Abstract
Background: Recurrence and metastasis are the most common reasons for the treatment failure of epithelial ovarian carcinoma (EOC). WW domain-containing oxidoreductase (WWOX) is a tumor suppressor, which causes down- or lost-expression and is able to promote cell infiltration and progression in several human malignant tumors. Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), an important marker of cancer stem cells (CSCs), has been considered a useful biomarker of tumor metastasis and patient prognosis. Vasohibin-1 (VASH1), also known as angiogenesis inhibiting protein-1, can be used as a biological marker for early infiltration and metastasis in many cancers. However, the correlations of WWOX, LGR5, and vasohibin-1 in EOC are still unclear. In this study, we analyzed the relationships of these three markers, as well as their respective correlations with clinicopathological characteristics, to determine whether they are useful biomarkers for the improvement and prognosis of EOC patients. Methods: The positive rates of WWOX, LGR5, and vasohibin-1 in 210 whole tissue samples of EOC were detected by immunohistochemistry. Clinical data was also collected. Results: The expressions of LGR5 and vasohibin-1 were significantly higher in EOC tissues than the levels in benign ovary tumors. However, WWOX expression was significantly lower in EOC tissues than the levels in benign ovary tumors. The investigation of the associations between WWOX, or LGR5, or vasohibin-1 positive rates with the clinicopathological characteristics of EOC showed associations between the positive rates of each with grade of tumor, lymph node metastasis (LNM), implantation, and International Federation of Gynecology and Obstetrics (FIGO) stage. The overall survival (OS) time of patients with LGR5-positive or vasohibin-1-positive EOC tissues was significantly shorter than that of those who were negative. On the contrary, the OS time of patients with WWOX-positive EOC tissues was significantly higher than the OS time of those who were negative. Importantly, a multivariate analysis indicated that the high level of WWOX, LGR5, and vasohibin-1, as well as implantation, LNM and FIGO stage could be independent prognostic biomarkers for OS in EOC patients. Conclusions: The expressions of WWOX, LGR5, and vasohibin-1 may represent useful promising biomarkers for metastasis and prognosis, as well as potential therapeutic targets in EOC.
Keywords: Epithelial ovarian carcinoma, WWOX, LGR5, vasohibin-1, prognosis
Introduction
Ovarian cancer, which has the highest incidence of all the gynecologic cancers, is the fifth-leading cause of cancer deaths in women [1]. Worldwide, about 200,000 women are diagnosed with ovarian cancer annually, and 125 000 women die of the disease [2]. It is estimated that there were about 52,100 new cases of ovarian cancer in China in 2015, and about 22,500 cases of patients died from this cancer [3]. Among all of the ovarian cancers, epithelial ovarian cancer (EOC) is the most common histological type, accounting for 85% to 90% of cases approximately, including the subtypes of serous carcinoma, mucous carcinoma, endometrial carcinoma and clear cell carcinoma [4]. It is difficult to diagnose EOC at the early stages due to its insidious onset, and more than 70% of all EOC patients are at the advanced stages when diagnosed. About 50% of all EOC patients also have abdominal diffusion and ascites, which results in a 5-year survival rate of less than 30% [5]. But the detailed pathogenesis of EOC remains unclear. Thus, it is urgent to explore novel and effective candidate biomarkers for EOC, in order to better predict patient prognosis.
Carcinogenesis and tumor development involves multiple steps and complex genetic changes. During the process, both oncogene activation and tumor suppressor gene inactivation play key roles. WW domain-containing oxidoreducatase (WWOX) is a recently discovered tumor suppressor, which resides in human autosomal common 16q23-24 with fragile sites FRA16D. The gene encodes a protein containing a short-chain dehydrogenase/reductase domain (SDR) and two N-terminal WW domains [6]. WWOX expression is always reduced or absent in several human malignant tumors [7]. The patterns of its inactivation usually include loss of heterozygosity [8], translocations [9], promoter hypermethylation [10], and so on. Previous studies have shown that downexpression of WWOX can not only promote the proliferation and inhibit the apoptosis of tumor cells [11], but it can also initiate the process of tumor angiogenesis through several molecules and pathways [12,13], then cause tumor infiltration and progression in the end.
Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5), also known as GPR49, is one of the members of the glycoprotein hormone receptors family. LGR5 has seven transmembrane domains and contains 17 leucine-rich repeats. LGR5, which is an important marker of cancer stem cells (CSCs), is related to the angiogenesis of tumors [14]. Recent studies have demonstrated that LGR5 is overexpressed in cervical cancer [15], gastric cancer [16], ovarian cancer [17], and colorectal cancer [18]. Liu [19] et al. reported that silenced expression of LGR5 could inhibit proliferation and migration in EOC cell lines. Moreover, LGR5 expression was also involved in the tumorigenesis and angiogenesis of EOC, which was induced via the Notch1 pathway.
