Abstract
Cationic polymeric liposomes (CPLs) were successfully prepared using a tumor-targeting EGF protein modification for the systemic delivery of the p53 gene in ovarian cancer. These functional CPL nanoparticles (NPs) can be used as effective gene delivery vectors and are composed of an EGF derivative (EGF-GHDC), cholesterol, and DOPE, which have lower gene transfection efficiencies compared with Lipofectamine 2000. The increased therapeutic efficiency of p53 gene-loaded EGF-CPL was tested in SKOV3 cells and compared with p53 gene-loaded CPL and free p53 gene in solution. In addition, EGF-CPL (effective diameter: 95.01±4.05 nm; polydispersity index: 0.270) significantly enhanced luciferase gene expression in vivo 3 days post-injection of pGL-3/NPs complexes. In conclusion, EGF-targeted CPL has the potential for use as an effective drug delivery system.
Keywords: Cationic liposomes, EGF, tumor targeting, gene delivery
Introduction
Gene therapy involves the therapeutic use of genes to fundamentally treat human diseases such as cancer and inherited genetic disorders. One of the major challenges to the success of cancer gene therapy is the formulation of a highly efficient method of therapeutic gene delivery to a specific target site. Compared with viral vectors [1-4], the superiority of nonviral vectors is reflected in their safety and pharmaceutical features, such as low immunogenicity, low toxicity and large-scale construction; however, they tend to show poor active tumor targeting and lower transfection efficiencies in vivo [5,6]. Cationic polymers and liposomes have emerged as important nonviral gene delivery tools and have been used to transfect various cell types and deliver cancer vaccines [7-9]. Extensive efforts have been made to modify gene carriers using target-specific receptors, including epidermal growth factor receptor (EGFR) [10,11], human epidermal growth factor receptor-2 (HER2) [12], and folate [13], to achieve highly tumor-targeted gene delivery systems. Epidermal growth factor (EGF) is one ligand involved in the ErbB pathway that is being investigated for use in ovarian cancer targeted therapy [14].
The present study used the EGF ligand-mediated cationic polymeric liposome (CPL) [15,16] gene delivery vectors for the therapeutic delivery of the p53 gene to ovarian carcinoma. Previous studies have confirmed that functional CPLs, formed from octadecyl quaternized carboxymethyl chitosan (OQCMC) and cholesterol (Chol), have a stable lipid bilayer structure and good drug delivery characteristics [17]. In the current study, EGF was coupled to CPL vectors to form EGF-targeted gene and drug vectors, which were used to deliver the p53 gene for the treatment of ovarian cancer. The physiochemical characteristics of the p53 DNA-loaded EGF-CPL complexes were analyzed by UV and included particle size, zeta potential measurements, TEM, and AFM. Moreover, we discuss the therapeutic effects and gene transfection efficiency of p53-loaded EGF-CPLs.
Materials and methods
Materials
Anti-EGF antibody (ab9695) was purchased from Abcam (Cambridge, UK). Glycidyl hexadecyl dimethylammonium chloride (GHDC) was a gift from the XF Liang laboratory (State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute). Molecular biology grade reagents, including L-α-phosphatidylethanolamine (DOPE), N,N-dimethyltetradecylamine, pancreatic DNase I (specific activity of 2000 Kunitz units/mg) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MT), were obtained from Sigma (St. Louis, MO, USA). All other chemicals were of reagent grade and used without further purification. A luciferase activity assay kit was obtained from Promega (Madison, USA).
SKOV3 cells were purchased from the American Type Culture Collection (Rockville, MD) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) at 37°C and 5% CO2. The plasmid DNAs were propagated in selective Luria-Bertani (LB) medium, centrifuged, and extracted from the cell pellets using the Qiagen Endofree Mega Plasmid Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Female BALB/C mice (aged 6-8 weeks; weighing 18-22 g) were purchased from National Rodent Laboratory Animal Resources, Shanghai Branch (Shanghai, China) and maintained in a pathogen-free environment under controlled temperatures.
