Abstract
The aim of the present study was to analyze the effects of Atractylodesin III (codonopsis pilosula) extract that maintains mitochondrial function, up-regulates Bcl-2, inhibits Caspase-3 activity, and ultimately leads to cardiomyocyte apoptosis in a rat model of acute myocardial infarction (AMI). 30 male Sprague-Dawley rats aged 6 months, weighed 150-200 g were randomly divided into sham operation group (SOG), model group (MG) and intervention group (IG). The IG was intragastrically administered with atractylodesin III (30 mg/kg/d) for 7 days. The model group was treated with (30 mg/kg/d) of sterile saline. After 4.5 h, the heart samples from each group were taken, the myocardial infarct size was detected by 2, 3, 5-triphenyltetrazolium chloride (TTC) staining.After ematoxylin & Eosin (HE) staining apoptosis indices were determined by Terminal deoxynucleotidyl transferase TdT-mediated dUTP Nick-End Labeling (TUNEL) method. Apoptosis-related genes and protein including Bcl-2, Bax, and Caspase-3 expression were determined by quantitative real time polymerase chain reaction (qRT-PCR) and western blot respectively. The infarct size and apoptotic index of the MG were significantly higher than SOG. However, infarct size and apoptotic index were reduced in IGcompared to MG (P < 0.05). The levels of Bax and Caspase-3 in the MG were significantly higher, while Bcl-2 and Bcl-2/Bax were lower than those in the SOG. The IG has lower levels of Bax and Caspase-3, higher levels of Bcl-2 and Bcl-2/Bax (P < 0.05) compared to MG. Atractylodesin III decreased apoptosis of myocardial cells in AMI, up-regulated Bcl-2 expression, and inhibited Bax and Caspase-3 activity.
Keywords: Atractylenolide III, AMI, apoptosis, mitochondrial function, caspase-3
Introduction
Acute myocardial infarction (AMI) is a cardiovascular disease with high morbidity, disability, and mortality worldwide. Arrhythmias and heart failure of post-infarction are also important causes of death [1]. Acute blood flow occlusion of the coronary artery leads to myocardial ischemia and hypoxia damage, which in turn induces inflammatory reaction, apoptosis, energy metabolism disorder, and other internal environmental imbalances, eventually leading to the occurrence of AMI. The apoptosis process occurs throughout the whole process of AMI, and can be reversed by early intervention which also savies ischemic myocardium, improving cardiac function and prognosis [2,3].
Atractylodesin III is categorized in TCMs as a tonic herb and used to invigorate the stomach and spleen by removing wetness and treating gastrointestinal disorders. It has also many other pharmacologic effects including anti-inflammation, anti-tumor, and immunoregulatory properties [4-6]. Morever, atractylon (AT), atractylenolides II (AT-II) and III (AT-III) are the main sesquiterpenoids in the A. ovata vital oils (ARO). Previously, Atractylodesin III has also been reported to have strong anti-oxidative effects [7].
Traditional Chinese medicine has the characteristics of a wide range of resources, simple processing, and impartial drug effects with highly economic benefits. However, Chinese herbal medicines are often complex in composition, and the mechanism of action of single components cannot be clarified, which makes their promotion and application limited [8]. Codonopsis pilosula is a popular medicinal herb of perennial species of flowering plant in the bellflower family. Its functions are to strengthening the spleen and benefiting the lungs, nourishing the blood and produced fluid. The main component of codonopsis pilosula includes polysaccharides, alkyne, atractylenolide III, saponins, and flavonoids [9,10]. Atractylenolide III has better anti-inflammatory effects and more stable components [11,12], so it is often used as an important indicator for the quality evaluation of codonopsis pilosula.
Thus the present study focused on analyzing the important effects of atractylodesin III of codonopsis pilosula extract by maintaining mitochondrial function, up-regulating Bcl-2 expression, and inhibiting Bax, and Caspase-3 activity for reversing cardiomyocyte apoptosis in AMI rat models.
Materials and methods
Animals
A total of 30 male Sprague-Dawley (SD) rats, 6-month-old, specific pathogen free (SPF)-grade, weighing 150-200 g were provided by the Zhejiang Experimental Animal Center. All the rats were given the same and suitable environment and adequate food 7 days before the starting of the experimental procedures.