Angiogenesis is a necessary condition for the growth and progression of malignant tumors and also plays a key role in the evolution of EOC. Vasohibin-1 (VASH1), also known as angiogenesis inhibiting protein-1, is a kind of angiogenesis inhibiting factor synthesized by endothelial cells. Vasohibin-1 can be induced by factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2); then it inhibits the proliferation of endothelial cells and the formation of new blood vessels [20]. Vasohibin-1 can inhibit the growth of ovarian cancer and peritoneal dissemination, further prolonging the survival time of the host, through inhibiting angiogenesis as demonstrated in a mouse model with the ovarian cancer cell line SKOV3 [21]. However, vasohibin-1 expression is up-regulated in a variety of malignancies. It is likely a positive feedback effect against angiogenic stimulating factors, such as VEGF. Therefore, vasohibin-1 may be chosen as a candidate target for antiangiogenic therapy in EOC.
At present, studies about the expression of WWOX, LGR5 and vasohibin-1 in association with invasion and progression in EOC have not been reported widely. In this study, we detected the internal connection of these factors and explored their relationship with invasion, metastasis and prognosis in EOC.
Materials and methods
Patients and tissue samples
210 samples of EOC tissues from patients were collected at Department of Pathology of the First Affiliated Hospital of Bengbu Medical University. All the samples were collected from January 2009 to December 2012. Patients who had not received preoperative chemo- or radiotherapy, or other anti-cancer therapy were selected. 70 cases of ovarian benign epithelial tumors were collected as a control group. The pathological, clinical, and follow-up data were also collected. The calculation of survival time was from the date of surgery to the patient’s death, or to December 2017 (range: 6-105 months). The ages of the patients ranged from 28 to 81 years, and the mean age was 58.3 ± 10.7 years. According to the 2015 version of the International Federation of Gynecology and Obstetrics (FIGO), all samples were evaluated with tumor-node-metastasis (TMN) stage. Pathological grades of EOC were determined according to World Health Organization standards. All the patients in the study signed a written informed consent, and this experiment was conducted after receiving approval from the Ethics Committee of Bengbu Medical University. Additional patient characteristics are shown in Table 1.
Table 1.
Characteristics of patients
| Patients characteristics | Frequency (n) | Percentage (%) |
|---|---|---|
| Age | ||
| < 55 | 104 | 49.5 |
| ≥ 55 | 106 | 50.5 |
| Size | ||
| < 8.0 | 123 | 58.6 |
| ≥ 8.0 | 87 | 41.4 |
| Location | ||
| Left | 89 | 42.4 |
| Right | 87 | 41.4 |
| Bilateral | 34 | 16.2 |
| Type | ||
| Serous | 140 | 66.7 |
| Mucinous | 42 | 20.0 |
| Endometrial | 16 | 7.6 |
| Clear cell | 12 | 5.7 |
| Grade | ||
| Well | 92 | 43.8 |
| Moderate + poor | 116 | 55.2 |
| FIGO stage | ||
| I + II | 101 | 48.1 |
| III + IV | 109 | 51.9 |
| Implantation | ||
| No | 118 | 56.2 |
| Yes | 92 | 43.8 |
| LNM | ||
| No | 148 | 70.5 |
| Yes | 62 | 29.5 |
| Ascite | ||
| No | 126 | 60.0 |
| Yes | 84 | 40.0 |
Immunohistochemical analysis
Immunohistochemical staining of WWOX, LGR5, and vasohibin-1 was performed according to the ElivisionTM Plus detection kit instructions (Lab Vision, USA). All the EOC and ovarian benign tumor tissues were fixed in 4% buffered formalin solution and embedded in paraffin. Continuous 4-μm-thick sections were cut. All sections were deparaffinized and dehydrated in a solution of xylene and graded alcohol and then washed with PBS (pH 7.2) for about 10 min. After antigen repair in the citrate buffer (pH 6.0) at 95°C for 20 min, the endogenous peroxidase activity of all tissues was blocked by 3% H2O2 for 10 min at room temperature (RT). After washing in PBS several times, the sections were blocked with goat serum for 30 min at room temperature, then we incubated them with rabbit polyclonal antibody against human WWOX (Abcam, Cambridge, MA, USA), mouse monoclonal antibody against human LGR5 (Abcam, Cambridge, MA, USA) and vasohibin-1 (Abcam, Cambridge, MA, USA) for 1 h at 37°C. We added a polymer enhancer (reagent A) to the slides at room temperature for 20 minutes. After washing with PBS, goat anti-mouse antibody (reagent B) was added to every section for 30 minutes at room temperature. Lastly, all a freshly prepared diaminobenzidine (DAB) solution was added to the slides, which then were counterstained with hematoxylin, and then they were dehydrated, air-dried and mounted. WWOX+ and vasohibin-1+ stains were mainly seen in the tumor cell cytoplasms. LGR5+ stains were seen in the tumor cell membranes and cytoplasms.