Preparation and characterization of CPL and EGF-CPLs
First, 0.1 mg anti-EGF antibody was dissolved in a 10 mL mixture of deionized water saturated with isopropanol; 1.0 mg GHDC was added slowly to the EGF solution, followed by maintenance of the reaction at 4°C for 24 h with stirring to obtain the hexadecyl quaternized EGF-GHDC. The sample was dialyzed (molecular weight cut-off of 1000 Da) against deionized water for 24 h and lyophilized to form a white powder. After lyophilization, the weights of the conjugates were measured to calculate the conjugation ratio. The EGF nanovesicles were prepared via the reverse-phase evaporation (REV) method. EGF-CPLs, EGF-GHDC, DOPE and cholesterol were dissolved in dichloromethane at room temperature to obtain the organic phase. Hydrophobic cargos may have been added to the organic phase, such as anticancer drugs (e.g., paclitaxel) or lipid-soluble QDs. Following sufficient dissolution, the aqueous phase and organic phase were mixed and sonicated using an ultrasonic probe at 100 W output for 3.0 min. The organic solvents were evaporated to form a gel-like, highly concentrated EGF liposome suspension on a rotary evaporator that could be diluted with a suitable aqueous buffer solution.
The average particle size and charge of CPLs and EGF-CPLs were determined using quasielastic laser light scattering with a Malvern Zetasizer (Nano-ZS 90, Malvern Instruments Limited, United Kingdom) and reported as the mean ± SE (n=3). The images were captured with an atomic force microscope (BioScope SPM, DI, USA) using scan rates between 0.5 and 1 Hz.
Preparation of gene-loaded EGF-CPLs
The EGFP and p53 plasmids were transformed and amplified in E. coli DH5α competent cells (TIANGEN, Beijing, China) and purified using the EndoFree Plasmid Maxi Kit (Qiagen). The gene-loaded cationic EGF-CPL NPs were obtained utilizing electrostatic attraction between different blank EGF-CPLs and the anionic plasmid DNA. In brief, the reporter gene pEGFP solution was added to the EGF-CPL solution at a fixed weight ratio (CPL: DNA, w/w) and then maintained at room temperature for 20 min; the resultant EGF-CPL/DNA complexes were used directly for subsequent studies.
DNA stability study
Different CPL NPs were formed at 40 µg/mL in a total volume of 200 µL of final plasmid DNA. DNase I was added to the CPLs at 1 unit/µg of DNA, incubated at 37°C for 30 min and inactivated by adding ten microliters of an EDTA solution (0.25 M). CPLs were disassembled by adding 5 IU of heparin and 10% Triton X-100 and then analyzed by agarose gel electrophoresis. In parallel, the following were also analyzed using gel electrophoresis, followed by a comparison of plasmid integrity to untreated DNA: untreated EGFP plasmid (pEGFP), pEGFP incubated with DNase I, CPLs in the absence of DNase I and CPLs incubated with DNase I.
In vitro gene expression and transfer
SKOV3 cells were seeded at 2.0×104 cells per well in a 24-well plate and incubated in DMEM supplemented with 10% serum at 37°C. When the cells reached 70-80% confluence, wells were transfected with different CPL/DNA complexes and naked plasmid with 1 µg DNA per well at different molar ratios. After a 48 h-incubation at 37°C, the enhanced green fluorescent protein (EGFP) was qualitatively visualized using a fluorescent microscope (Leica) and the percentage of transfected cells (transfection efficiency) and the total green fluorescence intensity were measured using a FACSCalibur flow cytometer (EPICS XL-MCL ADC). Transfection efficiency was expressed as the mean ± SE of four measurements and the total green fluorescence intensity as an indicator of overall EGFP expression levels. The animal experiments were conducted according to national regulations and with the approval of the Animal Center of Shanghai Jiaotong University and Use Committee and the local animal experiments ethical committee. Subcutaneous ovarian cancer tumors were induced by inoculation of 1×107 mouse SKOV3 cells in the flank. Groups of female BALB/c nude mice (n=4) were administered 40 µg of pGL-3 luciferase plasmid DNA (controls) when the tumor volume reached 0.5-1 cm3, or pGL-3 luciferase plasmid formulated with Lipofectamine 2000/DNA, CPLs/DNA or EGF-CPLs/DNA complexes. The mice were euthanized 3 days after injection, and luciferase levels in the tumor, heart, spleen, liver and lung were measured, as previously described [18].
