Abstract
Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channel activity plays a crucial role in the progress of peripheral neuropathic pain (PNP). However, the mechanism of HCN2 channels on PNP remains unclear. Here, we investigated the effects of HCN2 channel expression on the mechanical allodynia and thermal hyperalgesia, the local inflammatory response, the activation of astrocytes, microglia and transcription factor NF-κB in mice with spared sciatic nerve injury (SNI). The present study showed that the expression of HCN2 channels was increased in L4-L5 ipsilateral spinal dorsal horns, accompanied by a decreased paw mechanical withdrawal threshold (MWT) and paw withdrawal latency (PWL) in SNI mice. After intrathecal injection of ZD-7288 and si-HCN2, both MWT and PWL were significantly increased, while the level of pro-inflammatory factors TNF-α, IL-1β and MCP-1 were decreased in L4-L5 ipsilateral spinal dorsal horn. Furthermore, the inhibition of HCN2 channels reduces the activated astrocytes and microglia, and suppressed NF-κB p65 activation and nuclear translocation. In conclusion, the present study suggests that decreased HCN2 channel expression attenuates neuropathic pain by inhibiting pro-inflammatory reactions and NF-κB activation.
Keywords: HCN2 channels, neuropathic pain, inflammation, NF-κB
Introduction
Peripheral neuropathic pain (PNP) is a common and complicated disease affecting the somatosensory system and patients’ quality of life [1]. Furthermore, the mechanisms of PNP are rather complex, and the current treatment for PNP only partly relieves the pain [2]. Therefore, exploring the pathogenesis and novel therapeutic approaches to overcome the limitations of the traditional treatments for PNP are very important. Recent studies showed that the HCN ion channel family plays important roles in the pacemaker potential of the heart, in both inflammatory and neuropathic pain, and in neuronal cells [3,4]. Specially, HCN2 channels are involved in the progress of several diseases [5], including PNP [6], but the mechanism of PNP remains unclear. The pro-inflammatory factors have been considered to be an injury mechanism for PNP [7]. Blocking the inflammatory cascade by including TNF-α, IL-1β and MCP-1 is beneficial to attenuate neuropathic pain by increasing MWT and PWL [8]. However, whether HCN2 channels are involved in the progress of PNP by regulating the inflammatory factors is unknown. Many studies indicated that the astrocytes and microglia in ipsilateral spinal dorsal horns were activated in a PNP animal model, accompanied by a large release of inflammation factors [9,10]. Thus, further study is warranted to identify whether there is a cross-talk between HCN2 channels, pro-inflammation factors, astrocytes, and microglia in the progress of PNP.
In this study, we first established the PNP animal models, and then ZD-7288, a HCN2 channels blocker, and an adenovirus vector with si-HCN2 were intrathecally injected in the models to explore the effects of HCN2 channel expression on the mechanical allodynia and thermal hyperalgesia, the local inflammatory response, the activation of astrocytes, microglia. and the molecular mechanism of PNP.
Materials and methods
Animals
All protocols were approved by the Institutional Animal Experimental Ethics Committee of Hubei University for Nationalities. The present studies were performed on male wild-type C57BL/6 mice (22 ± 2 g) purchased from Hubei Provincial Center for Disease Control and Prevention. All mice were maintained under standard housing conditions with a 12-h light/dark cycle and provided with free access to food and water.
Animal model of neuropathic pain induced by spared sciatic nerve injury (SNI)
The SNI animal models were established as described previously [8]. Briefly, the mice were anesthetized with hydrochloride plus xylazine (hydrochloride: 90 mg/kg body weight; xylazine: 5 mg/kg). The left hindlimb skin on the lateral surface of the thigh was incised and the biceps femoris muscle was separated to expose the sciatic nerve and its three terminal branches (the tibial, common peroneal and sural nerves). The tibial and the common peroneal nerves were ligated with 5.0 silk and severed distal to the ligation, 2-4 mm of the distal nerve segment was removed. In the rats of the sham-operation group, the surgical operation was identical, except that the common peroneal and tibial nerves were not ligated or sectioned. During the progress of the operation, we tried to protect the sural nerve from any touch or injury. After the operation, the muscle and skin were closed in two layers.
Construction of adeno-associated virus (AAV) si-HCN2
Four siRNA oligos against HCN2 channels and one siRNA oligo control (non-specific sequence; TTCAGACTCGTACGTGAAT) were designed using web-based software (Genescript). After a blast-search (NCBI database), one siRNA candidate (siRNA-HCN2: CGUGGUCUCGGACACUUUCTT) was selected. Each oligo consists of 19 base-pair sense and antisense sequences, a 9 base-pair hairpin loop, TTCAAGAGA sequences, and a poly (T) termination signal. Sense region (5’) was attached with a BamHI restriction enzyme site, and antisense region (3’) was attached with a PacI restriction enzyme site. pAV-4in1siRNA-GFP was purchased form the Vigene company (Shandong, China). Each siRNA oligo was annealed and cloned into BamHI (5’)-PacI (3’) sites (New England Biolabs) of pAV-4in1siRNA-GFP vector.