Evaluation of staining
The results of the immunohistochemical expression were evaluated by two independent observers who were blinded to the clinical and follow-up data of all the patients. In order to prevent any intratumoral heterogeneity of antibody expression, ten high-power-fields (HPF) were selected randomly from each EOC section and analyzed. Expressions of the three markers were scored mainly with staining intensity (none: 0; weak: 1; moderate: 2; strong: 3) and extent (positive cells < 11%: 1; positive cells 11-50%: 2; positive cells 51-75%: 3; positive cells > 75%: 4). The final score was the product of intensity and extent which ranged from 0 to 12. Scores ≥ 3 were considered positive.
Statistical analysis
To determine the associations of WWOX/LGR5/vasohibin-1 expression and their correlation with clinical clinicopathological characteristics, we used Fisher’s exact test or a chi-squared test. To determine the correlations between WWOX, or LGR5, or vasohibin-1, we used Spearman’s coefficient test. Survival analysis was assessed using a log-rank test with the Kaplan-Meier method. A Cox regression model for multivariate analysis was used to assess the independent prognostic factors of EOC. All the statistical analyses for the experimental data were calculated with SPSS 19.0 software for Windows (Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Associations between expressions of WWOX/LGR5/vasohibin-1 and clinicopathological characteristics
In order to evaluate the values of WWOX, LGR5, and vasohibin-1 expressions in EOC, their immunohistochemical results were evaluated for tissues of both EOC and control samples. Then all the data were compared to the clinicopathological characteristics of the patients.
The positive rate of WWOX expression was significantly lower in the EOC groups (32.4%, 68/210) than it was in the control tissues (85.7%, 60/70; P < 0.001; Figure 1A and 1B). The positive rate of WWOX expression had an inverse association with histological grade, FIGO stage, implantation, and lymph node metastasis in EOC (P < 0.05). However, no relationship was found with patient age, tumor size, location, histological type, or ascites (Table 2).
Figure 1.
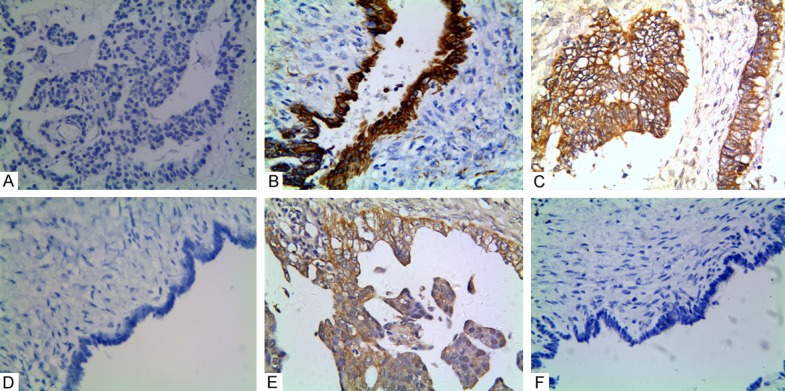
Positive staining of WWOX, or LGR5, or vasohibin-1 in epithelial ovarian carcinoma or the control tissue. A. Negative staining of WWOX in the EOC tissue (400 × magnification); B. Positive staining of WWOX in the cytoplasm of the control cells; C. Positive staining of LGR5 in the membranes and cytoplasms of cancer cells (400 magnification); D. Negative staining of LGR5 in the control tissue (100 × magnification); E. Positive staining of vasohibin-1 in the EOC tissue (400 × magnification); F. Negative staining of vasohibin-1 in the cytoplasms of the control cells (100 × magnification).
Table 2.
The correlation between WWOX, LGR5, vasohibin-1 and clinicopathological characteristics in epithelial ovarian carcinoma
| Variable | WWOX | P | LGR5 | P | Vasohibin-1 | P | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Positive | Negative | Positive | Negative | Positive | Negative | ||||
| Age | 0.377 | 0.400 | 0.889 | ||||||
| < 55 | 37 | 67 | 58 | 46 | 58 | 46 | |||
| ≥ 55 | 31 | 75 | 66 | 40 | 61 | 45 | |||
| Location | |||||||||
| Left | 28 | 61 | 0.075 | 55 | 34 | 0.130 | 52 | 37 | 0.392 |
| Right | 34 | 53 | 45 | 42 | 45 | 42 | |||
| Bilateral | 6 | 28 | 24 | 10 | 22 | 12 | |||
| Size | |||||||||
| < 8.0 | 45 | 78 | 0.123 | 76 | 51 | 0.776 | 67 | 60 | 0.200 |
| ≥ 8.0 | 23 | 64 | 48 | 35 | 52 | 31 | |||
| Type | 0.184 | 0063 | 0.183 | ||||||
| Serous | 39 | 101 | 91 | 49 | 86 | 54 | |||
| Mucinous | 17 | 25 | 21 | 21 | 18 | 24 | |||
| Endometrial | 8 | 8 | 8 | 8 | 8 | 8 | |||
| Clear cell | 4 | 8 | 4 | 8 | 7 | 5 | |||
| Grade | < 0.001 | < 0.001 | < 0.001 | ||||||
| Well | 46 | 48 | 41 | 53 | 34 | 60 | |||
| Moderate + poor | 22 | 94 | 83 | 33 | 85 | 21 | |||
| FIGO stage | < 0.001 | < 0.001 | < 0.001 | ||||||
| I + II | 63 | 38 | 33 | 68 | 22 | 79 | |||
| III + IV | 5 | 104 | 91 | 18 | 97 | 12 | |||
| Implantation | < 0.001 | < 0.001 | < 0.001 | ||||||
| No | 61 | 57 | 49 | 69 | 40 | 78 | |||
| Yes | 7 | 85 | 75 | 17 | 79 | 13 | |||
| LNM | < 0.001 | < 0.001 | < 0.001 | ||||||
| No | 66 | 82 | 73 | 75 | 60 | 88 | |||
| Yes | 2 | 60 | 51 | 11 | 59 | 3 | |||
| Ascite | 0.369 | 0.887 | 0.672 | ||||||
| No | 44 | 82 | 75 | 51 | 73 | 53 | |||
| Yes | 24 | 60 | 49 | 35 | 46 | 38 | |||
The rate of LGR5+ expression in the EOC tissues (59.0%, 124/210) was significantly higher than it was in the control tissues (7.1%, 5/70; P < 0.001; Figure 1C and 1D). The positive rate of MACC1+ expression was related with grade, implantation, LNM and FIGO stage, but not with age, tumor location, size, type, or ascites (Table 2).