In vitro cytotoxicity assays
SKOV3 cells were seeded at 2×104 cells per well in 96-well plates and cultured in 1640 medium plus 10% serum. The cells were treated with phosphate buffered saline (PBS), Lipofectamine 2000 (Lipo 2000), CPL, Lipo 2000/DNA, CPL/DNA or EGF-CPL complexes, which were prepared at a dose of 0.6 µg at an NPs:DNA weight ratio of 6:1 DNA per well. After 48 h, 20 µL of MTT solution (5 mg/mL) was added to each well for a final concentration of 0.5 mg/mL, followed by incubation at 37°C for 4 h. Media were aspirated from each well, followed by the addition of 150 µL of dimethyl sulfoxide (DMSO) and pipetting to dissolve the crystals. The plate was then replaced and incubated at 37°C for 5 min and measured by absorbance at 550 nm. The absorbance for experimental groups is expressed as the percent of the control group, where the control is defined as 100% viable.
Statistical analysis
Statistical analyses were performed using Student’s t-test and all data are expressed as the mean ± standard error of the mean (SEM). The differences were considered significant for p values <0.05.
Results and discussion
Preparation of CPL nanoparticles and their conjugation with EGF
Using an insertion and assembly method of the precursor, EGF-GHDC was synthesized first and the EGF-CPLs were assembled later from TQCMC, DOPE, Chol and EGF-GHDC (Figure 1) by liposome preparation methods (REV). The prepared oligopeptide surfactant was characterized by H-NMR, and the relative content of oligopeptide in EGF-GHDC was calculated from the weight ratio of peptide to the total modified conjugates. In this study, the content of EGF in the conjugates was ~37.88±10.78% wt % and the EGF content in the CPL NPs could be controlled by varying the weight ratio of EGF-GHDC to CPL in the feed.
Figure 1.
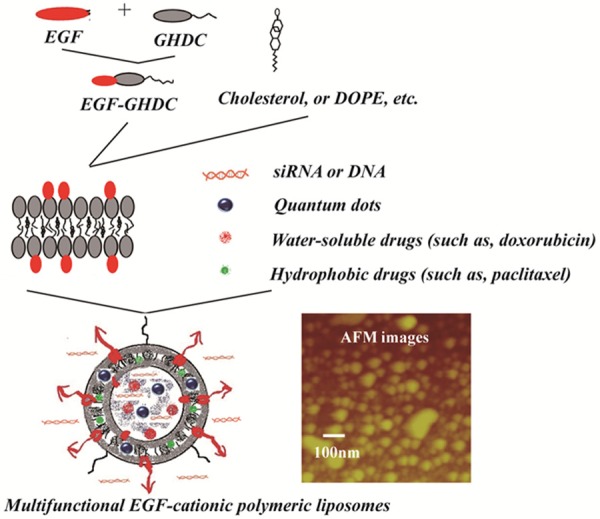
Schematic of the designed EGF modified CPLs performed by cationic polymeric lipids and lipid components.
Characterization of EGF-CPLs
UV-Visible spectral analysis was used to determine the exact content of EGF on the CPL NPs (Figure 2A), and obvious differences were observed in the UV absorption patterns of CPLs and EGF-CPLs. EGF had an absorption peak at 275 nm in the UV spectrum. EGF-CPLs exhibited a similar peak but displayed a bathochromic shift at 277 nm, which could be attributed to the addition of EGF-GHDC in the CPL NPs. In contrast, the CPL NPs did not demonstrate any distinct absorption peaks from 200 to 600 nm. The above result indicated that the inserted EGF-GHDC units caused differences between the UV spectra of the CPLs and EGF-CPLs. The size and zeta potential values were approximately 50~200 nm and 10~50 mV, respectively. The EGF-CPLs were spherical in shape with a generally narrow particle size distribution. However, the spherical shape became increasingly irregular with a slight increase in diameter (Figure 2B). The hydrodynamic diameter of EGF-CPL was 95.01±4.053 nm with a corresponding polydispersity index (PDI) of 0.270, which is larger than the TEM diameter for the hydration of polymeric liposome bilayers associated with the CPLs.
Figure 2.