Intrathecal injection
An intrathecal injection of artificial cerebrospinal fluid (ACSF, Sigma, USA) and ZD-7288 (HCN channels blocker, Tocris Bioscience, Britain) were performed using a modified version of a previously described method [9]. In brief, the mouse was held firmly by the pelvic girdle in one hand at an angle of about 20 degrees above the vertebral column. The microsyringe (10 μl) was inserted into the tissue so that it slipped into the groove between the spinous and transverse processes. A sudden slight flick of the tail or paw indicated successful entry into the dorsal subarachnoid space. Then, 5 μl of ACSF (5 μl) and ZD-7288 (10 ug/kg/5 ul) was slowly injected over a 1-min period, and the needle was left in place for a further 5 s. The concentration of drugs was indicated as previous studies described. No abnormal behavioral consequences of the injections were observed.
Treatment and groups
The mice were randomly divided into 6 groups: (1) Sham group; (2) SNI group; (3) SNI+ACSF group; (4) SNI+ZD-7288; (5) SNI+AAV-Vector; (6) SNI+AAV-HCN2. SNI mice: mice received the surgery of spared sciatic nerve injury; Sham mice: received the same operation, but the common peroneal and tibial nerves were not ligated. A 5 μL volume of ZD-7288 (10 ug/kg) and the same volume ACSF was intrathecally administered on day 3, 4, 5 after surgery in SNI+ACSF and SNI+ZD-7288. An intrathecal injection of the AAV-vector and AAV-HCN2 were performed 2 weeks before the surgery.
Behavioral tests
The paw mechanical withdrawal threshold (MWT) and the paw withdrawal thermal latency (PWL) were measured on the day before surgery (day 0) and on day 1 to day 7 after surgery. The mice were placed in individual plastic boxes (12×20×15 cm) on a metal mesh floor (for MWT) or on a glass floor (for PWL) and allowed to acclimatize for 1 h. An Electron Von Frey (IITC life science, USA) was used to assess the MWT for mice. A probe was applied with increasing force to the plantar surface of left hind paw. The Hargreaves test (BW-Plantar 390, USA) was used to detect the PWL for mice. A plantar test apparatus was applied to produce a beam of radiant heat to stimulate the mid-plantar surface of the hind paws. Brisk withdrawal or paw flinching was considered a positive response and we recorded the readout. The MWT and PWL tests were repeated three times on the same paw at an interval of 5 min and the mean was calculated.
Western blot analysis
The mice were sacrificed for extraction of the L4-L5 ipsilateral spinal dorsal horn on day 7 after the surgery. A western blot was performed as described previously [11] The total protein of the ipsilateral spinal dorsal horn was extracted using a cold RIPA buffer with PMSF. A BCA protein assay kit (Beyotime Biotechnology, China) was used to detect protein concentrations. Equal proteins were separated by electrophoresis within SDS-PAGE and transferred onto the polyvinylidene difluoride (PVDF) membranes (Millipore, USA). The membranes were blocked with 5% non-fat milk for 60 min at room temperature and then incubated with the following primary antibodies against HCN2 channels (#ab65707, Abcam, Cambridge, MA), p65 (#ab16502, Abcam, Cambridge, MA) and GAPDH (#ab8245, Abcam, Cambridge, MA) overnight at 4°C. Then the membranes were incubated with a goat-anti-mouse or goat-anti-rabbit antibody (1:4000, Antgene Biotechnology) for 60 min at room temperature. The labeled protein was detected with the chemiluminescence system (UVP Lab-Works, Upland, CA). The signal intensity was then analyzed with the Image J Software.
Enzyme-linked immunosorbent assay (ELISA)
The protein level of TNF-α, IL-1β, and MCP-1 of the L4-L5 ipsilateral spinal dorsal horns were tested using an ELISA Kit (R&D Systems, Inc., USA) according to the manufacturer’s instructions. The absorbance was detected at 450 nm with a Microplate Reader (ThermoFisher, USA).
Immunohistofluorescence staining
The tissues of the L4-L5 ipsilateral spinal dorsal horns were embedded with paraffin. The immunohistofluorescence staining in the paraffin embedded sections was performed as described previously [11]. The sections were stained with a primary antibody as follows: GFAP (#ab7260, Abcam, Cambridge, MA), IBA1 (#ab5076, Abcam, Cambridge, MA), and p65. The epifluorescence (Olympus, Japan) was used to capture the images.
