Abstract
Nasopharyngeal carcinoma (NPC) is a respiratory malignant epithelial carcinoma. Research has indicated that bactericidal/permeability-increasing fold-containing protein B1 (BPIFB1), mostly secreted by nasopharyngeal epithelia, is dysregulated in patients with NPC. This study aimed to explore the effects of BPIFB1 inviability, proliferation, apoptosis and its molecular mechanism. To confirm the effects of BPIFB1 on NPC cells, BPIFB1 was overexpressed or silenced in NPC-KT cells after being transfected with BPIFB1 or siBPIFB1 plasmids. The results showed that BPIFB1 overexpression could induce apoptosis and DNA damage in NPC-KT cells, and silenced BPIFB1 had the opposite effects. BPIFB1 overexpression can inhibit the cell cycle by being arrested at the G0/G1 phase and by regulating the MEK/ERK signaling pathway. MEK inhibitor U0126 was used to confirm the effects of BPIFB1 on the MEK/ERK pathway, and U0126 can inverse the effects of siBPIFB1. Additionally, BPIFB1 can enhance the anti-proliferative effect of chemotherapy drugs on NPC-KT cells. All the results indicated that BPIFB1 could be a potential target for the treatment of NPC.
Keywords: Nasopharyngeal carcinoma, BPIFB1, MEK/ERK pathway, cell cycle, apoptosis, proliferation
Introduction
Nasopharyngeal carcinoma (NPC), a malignant epithelial carcinoma, occurs in the head and neck region with a remarkable racial and geographical incidence [1]. The disease is rare globally but occurs more commonly in Southeast Asia and Southern China and with a gender imbalance (the male-to-female ratio is about 3:1), which indicates that genetic, ethnic, and environmental factors play an important role in the disease [2,3]. The primary treatment of NPC is radiotherapy (RT), as it is relatively sensitive to ionizing radiation for patients in the disease’s early stages. For late stage patients, chemoradiotherapy (CCRT) is superior to RT alone and leads to a better prognosis [4,5]. Although radiotherapy or chemoradiotherapy can control local NPC, a large number of patients in stages III and IV still experience local recurrence or distant metastasis [6]. Therefore, more studies on the mechanisms of NPC progression are essential to develop new targets and novel therapies for treating NPC.
Bactericidal/permeability-increasing (BPI)-fold-containing family B member 1 (BPIFB1), as the so called long-palate lung and nasal epithelium clone 1 (LPLUNC1), is secreted by nasopharyngeal epithelia or minor mucosal glands of the respiratory and upper digestive tracts [7,8]. Many studies have confirmed that BPIFB1 is dysregulated in patients with respiratory system disease. Researchers showed that BPIFB1 is downregulated in tumor tissues, negatively correlates with the severity of NPC [9], and, more importantly, relates with prognosis in NPC patients [10]. The previous studies indicated that BPIFB1 plays an important role in NPC progression [11] and could be a potential target for NPC treatment, but the details of its mechanisms remain unclear [12]. This research aimed to investigate the effects of BPIFB1on NPC cells and its related mechanisms.
Materials and methods
Cell culture
NPC-KT is an EBV-positive epithelial cell line derived from EBV-negative nasopharyngeal epithelial Ad-AH cells and EBV-positive NPC tissue, and our supply was kindly provided by Wuhan University. The NPC-KT cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) containing 10% fetal bovine serum (FBS), supplemented with penicillin and streptomycin, at 37°C with 5% CO2 [13].
Plasmid transfection
The BPIFB1 overexpressed mimics, BPIFB1 silencing siRNA (si-BPIFB1) and the separated negative control were purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China) and transfected into NPC-KT cells using the Lipo2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The sequences were cited as described [14].
Assay of cell proliferation
Cell proliferation was determined by MTT assay. The cells were seeded in a 96-well culture plate. Then 10 μL of MTT solution was added to a 100 μL medium and incubated at 37°C for 4 h. Then we added 200 µL DMSO to each well. Next, we incubated the plate at 37°C for 10 min and read the absorbance at 490 nm using an ELISA plate reader (Beckman, Miami, FL).