In the EOC group, vasohibin-1+ expression was also significantly higher (56.7%, 119/210) than the positive rate in the control group (8.6%, 6/70; P < 0.001; Figure 1E and 1F). Vasohibin-1+ expression was associated with tumor grade, FIGO stage, LNM, and implantation as well, but had no association with patient age, tumor size, location, type, or ascites (Table 2).
Univariate and multivariate analyzes
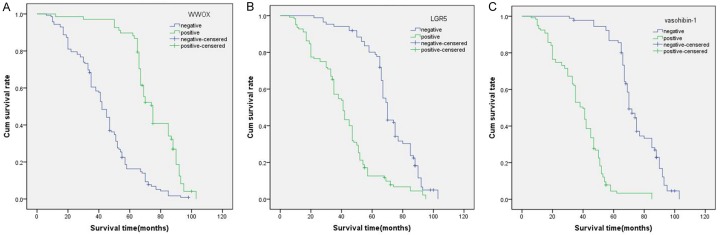
Follow-up data showed that in the EOC group, the overall survival (OS) time for patients with WWOX- samples was significantly shorter (42.73 ± 19.4 months) than it was for those with WWOX+ samples (73.65 ± 15.9 months; log-rank = 70.073, P < 0.001; Figure 2A). Also, OS time was significantly reduced in EOC patients with LGR5+ samples (40.84 ± 19.6 months) when compared with LGR5- samples (69.9 ± 16.666 months) (log-rank = 65.146, P < 0.001) (Figure 2A). Similarly, the OS time of vasohibin-1+ patients (37.45 ± 16.1 months) were significantly shorter than those who were vasohibin-1- (72.74 ± 14.5 months; log-rank = 153.188, P < 0.001; Figure 2B). In other univariate analyses, the OS for the EOC patients had a significant relationship with histological grade (P < 0.001, log-rank = 50.142), FIGO stage (P < 0.001, log-rank = 150.172), implantation (P < 0.001, log-rank = 92.739) and LNM (P = 0.002, log-rank = 141.633; Table 3).
Figure 2.
Kaplan-Meier analysis of the survival rate of patients with epithelial ovarian carcinoma. The y-axis represents the percentage of patients; the x-axis represents their survival in months. A. Overall survival of all patients in relation to WWOX (log-rank = 70.073, P < 0.001); B. Overall survival of all patients in relation to LGR5 expression (log-rank = 65.146, P < 0.001); C. Overall survival of all patients in relation to vasohibin-1 expression (log-rank = 153.188, P < 0.001). In A analyses, the green line represents patients with positive expression of WWOX with a trend of better survival time than the blue line representing the negative WWOX group. In the B and C analyses, the green line represents patients with positive expression of LGR5, or vasohibin-1 with a trend of worse survival time than the blue line representing the negative LGR5, or vasohibin-1 group.
Table 3.