Data analysis
All data in this study were presented as the mean ± SD. Statistical analyses of the results were evaluated using the unpaired Student’s t-test in behavioral experiments, an ELISA assay and a protein quantitative experiment. All P values given were based on two-tailed tests. P<0.05 was considered to indicate statistical significance.
Results
The expressions of the HCN2 channels were increased in the L4-L5 ipsilateral spinal dorsal horns of the SNI mice
The SNI model is a well-established and widely used preclinical model of neuropathic pain. HCN2 channel expression in the L4-L5 ipsilateral spinal dorsal horns was detected in the sham mice and SNI mice. As shown in Figure 1, compared with the sham mice, the expression of the HCN2 channels was increased significantly in the SNI mice (P<0.001). In order to determine the effects of the HCN2 channels on the neuropathic pain of SNI mice, an intrathecal injection of ZD-7288, a special blocker for the HCN channel, and of an adeno-associated virus (AAV) vector with si-HCN2 were performed, respectively. The results showed that both ZD-7288 and si-HCN2 could reduce the expression of the HCN2 channels (P<0.01, P<0.001). Moreover, the effect of si-HCN2 was better than ZD-7288 (P<0.01). Since changes in HCN2 channel expression affects the HCN channel activity, the expression of the HCN2 channels increased in the L4-L5 ipsilateral spinal dorsal horn may contribute to persistent neuropathic pain after SNI.
Figure 1.
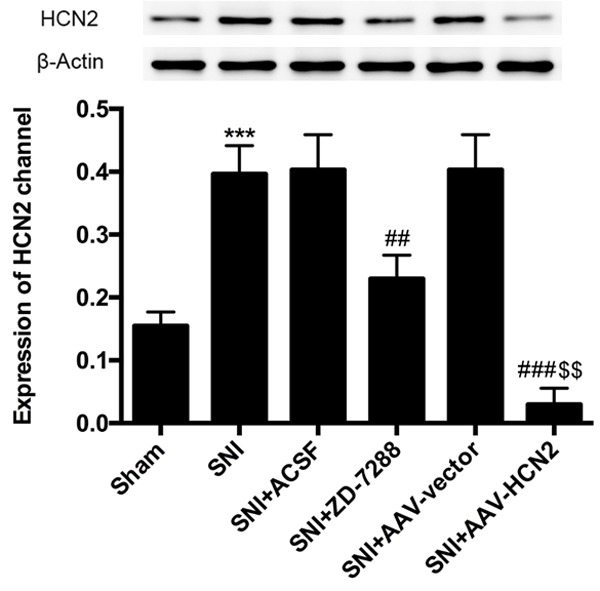
HCN2 channel expression in L4-L5 ipsilateral spinal dorsal horns was increased significantly after SNI and was decreased after treatment with an intrathecal injection of ZD-7288 and si-HCN2. Data are shown as the mean ± SD. ***P<0.001 vs. Sham group; ##P<0.01, ###P<0.001 vs. SNI group; $$P<0.01 vs. SNI+ZD-7288 group.
The inhibition of HCN2 channels significantly increased the MWT and PWL in SNI mice
The mechanical allodynia and thermal hyperalgesia were identified by the decreases of the MWT and PWL. We showed a sharp decline of ipsilateral MWT and PWL from the first day after surgery, and the lowest values were reached on day 3 after surgery (Figure 2, P<0.001), and then persistently maintained stable for a long time, while there was no change in the MWT (Figure 2A and 2C) or the PWL (Figure 2B and 2D) in the sham mice.
Figure 2.
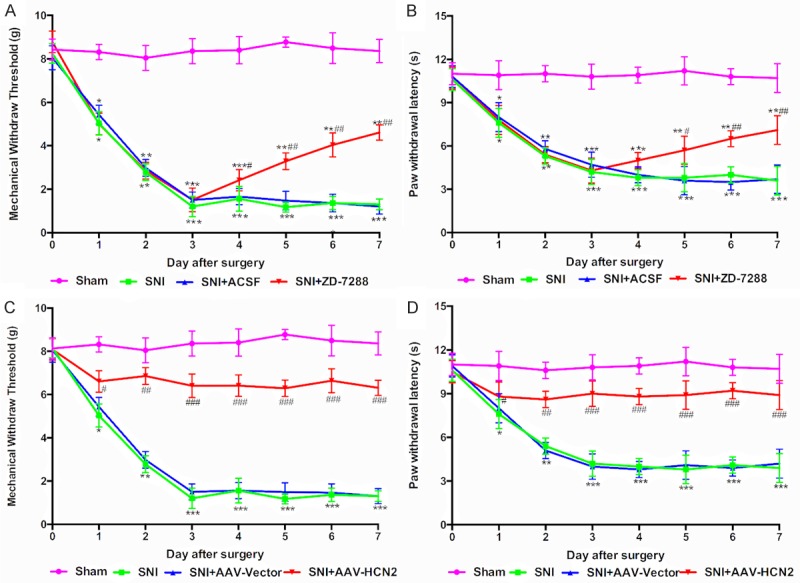
Inhibition of HCN2 channels significantly increased the MWT and PWL in SNI mice. The paw mechanical withdrawal threshold (MWT, A and C) and the paw withdrawal thermal latency (PWL, B and D) decreased after SNI in mice. The intrathecal injection of ZD-7288 increased the MWT (A) and PWL (B). Pre-treatment with si-HCN2 recovered the decreased MWT (C) and PWL (D) after SNI. Data are shown as the means ± SD. *P<0.05, **P<0.01, ***P<0.001 vs. Sham group; #P<0.05, ##P<0.01, ###P<0.001 vs. SNI group.