Cell cycle analysis
The cell cycle analysis was measured by flow cytometry. Briefly, the cells were fixed in cold, 70% alcohol at 4°C overnight, treated with 0.25% Triton X-100 for 5 min, stained in a propidium iodide solution at a final concentration of 50 μg/mL (Sigma-Aldrich, St. Louis, Mo. USA) for 1 h at 37°C. The cell cycle analysis was performed by a FACS can flow cytometer (BD Biosciences, Bedford, MA).
Hoechst stains
The blue fluorescent Hoechst dye (Sigma-Aldrich Chemical Co., St Louis, USA) creates cell permeable nucleic acid stains and is sensitive to DNA conformation and the chromatin state and thus can be used to detect nuclear damage. NPC-KT cells were washed or resuspended in a PBS solution (pH 7.4) and then stained in the Hoechst staining solution (1 μg/mL) for 20 min. Then the cells were examined under an inverted fluorescence microscope.
RNA isolation and qRT-PCR
Total RNA was isolated from NPC-KT cells using the TRIzol reagent (Invitrogen, Breda, Netherlands) and transcribed into cDNA using a First Strand cDNA Synthesis kit (Promega, San Luis Obispo, CA, USA) according to the manufacturer’s instructions. Real-time quantitative PCR (RT-qPCR) was performed using specific primers and a SYBR green PCR kit (GenePharma, Shanghai, China) and GAPDH was used as an internal control. The sequences of the primers are described elsewhere [15].
Protein extraction and Western blotting
The pretreated NPC-KT cells were lysed in a cold lysis buffer (RIPA solution with phosphatase and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, Mo. USA) and centrifuged at 12,000 g for 10 min. Protein concentrations were measured using the Bradford method (Pierce® BCA Protein Assay Kit, Thermo Scientific, Rockford, USA) and degenerated at 95°C. Equal amounts of proteins were separated on Invitrogen™NuPAGE™Bis-Tris gels (10%) and transferred onto PVDF membranes (Millipore, MA). After blocking, primary and secondary antibody incubation (primary antibody: BIPFB1, caspase-3,Bcl-2, Bax, CyclinD1, Cyclin B1, MEK, p-MEK, ERK, p-ERK, GADPH (Cell Signaling Technology, Inc. Beverly, MA) at 1:1,000 dilution; secondary antibody at 1:10000 dilution), the proteins were visualized using an ECL system according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using one-way ANOVA with Tukey’s test using Prism 6 (GraphPad Software, Inc). Data are presented as the mean ± standard deviation (SD) and P<0.05 was considered statistically significant.
Results
BPIFB1 promoted the apoptosis of NPC-KT cells
To investigate the effects of BPIFB1 on the apoptosis of NPC-KT cells, BPIFB1 was overexpressed or silenced by the BPIFB1 plasmid or siRNA. As shown in Figure 1, nuclear damage, cell apoptosis, and the apoptosis protein level (Bax, Bcl, Caspase 3) were examined. The transfection efficiency was confirmed by Western blotting and RT-PCR analysis. The BPIFB1 mRNA (Figure 1A) and the protein level (Figure 1B) in the BPIFB1 group were remarkably increased and in the siBPIFB1 group they were decreased compared with the negative control. Hoechst staining results (Figure 1C) indicated that the cell proportion with DNA damage (arrows in Figure 1C) was increased when BPIFB1 was overexpressed and decreased when BPIFB1 was silenced. Furthermore, the apoptosis results (Figure 1D) measured by flow cytometry were in accordance with the Hoechst staining results. The amount of apoptotic cells was increased when BPIFB1 was overexpressed and decreased when BPIFB1 was silenced. Apoptotic protein expressions were measured by Western blotting. As shown in Figure 1E, BPIFB1 overexpression increased the expression of the pro-apoptosis protein Bax and increased caspase 3, anti-apoptosis Bcl-2, and in siBPIFB1 the opposite was the case. All the results demonstrated that BPIFB1 promoted the apoptosis of NPC-KT cells.