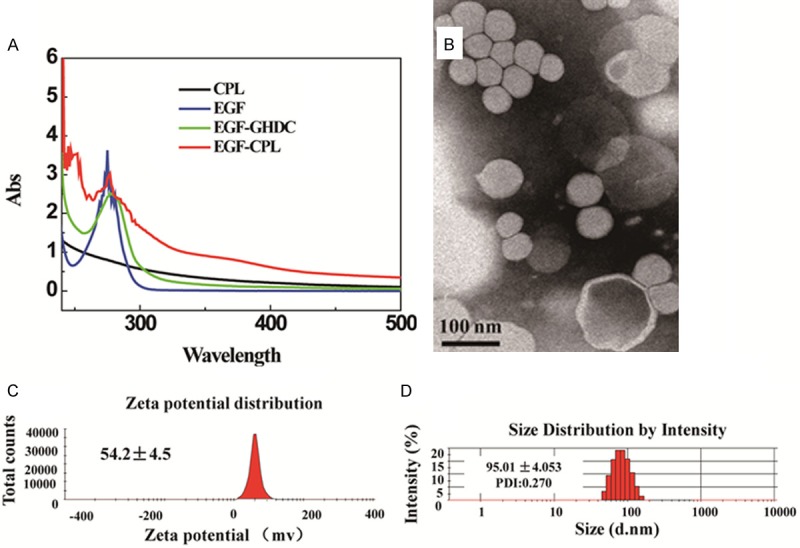
(A) Ultraviolet spectra analysis of CPL and EGF-CPL. (B) Transmission electron micrographs of EGF-CPL. (C) Zeta potential distribution and (D) Particle size distribution based intensity of EGF-CPL.
Characterization of EGF-CPL as gene delivery vectors
Agarose gel electrophoresis was performed at weight ratios ranging from 0 to 13 to investigate the DNA condensation capacity of EGF-CPLs. The mobility of the plasmid was completely retarded at ratios higher than 4 (for EGF-CPL) and 9 (CPL), indicating that all the CPL NPs could bind DNA strongly, as shown in Figure 3A. It was also observed that the capacity of the EGF-CPLs to condense DNA was increased upon the addition of EGF-GHDC in the NPs compared to the CPLs.
Figure 3.
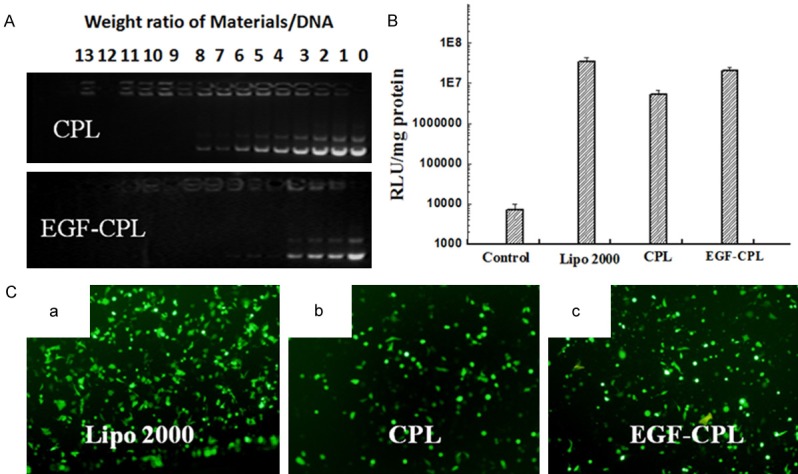
(A) Agarose gel electrophoresis analysis of CPL and EGF-CPL combined with DNA at various weight ratios. Lane 1: DNA control; lanes 2-14: NPs/DNA weight ratios of 1:1 to 13:1. In vitro luciferase expression (B) and EGFP expression of fluorescent intensity (C) in SKOV3 cells. Different wells of cells were transfected with naked plasmid, lipo 2000/DNA complexes, CPL/DNA complexes and EGF-CPL/DNA complexes at varied weight ratios of total CPLs/DNA of 3/1, 6/1 and 9/1 at a dose of 1 μg of DNA per well. The EGFP plasmid was used; at 48 h, fluorescent microscopy was performed.
Luciferase expression experiments were carried out to quantitatively test the gene transfection efficiency of EGF-modified liposomes and EGF-free liposomes. As shown in Figure 3B, the luciferase expression mediated by EGF-CPLs/PGL-3 and CPLs/PGL-3 complexes was influenced by SKOV3 cell lines. The expression of luciferase-mediated by the EGF-CPLs was 4-fold higher than the expression mediated by CPLs. These results suggested that EGF-CPLs can selectively deliver genes to cells overexpressing EGF.