To determine whether increased HCN2 channel expression in ipsilateral spinal dorsal horns contributes to the maintenance of neuropathic pain, the mice were treated with ZD-7288 on day 3 after surgery. The result showed that, after the intrathecal injection of ZD-7288 in the SNI mice for 3 consecutive days, the MWT and PWL increased steadily from day 5 to day 7 (Figure 2A and 2B, P<0.05, P<0.01), while the ACSF barely affected the neuropathic pain induced by SNI. To verify the role of HCN2 channels in the SNI induced neuropathic pain; si-HCN2 was designed to inhibit the expression of HCN2 channels. It was shown that there was only a slightl decrease of the MWT (Figure 2C) and PWL (Figure 2D) after pre-treatment of si-HCN2 and then maintained a relative high level after SNI, which indicated that mice were insensitive to the mechanical and thermal stimulus compared with the SNI group (Figure 2C and 2D, P<0.001), which is consistent with the previous results of the suppression efficiency measurements of HCN2 channel expression. No effect of the AAV-vector on pain behavior was observed. These data indicated that the inhibition of the HCN2 channel plays an important role in the therapeutic effect on neuropathic pain. However, the mechanism by which HCN2 channels silence attenuating neuropathic pain is still unknown.
HCN2 channel inhibition attenuated the levels of TNF-α, IL-1β, and MCP-1 in ipsilateral spinal cord horns
It is understood that many inflammatory factors play important roles in the progress of neuropathic pain, so the levels of TNF-α, IL-1β, and MCP-1 were measured by an ELISA assay in the ipsilateral spinal dorsal horn on day 7 after the intrathecal injection of ZD-7288 and si-HCN2. Compared with the sham mice, the levels of TNF-α (Figure 3A), IL-1β, (Figure 3B) and MCP-1 (Figure 3C) were significantly increased in ipsilateral spinal dorsal horns in SNI mice (P<0.001). The results indicated that SNI could induce local inflammation in ipsilateral spinal dorsal horns. After inhibition of HCN2 channel expression with ZD-7288 and si-HCN2, the levels of TNF-α, IL-1β, and MCP-1 were significantly decreased in the ipsilateral spinal dorsal horns in SNI mice (Figure 3, P<0.01, P<0.001). These data suggest that the down-expression of HCN2 channels relieves neuropathic pain by reducing the local inflammation factors, including TNF-α, IL-1β, and MCP-1.
Figure 3.
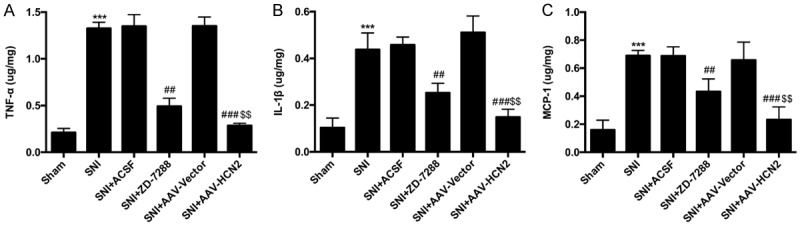
Increased production of pro-inflammatory factors induced by SNI were suppressed after HCN2 channel inhibition in L4-L5 ipsilateral spinal dorsal horns. The level of the pro-inflammatory factors TNF-α (A), IL-1β (B) and MCP-1 (C) after SNI increased dramatically, but they were reduced after the HCN2 channels expression decreased in the L4-L5 ipsilateral spinal dorsal horns. Si-HCN2 showed a higher effect on the reduction of the pro-inflammatory factors, which indicated that the activity of the HCN2 channels is indeed involved in the inflammatory response. The data were shown as the means ± SD. ***P<0.001 vs. the sham group, ##P<0.01, P<0.001 vs. the SNI group, $$P<0.01 vs. SNI+ZD-7288 group.
