Abstract
Radioresistance remains a challenge during nasopharyngeal carcinoma (NPC) radiotherapy. Numerous studies suggest that the miRNAs may play important roles in the regulation of radioresistance. miRNA-17-5p, which is located within the miR-17-92a cluster, could modulate tumor progression in different tissues by targeting multiple tumor associated genes. However, whether it is correlated with the radioresistance of tumor cells has not yet been elucidated. In our study, we have observed increasing miR-17-5p expression in radioresistant NPC tissues. The functional experiments suggested that miR-17-5p could clearly promote NPC cell proliferation and the cell cycle even after X-ray irradiation. Irradiation leads to tumor cell damage and death via ROS generation. The overexpression of miR-17-5p could protect NPC cells from apoptosis induced by irradiation. In addition, an in vivo experiment indicated that miR-17-5p promoted tumor growth with radiotherapy using the xenograft tumor model. A bioinformatics analysis and reporter assay were carried out to demonstrate that PTEN, which is a key regulator of AKT phosphorylation, is a target of miR-17-5p. The overexpression of miR-17-5p directly suppresses the mRNA and protein expression of PTEN. In addition, the rescue experiments showed that the AKT inhibitor can diminish the proliferation, promotion, and apoptosis inhibition effects on radioresistant NPC cells mediated by miR-17-5p. In conclusion, our findings demonstrated that miR-17-5p can enhance the radioresistance of NPC through the PTEN/AKT pathway, which is a biomarker of radioresistant NPC and a potential target for new therapeutic strategies.
Keywords: Nasopharyngeal carcinoma, radioresistance, miR-17-5p, PTEN, AKT, ROS
Introduction
Nasopharyngeal carcinomas (NPCs), which occur frequently in East and Southeast Asia, the Middle East, North Africa, and the Arctic [1], differ from other head and neck malignancies in their pathogenesis, clinical progression, and management strategy [2,3]. Radiotherapy is the primary and most effective strategy for treating NPCs, and NPCs at the early stage can be treated with radiotherapy alone with five year survival rates of more than 90% [4]. However, NPC patients often are diagnosed at advanced stages with only a less than 50% survival rate over 5 years. A major obstacle to achieving long-term survival is radioresistance [5]. Since NPC radioresistance is complicated, it is important that studies reveal the mechanisms underlying this process, even though several molecules have already been shown to affect NPC radioresistance [6].
The microRNAs (miRNAs) are highly conserved, endogenous, small noncoding RNAs which contain 19-21 nucleotides (nt) and suppress gene expression post-transcriptionally by targeting the 3’ untranslated regions of mRNAs [7]. Increasing evidence shows that miRNAs can regulate the molecular pathogenesis of cancer as oncogenes or anti-oncogenes. Recent studies have shown that certain miRNAs are intimately involved in NPC tumorigenesis and are associated with NPC radioresistance [8-10]. However, the miRNAs often work as a network. Individual miRNAs have multiple targets, and one gene could also be modulated by variant miRNAs. The functional diversity of miRNAs makes them able to be considered as early diagnosis biomarkers or new therapeutic targets.
miRNA-17-5p, miR-18a, and miR-20a are all located within the miR-17-92a cluster, which plays important roles in tumor progression by targeting multiple tumor-associated molecules [11]. Several studies have indicated that miR-17-5p plays significant roles in the cell cycle regulation of cancer cells in different tissues, such as breast cancer [12], hepatocellular carcinoma, [13] and ovarian carcinoma [14]. In the present study, we found that the miR-17-5p expression was significantly upregulated in the NPC tissues of radioresistant patients. Further study showed that miR-17-5p promotes NPC radioresistance through the PTEN/AKT signaling pathway. These findings suggest that the miR-17-5p is a potential treatment target and provide new therapeutic strategies for NPC radioresistance.
Materials and methods
Human tissue samples
All human NPC tissues were obtained from patients undergoing surgery for nasopharyngeal carcinoma in the Department of Maxillofacial Surgery, People’s Liberation Army 113th Hospital. The tumor samples were classified according to the patients’ sensitivity to the subsequent radiotherapy. The patients’ detailed clinical information is provided in Table S1. Written informed consent conforming to the tenets of the Declaration of Helsinki was obtained from each participant, and the study procedures were approved by the Institutional Review Board of the hospital.
Tumor xenograft
Eight week-old nude mice (BALB/cA-nu) were purchased from the Beijing Laboratory Animal Research Center (Beijing Academy of Science and Technology, Beijing, China) and maintained in specific pathogen-free conditions. Six mice which all weighed close to 25 g were randomly divided into two groups. CNE2 cells were transfected with a miR-17-5p mimic or a negative control and injected subcutaneously on one side of the lateral back of each mouse with 2 × 106 cells in two groups. Seven days after the injections, the mice were subjected to radiotherapy. Tumor growth was monitored by measuring tumor length (L) and width (S) with a sliding caliper (tumor size = 0.51 × L × W2). All animal experiments were approved by the Animal Experiment Administration Committee of People’s Liberation Army 113th Hospital, and in accordance with the recommendations of Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23, revised 1985).