Reagents
Atractylodesin III was purchased from Shanghai First Biochemical Pharmaceutical Co., Ltd., TTC reagent was purchased from Jiangsu Biyuntian Technology Co., Ltd., TUNEL kit was purchased from Sigma (St. Louis, MO, USA), Protein Quantitation Kit (BCA method) was purchased from R&D Corporation (Minneapolis, MN, USA); rabbit anti-mouse Bcl-2, Bax, and Caspase-3 monoclonal antibodies were purchased from Invitrogen Corporation (Carlsbad, California, USA) and PVDF membranes were purchased from Santa Cruz Biotechnology (CA, USA).
Experimental methods
All experimental rats were randomly divided into sham operation group, model group, and intervention group (10 rats each group). The AMI model: SD rats were anesthetized by 10% chloral hydrate. Then we cut the left front chest skin, opened 3~4 ribs, and exposed the heart. A needle was used to pass through the lower edge of the left atrial appendage and the pulmonary artery and ligated at the beginning of the left anterior descending coronary artery. After that, the left ventricular wall became pale, the wall motion was weakened, and the ST segment of the electrocardiogram (ECG) showed significant elevation, which indicated the success of model making of AMI. After 20 minutes of ischemia, the thoracic gas was squeezed out, and the chest wall was sutured layer by layer. After the animal resumed spontaneous breathing, the cannula was pulled out and the ventilator was stopped. The sham operation group only performed the thoracotomy and did not ligate their coronary artery. The intervention group was intragastrically administeredatractylenolide III (30 mg/kg/d) for 7 days before operation. The model group was given the same amount of sterile physiological saline. Cardiac specimens were taken 4.5 h after operation, and myocardial infarct size was quantitatively detected by triphenyltetrazolium chloride (TTC) staining. The principle of TTC staining is that TTC react with succinate dehydrogenase in cells to form red insoluble matter. TTC enter the normal cells and bind to dehydrogenase in red, while dead cells will not be stained by TTC due to the lack of dehydrogenase. Then cardiac specimens were cut into 4 μm-thick coronal sections for Hematoxylin & Eosin (HE) staining.
TUNEL method
Apoptosis index was determined by TUNEL method. Briefly, Paraffin sections of about thickness 4 μm were made and the procedures were strictly applied in accordance with the kit requirements. Each paraffin block was acquired 6 sections, randomly selected 6 fields of view. Negative cell nuclei were stained blue, while positive nuclei were brown. Images were analyzed using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA). To show the expression of apoptotic index the following formula was used: number of apoptotic cells/total number of observed cells × 100%.
RNA extraction and qRT-PCR
Total RNA was isolated from myocardial tissue using Trizol reagent, and the RNA was reverse-transcribed into cDNA with a Toyobo First Strand cDNA Synthesis Kit. The relative mRNA expression levels were detected by using a Biomiga SYBR qPCR mix kit and ABI 7500 real-time reverse transcription PCR (qRT-PCR). Data were presented as 2-ΔΔct. The primer sequences of Bax, Bcl-2, and Caspase-3 that were used for qRT-PCR were presented in Table 1.
Table 1.
Primer sequences for qRT-PCR used in the present study
| Genes | Primers 5’-3’ (forward, reverse) |
|---|---|
| Bax | Forward: 5’-CGGCGAATTGGAGATGAACTGG-3’ |
| Reverse: 5’-CTAGCAAAGTAGAAGAGGGCAACC-3’ | |
| Bcl-2 | Forward: 5’-TGTGGATGACTGACTACCTGAACC-3’ |
| Reverse: 5’-CAGCCAGGAGAAATCAAACAGAGG-3’ | |
| Caspase-3 | Forward: 5’-GTGGAACTGACGATGATATGGC-3’ |
| Reverse: 5’-CGCAAAGTGACTGGATGAACC-3’ | |
| β-actin | Forward: 5’-AAGATCCTGACCGAGCGTGG-3’ |
| Reverse: 5’-CAGCACTGTGTTGGCATAGAGG-3’ |
Western blot analysis
The expression levels of apoptotic proteins including Bcl-2, Bax, and Caspase-3 were determined by western blot (WB). The myocardial tissue was ground in liquid nitrogen, and RIPA cell lysate was added. After homogenization, the supernatant was obtained and the protein concentration was determined by BCA method and stored at -80°C. Each group of samples was dose-normalized to 50 μg, and transferred to PVDF membrane by SDS-PAGE gel electrophoresis. Then the PVDF membrane was soaked in skim milk powder solution and sealed at room temperature for 4 h. The primary antibody with a dilution of (1:1000) was added overnight at 4°C. TBST was used to wash the membrane 3 times 10 min each. After that we added the secondary antibody with a dilution of (1:500) for 1.5 h at room temperature and again washed with TBST 3 times. β-actin is used as an internal reference, and the ratio result indicates the relative expression level of each target protein. The protein bands for Bcl-2, Bax, and Caspase-3 were detected with a chemiluminescence western blot detection system.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software. The data were expressed as mean ± standard deviation. The comparison between groups was performed by single factor ANOVA analysis. The comparison between the two groups was performed by LSD-t test. P < 0.05 was considered significant.