Decreased HCN2 channel expression inhibits the activation of astrocyte and microglia in ipsilateral spinal dorsal horns
It is believed that the activation of astrocytes and microglia are involved in the progression of neuropathic pain. GFAP and IBA1 were hallmark proteins for activated astrocytes and microglia, respectively. Accordingly, we determined the effects of HCN2 channel expression on the activation of astrocytes and microglia in ipsilateral spinal dorsal horns by immunofluorescence staining. As Figure 4 shows, compared with the sham mice, the activated astrocytes (Figure 4A) and microglia (Figure 4B) were increased in the SNI mice. Conversely, after the intrathecal injection of ZD-7288 and si-HCN2, the activated astrocytes and microglia were decreased in the SNI mice compared to the control mice. These results indicate that decreased HCN2 channel expression alleviates neuropathic pain by inhibiting the activation of astrocytes and microglia in ipsilateral spinal dorsal horns.
Figure 4.
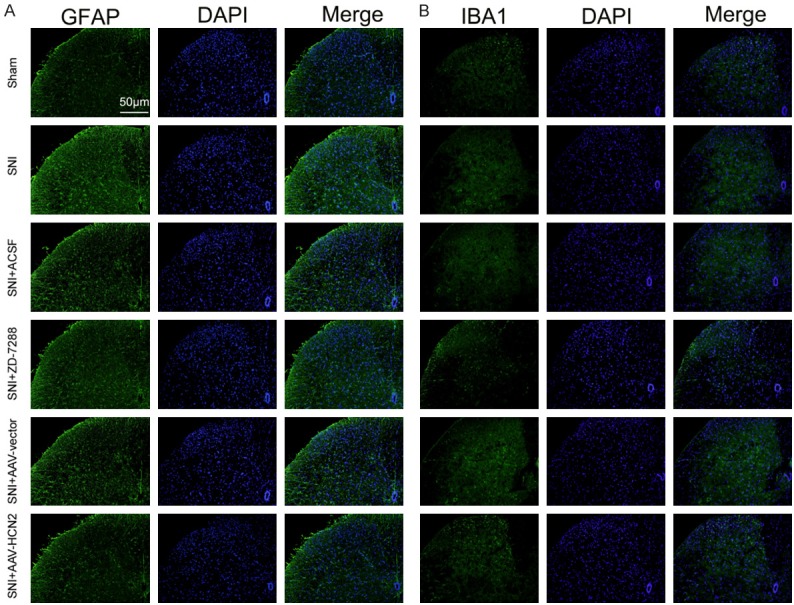
Decreased HCN2 channel expression inhibited the activation of astrocytes and microglia in ipsilateral spinal dorsal horns. Representative immunofluorescent staining of GFAP (A) and IBA1 (B) to detect activated astrocytes and microglia, respectively. Treatment with ZD-7288 and si-HCN2 obviously inhibited the activation of astrocytes and microglia in L4-L5 ipsilateral spinal dorsal horns.
Decreased HCN2 channel expression suppresses nuclear factor-κB (NF-κB) activation
Recent studies indicated that NF-κB is one of the critical transcription factors involved in the progression of neuropathic pain through the activation of astrocytes and microglia, and the release of inflammation factors. Therefore, immunofluorescence staining and western blot were further performed to determine the role of HCN2 channels on the activation of NF-κB in ipsilateral spinal dorsal horn. As shown in Figure 5A, SNI promotes NF-κB p65 translocating to the nuclei in a manner that was suppressed by the intrathecal injection of ZD-7288 and si-HCN2. In addition, the western blot results showed that the expression of cytoplasmic p65 was decreased, while the nuclear p65 expression was increased in ipsilateral spinal dorsal horns (Figure 5A and 5B). In contrast, the nuclear-cytoplasmic ratio of p65 was reduced after the intrathecal injection of ZD-7288 and si-HCN2 (Figure 5B, P<0.01, P<0.001). Moreover, the phosphorylation of p65 at Ser 536 was increased in SNI mice, while the p-p65/p65 ratio was decreased by ZD-7288 and si-HCN2 treatment when compared with the SNI mice (Figure 5C, P<0.05). These results show that HCN2 channel expression is involved in neuropathic pain by regulating the activity of NF-κB.
Figure 5.