SKOV3 cells were also chosen to study the gene transfection efficiency of EGF-modified CPLs and CPLs (at different NPs: DNA weight ratios of 4:1 and 9:1, respectively). As shown in Figure 3C, in EGF-overexpressing SKOV3 cells, EGF-CPLs displayed higher GFP fluorescence than did the EGF-free CPLs. This implied that the enhanced fluorescence expression of EGF-CPLs by SKOV3 was due to EGF-mediated endocytosis.
Figure 4A shows the cell uptake efficiency of FITC-labeled CPL and EGF-CPL. The EGF-CPL showed higher FITC fluorescence intensity in the SKOV3 cells. In addition, the average fluorescence intensities (geometric mean) treated with EGF-CPL were almost twice that of the cells treated without EGF modification, indicating that EGF modification can enhance the cell uptake efficiency of CPL NPs.
Figure 4.
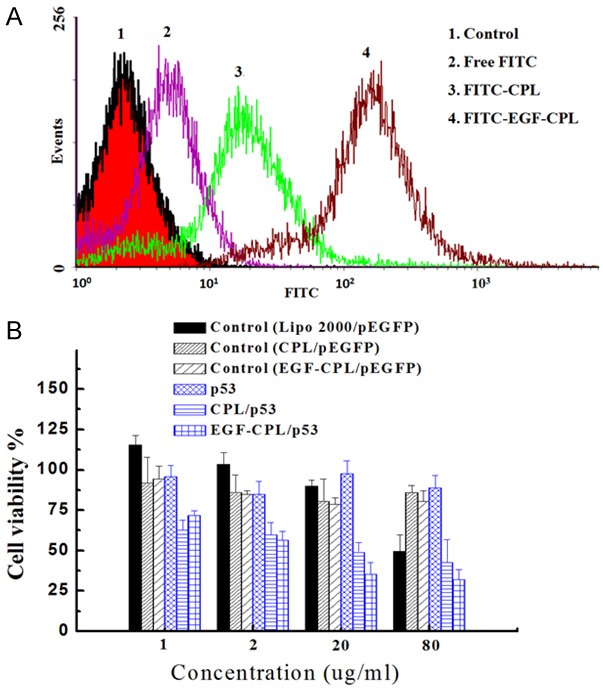
A. Flow cytometry analysis of SKOV3 cells incubated with the control, free FITC, FITC-CPL and FITC-EGF-CPL for 20 min at the same NP concentrations (20 μg/mL). B. The cytotoxicity of CPL and EGF-CPL were measured using MTT assays after a 48-h incubation with SKOV3 cells, enhancing the inhibition effect on cell growth with CPL/P53 and EGF-CPL/P53 complexes in the SKOV3 cells. Each point represents the mean ± SD of three experiments.
Cytotoxicity and in vitro growth inhibition of SKOV3 cells
Cytotoxicity is a significant parameter of nonviral vectors. The MTT assay comparing CPLs/DNA and EGF-CPLs/DNA was performed in SKOV3 cells with the Lipo 2000/pDNA complex used as a control. As shown in Figure 4B, all samples displayed high cell viabilities at NP concentrations of 1 and 2 μg/ml after 48 h incubation. Compared to Lipo 2000/pEGFP complexes, the CPLs/pEGFP and EGF-CPLs/pEGFP complexes also exhibited lower cytotoxicities after 48 h incubation at higher NP concentrations of 20 and 80 μg/ml. Furthermore, the growth inhibition of SKOV3 cells by CPLs/p53 gene and EGF-CPLs/p53 gene after 48 h incubation at different NP concentrations (from 1 to 80.00 µg/ml) were observed. All NPs demonstrated dose-dependent cell growth inhibition rates and cytotoxicity in the SKOV3 cells after a 48 h incubation. Distinct differences in the growth inhibition of the SKOV3 cells could not be found at lower NP concentrations of 1.0 and 2.0 µg/ml. At increased NP concentrations of 20.0 and 80.0 µg/ml, CPLs/p53 and EGF-CPLs/p53 both significantly enhanced the cytotoxicity [P<0.05].