Results
Survival of each group
All the sham-operated groups survived. There were two deaths in the model group and one death in the intervention group. Eight ventricular specimens from each group were taken for the next experiment.
Comparison of infarct size and apoptotic index in each group
The infarcted area of each group was shown in Figure 1 and Table 2. The apoptosis index of cells in each group was observed by TUNEL staining (Figure 2). The infarction area and the apoptosis index of MG group was obviously higher than that of SOG. Infarct size and apoptosis index in the IG group was lower than that of MG group and showed a significant difference (P < 0.05).
Figure 1.

Myocardial infarction area was shown by TCC staining. Myocardial infarction area is in white, normal myocardium is in red (MG represents model group; IG Intervention group; SOG Sham operation group).
Table 2.
Comparison of infarction size and apoptosis index of each group (%)
| Group | Sham operation group | Model group | Intervention group |
|---|---|---|---|
| Infarct size | 0.08 ± 0.01 | 28.9 ± 4.7a | 11.5 ± 2.6a,b |
| Apoptotic index | 0.04 ± 0.01 | 56.6 ± 12.3a | 34.5 ± 7.6a,b |
compared with the sham group (P < 0.05);
compared with the model group, (P < 0.05).
Figure 2.
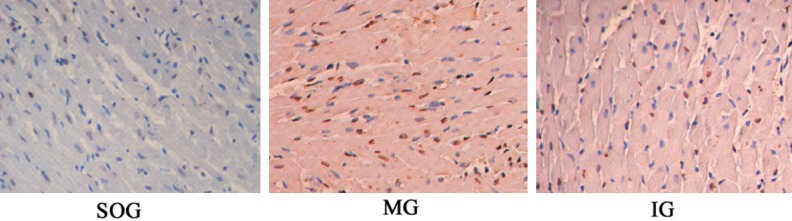
Apoptosis index was determined by TUNEL method in each group (400 ×, MG represents model group; IG Intervention group; SOG Sham operation group).
Myocardial cell morphology was showed in (Figure 3). No abnormalities were found in the myocardial tissue in the SOG group. The myocardial fibers were arranged in parallel, clear and orderly, and the myocardial cells were distributed uniformly with normal morphology and complete cell membrane structure. The myocardial tissue of MG group showed obvious pathological morphologic changes such as the myofiber arrangement was disordered and interstitial edema was denatured. The myocardial cells were swollen and showed vacuolar degeneration and the nuclear stain was uneven. The morphologic changes of myocardial tissue in the IG group were significantly less than in the SOG group.
Figure 3.
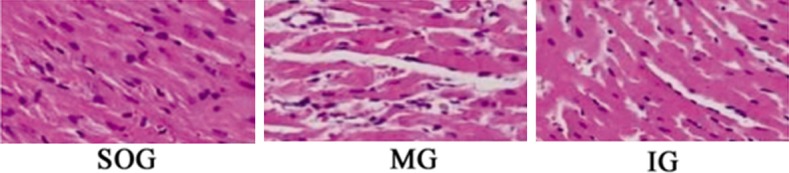
Myocardial cell morphology is shown by HE staining (400 ×, MG represents model group; IG Intervention group; SOG Sham operation group).
Effect of atractylodesin III on apoptosis gene mRNA relative expression levels in each group
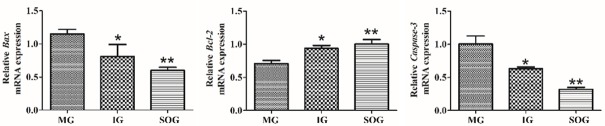
As shown in Figure 4, Bax relative mRNA expression was increased (P < 0.05) and highly significantly increased (P < 0.01) in the model group and intervention group compared with the sham group whereas Bcl-2 expression were significantly decreased (P < 0.05) in the model group and intervention group and highly significantly increased in sham group. Caspase-3 expression was higher in the model group than in the intervention and sham group presented in Figure 4).
Figure 4.