Cell culture and transfection
The radiosensitive human NPC cell line CNE2 was cultured in RPMI-1640 medium (Gibico, Waltham, MA) supplemented with 10% fetal bovine serum (FBS) (Gibico), 2 mM glutamine (Gibico) and 1% penicillin/streptomycin (Gibico). To perform the irradiation treatment, the cells in a logarithmic growth phase were irradiated by X-ray with doses of 10 Gy in a 15 cm × 15 cm radiation field at 100 cm of source-skin distance and 285 cGy/min rate by the accelerator (SIEMENS Corporation, Germany).
The NPC cells at a confluence of 70% to 80% were prepared to be transfected with miRNA by using LipofectamineTM 2000 (Invitrogen). The oligonucleotides were chemically synthesized as a commercial service provided by GenePharma Company (GenePharma, Shanghai, China) and transfected into NPC cells at a final concentration of 50 nmol/L according to the manufacturer’s instructions. The treated cells were cultured in a complete medium for definite periods of time and then harvested for further experiments. All the cells were incubated at 37°C in an atmosphere of 5% CO2.
Real-time PCR
The total RNAs were extracted from human tissue specimens of both radiosensitive and radioresistant patients (15 patients each) or cell lines with TRizol reagent (Invitrogen, Waltham, MA). The cDNA was reverse-transcribed using a TaqMan MicroRNA Reverse Transcription kit (ThermoFisher Scientific, Waltham, MA). Real-time PCR was performed to detect the miR-17-92a clusters’ and the downstream molecules’ expression levels by using the TaqMan Fast Universal PCR Master Mix (ThermoFisher Scientific) and the CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The primers specific for mature miRNAs and PTEN were synthetized by Qiagen (Qiagen, Venlo, Netherlands) and are shown in Table S2. Their mRNA levels were measured with U6 or GAPDH as internal controls, respectively.
Cell cycle
The cell cycle distribution was determined using a BD Accuri™ C6 Plus Flow Cytometer (BD, Franklin Lakes, NJ). Briefly, the cells were collected and fixed in ice cold ethanol (70% PBS) overnight at 4°C. The cells were treated with 20 g/ml RNase A (Sigma, St. Louis, MO) for 1 h at 37°C to degrade the RNA and then were incubated with 50 μg/ml propidium iodide (Sigma) in the dark. The DNA content was analyzed by flow cytometry and all phases of the cell cycle were analyzed by proper gating on the distribution plot.
Cell proliferation
The cell proliferation was detected by both colony formation ability and MTT analysis after the miRNA was overexpressed. The cells transfected with miRNA mimics or control oligonucleotides were seeded at a density of 1000 cells/well into the 6-well plate and incubated for 3 hours to allow attachment before variant doses of radiation treatment. The radiated cells were further incubated for 3 weeks until the control cells formed colonies. After they were removed from the medium and rinsed in PBS, the colonies were fixed and stained with 0.5% crystal violet solution (Sigma) for 2 hours at room temperature. Then the samples were washed completely with double distilled water and examined by a light microscope (Olympus, Tokyo, Japan) to count the number of colonies.
Similarly, the miR-17-5p overexpressed or negative control CNE2 cells were seeded into a 96-well plates and evaluated cell proliferation at 24, 48, 72, and 96 h after X-ray irradiation using the methyl thiazolyl tetrazolium (MTT) reagent (5 mg/ml in phosphate-buffered saline) (Sigma). After incubation for 4 h at 37°C, the supernatant was carefully removed, and the precipitation was dissolved in DMSO (Sigma). Spectrophotometric absorbance was measured at the wavelength of 570 nm by a microplate reader (BioTek Instruments Inc., Winooski, VT).
Cell apoptosis
The cell apoptosis was examined by both Tunel staining and flow cytometry analysis. For the Tunel staining, the cells with different treatments were seeded in 96-well plates. After overnight incubation, the TUNEL assay was performed using the Click-iT® TUNEL Alexa Fluor® Imaging Kit (Invitrogen) in accordance with the manufacturer’s protocol. In brief, the cells were fixed with 4% paraformaldehyde and permeabilized with Triton X-100 (0.25% in PBS) for 20 min and washed with PBS. Then the cells were treated with a terminal deoxynucleotidyl transferase (TdT) reaction buffer for 10 min at room temperature. The TdT reaction cocktail was added to incubate the cells in a humidified chamber at 37°C for 60 min. The cells was incubated with the Click-iT reaction mixture for 30 min and counterstained with Hoechst (Thermo Fisher Scientific) for 15 min at room temperature. The TUNEL-positive cells were counted in random fields for each well.
Meanwhile, the apoptosis was also examined using a Dead Cell Apoptosis Kit with FITC-Annexin V and PI (ThermoFisher Scientific). Briefly, FITC-Annexin V and propidium iodide were added into the single cell suspension and incubated at room temperature for 15 min. Afterwards, 400 μl of 1 × Annexin-binding buffer was added and mixed gently for further analysis by flow cytometry.
