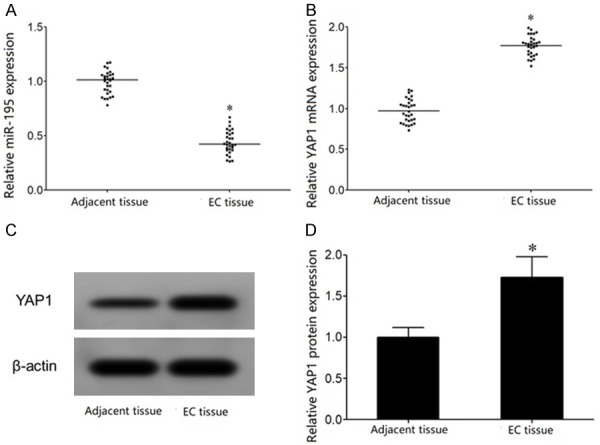
miR-195 is reduced, while YAP1 expression is elevated in EC tissue. A. qRT-PCR detection of miR-195 expression in EC tissue; B. qRT-PCR detection of YAP1 mRNA expression in EC tissue; C. Western blot detection of YAP1 protein expression in EC tissue; D. YAP1 protein expression analysis. *P < 0.05, compared with adjacent tissue.
The targeted regulatory relationship between miR-195 and YAP1
MicroRNA.org online prediction showed the complementary binding site between miR-195 and the 3’-UTR of YAP1 mRNA (Figure 2A). Dual luciferase reporter gene assay exhibited that 0miR-195 mimic transfection significantly reduced the relative luciferase activity in HEK293T cells transfected by pMIR-YAP1-WT but not by pMIR-YAP1-MUT, confirming that YAP1 was the target gene of miR-195 (Figure 2B).
Figure 2.

The targeted regulatory relationship between miR-195 and YAP1 mRNA. A. The complementary binding site between miR-195 and the 3’-UTR of YAP1 mRNA. B. Dual luciferase reporter assay. *P < 0.05, compared with miR-NC.
MiR-195 and YAP1 mRNA expressions changed in EC cells
qRT-PCR exhibited that miR-195 expression was significantly reduced, whereas YAP1 mRNA level was enhanced in EC706 cells compared with HEEC cells (Figure 3A, 3B). In addition, western blot analysis revealed that YAP1 protein expression was significantly upregulated in EC706 cells compared with HEEC cells (Figure 3C, 3D).
Figure 3.
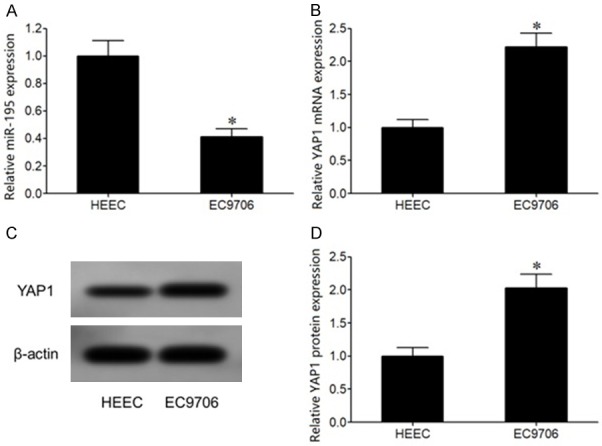
miR-195 and YAP1 mRNA expressions changed in EC cells. A. qRT-PCR detection of miR-195 expression in EC cells; B. qRT-PCR detection of YAP1 mRNA expression in EC cells; C. Western blot detection of YAP1 protein expression in EC cells; D. YAP1 protein expression analysis. *P < 0.05, compared with HEEC cells.
miR-195 mimic transfection suppressed EC9706 cell proliferation and promoted apoptosis
qRT-PCR showed that miR-195 mimic transfection significantly upregulated miR-195 expression and declined YAP1 mRNA level in EC9706 cells compared with miR-NC group (Figure 4A, 4B). Western blot demonstrated that miR-195 mimic transfection obviously reduced YAP1 protein levels in EC9706 cells (Figure 4C, 4D). Flow cytometry revealed that transfection of miR-195 mimic markedly enhanced cell apoptosis, while inhibited cell proliferation in EC9706 cells (Figure 4E, 4F).
Figure 4.