Effect of Atractylodesin III on apoptotic gene mRNA relative expression in Sprague-Dawley rats. MG represents model group; IG Intervention group; SOG Sham operation group. *(P < 0.05) shows significant difference **(P < 0.01) shows highly significant difference.
Comparison of apoptotic protein expression levels in each group
As shown in Table 3 and Figure 5, Bax and Caspase-3 levels in the model group were significantly higher than in the sham group whereas, Bcl-2 and Bcl-2/Bax levels were decreased. Bcl-2 and bcl-2/Bax levels were higher in the intervention group than in the model group, and Bax and Caspase-3 levels were decreased significantly (P < 0.05).
Table 3.
Comparison of apoptotic protein expression levels in each group
| Group | Sham operation group | Model group | Intervention group |
|---|---|---|---|
| Bcl-2 | 0.85 ± 0.19 | 0.59 ± 0.13a | 0.72 ± 0.16a,b |
| Bax | 0.22 ± 0.09 | 0.39 ± 0.12a | 0.28 ± 0.11a,b |
| Bcl-2/Bax | 3.83 ± 0.56 | 1.47 ± 0.38a | 2.46 ± 0.64a,b |
| Caspase-3 | 0.19 ± 0.08 | 0.27 ± 0.09a | 0.22 ± 0.07a,b |
comparison of intervention group with the sham group (P < 0.05);
shows its comparison with the model group (P < 0.05).
Figure 5.
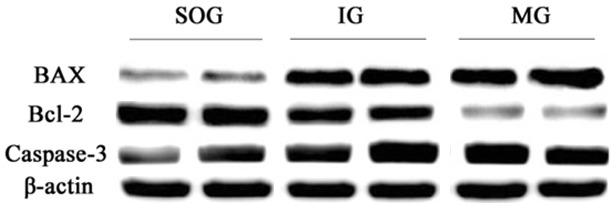
Apoptosis protein expression levels of each group by western blot. (MG represents model group; IG Intervention group; SOG Sham operation group).
Discussion
China is the main producer of codonopsis, especially in Gansu Province. Its export volume accounts for 80% of the total national production. The toxicity of codonopsis pilosula is small, and its tonic effects are moderate. In clinical practice, codonopsis pilosula is an essential herbal medicine for invigorating spleen-stomach and replenishing qi, alleviating gastrointestinal dysfunction, enhancing the immune function of body, increasing the content of erythrocyte and hemoglobin. At the same time, codonopsis pilosula has sedative, hypnotic and anticonvulsant effects applying on the central nervous system [13,14]. Therefore, in-depth exploration of the pharmacodynamic importance of codonopsis pilosula, especially the mechanism of action of a single component, is of great significance for the promotion of application of traditional Chinese medicine.
Our study shows that the infarct size and apoptotic index of the model group were significantly higher and triggered and activated the inflammatory signal transduction pathway, leading to a cascade of inflammatory reaction, which in turn induced activation of multiple cell-death pathways that allowed progressive apoptosis of cardiomyocytes [15-17]. Myocardial infarction is the most common cause of cardiac injury and results in acute loss of a large number of myocardial cells. Increases myocardial tissue damage ultimately leads to ventricular remodeling [18,19].
In the current study, expression of the caspase-3 gene as well as bax, and bcl-2, gene in the myocardial tissue of rats were altered significantly when compared among all the groups. However, levels of caspase-3 and bax mRNA did not decrease. This also may be considered helpful to protecting cardiac function in rats. It is known that cardiac myocyte apoptosis is related to increased DNA damage [20]. In such a context, our results shows unchanged expression of these 3 genes may presumably reflect no significant DNA damage in myocardial tissue of rats and shows that Atractylodesin III decreased the degree of apoptosis of myocardial cells. These results are same as the previous observations in hearts of GHRKO mice which revealed mechanisms possibly responding to the process of apoptosis and, thus, apparently defending the heart from aging [21,22].
Mitochondria are the major regulators involved in apoptosis [23,24]. Apoptosis is mainly mediated by both endogenous and exogenous pathways. The mitochondrial pathway is composed of Bcl-2 and Bax family members. B-cell lymphoma 2 (Bcl-2) and Bax bind to the outer membrane or cytoplasm of the mitochondria which are activated by intracellular death signals. This induces Bax oligomerization and it is inserted into the mitochondrial membrane, causing changes in mitochondrial permeability, loss of transmembrane potential, and release of proteins such as cytochrome C [25,26]. It binds to the apoptosis-promoting factor Apaf-1 and oligomerizes, recruits and activates the Caspase family. Caspase-3 is the last performer of apoptosis, initiates the Caspase cascade reaction, and cleaves specific substrates to cause apoptosis [27,28]. It is generally believed that the Bcl-2/Bax ratio determines the direction of apoptosis. Bcl-2 plays an anti-apoptotic role but Bax promotes apoptosis [29,30]. Similarly our study shows that the levels of Bax and Caspase-3 in the model group were significantly higher than those in the sham operation group; Bcl-2 and Bcl-2/Bax were lower. However, the expression of Bcl-2 and Bcl-2/Bax were higher in the intervention group than in the model group, while Bax and Caspase-3 levels were lower.