ROS generation assay
The collected cells were stained by 2’,7’-dichlorofluorescein diacetate (DCFDA) (Abcam, Cambridge, MA) and incubated for 30 min at 37°C. Then the flow cytometry was used to measure the fluorescence intensity.
Luciferase report assay
The fragments of wild type and mutated 3’-UTRs of PTEN were amplified by PCR from human cDNA library and inserted into a pMir-Report vector (Ambion, Waltham, MA). The primers are shown in Table S2. CNE2 cells were prepared and the luciferase reporter plasmids bearing 3’-UTRs of PTEN were transfected with miR-17-5p oligonucleotides and a pRL-TK vector. The cells were harvested and lysed with a lysis buffer 24 h later (Promega, Madison, WI). The relative luciferase activity was read out using the Dual Luciferase Reporter Assay System (Promega, Madison, WI, USA) and normalized by the relative activity of Renilla. Each experiment was performed at least five times and the data were analyzed with the Student’s t-test.
Western blotting analysis
For Western blotting analysis, the cells were harvested and lysed on ice for 30 min within the RIPA buffer supplemented with protease inhibitors. The cells lysates were centrifuged, and the supernatants were collected as total proteins. After the concentrations of protein samples were determined by the BCA method (Beyotime, Haimen, China), an equal amount of each sample was separated by SDS-PAGE and transferred onto a PVDF membrane. The membranes were then incubated with primary antibodies for PTEN, total AKT, phospho-AKT (Abcam, Cambridge, MA), and β-actin (Boster Bio Tec, Wuhan, China) at the indicated dilution. After washing three times, the membranes were incubated with an HRP conjugated secondary antibody and visualized with an ECL detection system. The protein expression was measured by ImageJ software.
Statistical analyses
The experiments were repeated independently at least three times, and the data was presented as the mean ± standard deviation. A Student’s-t test or a one-way ANOVA with Turkey’s multiple comparison test was performed for the comparisons between groups. The statistical results are expressed as the mean ± SEM. P < 0.05 was considered significant and P < 0.01 was considered strong significance.
Results
The miR-17-5p expression was significantly upregulated in radioresistant NPCs
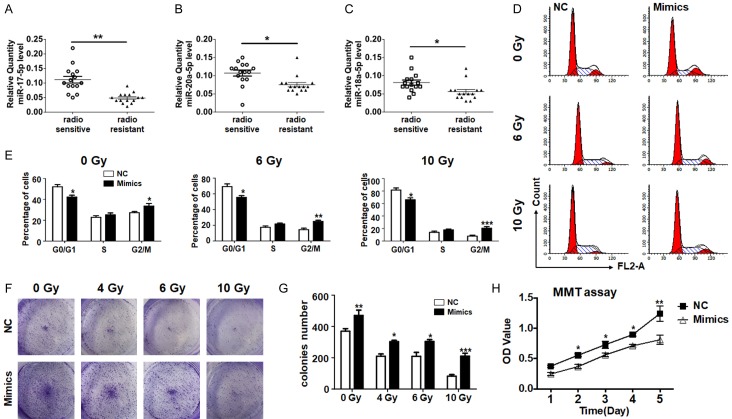
The miRNA array analysis from several groups indicated that the miR-17-92a clusters, as onco-miRs, were increased in different tumor tissues and modulated tumor development [15-17]. It should be noted that miR-17, miR-18a, and miR-20a, located within the miR-17-92a cluster, share similar expressions and are highly abundant in tumors [15]. In order to validate the roles of these miRNAs on tumor cell radioresistance, we measured the expressions of these three miRNAs in frozen NPC tissues from both radiosensitive and radioresistant patients using RT-PCR. All the examined miRNAs were increased in the radioresistant patients, among which miR-17-5p was altered most obviously more than twofold higher than the control samples (Figure 1A-C). These results suggest that the miR-17-92a cluster, especially miR-17-5p, displays different expressions in radiosensitive and radioresistant NPC tissues and might participate in NPC radioresistance modulation.
Figure 1.
MiR-17-5p decreased expression in radioresistant NPCs and elevated the proliferation of NPC cells after irradiation. A-C. miR-17, miR-18a, and miR-20a expression in radiosensitive and radioresistant NPCs, and each group contained 15 samples. D, E. NPC cells were transfected with miR-17-5p mimics or a negative control (NC) and treated with different does of irradiation. The cell cycle was measured and the percentage of each phase was calculated (n = 5). F, G. NPC cells transfected with miR-17-5p mimics or a negative control were treated with different doses of radiation. The colonies numbers were counted and analyzed after 3 weeks (n = 5). H. The growth curve of NPC cells transfected miR-17-5p followed by 10 Gy irradiation was determined by MTT assay in the initial 5 days (n = 5). *: P < 0.05, **: P < 0.01.