Figure 1.
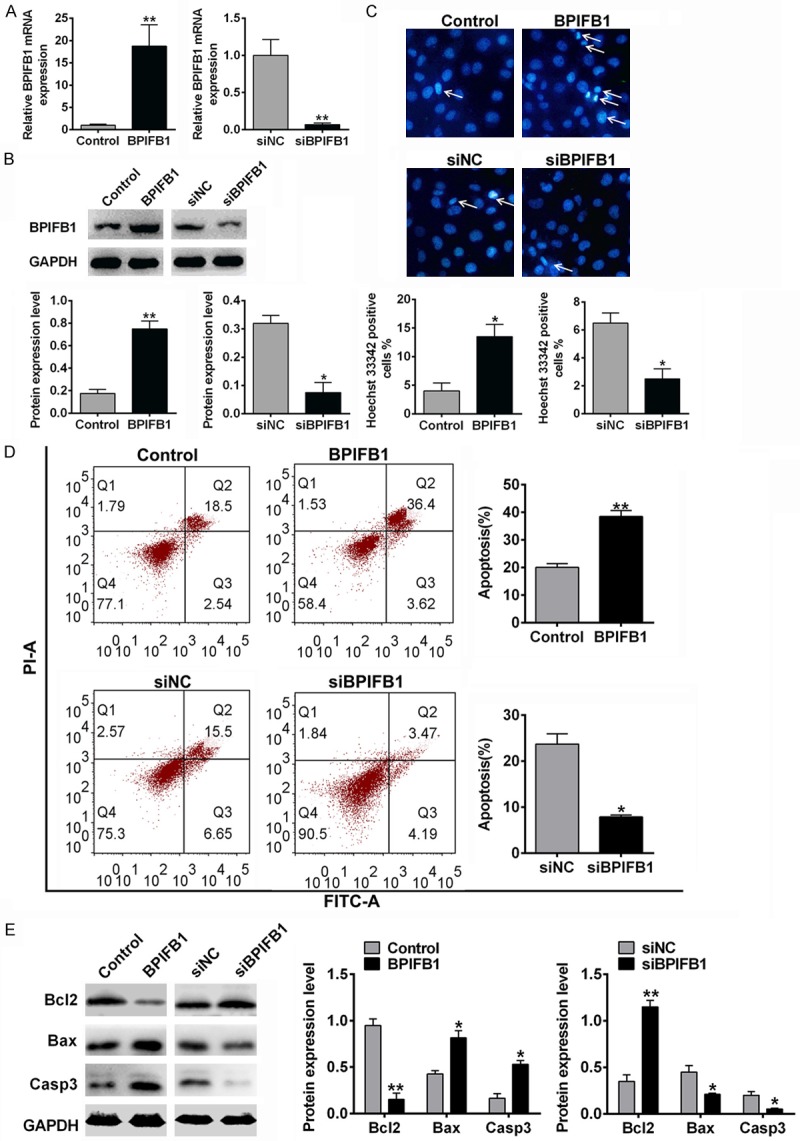
The effects of BPIFB1 on the cell apoptosis of NPC-KT cells. NPC-KT cells were transfected with BPIFB1 mimics or siRNA and the negative control (A, B) BPIFB1 expression of mimics or siRNA transfected NPC-KT cells was measured by PCR (A) and Western blotting (B). (C) DNA damage was measured by Hoechst staining. (D) The cell apoptosis was analyzed by flow cytometry. (E) The apoptosis related protein expression was assessed by Western blotting. Data were presented as the mean ± SD. n=3, *P<0.05, **P<0.01 vs. Control.