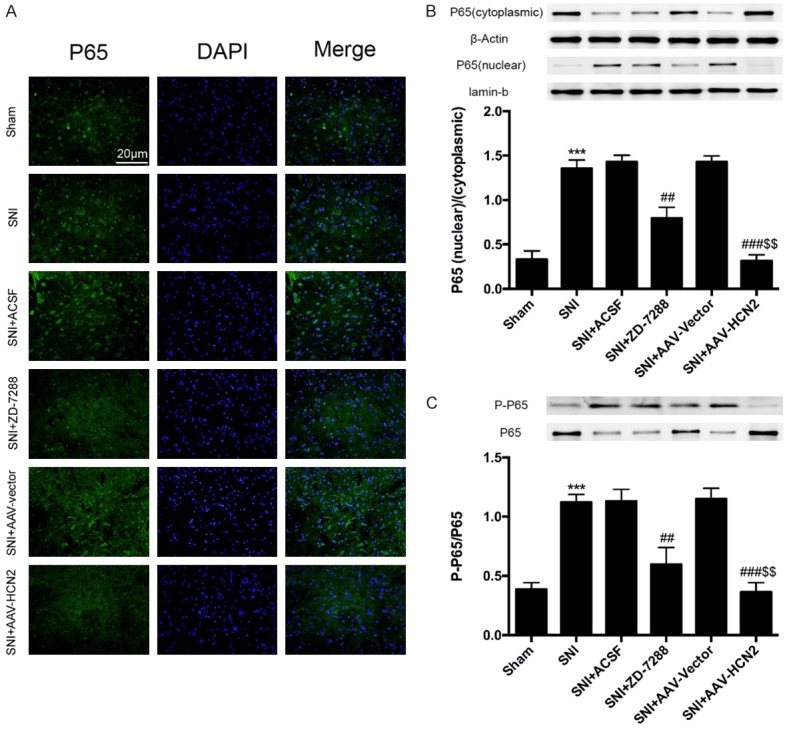
Decreased HCN2 expression suppressed nuclear factor-κB (NF-κB) activation. Immunofluorescent staining of P65 in ipsilateral L4-L5 spinal cord dorsal horns was performed to investigate the effects of HCN2 expression on NF-κB activation (A). Western blotting was used to analyze nuclear and cytoplasmic p65 (B) and P65 phosphorylation (C). Blocking the activity of HCN2 channels sharply reduced p65 nuclear translocation and phosphorylation. Data are shown as the means ± SD. ***P<0.001 vs. Sham group; ##P<0.01, ###P<0.001 vs. SNI group; $$P<0.01 vs. SNI+ZD-7288 group.
Discussion
The HCN ion channel includes four isoforms, from HCN1 to HCN4, each with a distinct distribution or expression throughout the body. HCN1 channels are mainly expressed in the heart and brain [12], while the HCN3 channels is substantially expressed in the central nervous system (CNS) [13]. HCN2 channels and HCN4 channels are both mainly expressed in the CNS and heart [14]. The HCN ion channels are activated at hyperpolarized membrane potentials, providing a depolarizing inward current and consequently promoting the activation of other voltage-gated channels. A large number of studies indicated that HCN channels are involved in a wide range physiological and pathological processes, including the secretion of pancreatic cells, vision, sinoatrial node function, memory learning, taste perception and PNP [15,16]. Essentially, HCN2 channels play important roles in the regulation of mechanical allodynia and thermal hyperalgesia and participate in the disease onset and progression of PNP [6]. The present study revealed the change of HCN2 channel expression in ipsilateral spinal dorsal horns of a PNP mice model induced with SNI and the mechanisms of HCN2 channel inhibition on relieving the neuropathic pain.
Preclinical studies in PNP animal models indicated that PNP is due, at least in part, to the abnormal hyperexcitability of ipsilateral spinal dorsal horn neurons [17]. Our results showed that HCN2 channel expression was significantly increased in the L4-L5 ipsilateral spinal dorsal horns of SNI mice compared with the sham mice. MWT and PWL were used to assess the degree of neuropathic pain. In the SNI mice, we showed a sharp decline of ipsilateral MWT and PWL from the first day after surgery, which reached the lowest values on the day 3 after the surgery, and then maintained a stable level for a long time. Our results are consistent with previous studies [6]. These findings suggest that increased HCN2 channel expression is involved in the progress of PNP. Therefore, inhibiting the activity of the HCN2 channels during the initiation and maintenance progress of PNP might be a promising therapeutic approach.
In order to investigate the effects and mechanisms of HCN2 channel expression on PNP, the SNI mice were treated with ZD-7288, a blocker for HCN2 channels, and an adenovirus vector with si-HCN2. We showed that inhibiting the activity or decreasing HCN2 channel expression significantly increased ipsilateral MWT and PWL, and relieved the neuropathic pain gradually. Blocking HCN2 channels also attenuates chronic visceral pain in irritable bowel syndrome [18]. A recent study revealed that HCN2 channels contribute to the generation and progress of diabetic neuropathic pain [19]. These results indicated that HCN2 channels contribute to the modulation of both the somatosensory and visceral sensory system. This study further confirms that HCN2 channels are involved in the progress of PNP, but the mechanisms are still unclear.