The influence of miR-17-5p on CNE2 proliferation after irradiation
We wondered whether miR-17-5p was involved in cell proliferation regulation and examined the cell cycles of NPC cells with different treatments. The results showed that the cell cycle of the NPC cells was blocked at the G0/G1 phase with irradiation doses raising (Figure 1D, 1E). Meanwhile miR-17-5p accelerated NPC cell division and the cell cycle impairment mediated by variant does of irradiation could also be partially retrieved by miR-17-5p transfection (Figure 1D, 1E). The data indicated that miR-17-5p could promote the cell cycle of NPC cells.
A colony formation assay and an MTT experiment were carried out to determine the NPC cell proliferation. Irradiation with x-rays reduced the colony formation of CNE2 cells significantly and the number of colonies number in an irradiation dose dependent way. In addition, miR-17-5p overexpression could increase the number of colonies compared with the oligonucleotide control group under all doses of irradiation (Figure 1F, 1G). The MTT experiment also showed that the viability of cells transfected with miR-17-5p was much higher than the control cells in the initial 5 days (Figure 1H). These data suggest that miR-17-5p promotes NPC cell proliferation.
miR-17-5p inhibited CNE2 cell apoptosis after irradiation via blocking ROS generation
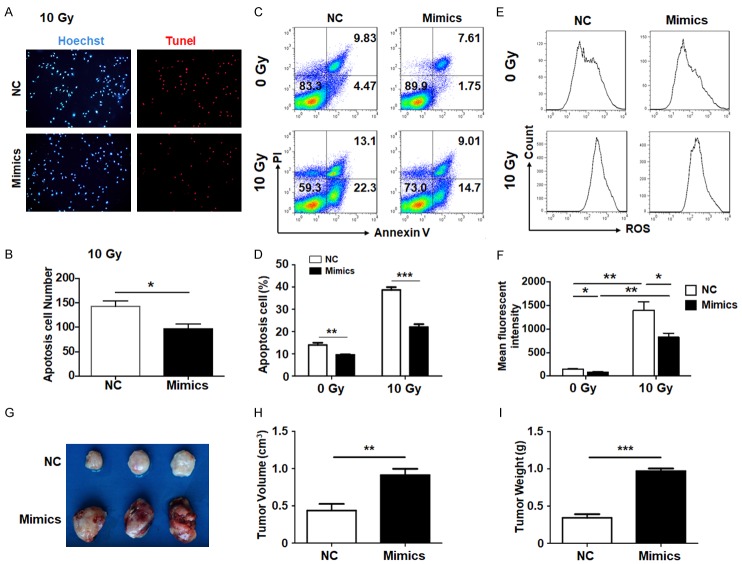
Increasing proliferation was always accompanied with avoiding apoptosis, which is one of the main hallmarks of malignant tumors. Different experiments were carried out to detect the effect of miR-17-5p on NPCs apoptosis. The TUNEL staining results showed that cell apoptosis was frequent in one field after irradiation. However, when miR-17-5p was transfected, the apoptosis rate was significantly decreased (Figure 2A, 2B). Meanwhile, the Annexin V/PI measurement by flow cytometry also reached the same conclusion. The apoptosis of the NPC cells was inhibited by forced miR-17-5p overexpression, when the CNE2 cells were treated with or without x-ray irradiation (Figure 2C, 2D). These data indicate that miR-17-5p reduced the apoptosis of NPC cells induced by irradiation.
Figure 2.
Cell apoptosis mediated by ROS was attenuated and tumorigenesis was promoted by miR-17-5p. (A-D). NPC cells were transfected with miR-17-5p mimics or a negative control (NC) and followed by 10 Gy irradiation. TUNEL (A, B) and Annexin V/PI (C, D) staining were performed to detect the apoptotic cells (n = 5). (E, F) The NPC cells were treated as (A) and the non-irradiation groups as controls. The ROS generation was analyzed with a mean fluorescence index by flow cytometry (n = 5). (G-I) The NPC cells were stably transfected with miR-17-5p or a negative control (NC) and inoculated subcutaneously into the back of mice followed by irradiation treatment. The tumors’ volume (H) and weight (I) were determined (n = 3). *: P < 0.05, **: P < 0.01.
Cell apoptosis induced by irradiation can be viewed as a series of cascading events, which begins with cellular damage, is followed by the activation of cell death regulatory genes and ends with cell destruction and the apoptotic corpses’ removal. It has been demonstrated that irradiation could induce ROS accumulation and result in DNA damage and cell injury [18,19]. In some models, the miR-17-92 cluster promoted tumor progression by cancelling ROS generation [20]. To figure out the mechanism of the protective effect against apoptosis during irradiation by miR-17-5p, intracellular ROS generation was determined by flow cytometry. The result indicated that ROS generation, which was greatly induced by X-ray irradiation, could be sharply decreased by miR-17-5p transfection (Figure 2E, 2F). The above data demonstrate that miR-17-5p could protect NPC cells from apoptosis by inhibiting the production of ROS.