In vivo analysis of delivered genes by EGF-CPL
The consequent in vivo luciferase activity and Chol biodistribution of the nontargeted CLs and EGF-CPL/pGL3 were quantitatively analyzed at 3 days post injection (Figure 5). The CPLs with EGF modification exhibited the strongest tumor-targeting capacity, based on an analysis of Chol biodistribution. The tumor Chol content in the EGF-CPL group was consistently higher than that of the Lipo 2000 and CPL groups and exhibited distinct differences at 3 days post injection (EGF-CPL vs CPL and EGF-CPL vs Lipo 2000, P<0.05).
Figure 5.
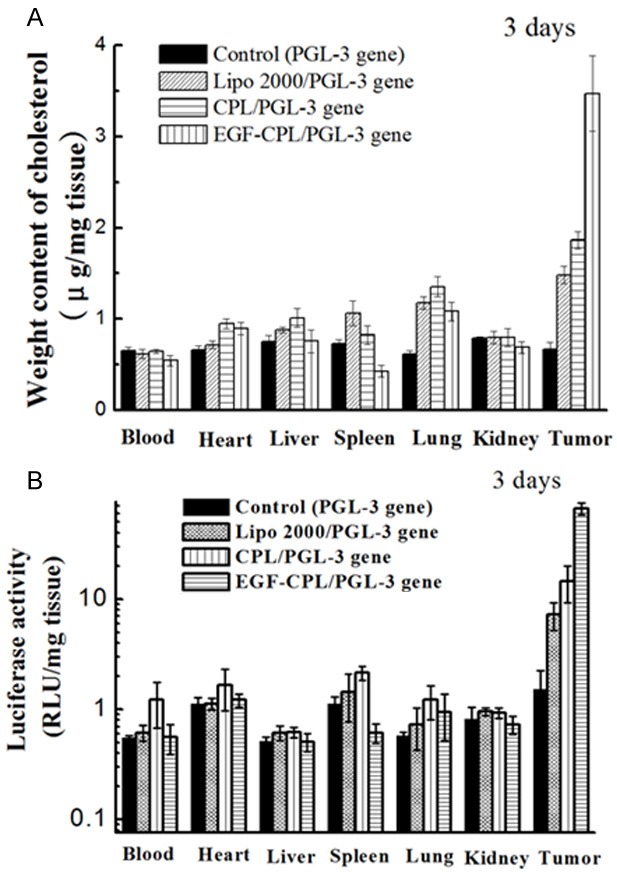
In vivo Chol distribution (A) and luciferase expression (B) of Lipo 2000/pGL-3, CPL/pGL-3, and EGF-CPL/pGL-3 complexes 3 days after administration to tumour-bearing nude mice via tail vein injection.
The Lipo 2000/pGL3, CPL/pGL3 and EGF-CPL/pGL3 complexes were formulated at an optimal weight ratio of 5:1 (material to plasmid), contained 40 μg of plasmid, and were injected into tumor-bearing mice via the tail vein. Three days after tail vein injection, the tumor luciferase expression induced by the EGF-CPL/pGL3 complexes was approximately three-fold higher than that of the CPL/pGL3 complexes. The observed trend in the cholesterol content was consistent with that of luciferase expression. These data demonstrated that EGF can be specifically targeted to ovarian cancer, reducing the circulation of the EGF-CPL/pGL3 complexes and subsequently promoting gene expression in vivo.
Conclusions
By combining the superiority of the EGF protein and cationic liposomes, we have developed an efficient targeted gene delivery system. Stable EGF-CPLs/DNA complexes proved to have a high gene transfection efficiency in SKOV3 cells. The EGF-CPLs offer significant advantages, including small and stable particle sizes to improve the reproducibility of transfection efficacy, decreased cytotoxicity, and efficient transfection of SKOV3 cells in vitro and in vivo. The results demonstrate that the EGF-CPL vector can effectively deliver the p53 gene into target tumors and inhibit tumor growth in vitro. Our findings clearly support the potential of nonviral gene delivery strategies for the treatment of malignant tumors.
Acknowledgements
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Hospital horizontal subject (2015hx1).
Disclosure of conflict of interest
None.