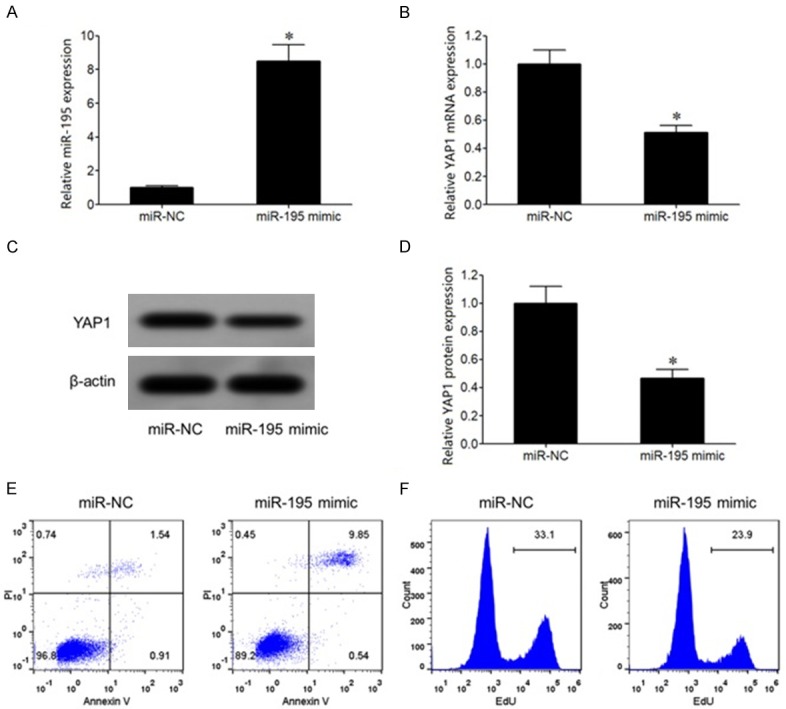
miR-195 mimic transfection suppressed EC9706 cell proliferation and promoted apoptosis. A. qRT-PCR detection of miR-195 expression in EC cells; B. qRT-PCR detection of YAP1 mRNA expression in EC cells; C. Western blot detection of YAP1 protein expression in EC cells; D. YAP1 protein expression analysis; E. Flow cytometry detection of cell apoptosis; F. Flow cytometry detection of cell proliferation. *P < 0.05, compared with miR-NC group.
Discussion
Hippo-YAP is a newly discovered intracellular signaling pathway that is regulated by a variety of upstream tumor suppressors and downstream cancer-promoting factors, which can regulate cell proliferation, migration, and apoptosis. The Hippo-YAP pathway also plays an important regulatory role in tumor biology. Hippo-YAP pathway abnormality is associated with the occurrence, progression and drug resistance of various tumors, such as pancreatic cancer [15], ovarian cancer [16] colorectal cancer [17], prostate cancer [18], and gastric cancer [19]. YAP1 gene is located at chromosome 11q13 and encodes a protein of 65 kD size [20]. YAP1 is an important regulator of the Hippo-YAP signaling pathway and plays a regulatory role by binding with transcription factors to regulate the transcription and expression of various genes. It was reported that YAP1 is a cancer-promoting factor, which is abnormally elevated in various tumors, such as gastric cancer [2], non-small cell lung cancer [21], and breast cancer [22-24]. It was showed that increased expression of YAP1 is associated with the development, progression and drug resistance of esophageal cancer [5-7]. Moreover, it was revealed that the expression of miR-195 in tumor tissues of patients with esophageal cancer was significantly reduced, suggesting that miR-195 may play a role as a tumor suppressor gene in the development of esophageal cancer [11-14]. Bioinformatics analysis revealed a targeted relationship between miR-195 and the 3’-UTR of YAP1. This study investigated the role of miR-195 in regulating YAP1 expression and affecting proliferation and apoptosis of esophageal cancer cells.
Dual luciferase reporter gene assay exhibited that miR-195 mimic transfection significantly reduced the relative luciferase activity in HEK293T cells transfected by pMIR-YAP1-WT but not by pMIR-YAP1-MUT, confirming that YAP1 was the target gene of miR-195. In this study, miR-195 expression was significantly decreased, while YAP1 level was elevated in tumor tissues of patients with esophageal cancer compared with adjacent tissues. Consistent with this, in vitro investigation also exhibited that miR-195 expression was significantly reduced, whereas YAP1 mRNA level was enhanced in EC706 cells compared with HEEC cells. It was suggested that the decreased expression of miR-195 may play a role in up-regulating YAP1 expression and promoting the pathogenesis of esophageal cancer. Li et al. [11] demonstrated that the expression of miR-195 in tumor tissues of patients with esophageal cancer was significantly lower than that of normal esophageal epithelial tissues. Sun et al. [12] reported that compared with adjacent tissues, the expression of miR-195 in tumor tissues of patients with esophageal cancer was apparently decreased, while the expression of target gene CDC42 was markedly elevated with negative correlation. Decreased miR-195 expression was related to clinical stage and lymph node metastasis. Lower miR-195 expression was associated with poor overall survival rate and disease-free survival rate. Fu et al. [14] showed that compared with adjacent tissues, the expression of miR-195 in esophageal cancer tissues was reduced for 4.141 times. Fu et al. [13] proved that the expression of miR-195 in tumor tissues of patients with esophageal cancer was significantly lower than that of mR-195 in adjacent tissues for 2.63 times. This study showed that the decreased expression of miR-195 was associated with esophageal cancer, which was similar to the above results.