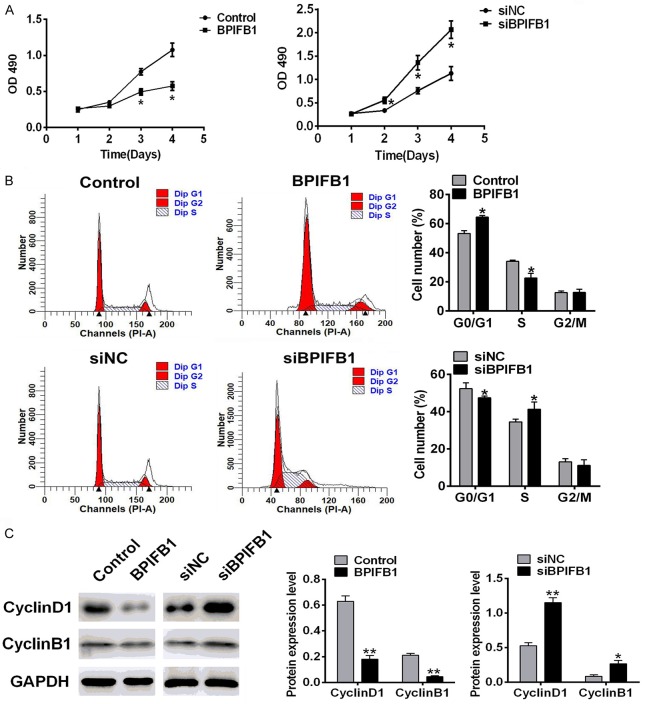
BPIFB1 inhibited the proliferation of NPC-KT cells
To evaluate the effects of BPIFB1 on the proliferation of NPC-KT cells, the cell viability, cell cycle, and cyclin were investigated. As shown in Figure 2A, the MTT results demonstrated that BPIFB1 overexpression (BPIFB1 group) could inhibit the proliferation of NPC-KT cells, and BPIFB1 silencing (siBPIFB1) promoted proliferation. To explore the mechanism by which BPIFB1 inhibited cell proliferation, we evaluated the cell cycle by flow cytometry. The amount of the cells in the G0/G1, S, and G2/M phases is illustrated in Figure 2B. The fraction in theG0/G1 phase was higher in the BPIFB1 overexpression group, and the fraction in the S phase was lower. The result of the BIPFB1 group was in the opposite. Furthermore, cell cycle proteins (cyclin D1 and cyclin B1) were measured by Western blotting. As shown in Figure 2C, the level of cyclins was decreased when BIPFB1 was overexpressed and increased when BIPFB1 was silenced. All the results demonstrated that BPIFB1 inhibits the cell cycle of NPC-KT cells.
Figure 2.
The effect of BPIFB1 on the cell cycle of NPC-KT cells. NPC-KT cells were transfected with BPIFB1 mimics or siRNA and the negative control. A. The proliferation was assessed using an MTT assay. B. The cell cycle distribution was analyzed by flow cytometry. C. The protein levels of the cyclins were analyzed by Western blotting. Data are the means ± SD, n=3, *P<0.05, **P<0.01 vs. Control.
BPIFB1 inhibited the activation of the MEK/ERK signaling pathway
The MEK/ERK signaling pathway plays an important role in NPC cell viability, proliferation, and chemoresistance. To investigate the effects of BPIFB1 on the MEK/ERK pathway, the expression of phosphorylated MEK (p-MEK), ERK (p-EKR), and total MEK, ERK were analyzed by Western blotting. To demonstrate the activation of ERK and MEK, p-ERK/ERK and p-MEK/MEK were used. As shown in Figure 3, p-MEK/MEK and p-ERK/ERK were decreased in the BPIFB1 overexpressed group, and increased in BPIFB1 silenced group, which indicated that BPIFB1 could inhibit the activation of the MEK/ERK signaling pathway.
Figure 3.