Several studies reported that many inflammatory factors mediate neuropathic pain by directly or indirectly binding to the neuron following different injuries in the nervous system [20]. The local cytokines such as IL-17 and IL-1β are responsible for the initiation and maintenance of chronic neuropathic pain [21]. The exogenous pro-inflammatory factors IL6 and TNF-α can induce pain hypersensitivity [22]. Furthermore, IL-10, an anti-inflammatory factor, could inhibit the progress of neuro-inflammation by reducing the level of pro-inflammatory factors [23]. To further investigate the effects of HCN2 channels on the synthesis of inflammatory factors, we measured the levels of TNF-α, IL-1β, and MCP-1 in the ipsilateral spinal dorsal horns on day 7 after surgery. Our results indicated that inhibition of HCN2 channels decreased the expression of SNI-induced TNF-α, IL-1β, and MCP-1 in ipsilateral spinal dorsal horns on day 7 after surgery. Bringing together our results and those of recent studies, we indicated that the inhibition of HCN2 channels could relieve PNP by reducing the expression of local pro-inflammatory factors. However, the source of the pro-inflammatory factors in ipsilateral spinal dorsal horn is unclear.
Recent studies showed that the activation of astrocytes and microglia in ipsilateral spinal dorsal horn contribute to the initiation and development of PNP [9]. Moreover, the activation of astrocytes and microglia in ipsilateral spinal dorsal horns is concurrent with the release of the pro-inflammatory factors after nerve injury [24]. Therefore, we plan to further explore the cross-talk of HCN2 channels, the activation of astrocytes and microglia, and PNP. In our study, blocking the HCN2 channels by ZD-7288 and si-HCN2 could inhibit the activation of astrocytes and microglia and lessen the degree of PNP. However, whether the pro-inflammatory factors in ipsilateral spinal dorsal horns are derived from the activated astrocytes and microglia remains to be further studied.
NF-κB, an important nuclear transcription factor, has been linked to several diseases including cancer, autoimmune disease, septic shock, inflammatory response and PNP [25]. Activated NF-κB p65 translocates into the nucleus, where it regulates the transcription of downstream genes. Moreover, activated NF-κB is involved in the activation of astrocytes and microglia, and the inflammatory response [26]. Reciprocally, the pro-inflammatory factors also could activate NF-κB signal pathway [26]. Our results showed that inhibiting the activity of the HCN2 channels suppresses the nuclear translocation and phosphorylation of NF-κB, which suggests that NF-κB inhibition is a protective mechanism for blocking HCN2 channels against PNP. However, this potential molecular mechanism needs further investigation.
There are some limitations should be discussed in this study. First, even though the siRNA for HCN2 channels significantly inhibited the expression of the HCN2 channels, the use of HCN2 knockout mice would be a better way to verify the role of the HCN2 channels in the inflammatory response and the NF-κB signaling pathway. Second, as can be seen in the results, pre-treatment with si-HCN2 could not completely alleviate the mechanical allodynia and thermal hyperalgesia, indicating that other factors or the signaling pathway must be involved in neuropathic pain, discoveries that will be determined in future studies.
In conclusion, our studies indicate that the HCN2 channels play a critical role in the initiation and progress of PNP. Decreased HCN2 channel expression attenuates neuropathic pain by inhibiting pro-inflammatory reactions and NF-κB activation. Specific inhibition of the HCN2 channels possibly represents a novel target for PNP treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81560675). All animal experiments were approved by the Hubei University for Nationalities Committee on the Ethics of Animal Experiments (IRB). All treatments were in accordance with the US Public Health Service Policy on the Use of Laboratory Animals. Surgery was performed using hydrochloride plus xylazine anesthesia.
Disclosure of conflict of interest
None.