In summary, Atractylenolide III reduced the degree of apoptosis of myocardial cells in AMI, up-regulated Bcl-2, and inhibited Caspase-3 activity. The chemical composition of traditional Chinese medicine is complex and diverse, and the therapeutic effect is the result of multiple components and multiple targets. Although the effect of codonopsis pilosula on AMI or other types of coronary heart disease is closely related to Atractylodes III we do not know their synergy with other multiple components. So in clinical practice, it is often not a single flavor of codonopsis used in cardiovascular diseases, but also necessary to synergize the effect with other kinds of Chinese medicinal ingredients. Further analysis is needed to elucidate how to efficiently extract the content of Atractylenolide III and whether different concentrations have different results on the apoptosis of AMI cells.
Disclosure of conflict of interest
None.
References
- 1.Numao Y, Suzuki S, Kano H, Yajima J, Oikawa Y, Matsuno S, Arita T, Yagi N, Semba H, Kato Y, Otsuka T, Uejima T, Yamashita T. Eleven-year temporal trends of clinical characteristics and long-term outcomes in patients undergoing percutaneous coronary intervention for acute coronary syndrome in the Shinken database. Heart Vessels. 2018 doi: 10.1007/s00380-018-1229-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Li C, Meng H, Guo D, Zhang Q, Lu W, Wang Q, Wang Y, Tu P. BYD ameliorates oxidative stress-induced myocardial apoptosis in heart failure post-acute myocardial infarction via the P38 MAPK-CRYAB signaling pathway. Front Physiol. 2018;9:505. doi: 10.3389/fphys.2018.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou L, Guo J, Xu F, Weng X, Yue W, Ge J. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018;113:28. doi: 10.1007/s00395-018-0685-y. [DOI] [PubMed] [Google Scholar]
- 4.Lee JC, Lee KY, Son YO, Choi KC, Kim J, Kim SH, Chung GH, Jang YS. Stimulating effects on mouse splenocytes of glycoproteins from the herbal medicine atractylodes macrocephala Koidz. Phytomed. 2007;14:390–395. doi: 10.1016/j.phymed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Huang HL, Chen CC, Yeh CY, Huang RL. Reactive oxygen species mediation of baizhu-induced apoptosis in human leukemia cells. J Ethnopharmacol. 2005;97:21–29. doi: 10.1016/j.jep.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 6.Prieto JM, Recio MC, Giner RM, Máñez S, Ginerlarza EM, Ríos JL. Influence of traditional Chinese anti-inflammatory medicinal plants on leukocyte and platelet functions. J Pharm Pharmacol. 2003;55:1275–1282. doi: 10.1211/0022357021620. [DOI] [PubMed] [Google Scholar]
- 7.Wang CC, Lin SY, Cheng HC, Hou WC. Pro-oxidant and cytotoxic activities of atractylenolide I in human promyeloleukemic HL-60 cells. Food Chem Toxicol. 2006;44:1308–1315. doi: 10.1016/j.fct.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Wang ZF, Zhong L, Xiao BJ. Effect of rich selenium banqiao codonopsis pilosula on anti-oxidation of myocardial mitochondria of rats in exhausting exercise. Chinese J Appl Physiol. 2013;29:177. [PubMed] [Google Scholar]
- 9.Xia ZD, Wang ZF, Zhong L, Yang FM. Experimental study on anti-fatigue and anti-hypoxia effect of rich selenium-banqiao-codonopsis pilosula. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2014;30:156–158. [PubMed] [Google Scholar]
- 10.Tan WB, Li FQ. Protective effects and mechanisms of rich selenium Banqiao codonopsis pilosula on myocardial ischemia/reperfusion injury in rats. Article in Chinese. 2015;31:147–149. [PubMed] [Google Scholar]
- 11.Shergis JL, Liu S, Chen X, Zhang AL, Guo X, Lu C, Xue CC. Dang shen [codonopsis pilosula (Franch.) nannf] herbal formulae for chronic obstructive pulmonary disease: a systematic review and meta-analysis. Phytother Res. 2015;29:167–186. doi: 10.1002/ptr.5248. [DOI] [PubMed] [Google Scholar]
- 12.Lin LC, Tsai TH, Kuo CL. Chemical constituents comparison of codonopsis tangshen, codonopsis pilosula var. Modesta and codonopsis pilosula. Nat Prod Res. 2013;27:1812–1815. doi: 10.1080/14786419.2013.778849. [DOI] [PubMed] [Google Scholar]