NPC cells were stably transfected with miR-17-5p or a vehicle and inoculated subcutaneously into the abdomen of mice followed by the irradiation treatment. It was notable that the NPCs with the miR-17-5p overexpression grew much faster than the control group in both volume and weight (Figure 2G-I), which indicated that miR-17-5p could also accelerate tumor growth in vivo.
MiR-17-5p suppressed PTEN expression and promoted downstream AKT phosphorylation
It is known that miRNAs regulate gene expression mainly through post transcriptional modification. To identify targets regulated by miR-17-5p that modulate NPC cell function, we predicted the target genes using several bioinformatic algorithms (Target Scan, PicTar, and miRDB). The prediction results showed that 3’-UTR of PTEN harbored two recognized sites of miR-17-5p, which indicated PTEN was a candidate target of miR-17-5p. The bioinformatics analysis displayed that the 3’ UTR of PTEN harbored two conserved miR-17-5p recognized sites, which were located from 2236 to 2242 bp and from 3138 to 3144 bp (Figure 3A). We constructed the reporter assays vectors, including the wild type fragment (con), the first site mutated fragment (mut1), the second site mutated fragment (mut2), and both sites mutated fragment (mut3). We found that the activation of luciferase with the wild type fragment was repressed by the miR-17-5p mimics; however, the fragments with mutations from either site would lose their suppressing function (Figure 3B). The results indicated miR-17-5p modulated PTEN expression and was dependent on both of the two recognized sites in 3’ UTR.
Figure 3.
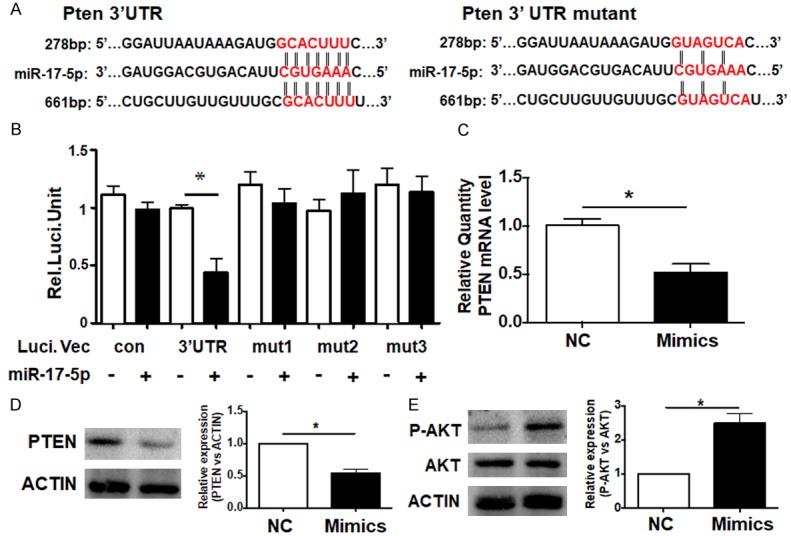
miR-17-5p suppressed PTEN/AKT signaling pathway. A. The 3’ UTR of PTEN matched with the recognition site of miR-17-5p. The seed sequence is marked in red. B. The plasmids with the 3’ UTR of PTEN (3’ UTR), the mutated 3’ UTR regions (mut1, mut2 and mut3) or the vehicle (con) were co-transfected into NPC cells with an miR-17-5p mimic or control respectively. 24 h after transfection, luciferase activity was determined (n = 5). C and D. The mRNA and protein levels of PTEN were measured after miR-17-5p overexpression (n = 5). E. The activation of the AKT signal was detected by western blot (n = 5). *: P < 0.05.
To confirm the target of miR-17-5p, RT-PCR and western blot were performed to measure the PTEN expression. It was clear that miR-17-5p inhibited PTEN expression at both the mRNA and protein levels (Figure 3C, 3D). The PTEN/PI3K/AKT pathway is critically involved in multiple cellular events including proliferation, survival, and apoptosis [21]. In many cells and models, PTEN suppression always induces the activation of AKT signaling [22]. Therefore, the total AKT and phosphorylated AKT (p-AKT) levels were also determined in CNE2 cells overexpressing miR-17-5p, which suggests that miR-17-5p significantly promotes AKT activation (Figure 3E). The above data demonstrated miR-17-5p could specifically repress PTEN expression and then activate AKT signaling.