To further investigate the effect of miR-195 on the biological effects of esophageal cancer cells, this study overexpressed miR-195 in esophageal cancer cells, and observed that transfection of miR-195 mimic significantly increased the expression of YAP1 in esophageal cancer EC9706 cells, reduced cell proliferation and enhanced cell apoptosis. It indicated that miR-195 exerts a tumor suppressor effect that attenuates the malignant biological characteristics of esophageal cancer cells possibly through targeting YAP1. Li et al. [11] demonstrated that transfection of miR-195 mimic in esophageal cancer EC109 and EC9706 cells significantly increased miR-195, downregulated its target gene HMGA2, and inhibited esophageal cancer EC109 and EC9706 cells proliferative activity and promoted cell apoptosis. Fu et al. [13] revealed that transfection of miR-195 mimic significantly reduced the proliferation activity, inhibited clonal formation and invasion ability of esophageal cancer Eca109 and TE13 cells. Furthermore, miR-195 induced Ec109 cell cycle G1 phase arrest by targeting the cell cycle regulatory gene CDC42. Zhao et al. [3] demonstrated that the expression of YAP1 in esophageal cancer tissues was significantly higher than that in adjacent tissues, and was related to the clinical stage and lymph node metastasis. Knockdown of YAP1 expression in esophageal cancer cells apparently attenuated cell proliferation and invasion, and induced apoptosis. Song et al. [6] showed that compared with chemotherapy-sensitive esophageal cancer tissues, the expression of YAP1 was markedly elevated in chemotherapy-resistant esophageal cancer tissues. Knockdown of YAP1 in vitro obviously increased the drug sensitivity of esophageal cancer cells to 5-FU and docetaxel. This study combined the targeted regulatory relationship between miR-195 and YAP1, revealing that miR-195 plays a role in targeting the inhibition of YAP1 and attenuating the malignant biologic characteristics of esophageal cancer cells.
Conclusion
Decreased expression of miR-195 plays a regulatory role in increasing YAP1 expression and promoting the pathogenesis of esophageal cancer. Elevation of miR-195 inhibits the expression of YAP1, restrains cell proliferation, and promotes apoptosis.
Disclosure of conflict of interest
None.
References
- 1.Ji L, Cao XF, Wang HM, Li YS, Zhu B, Xiao J, Wang D. Expression level of beta-catenin is associated with prognosis of esophageal carcinoma. World J Gastroenterol. 2007;13:2622–2625. doi: 10.3748/wjg.v13.i18.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu L, Gao C, Feng B, Wang L, Tian X, Wang H, Ma D. Distinct prognostic values of YAP1 in gastric cancer. Tumour Biol. 2017;39:1010428317695926. doi: 10.1177/1010428317695926. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J, Li X, Yang Y, Zhu D, Zhang C, Liu D, Wu K, Zhao S. Effect of YAP1 silencing on esophageal cancer. Onco Targets Ther. 2016;9:3137–3146. doi: 10.2147/OTT.S102338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzetto E, Brenca M, Boeri M, Verri C, Piccinin E, Gasparini P, Facchinetti F, Rossi S, Salvatore G, Massimino M, Sozzi G, Maestro R, Modena P. YAP1 acts as oncogenic target of 11q22 amplification in multiple cancer subtypes. Oncotarget. 2014;5:2608–2621. doi: 10.18632/oncotarget.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song S, Ajani JA, Honjo S, Maru DM, Chen Q, Scott AW, Heallen TR, Xiao L, Hofstetter WL, Weston B, Lee JH, Wadhwa R, Sudo K, Stroehlein JR, Martin JF, Hung MC, Johnson RL. Hippo coactivator YAP1 upregulates SOX9 and endows esophageal cancer cells with stem-like properties. Cancer Res. 2014;74:4170–4182. doi: 10.1158/0008-5472.CAN-13-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, Wu TT, Johnson RL, Hung MC, Ajani JA. The hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21:2580–2590. doi: 10.1158/1078-0432.CCR-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Fu X, Deng Y. Histone demethylase JMJD1C regulates esophageal cancer proliferation Via YAP1 signaling. Am J Cancer Res. 2017;7:115–124. [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Xu Z, Wang N, Xu T, Zhu M. MicroRNA and gene networks in human diffuse large B-cell lymphoma. Oncol Lett. 2014;8:2225–2232. doi: 10.3892/ol.2014.2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang K, Wang YW, Wang YY, Song Y, Zhu J, Si PC, Ma R. Identification of microRNA biomarkers in the blood of breast cancer patients based on microRNA profiling. Gene. 2017;619:10–20. doi: 10.1016/j.gene.