Results of univariate analyses of overall survival time (OST)
| Variable | n | Mean OS (months) | Log-rank | P value |
|---|---|---|---|---|
| WWOX | 70.073 | < 0.001 | ||
| Negative | 142 | 42.73 ± 19.350 | ||
| Positive | 68 | 73.65 ± 15.872 | ||
| LGR5 | 65.146 | < 0.001 | ||
| Negative | 86 | 69.9 ± 16.666 | ||
| Positive | 124 | 40.84 ± 19.579 | ||
| Vasohibin-1 | 153.188 | < 0.001 | ||
| Negative | 91 | 72.74 ± 14.486 | ||
| Positive | 119 | 37.45 ± 16.084 | ||
| Age | 0.174 | 0.677 | ||
| < 55 | 104 | 53.27 ± 24.418 | ||
| ≥ 55 | 106 | 52.25 ± 22.291 | ||
| Location | 5.468 | 0.065 | ||
| Left | 89 | 49.45 ± 24.774 | ||
| Right | 87 | 58.31 ± 22.086 | ||
| Bilateral | 34 | 47.09 ± 19.765 | ||
| Size | 1.926 | 0.165 | ||
| < 8.0 | 127 | 54.01 ± 24.317 | ||
| ≥ 8.0 | 83 | 50.80 ± 21.701 | ||
| Type | 3.200 | 0.362 | ||
| Serous | 140 | 50.84 ± 23.549 | ||
| Mucinous | 42 | 59.31 ± 21.618 | ||
| Endometrial | 18 | 55.44 ± 24.514 | ||
| Clear cell | 12 | 48.33 ± 22.785 | ||
| Grade | 50.142 | < 0.001 | ||
| Well | 94 | 65.84 ± 20.036 | ||
| Moderate + poor | 116 | 42.12 ± 20.236 | ||
| FIGO stage | 150.172 | < 0.001 | ||
| I + II | 101 | 70.19 ± 16.132 | ||
| III + IV | 109 | 36.57 ± 16.205 | ||
| Implantation | 92.739 | < 0.001 | ||
| No | 118 | 65.24 ± 19.269 | ||
| Yes | 92 | 36.71 ± 17.578 | ||
| LNM | 141.633 | < 0.001 | ||
| No | 148 | 62.17 ± 19.750 | ||
| Yes | 62 | 30.23 ± 13.858 | ||
| Ascite | 1.799 | 0.180 | ||
| No | 126 | 53.73 ± 23639 | ||
| Yes | 84 | 51.25 ± 22.886 |
Multivariate analysis showed that a negative expression of WWOX, a positive expression of LGR5 and vasohibin-1, FIGO stage, implantation and LNM were independent prognostic factors for EOC (Table 4).
Table 4.
Results of multivariate analyses of overall survival time (OST)
| Variable | B | SE | P | RR | 95% CI |
|---|---|---|---|---|---|
| WWOX | -0.507 | 0.208 | 0.015 | 0.602 | 0.401-0.905 |
| LGR5 | 0.495 | 0.180 | 0.006 | 1.640 | 1.152-2.334 |
| Vasohibin-1 | 0.916 | 0.254 | < 0.001 | 2.500 | 1.520-4.110 |
| FIGO stage | 0.639 | 0.234 | 0.006 | 1.895 | 1.198-2.997 |
| Implantation | 0.532 | 0.225 | 0.018 | 1.703 | 1.095-2.648 |
| LNM | 0.720 | 0.262 | 0.006 | 2.055 | 1.229-3.436 |
Correlation of WWOX, LGR5 and vasohibin-1 in EOC
Spearman association coefficient analysis showed that there was a negative correlation between expressions of WWOX and LGR5 in EOC (r = -0.603, P < 0.001). And there was also a negative correlation between expressions of WWOX and vasohibin-1 (r = -0.607, P < 0.001). There was a positive association between LGR5 expression and vasohibin-1 expression (r = 0.601, P < 0.001; Table 5).
Table 5.
Correlation among WWOX, LGR5, and vasohibin-1 in ovarian epithelial carcinoma
| Variable | WWOX | r | P | LGR5 | r | P | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Negative | Positive | Negative | Positive | |||||
| LGR5 | -0.603 | < 0.001 | ||||||
| Negative | 29 | 57 | ||||||
| Positive | 113 | 11 | ||||||
| Vasohibin-1 | -0.607 | < 0.001 | 0.601 | < 0.001 | ||||
| Negative | 32 | 59 | 68 | 23 | ||||
| Positive | 110 | 9 | 18 | 101 | ||||
Discussion
As a common gynecological malignant tumor, EOC, which is characterized by poor prognosis and high mortality, seriously threatens women’s health. Therefore, it is urgent to find effective and valuable biomarkers to evaluate EOC biological behavior. WWOX, a tumor suppressor gene, is abnormally expressed in a wide variety of cancers. It has been reported that the loss or reduction of WWOX expression is related to the unfavorable clinical prognosis of many human tumors [22-25]. In addition, low expression of WWOX is related to aggressive tumor phenotypes, including poor differentiation, microvascular invasion, and advanced stages [26]. The current study showed that a lower expression of WWOX promoted EOC progression, invasion, and metastasis. Moreover, a Kaplan- Meier survival indicated that EOC patients of the WWOX+ group had significantly higher survival time than did the WWOX- group. Our results demonstrated that down-regulation of WWOX should promote EOC progression and metastasis as well as confirm the previous findings.
Cancer Stem Cells (CSCs) are a small cluster of cells, which have potential ability of unlimited proliferation and determine the occurrence, development, and invasion of tumors. At the same time, they are also the cause of tumor recurrence, metastasis, and treatment failure. LGR5 is usually recognized as a marker of CSCs, which is the target gene of the Wnt/β-catenin signaling pathway. Recently, several studies showed that LGR5 overexpression participates in the occurrence and progression of various malignant tumors [18,19,27,28]. It was found in this study that the positive rate of LGR5 in EOC tissues was significantly higher than it was in control tissues. It is suggested that a high expression of LGR5 in cancer tissues was likely to have commonality. Meanwhile, this study also showed that the positive rate of LGR5 was associated with histological grade, the number of lymph node metastases, TNM stage, implantation, and the poor prognosis of EOC patients. Our results were similar to those of previous studies.