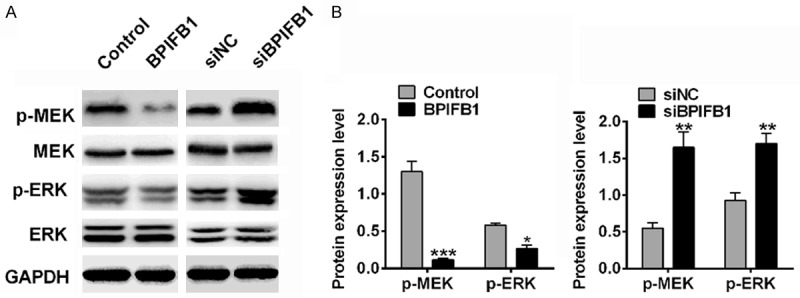
BPIFB1 inhibited the phosphorylation of ERK and MEK. A. The expression of ERK, p-ERK, MEK and p-MEK protein. B. Gray analysis of protein expression. Data are means ± SD, n=3, *P<0.05, **P<0.01 vs. Control.
BPIFB1 reduced NPC-KT cell viability via the MEK/ERK signaling pathway
To confirm that BPIFB1 inhibited cell viability through the MEK/ERK signaling pathway, siBPIFB1 was transfected into NPC-KT cells, and the MEK inhibitor U0126 was added to the medium in another group. As in the previous results, in the siBPIFB1 cells, NPC-KT cell viability increased (Figure 4A), DNA damage decreased (Figure 4B), the proportion of apoptotic cells decreased (Figure 4C), mitosis was enhanced (Figure 4D), and pERK/ERK and pMEK/MEK increased (Figure 4E). All the effects were inversed by the MEK inhibitor U0126. These results suggest that BPIFB1 reduced NPC-KT cell viability and proliferation via the MEK/ERK signaling pathway.
Figure 4.
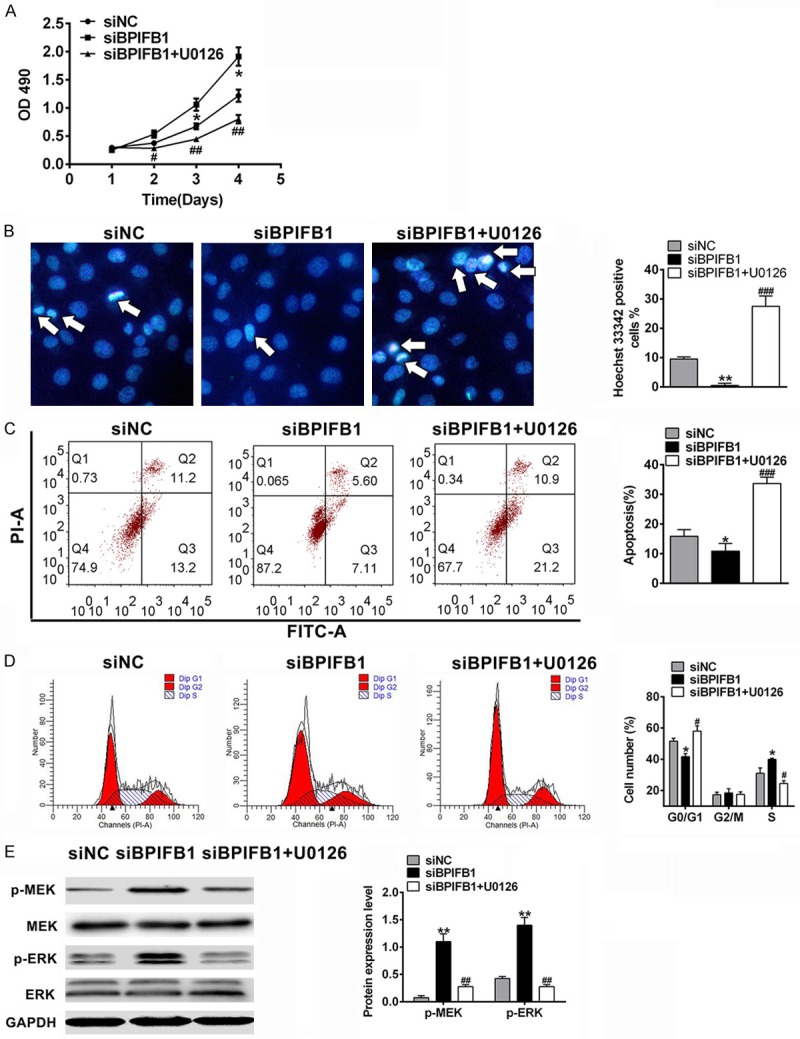
BPIFB1 regulated cell viability of NPC-KT cells via the MEK/ERK signaling pathway. NPC-KT cells were transfected with siRNA and/or treated with the MEK inhibitor U0126. A. The proliferation was assessed by MTT assay. B. DNA damage was measured by Hoechst staining. C. The cell apoptosis was analyzed by flow cytometry. D. The cell cycle distribution was analyzed by flow cytometry. E. The protein levels of MEK and p-ERK were analyzed by Western blotting. Data are the means ± SD, n=3, *P<0.05, **P<0.01 vs. Control, #P<0.05, ##P<0.01 vs. Model.