The miR-17-5p-PTEN-AKT axis is involved in cell proliferation and apoptosis regulation after irradiation
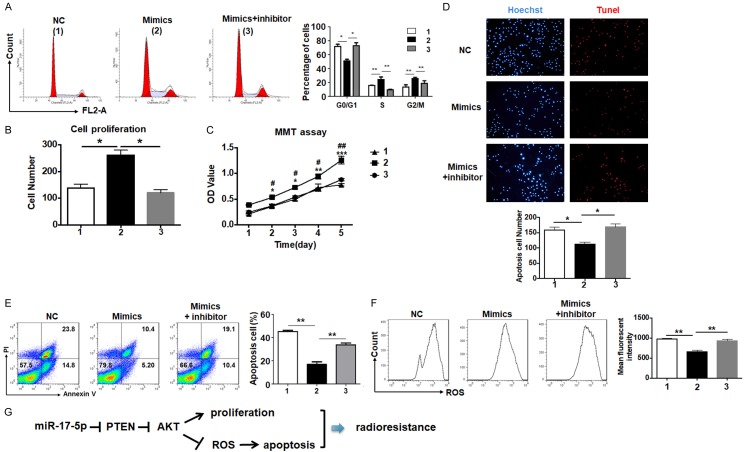
To validate miR-17-5p regulated NPC cells’ radioresistance by targeting PTEN, we measured the cell cycle of the CNE2 cells after miR-17-5p overexpression in and without the presence of the AKT inhibitor. The result showed that the AKT inhibitor could totally rescue the promotion of the cell cycle mediated by miR-17-5p (Figure 4A). Meanwhile, the colony formation and MTT assay also led to the same conclusion that NPC cells’ proliferation motivated by miR-17-5p was significantly reduced by the AKT inhibitor (Figure 4B, 4C).
Figure 4.
Mir-17-5p’s effect on NPC cell radioresistance was retrieved by the AKT signaling blockade. A. CNE2 cells overexpressed miR-17-5p in the presence of the AKT inhibitor were detected in the cell cycle using flow cytometry (n = 5). B. The number of colonies were counted in the CNE2 cells with same treatment as above (n = 5). C. CNE2 cells with same treatment as above were cultured in 96-well plates and the growth curve was measured (n = 5). D. The apoptosis of CNE2 cells treated as above was detected using TUNEL staining (n = 5). E. The apoptosis of the CNE2 cells treated as above was determined with Annexin V/PI staining followed by flow cytometry analysis (n = 5). F. The intracellular ROS generation was detected by flow cytometry and quantified by a mean fluorescence index (n = 5). Note: 1: NC; 2: mimics; 3: mimics + AKT inhibitor. G. The schematic diagram of miR-17-5p protecting the NPC cells from irradiation damage. *: P < 0.05, **: P < 0.01.
Moreover, the TUNEL and Annexin V/PI staining were carried out to illustrate the apoptosis affected by the AKT inhibitor. The TUNEL positive cells were rare in the miR-17-5p transfected group even with irradiation. However, the apoptotic rate recovered to a normal level when the AKT inhibitor was added (Figure 4D). The Annexin V/PI measurement also suggested that the AKT inhibitor retrieved apoptosis (Figure 4E). As the main factor to induce cell apoptosis by irradiation, ROS generation was also rescued by the AKT signal blockade (Figure 4F). As the above data showed that the additional AKT inhibitor could totally diminish the effects on NPC cells mediated by miR-17-5p, it is confirmed that miR-17-5p could enhance the radioresistance of NPCs through the PTEN-AKT signaling pathway (Figure 4G), which may be a potential target to develop new therapeutic strategies.
Discussion
NPCs are radiosensitive at the early stages with a greater than 90% survival rate for five years, which declines with tumor progression to only 30-50%. Unfortunately, quite a lot of patients have been in the advanced stages at the time of their initial diagnosis. The progressive NPCs with malignant proliferation and apoptotic resistance are always radioresistant [23-25]. Especially for recurrent NPC, re-irradiation is associated with severe complications, which could even be the primary cause of death [26]. Therefore, a further understanding of the molecular mechanisms involved in NPC radioresistance is necessary and will provide better strategies for NPC therapy.
Accumulating evidence suggests that multiple miRNAs play important roles in tumor development and radioresistance regulation, including miR-100, miR-21, and miR-95 [27-29]. In fact, numerous miRNAs are involved in the modulation of multiple tumor suppressors and oncogenes [30]. In this study, we found that the miR-17-5p expression was significantly upregulated in radioresistant NPC tissues. This suggests that it might play a crucial role in the regulation of radioresistance. Further studies should confirm that miR-17-5p protects NPC cells from radiation injury by promoting proliferation and inhibiting apoptosis. The death and apoptosis of tumor cells could result from many factors, such as the effect of NK cells and T cells. Also, the ROS generation mediated by irradiation could induce tumor cell injury, and it has been considered as an indication of the initiation of apoptosis [18,31,32]. Our results demonstrated that miR-17-5p obviously decreased ROS production after irradiation, which should be the mechanism miR-17-5p uses to protect NPC cells from damage. The in vivo experiment also indicated that miR-17-5p attenuated the radiotherapy outcome of NPCs. These findings suggest that miR-17-5p should be a key factor in the regulation of NPC radioresistance.