Angiogenesis is the formation of a neovascularization network which is necessary for tumor growth. Through this process, the body’s blood supply is effectively established, and it also provides tumor cells with oxygen, nutrients and various growth factors. In particular, angiogenesis promotes tumor proliferation and dissemination rapidly. Angiogenesis is often regulated by an angiogenesis promotor and angiogenesis inhibitor and secreted by tumor cells or mesenchymal cells in the tumor microenvironment [29]. Vasohibin-1 was first discovered in vascular endothelial cells, and is considered a new negative-feedback regulator of angiogenesis. It is suggested that the gene may serve as a potential target for anti-tumor angiogenesis [30]. Recently, some reports revealed that vasohibin-1 overexpression is associated with infiltration depth, LNM and TNM stage in various malignant tumors such as colon cancer [31], breast cancer [32], prostate cancer [33], and hepatocellular carcinoma [34]. Our study proved that the overexpression of vasohibin-1 was positively related to tumor grade, FIGO stage, implantation, and LNM in EOC. In addition, our OS analysis indicated that the OS of vasohibin-1+ EOC patients was significantly shorter than it was for the vasohibin-1- group, as well as the OS of LGR5. In the process of tumor vascularization, although the main role of vasohibin-1 is to inhibit angiogenesis, the imbalance between vasohibin-1 and VEGF results in the formation of many new blood vessels with an incomplete structure or the lack of a basement membrane. Further, the significantly increased permeability of the vascular wall causes the invasion and metastasis of tumor cells easily [35]. Therefore, vasohibin-1 should be considered a biological marker for the prediction of early infiltration and metastasis in EOC, as well as for the prognosis of patients with EOC.
It is well known that the most common reason for the high mortality rate in malignant solid tumors is recurrence and metastasis. The FIGO stage is an important guide to decide clinical therapeutic strategies for EOC. But it does not clearly show the biological behavior of EOC. Therefore, finding effective biomarkers to predict tumor growth, metastasis, and patients’ prognosis for EOC is especially urgent. Multivariate Cox regression analysis demonstrated that the expression of WWOX, LGR5, and vasohibin-1, FIGO stage, LNM, as well as implantation, are all independent prognostic factors for EOC patients.
The tumor microenvironment is composed of a large number of microvessels and lymphatic vessels. Previous studies implied that WWOX can inhibit the Wnt/β-catenin pathway [36], and the downregulation of WWOX leads to the accumulation of β-catenin in cytoplasms [37]. Eventually, a high level of β-catenin causes the activation of the Wnt/β-catenin pathway. VEGF is considered a Wnt/β-catenin target, whose expression is up-regulated, thus, the vascular endothelial cells are promoted to proliferate in tumors [38]. At the same time, When LGR5 is activated by its ligand, the degradation of β-catenin is interfered. As its target gene, the levels of VEGF mRNA and protein were dramatically up-regulated by the accumulated β-catenin. Therefore, believe that LGR5 participates in angiogenesis by enhancing Wnt/β-catenin signaling [39]. Both WWOX and LGR5 can regulate tumor angiogenesis via the VEGF pathway. On the other hand, through the positive feedback effect, in tumor tissues, more and more vasohibin-1 are secreted by stimulating by VEGF. The endothelial cells of newborn blood vessels are imperfect in structure and function, and tumor cells can very easy pass through the vessels, a process that results in tumor invasion and metastasis. Moreover, some tumor cells with stem cell functions can also form blood vessels directly [40,41], further promoting the occurrence of metastasis.
Conclusion
This study found that WWOX, LGR5, and vasohibin-1 may play important roles in EOC progression. The combined detection of these indicators will be helpful in evaluating the metastasis and prognosis of EOC patients.