BPIFB1 enhanced the inhibition of the chemotherapeutic agent on the viability of NPC-KT cells
To investigate the role of BPIFB1 in chemoresistance, NPC-KT cells were transfected with a BPIFB1 plasmid and incubated with cisplatin (Cis, final concentration 2 μM) or Paclitaxel (Pct, final concentration 50 nM), and the proliferation was assessed using an MTT assay. As shown in Figure 5, Cis or Pct treated alone reduced NPC-KT cell viability, and BPIFB1 enhanced the inhibition effects on cancer cells, which partly indicated that BPBIFA1 could enhance the chemo-sensitivity of NPC-KT cells.
Figure 5.
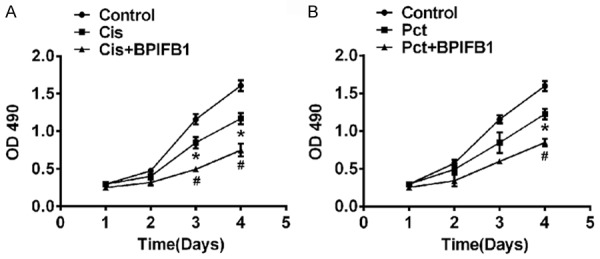
BPIFB1 enhanced the chemo-sensitivity of NPC-KT cells. NPC-KT cells were transfected with BPIFB1 plasmids and treated with cisplatin (Cis) (A) or Paclitaxel (Pct) (B), and the proliferation was measured using an MTT assay. Data are the means ± SD, n=3, *P<0.05, vs. Control, #P<0.05 vs. Pct or Cis group.
Discussion
BPIFB1 or LPLUNC1, secreted by nasopharyngeal epithelia or the minor mucosal glands of the respiratory and upper digestive tracts [8], has been proved to regulate host immune responses in respiratory diseases [16] and is upregulated in COPD and relevant to its severity [17], but it is downregulated in NPC tissues [18]. The effects and mechanism of BPIFB1 in NPC are reported as limited, and whether BPIFB1 downregulation is associated with the progression and prognosis of NPC has not been clarified. In this research, the effects and the molecular mechanisms of BPIFB1 on the proliferation, apoptosis, cell cycle, and chemoresistance of NPC cells were investigated. Our results indicated that the overexpression of BPIFB1 inhibited cell growth, promoted apoptosis through cell cycle arrest at the G0/G1 phase, and regulated the MEK/ERK signaling pathway. Additionally, BPIFB1 could enhance the inhibitory effects of chemotherapeutic agents on cancer cells, which suggests that BPIFB1 could be a potential target for the treatment of NPC.