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 10.Cheng N, Xu Y, Luo Y, Zhu L, Zhang Y, Huang K, Xu W. Specific and relative detection of urinary microRNA signatures in bladder cancer for point-of-care diagnostics. Chem Commun (Camb) 2017;53:4222–4225. doi: 10.1039/c7cc01007a. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wu D, Wang P, Li X, Shi G. miR-195 regulates proliferation and apoptosis through inhibiting the mTOR/p70s6k signaling pathway by targeting HMGA2 in esophageal carcinoma cells. Dis Markers. 2017;2017:8317913. doi: 10.1155/2017/8317913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun N, Ye L, Chang T, Li X, Li X. microRNA-195-Cdc42 axis acts as a prognostic factor of esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:6871–6879. [PMC free article] [PubMed] [Google Scholar]
- 13.Fu MG, Li S, Yu TT, Qian LJ, Cao RS, Zhu H, Xiao B, Jiao CH, Tang NN, Ma JJ, Hua J, Zhang WF, Zhang HJ, Shi RH. Differential expression of miR-195 in esophageal squamous cell carcinoma and miR-195 expression inhibits tumor cell proliferation and invasion by targeting of Cdc42. FEBS Lett. 2013;587:3471–3479. doi: 10.1016/j.febslet.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Fu HL, Wu DP, Wang XF, Wang JG, Jiao F, Song LL, Xie H, Wen XY, Shan HS, Du YX, Zhao YP. Altered miRNA expression is associated with differentiation, invasion, and metastasis of esophageal squamous cell carcinoma (ESCC) in patients from Huaian, China. Cell Biochem Biophys. 2013;67:657–668. doi: 10.1007/s12013-013-9554-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhou M, Wei H, Zhou H, He J, Lu Y, Wang D, Chen B, Zeng J, Peng W, Du F, Gong A, Xu M. Furin promotes epithelial-mesenchymal transition in pancreatic cancer cells via Hippo-YAP pathway. Int J Oncol. 2017;50:1352–1362. doi: 10.3892/ijo.2017.3896. [DOI] [PubMed] [Google Scholar]
- 16.Jeong W, Kim SB, Sohn BH, Park YY, Park ES, Kim SC, Kim SS, Johnson RL, Birrer M, Bowtell DS, Mills GB, Sood A, Lee JS. Activation of YAP1 is associated with poor prognosis and response to taxanes in ovarian cancer. Anticancer Res. 2014;34:811–817. [PMC free article] [PubMed] [Google Scholar]
- 17.Sun M, Song H, Wang S, Zhang C, Zheng L, Chen F, Shi D, Chen Y, Yang C, Xiang Z, Liu Q, Wei C, Xiong B. Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. J Hematol Oncol. 2017;10:79. doi: 10.1186/s13045-017-0445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collak FK, Demir U, Ozkanli S, Kurum E, Zerk PE. Increased expression of YAP1 in prostate cancer correlates with extraprostatic extension. Cancer Biol Med. 2017;14:405–413. doi: 10.20892/j.issn.2095-3941.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng J, Lei W, Xiang X, Zhang L, Yu F, Chen J, Feng M, Xiong J. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumour Biol. 2015;36:6823–6831. doi: 10.1007/s13277-015-3364-8. [DOI] [PubMed] [Google Scholar]
- 20.Jerhammar F, Johansson AC, Ceder R, Welander J, Jansson A, Grafstrom RC, Soderkvist P, Roberg K. YAP1 is a potential biomarker for cetuximab resistance in head and neck cancer. Oral Oncol. 2014;50:832–839. doi: 10.1016/j.oraloncology.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Huang C, Ma R, Yue J, Li N, Li Z, Qi D. MiR-497 suppresses YAP1 and inhibits tumor growth in non-small cell lung cancer. Cell Physiol Biochem. 2015;37:342–352. doi: 10.1159/000430358. [DOI] [PubMed] [Google Scholar]
- 22.Yu SJ, Yang L, Hong Q, Kuang XY, Di GH, Shao ZM. MicroRNA-200a confers chemoresistance by antagonizing TP53INP1 and YAP1 in human breast cancer. BMC Cancer. 2018;18:74. doi: 10.1186/s12885-017-3930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade D, Mehta M, Griffith J, Panneerselvam J, Srivastava A, Kim TD, Janknecht R, Herman T, Ramesh R, Munshi A. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget. 2017;8:98495–98508. doi: 10.18632/oncotarget.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu SJ, Hu JY, Kuang XY, Luo JM, Hou YF, Di GH, Wu J, Shen ZZ, Song HY, Shao ZM. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin Cancer Res. 2013;19:1389–1399. doi: 10.1158/1078-0432.CCR-12-1959. [DOI] [PubMed] [Google Scholar]