Acknowledgements
This work was supported by the Nature Science Key Program of College and University of Anhui Province (no. KJ2017A224).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012;109:3921–6. doi: 10.1073/pnas.1117135109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberaigner W, Minicozzi P, Bielska-Lasota M, Allemani C, de Angelis R, Mangone L, Sant M. Survival for ovarian cancer in Europe: the across-country variation did not shrink in the past decade. Acta Oncol. 2012;51:441–53. doi: 10.3109/0284186X.2011.653437. [DOI] [PubMed] [Google Scholar]
- 6.Płuciennik E, Nowakowska M, Gałdyszyńska M, Popęda M, Bednarek AK. The influence of the WWOX gene on the regulation of biological processes during endometrial carcinogenesis. Int J Mol Med. 2016;37:807–15. doi: 10.3892/ijmm.2016.2469. [DOI] [PubMed] [Google Scholar]
- 7.Schrock MS, Huebner K. WWOX: a fragile tumor suppressor. Exp Biol Med. 2015;240:296–304. doi: 10.1177/1535370214561590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finnis M, Dayan S, Hobson L, Chenevix-Trench G, Friend K, Ried K, Venter D, Woollatt E, Baker E, Richards RI. Common chromosomal fragile site FRA16D mutation in cancer cells. Hum Mol Genet. 2005;14:1341–9. doi: 10.1093/hmg/ddi144. [DOI] [PubMed] [Google Scholar]
- 9.Lewandowska U, Zelazowski M, Seta K, Byczewska M, Pluciennik E, Bednarek AK. WWOX, the tumour suppressor gene affected in multiple cancers. J Physiol Pharmacol. 2009;60(Suppl 1):47–56. [PubMed] [Google Scholar]
- 10.Baykara O, Demirkaya A, Kaynak K, Tanju S, Toker A, Buyru N. WWOX gene may contribute to progression of non-small-cell lung cancer (NSCLC) Tumour Biol. 2010;31:315–20. doi: 10.1007/s13277-010-0039-3. [DOI] [PubMed] [Google Scholar]
- 11.Hu BS, Tan JW, Zhu GH, Wang DF, Zhou X, Sun ZQ. WWOX induces apoptosis and inhibits proliferation of human hepatoma cell line SMMC-7721. World J Gastroenterol. 2012;18:3020–6. doi: 10.3748/wjg.v18.i23.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trisciuoglio D, Gabellini C, Desideri M, Ragazzoni Y, De Luca T, Ziparo E, Del Bufalo D. Involvement of BH4 domain of bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ. 2011;18:1024–35. doi: 10.1038/cdd.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen J, Xu Z, Li J, Zhang Y, Fan W, Wang Y, Lu M, Li J. Decreased WWOX expression promotes angiogenesis in osteosarcoma. Oncotarget. 2017;8:60917–32. doi: 10.18632/oncotarget.17126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao FJ, Chen JY, Wu HY, Shi J, Chen M, Fan XS, Huang Q. Lgr5 over-expression is positively related to the tumor progression and HER2 expression in stage pTNM IV colorectal cancer. Int J Clin Exp Pathol. 2014;7:1572–4. [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q, Cao HZ, Zheng PS. LGR5 promotes the proliferation and tumor formation of cervical cancer cells through the Wnt/beta-catenin signaling pathway. Oncotarget. 2014;5:9092–105. doi: 10.18632/oncotarget.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bu Z, Zheng Z, Zhang L, Li Z, Sun Y, Dong B, Wu A, Wu X, Wang X, Cheng X, Xing X, Li Y, Du H, Ji J. LGR5 is a promising biomarker for patients with stage I and II gastric cancer. Chin J Cancer Res. 2013;25:79–89. doi: 10.3978/j.issn.1000-9604.2013.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClanahan T, Koseoglu S, Smith K, Grein J, Gustafson E, Black S, Kirschmeier P, Samatar AA. Identification of overexpression of orphan G protein-coupled receptor GPR49 in human colon and ovarian primary tumors. Cancer Biol Ther. 2006;5:419–26. doi: 10.4161/cbt.5.4.2521. [DOI] [PubMed] [Google Scholar]
- 18.de Sousa e Melo F, Kurtova AV, Harnoss JM, Kljavin N, Hoeck JD, Hung J, Anderson JE, Storm EE, Modrusan Z, Koeppen H, Dijkgraaf GJ, Piskol R, de Sauvage FJ. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature. 2017;543:676–80. doi: 10.1038/nature21713. [DOI] [PubMed] [Google Scholar]
- 19.Liu W, Zhang J, Gan X, Shen F, Yang X, Du N, Xia D, Liu L, Qiao L, Pan J, Sun Y, Xi X. LGR5 promotes epithelial ovarian cancer proliferation, metastasis, and epithelial-mesenchymal transition through the Notch1 signaling pathway. Cancer Med. 2018;7:3132–42. doi: 10.1002/cam4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimizu K, Watanabe K, Yamashita H, Abe M, Yoshimatsu H, Ohta H, Sonoda H, Sato Y. Gene regulation of a novel angiogenesis inhibitor, vasohibin, in endothelial cells. Biochem Biophys Res Commun. 2005;327:700–6. doi: 10.1016/j.bbrc.2004.12.073. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi Y, Saga Y, Koyanagi T, Takei Y, Machida S, Taneichi A, Mizukami H, Sato Y, Matsubara S, Fujiwara H. The angiogenesis regulator vasohibin-1 inhibits ovarian cancer growth and peritoneal dissemination and prolongs host survival. Int J Oncol. 2015;47:2057–63. doi: 10.3892/ijo.2015.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lan C, Chenggang W, Yulan B, Xiaohui D, Junhui Z, Xiao W. Aberrant expression of WWOX protein in epithelial ovarian cancer: a clinicopathologic and immunohistochemical study. Int J Gynecol Pathol. 2012;31:125–32. doi: 10.1097/PGP.0b013e3182297fd2. [DOI] [PubMed] [Google Scholar]