Although the studies on BPIFB1 are limited, there are still studies on the effects and mechanisms of BPIFB1 on NPC. Wei et al. first demonstrated that BPIFB1 markedly inhibited NPC cell migration, invasion, and metastatic abilities via the inhibition of VTN (a BPIFB1-interacting protein), leading to the inactivation of the FAK/Src/ERK signaling pathway [19]. BPIFB1 also inhibited NPC cell growth by downregulating MAPK and the cyclin D1/E2F pathways [12], and the inflammation related signaling pathway (signal transducer and activator of transcription 3 (STAT3)) activity [20]. The previous studies were in accordance with our findings that BPIFB1 could regulate the cell cycle and the MAPK/EKR signaling pathway. The MAPK pathway is shared by four downstream cascades, including the Jun amino-terminal kinases (JNK1/2/3), the extracellular signal-related kinases (ERK1/2), p38 and ERK5, among which ERK is reported to be related to cell proliferation, migration, and apoptosis and plays an important role in tumor progression and cancer treatment [21]. In our research, BPIFB1 could inhibit NPC cell proliferation and promote cell apoptosis through the inactivation of the MEK/ERK signaling pathway.
Chemoresistance is a crucial challenges in the treatment of cancer and is related to poor prognosis in cancer patients [22]. NPC is relatively sensitive to radiotherapy, but for late stage patients, chemo- or radio resistance were still a challenge and affects the prognosis of NPC patients [4]. We et al. first demonstrated that BPIFB1 enhanced the sensitivity of NPC cells to ionizing radiation by inhibiting VTN (a BPIFB1-binding protein) expression [23]. Our results confirmed the finding that BPIFB1 overexpression could increase the chemosensitivity of NPC-KT cells. More interestingly, researchers found that BPIFB1 plays an important role in inducing innate immune responses. BPIFB1, along with PA-MSHA (Pseudomonas aeruginosa mannose sensitive hemagglutination) can activate CD14/TLR4/MyD88 and induce the upregulation of pro-inflammatory cytokines [24]. This research indicated that BPIFB1 may also regulate the inflammatory response in the tumor microenvironment and induce the apoptosis of cancer cells.
Conclusion
Our research demonstrated that BPIFB1 can affect cell proliferation and the cell cycle, promote the apoptosis of NPC cells by regulating the MEK/ERK signaling pathway, and enhance the inhibition effects of chemotherapeutic agents on cancer cells. The results indicated that BPIFB1 could be a potential target for the treatment of NPC.
Disclosure of conflict of interest
None.
References
- 1.Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet. 2005;354:2041–2054. doi: 10.1016/S0140-6736(05)66698-6. [DOI] [PubMed] [Google Scholar]
- 2.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 3.Lenz O, Fornoni A, Ijaz A, Tejada T. Role of inflammation in diabetic nephropathy. Current Diabetes Reviews. 2008;4:10–17. doi: 10.2174/157339908783502361. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S, Gupta SC, Tyagi AK, Aggarwal BB. Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 2014;32:1053–1064. doi: 10.1016/j.biotechadv.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Turgut F, Bolton WK. Potential new therapeutic agents for diabetic kidney disease. Am J Kidney Dis. 2010;55:928–940. doi: 10.1053/j.ajkd.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Lee FTH, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, Forbes JM. Interactions between angiotensin II and NF-κB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–2151. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- 7.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 8.Chiu J, Khan ZA, Farhangkhoee H, Chakrabarti S. Curcumin prevents diabetes-associated abnormalities in the kidneys by inhibiting p300 and nuclear factor-κB. Nutrition. 2009;25:964–972. doi: 10.1016/j.nut.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 9.He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. 2012;3:94–104. doi: 10.4239/wjd.v3.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brouwers O, Niessen PM, Miyata T, Østergaard JA, Flyvbjerg A, Peutz-Kootstra CJ, Sieber J, Mundel PH, Brownlee M, Janssen BJ, De Mey JG, Stehouwer CD, Schalkwijk CG. Glyoxalase-1 overexpression reduces endothelial dysfunction and attenuates early renal impairment in a rat model of diabetes. Diabetologia. 2014;57:224–235. doi: 10.1007/s00125-013-3088-5. [DOI] [PubMed] [Google Scholar]