References
- 1.Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158:261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vollert J, Maier C, Attal N, Bennett DL, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmuhlen J, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tolle TR, Treede RD, Baron R. Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain. 2017;158:1446–1455. doi: 10.1097/j.pain.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang YQ, Sun Q, Tu HY, Wan Y. Characteristics of HCN channels and their participation in neuropathic pain. Neurochem Res. 2008;33:1979–1989. doi: 10.1007/s11064-008-9717-6. [DOI] [PubMed] [Google Scholar]
- 4.Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA. Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain. 2014;155:1708–1719. doi: 10.1016/j.pain.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 5.DiFrancesco JC, DiFrancesco D. Dysfunctional HCN ion channels in neurological diseases. Front Cell Neurosci. 2015;6:174. doi: 10.3389/fncel.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 7.Backryd E, Ghafouri B, Larsson B, Gerdle B. Plasma pro-inflammatory markers in chronic neuropathic pain: a multivariate, comparative, cross-sectional pilot study. Scand J Pain. 2016;10:1–5. doi: 10.1016/j.sjpain.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Tang J, Zhu C, Li ZH, Liu XY, Sun SK, Zhang T, Luo ZJ, Zhang H, Li WY. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. J Neuroinflammation. 2015;12:57. doi: 10.1186/s12974-015-0279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki R, Matsuura Y, Ohtori S, Suzuki T, Kuniyoshi K, Takahashi K. Activation of astrocytes and microglia in the C3-T4 dorsal horn by lower trunk avulsion in a rat model of neuropathic pain. J Hand Surg Am. 2013;38:841–846. doi: 10.1016/j.jhsa.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Liang Y, Qiu Y, Du J, Liu J, Fang J, Zhu J, Fang J. Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation. Acupunct Med. 2016;34:40–47. doi: 10.1136/acupmed-2015-010773. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Yuan W, Wu L, Tang Q, Xia Q, Ji J, Liu Z, Ma Z, Zhou Z, Cheng Y, Shu X. PDGF-D promotes cell growth, aggressiveness, angiogenesis and EMT transformation of colorectal cancer by activation of Notch1/Twist1 pathway. Oncotarget. 2017;8:9961–9973. doi: 10.18632/oncotarget.14283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- 13.Stieber J, Stockl G, Herrmann S, Hassfurth B, Hofmann F. Functional expression of the human HCN3 channel. J Biol Chem. 2005;280:34635–34643. doi: 10.1074/jbc.M502508200. [DOI] [PubMed] [Google Scholar]
- 14.Elinder F, Mannikko R, Pandey S, Larsson HP. Mode shifts in the voltage gating of the mouse and human HCN2 and HCN4 channels. J Physiol. 2006;575:417–431. doi: 10.1113/jphysiol.2006.110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- 16.Maroso M, Szabo GG, Kim HK, Alexander A, Bui AD, Lee SH, Lutz B, Soltesz I. Cannabinoid control of learning and memory through HCN channels. Neuron. 2016;89:1059–1073. doi: 10.1016/j.neuron.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falnikar A, Hala TJ, Poulsen DJ, Lepore AC. GLT1 overexpression reverses established neuropathic pain-related behavior and attenuates chronic dorsal horn neuron activation following cervical spinal cord injury. Glia. 2016;64:396–406. doi: 10.1002/glia.22936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Lin C, Tang Y, Chen AQ, Liu CY, Lu DL. ZD 7288, an HCN channel blocker, attenuates chronic visceral pain in irritable bowel syndrome-like rats. World J Gastroenterol. 2014;20:2091–2097. doi: 10.3748/wjg.v20.i8.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsantoulas C, Lainez S, Wong S, Mehta I, Vilar B, McNaughton PA. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med. 2017;9:eaam6072. doi: 10.1126/scitranslmed.aam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu HM, Wang Q, Sun WB. Silencing of FKBP51 alleviates the mechanical pain threshold, inhibits DRG inflammatory factors and pain mediators through the NF-kappaB signaling pathway. Gene. 2017;627:169–175. doi: 10.1016/j.gene.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Shao Q, Li Y, Wang Q, Zhao J. IL-10 and IL-1beta mediate neuropathic-pain like behavior in the ventrolateral orbital cortex. Neurochem Res. 2015;40:733–739. doi: 10.1007/s11064-015-1521-5. [DOI] [PubMed] [Google Scholar]
- 22.Choi BM, Lee SH, An SM, Park DY, Lee GW, Noh GJ. The time-course and RNA interference of TNF-alpha, IL-6, and IL-1beta expression on neuropathic pain induced by L5 spinal nerve transection in rats. Korean J Anesthesiol. 2015;68:159–169. doi: 10.4097/kjae.2015.68.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine PG. IL10 and neuropathic pain. J Pain Palliat Care Pharmacother. 2008;22:26–27. doi: 10.1080/15360280801989286. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Peng L, Luo J, Yi H, Hu X. Intra-amygdala microinfusion of neuropeptide S attenuates neuropathic pain and suppresses the response of spinal microglia and astrocytes after spinal nerve ligation in rats. Peptides. 2016;82:26–34. doi: 10.1016/j.peptides.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caito SW, Yu Y, Aschner M. Differential inflammatory response to acrylonitrile in rat primary astrocytes and microglia. Neurotoxicology. 2014;42:1–7. doi: 10.1016/j.neuro.2014.02.006. [DOI] [PubMed] [Google Scholar]