- 23.Maeda N, Semba S, Nakayama S, Yanagihara K, Yokozaki H. Loss of WW domain-containing oxidoreductase expression in the progression and development of gastric carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010;457:423–32. doi: 10.1007/s00428-010-0956-y. [DOI] [PubMed] [Google Scholar]
- 24.Chiang MF, Chen ST, Lo CP, Sze CI, Chang NS, Chen YJ. Expression of WW domain-containing oxidoreductase WOX1 in human nervous system tumors. Anal Cell Pathol (Amst) 2013;36:133–47. doi: 10.3233/ACP-140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowakowska M, Płuciennik E, Wujcicka WI, Sitkiewicz A, Kazanowska B, Zielińska E, Bednarek AK. The correlation analysis of WWOX expression and cancer related genes in neuroblastoma- a real time RT-PCR study. Acta Biochim Pol. 2014;61:91–7. [PubMed] [Google Scholar]
- 26.Zhou C, Chen W, Sun J, Atyah M, Yin Y, Zhang W, Guo L, Ye Q, Dong Q, Shi Y, Ren N. Low expression of WW domain-containing oxidoreductase associates with hepatocellular carcinoma aggressiveness and recurrence after curative resection. Cancer Med. 2018;7:3031–43. doi: 10.1002/cam4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cammareri P, Rose AM, Vincent DF, Wang J, Nagano A, Libertini S, Ridgway RA, Athineos D, Coates PJ, McHugh A, Pourreyron C, Dayal JH, Larsson J, Weidlich S, Spender LC, Sapkota GP, Purdie KJ, Proby CM, Harwood CA, Leigh IM, Clevers H, Barker N, Karlsson S, Pritchard C, Marais R, Chelala C, South AP, Sansom OJ, Inman GJ. Inactivation of TGFβ receptors in stem cells drives cutaneous squamous cell carcinoma. Nat Commun. 2016;7:12493. doi: 10.1038/ncomms12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XL, Chen XZ, Wang YG, He D, Lu ZH, Liu K, Zhang WH, Wang W, Li CC, Xue L, Zhao LY, Yang K, Liu JP, Zhou ZG, Hu JK, Mo XM. Clinical significance of putative markers of cancer stem cells in gastric cancer: a retrospective cohort study. Oncotarget. 2016;7:62049–69. doi: 10.18632/oncotarget.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;8:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 30.Heishi T, Hosaka T, Suzuki Y, Miyashita H, Oike Y, Takahashi T, Nakamura T, Arioka S, Mitsuda Y, Takakura T, Hojo K, Matsumoto M, Yamauchi C, Ohta H, Sonoda H, Sato Y. Endogenous angiogenesis inhibitor vasohibin1 exhibits broad-spectrum antilymphangiogenic activity and suppresses lymph node metastasis. Am J Pathol. 2010;176:1950–8. doi: 10.2353/ajpath.2010.090829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Y, Shen Z, Ye Y, Jiang K, Zhang H, Shen C, Mustonen H, Puolakkainen P, Wang S. A novel molecular marker of prognosis in colorectal cancer: vasohibin-1. Med Oncol. 2014;31:816. doi: 10.1007/s12032-013-0816-0. [DOI] [PubMed] [Google Scholar]
- 32.Tamaki K, Moriya T, Sato Y, Ishida T, Maruo Y, Yoshinaga K, Ohuchi N, Sasano H. Vasohibin-1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci. 2009;100:88–94. doi: 10.1111/j.1349-7006.2008.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kosaka T, Miyazaki Y, Miyajima A, Mikami S, Hayashi Y, Tanaka N, Nagata H, Kikuchi E, Nakagawa K, Okada Y, Sato Y, Oya M. The prognostic significance of vasohibin-1 expression in patients with prostate cancer. Br J Cancer. 2013;108:2123–9. doi: 10.1038/bjc.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Tian X, Zhang C, Wang Q. Upregulation of vasohibin-1 expression with angiogenesis and poor prognosis of hepatocellular carcinoma after curative surgery. Med Oncol. 2012;29:2727–36. doi: 10.1007/s12032-011-0106-7. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Zhou K, Wang S, Shi Z, Yang Z. Recombinant adenovirus encoding vasohibin prevents tumor angiogenesis and inhibits tumor growth. Cancer Sci. 2010;101:448–52. doi: 10.1111/j.1349-7006.2009.01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. Inhibition of the Wnt/beta-catenin pathway by the WWOX tumor suppressor protein. APMIS. 2013;121:120–6. doi: 10.1038/onc.2009.120. [DOI] [PubMed] [Google Scholar]
- 37.Bouteille N, Driouch K, Hage PE, Sin S, Formstecher E, Camonis J, Lidereau R, Lallemand F. WWOX is a novel inhibitor of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2569–80. doi: 10.1038/onc.2009.120. [DOI] [PubMed] [Google Scholar]
- 38.Liebner S, Plate KH. Differentiation of the brain vasculature: the answer came blowing by the Wnt. J Angiogenes Res. 2010;2:1. doi: 10.1186/2040-2384-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 40.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 41.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C, Tabar V. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]