- 11.Varga N, Mozes J, Keegan H, White C, Kelly L, Pilkington L, Benczik M, Zsuzsanna S, Sobel G, Koiss R, Babarczi E, Nyiri M, Kovacs L, Attila S, Kaltenecker B, Geresi A, Kocsis A, O’Leary J, Martin CM, Jeney C. The value of a novel panel of cervical cancer biomarkers for triage of HPV positive patients and for detecting disease progression. Pathol Oncol Res. 2017;23:295–305. doi: 10.1007/s12253-016-0094-1. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Liao Q, Wei F, Li X, Zhang W, Fan S, Shi L, Gong Z, Ma J, Zhou M, Xiang J, Peng S, Xiang B, Deng H, Li Y, Xiong W, Zeng Z, Li G. LPLUNC1 inhibits nasopharyngeal carcinoma cell growth via down-regulation of the MAP kinase and cyclin D1/E2F pathways. PLoS One. 2013;8:e62869. doi: 10.1371/journal.pone.0062869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Shon E, Kim CS, Kim JS. Renal podocyte injury in a rat model of type 2 diabetes is prevented by metformin. Exp Diabetes Res. 2012;2012:210821. doi: 10.1155/2012/210821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Q, Zeng Z, Guo X, Li X, Wei F, Zhang W, Li X, Chen P, Liang F, Xiang B, Ma J, Wu M, Tang H, Deng M, Zeng X, Tang K, Xiong W, Li G. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the Stat3 activation. Oncogene. 2014;33:2098–2109. doi: 10.1038/onc.2013.161. [DOI] [PubMed] [Google Scholar]
- 15.Jensen TJ, Wozniak RJ, Eblin KE, Wnek SM, Gandolfi AJ, Futscher BW. Epigenetic mediated transcriptional activation of WNT5A participates in arsenical-associated malignant transformation. Toxicol Appl Pharmacol. 2009;235:39–46. doi: 10.1016/j.taap.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/β-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingg JM, Hasan ST, Meydani M. Molecular mechanisms of hypolipidemic effects of curcumin. Biofactors. 2013;39:101–121. doi: 10.1002/biof.1072. [DOI] [PubMed] [Google Scholar]
- 18.Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/β-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17:2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- 19.Wei F, Wu Y, Tang L, He Y, Shi L, Xiong F, Gong Z, Guo C, Li X, Liao Q, Zhang W, Zhou M, Xiang B, Li Y, Li G, Xiong W, Zeng Z. BPIFB1 (LPLUNC1) inhibits migration and invasion of nasopharyngeal carcinoma by interacting with VTN and VIM. Br J Cancer. 2018;118:233–247. doi: 10.1038/bjc.2017.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Q, Zeng Z, Guo X, Li X, Wei F, Zhang W, Chen P, Liang F, Xiang B, Ma J, Wu M, Tang H, Deng M, Zeng X, Tang K, Xiong W, Li G. LPLUNC1 suppresses IL-6-induced nasopharyngeal carcinoma cell proliferation via inhibiting the stat3 activation. Oncogene. 2014;33:2098–2109. doi: 10.1038/onc.2013.161. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600–604. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 22.Taylor S, Spugnini EP, Assaraf YG, Azzarito T, Rauch C, Fais S. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist Updat. 2015;23:69–78. doi: 10.1016/j.drup.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Wei F, Tang L, He Y, Wu Y, Shi L, Xiong F, Gong Z, Guo C, Li X, Liao Q, Zhang W, Ni Q, Luo J, Li Y, Peng C, Chen X, Li G, Xiong W, Zeng Z. BPIFB1 (LPLUNC1) inhibits radioresistance in nasopharyngeal carcinoma by inhibiting VTN expression. Cell Death Dis. 2018;9:432. doi: 10.1038/s41419-018-0409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou W, Duan Z, Yang B, Xiao C. The effective regulation of pro- and anti-inflammatory cytokines induced by combination of PA-MSHA and BPIFB1 in initiation of innate immune responses. Open Med (Wars) 2017;12:299–307. doi: 10.1515/med-2017-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]