Through the algorithm prediction and luciferase report assay, we have confirmed that PTEN is a specific target of miR-17-5p in the CNE2 cells. PTEN has been shown by many research groups to modulate the progression of multiple tumors, such as hepatocellular carcinoma [13] and glioblastoma [33]. On the other hand, miR-17-5p has far more than one target in different tumor cells. Yang et al. found that miR-17-5p inhibited TIMP3 expression to induce prostate tumor growth and invasion [34]. It also promoted breast cancer cell proliferation by targeting AIB1 [12]. These findings indicate that miR-17-5p works in a network, and the mathematical method may be applied to understand these complicated connections [35]. PTEN is a protein phosphatase, whose substrates include phosphatidyl-inositol, 3, 4, 5 triphosphate (PIP3). Increasing activation of PIP3 recruits AKT onto the membrane to be activated by other kinases. Numerous publications indicate that the PTEN/PI3K/AKT signaling pathway is involved in tumor development and acts as an important target in clinical medicine and pharmaprojects [36,37]. From our demonstration, the protective effects of miR-17-5p with irradiation are canceled by the AKT inhibitor, suggesting that the PTEN/AKT pathway may be the downstream signal cascade of miR-17-5p. It should be noted that miR-17-5p is not the only miRNA for PTEN silencing. For example, miR-21 could regulate the expression of PTEN in hepatocellular cancer [38]. As mentioned above, this regulation network of miRNAs enables an extensive supervision of gene expression, which may help to maintain homeostasis under different conditions [39].
It is unclear how the AKT pathway can help NPC cell survival during radiotherapy. One possible way may be the autophagy activation. Autophagy is known as a central procedure to clear damaged organelles or other intracellular parts [40]. The AKT activation will result in suppression on mammalian targets of rapamycin (mTOR) [41], which is precisely a trigger of autophagy signaling [42]. Therefore, we infer that the radiotherapy will directly cause intracellular organelle damage and the AKT mediated autophagy is activated in order to survive. The miR-17-5p may play a critical role in the PTEN/AKT/mTOR pathway to regulate autophagy activation.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Ningbo (2013A610215 and 2014A610230).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–1777. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 2.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. 2016;387:1012–1024. doi: 10.1016/S0140-6736(15)00055-0. [DOI] [PubMed] [Google Scholar]
- 3.Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Sze WM, Au JS, Leung SF, Leung TW, Chua DT, Zee BC, Law SC, Teo PM, Tung SY, Kwong DL, Lau WH. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 5.Kristensen CA, Kjaer-Kristoffersen F, Sapru W, Berthelsen AK, Loft A, Specht L. Nasopharyngeal carcinoma. Treatment planning with IMRT and 3D conformal radiotherapy. Acta Oncol. 2007;46:214–220. doi: 10.1080/02841860600635862. [DOI] [PubMed] [Google Scholar]
- 6.Guo Y, Zhu XD, Qu S, Li L, Su F, Li Y, Huang ST, Li DR. Identification of genes involved in radioresistance of nasopharyngeal carcinoma by integrating gene ontology and protein-protein interaction networks. Int J Oncol. 2012;40:85–92. doi: 10.3892/ijo.2011.1172. [DOI] [PubMed] [Google Scholar]
- 7.Eulalio A, Huntzinger E, Izaurralde E. Getting to the root of miRNA-mediated gene silencing. Cell. 2008;132:9–14. doi: 10.1016/j.cell.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc Natl Acad Sci U S A. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang M, Xiao J, Wang J, Zhou P, Wei T, Zhao T, Wang R. MiR-24 enhances radiosensitivity in nasopharyngeal carcinoma by targeting SP1. Cancer Med. 2016;5:1163–1173. doi: 10.1002/cam4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu JQ, Yi HM, Ye X, Zhu JF, Yi H, Li LN, Xiao T, Yuan L, Li JY, Wang YY, Feng J, He QY, Lu SS, Xiao ZQ. MiRNA-203 reduces nasopharyngeal carcinoma radioresistance by targeting IL8/AKT signaling. Mol Cancer Ther. 2015;14:2653–2664. doi: 10.1158/1535-7163.MCT-15-0461. [DOI] [PubMed] [Google Scholar]
- 11.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y, Takahashi T. A polycistronic microRNA cluster, miR-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 12.Hossain A, Kuo MT, Saunders GF. Mir-17-5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol. 2006;26:8191–8201. doi: 10.1128/MCB.00242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shan SW, Fang L, Shatseva T, Rutnam ZJ, Yang X, Du W, Lu WY, Xuan JW, Deng Z, Yang BB. Mature miR-17-5p and passenger miR-17-3p induce hepatocellular carcinoma by targeting PTEN, GalNT7 and vimentin in different signal pathways. J Cell Sci. 2013;126:1517–1530. doi: 10.1242/jcs.122895. [DOI] [PubMed] [Google Scholar]