- 13.Fu YP, Feng B, Zhu ZK, Feng X, Chen SF, Li LX, Yin ZQ, Huang C, Chen XF, Zhang BZ, Jia RY, Song X, Lv C, Yue GZ, Ye G, Liang XX, He CL, Yin LZ, Zou YF. The polysaccharides from codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules. 2018;23:1801. doi: 10.3390/molecules23071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Lv X, Huang W, Yao W, Gao X. Characterization and hypoglycemic effect of a neutral polysaccharide extracted from the residue of codonopsis pilosula. Carbohydr Polym. 2018;197:215–226. doi: 10.1016/j.carbpol.2018.05.067. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Xu Y, Wang J, Zhao W, Ruan H. Baicalin ameliorates isoproterenol-induced acute myocardial infarction through iNOS, inflammation and oxidative stress in rat. Int J Clin Exp Pathol. 2015;8:10139–10147. [PMC free article] [PubMed] [Google Scholar]
- 16.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 17.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, Criollo A, Nemchenko A, Hill JA, Lavandero S. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 20.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, Kittleson MM, Minhas KM, Dan EB, Wei C. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 21.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Bartke A. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40:679–684. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Kopchick JJ, Wang Z, Bartke A. Caloric restriction and growth hormone receptor knockout: effects on expression of genes involved in insulin action in the heart. Exp Gerontol. 2006;41:417. doi: 10.1016/j.exger.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Yang L, Yin J, Su D, Pan Z, Li P, Wang X. MicroRNA-15b deteriorates hypoxia/reoxygenation-induced cardiomyocyte apoptosis by downregulating Bcl-2 and MAPK3. J Investig Med. 2018;66:39–45. doi: 10.1136/jim-2017-000485. [DOI] [PubMed] [Google Scholar]
- 24.Yang Q, Cao Y. Study on mechanisms and myocardial protective effect of Qishen Yiqi dropping pills on rats with myocardial infarction. Article in Chinese. 2017;29:501–505. doi: 10.3760/cma.j.issn.2095-4352.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Wang Z, Feng SJ, Xu L, Shi HX, Chen LL, Yuan GD, Yan W, Zhuang W, Zhang YQ, Zhang ZM, Dong HY. PEDF improves cardiac function in rats with acute myocardial infarction via inhibiting vascular permeability and cardiomyocyte apoptosis. Int J Mol Sci. 2015;16:5618–5634. doi: 10.3390/ijms16035618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng L, Hong T, Lin J, Ding S, Huang Z, Chen J, Jia J, Zou Y, Wang TC, Yang X, Ge J. Histamine deficiency exacerbates myocardial injury in acute myocardial infarction through impaired macrophage infiltration and increased cardiomyocyte apoptosis. Sci Rep. 2015;5:13131. doi: 10.1038/srep13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen BD, Rampalli S, Burns LE, Brunette S, Dilworth FJ, Megeney LA. Caspase 3/caspase-activated DNase promote cell differentiation by inducing DNA strand breaks. Proc Natl Acad Sci U S A. 2010;107:4230–4235. doi: 10.1073/pnas.0913089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu W, Liu Q, Zhu S. Carvacrol protects against acute myocardial infarction of rats via anti-oxidative and anti-apoptotic pathways. Biol Pharm Bull. 2013;36:579–584. doi: 10.1248/bpb.b12-00948. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Yu J, Fordjour PA, Xing X, Gao H, Li Y, Li L, Zhu Y, Gao X, Fan G. Danshen injection prevents heart failure by attenuating post-infarct remodeling. J Ethnopharmacol. 2017;205:22–32. doi: 10.1016/j.jep.2017.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Ma LN, Li LD, Li SC, Hao XM, Zhang JY, He P, Li YK. Allicin improves cardiac function by protecting against apoptosis in rat model of myocardial infarction. Chin J Integr Med. 2017;23:589–597. doi: 10.1007/s11655-016-2523-0. [DOI] [PubMed] [Google Scholar]