References
- 1.Jia LJ, Hua ZC. Development of bacterial vectors for tumor-targeted gene therapy. Methods Mol Biol. 2009;542:131. doi: 10.1007/978-1-59745-561-9_7. [DOI] [PubMed] [Google Scholar]
- 2.Kiang A, Amalfitano A. Progress and problems when considering gene therapy for GSD-II. Acta Myol. 2007;26:49–52. [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Y, Yue Y, Bostick B, Duan D. Delivering large therapeutic genes for muscle gene therapy. 2010 [Google Scholar]
- 4.Clément N, Grieger JC. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol Ther Methods Clin Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Ren X, Wang H, Ma Y, Wang L, Shen Y, Oka K, Zhang Z, Zhang Y. Liver-specific expression of an exogenous gene controlled by human apolipoprotein A-I promoter. Int J Pharm. 2010;398:161–164. doi: 10.1016/j.ijpharm.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 6.Park KM, Na K. Development of effective non-viral gene delivery system from polyethylenimine (PEI) dervatives. PLoS Negl Trop Dis. 2008;6:164–175. [Google Scholar]
- 7.Liang XF, Shi BZ, Wang K, Fan ML, Jiao DJ, Ao JP, Song N, Wang C, Gu JR, Li ZH. Development of self-assembling peptide nanovesicle with bilayers for enhanced EGFR-targeted drug and gene delivery. Biomaterials. 2016;82:194–207. doi: 10.1016/j.biomaterials.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 8.Egorova AA, Kiselev AV. Peptide modules for overcoming barriers of nucleic acids transport to cells. Curr Top Med Chem. 2016;16:330–42. doi: 10.2174/1568026615666150812120755. [DOI] [PubMed] [Google Scholar]
- 9.Liang XF, Li XY, Chang J, Duan YR, Li ZH. Properties and evaluation of quaternized chitosan/lipid cation polymeric liposomes for cancer targeted gene delivery. Langmuir. 2013;29:8683–8693. doi: 10.1021/la401166v. [DOI] [PubMed] [Google Scholar]
- 10.Cattaneo MV. Ligand targeted nanocapsules for the delivery of RNAi and other agents. US. 2010 [Google Scholar]
- 11.Luo KQ, Yu T, Luo HW. Acetyltanshinone IIA (ATA) as anticancer agent. 2012 [Google Scholar]
- 12.Rugo HS. Dosing and safety implications for oncologists when administering everolimus to patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2016;16:18–22. doi: 10.1016/j.clbc.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Kim YK, Jiang HL, Choi YJ, Park IK, Cho MH, Cho CS. Polymeric nanoparticles of chitosan derivatives as DNA and siRNA carriers. Springer Berlin Heidelberg. 2011 [Google Scholar]
- 14.Miyata K, Yotsumoto F, Fukagawa S, Kiyoshima C, Ouk NS, Urushiyama D, Ito T, Katsuda T, Kurakazu M, Araki R. Serum heparin-binding epidermal growth factor-like growth factor (HB-EGF) as a biomarker for primary ovarian cancer. Anticancer Res. 2017;37:3955. doi: 10.21873/anticanres.11779. [DOI] [PubMed] [Google Scholar]
- 15.Xin L, Fan JC, Le YG, Zeng F, Cheng H, Hu XY, Cao JQ. Construction of METHFR shRNA/5-fluorouracil co-loaded folate-targeted chitosan polymeric nanoparticles and its anti-carcinoma effect on gastric cells growth. Journal of Nanoparticle Research. 2016;18:1–12. [Google Scholar]
- 16.Parida UK, Rout N, Bindhani BK. In vitro properties of chitosan nanoparticles induce apoptosis in human lymphoma SUDHL-4 cell line. Advances in Bioscience & Biotechnology. 2013;4:1118–1127. [Google Scholar]
- 17.Fatouros DG, Lamprou DA, Urquhart AJ, Yannopoulos SN, Vizirianakis IS, Zhang S, Koutsopoulos S. Lipid-like self-assembling peptide nanovesicles for drug delivery. ACS Appl Mater Interfaces. 2014;6:8184. doi: 10.1021/am501673x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel AK, Shah RK, Parikh IK, Joshi CG. Goat activin receptor type iib knockdown by artificial microRNAs in vitro. Appl Biochem Biotechnol. 2014;174:424–436. doi: 10.1007/s12010-014-1071-3. [DOI] [PubMed] [Google Scholar]