- 14.Fang Y, Xu C, Fu Y. MicroRNA-17-5p induces drug resistance and invasion of ovarian carcinoma cells by targeting PTEN signaling. J Biol Res. 2015;22:12. doi: 10.1186/s40709-015-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molinari C, Salvi S, Foca F, Teodorani N, Saragoni L, Puccetti M, Passardi A, Tamberi S, Avanzolini A, Lucci E, Calistri D. miR-17-92a-1 cluster host gene (MIR17HG) evaluation and response to neoadjuvant chemoradiotherapy in rectal cancer. Onco Targets Ther. 2016;9:2735–2742. doi: 10.2147/OTT.S105760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S, Liu L, Chang EB, Wang JY, Raufman JP. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol Cancer. 2015;14:180. doi: 10.1186/s12943-015-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo J, Mei Y, Li K, Huang X, Yang H. Downregulation of miR-17-92a cluster promotes autophagy induction in response to celastrol treatment in prostate cancer cells. Biochem Biophys Res Commun. 2016;478:804–810. doi: 10.1016/j.bbrc.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50–64. doi: 10.1016/j.semcdb.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Liu Q, Sun Y, Lv Y, Le Z, Xin Y, Zhang P, Liu Y. TERT alleviates irradiation-induced late rectal injury by reducing hypoxia-induced ROS levels through the activation of NF-kappaB and autophagy. Int J Mol Med. 2016;38:785–793. doi: 10.3892/ijmm.2016.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebi H, Sato T, Sugito N, Hosono Y, Yatabe Y, Matsuyama Y, Yamaguchi T, Osada H, Suzuki M, Takahashi T. Counterbalance between RB inactivation and miR-17-92 overexpression in reactive oxygen species and DNA damage induction in lung cancers. Oncogene. 2009;28:3371–3379. doi: 10.1038/onc.2009.201. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Ramirez C, Canadas-Garre M, Molina MA, Faus-Dader MJ, Calleja-Hernandez MA. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics. 2015;16:1843–1862. doi: 10.2217/pgs.15.122. [DOI] [PubMed] [Google Scholar]
- 22.Carnero A, Paramio JM. The PTEN/PI3K/AKT Pathway in vivo, Cancer mouse models. Front Oncol. 2014;4:252. doi: 10.3389/fonc.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan L, Yi HM, Yi H, Qu JQ, Zhu JF, Li LN, Xiao T, Zheng Z, Lu SS, Xiao ZQ. Reduced RKIP enhances nasopharyngeal carcinoma radioresistance by increasing ERK and AKT activity. Oncotarget. 2016;7:11463–11477. doi: 10.18632/oncotarget.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di J, Cao H, Tang J, Lu Z, Gao K, Zhu Z, Zheng J. Rap2B promotes cell proliferation, migration and invasion in prostate cancer. Med Oncol. 2016;33:58. doi: 10.1007/s12032-016-0771-7. [DOI] [PubMed] [Google Scholar]
- 26.Zheng XK, Ma J, Chen LH, Xia YF, Shi YS. Dosimetric and clinical results of three-dimensional conformal radiotherapy for locally recurrent nasopharyngeal carcinoma. Radiother Oncol. 2005;75:197–203. doi: 10.1016/j.radonc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Li XH, Qu JQ, Yi H, Zhang PF, Yi HM, Wan XX, He QY, Ye X, Yuan L, Zhu JF, Li JY, Xiao ZQ. Integrated analysis of differential miRNA and mRNA expression profiles in human radioresistant and radiosensitive nasopharyngeal carcinoma cells. PLoS One. 2014;9:e87767. doi: 10.1371/journal.pone.0087767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang XD, Xu XH, Zhang SY, Wu Y, Xing CG, Ru G, Xu HT, Cao JP. Role of miR-100 in the radioresistance of colorectal cancer cells. Am J Cancer Res. 2015;5:545–559. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang S, Chen Y. Abnormal expression of miR-21 and miR-95 in cancer stem-like cells is associated with radioresistance of lung cancer. Cancer Invest. 2015;33:165–171. doi: 10.3109/07357907.2015.1019676. [DOI] [PubMed] [Google Scholar]
- 30.Kent OA, Mendell JT. A small piece in the cancer puzzle: microRNAs as tumor suppressors and oncogenes. Oncogene. 2006;25:6188–6196. doi: 10.1038/sj.onc.1209913. [DOI] [PubMed] [Google Scholar]
- 31.Lee CH, Yu HS. Role of mitochondria, ROS, and DNA damage in arsenic induced carcinogenesis. Front Biosci (Schol Ed) 2016;8:312–320. doi: 10.2741/s465. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Karakhanova S, Hartwig W, D’Haese JG, Philippov PP, Werner J, Bazhin AV. Mitochondria and mitochondrial ros in cancer: novel targets for anticancer therapy. J Cell Physiol. 2016;231:2570–2581. doi: 10.1002/jcp.25349. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Yang BB. Stress response of glioblastoma cells mediated by miR-17-5p targeting PTEN and the passenger strand miR-17-3p targeting MDM2. Oncotarget. 2012;3:1653–1668. doi: 10.18632/oncotarget.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang X, Du WW, Li H, Liu F, Khorshidi A, Rutnam ZJ, Yang BB. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucleic Acids Res. 2013;41:9688–9704. doi: 10.1093/nar/gkt680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai X, Wolkenhauer O, Vera J. Understanding microRNA-mediated gene regulatory networks through mathematical modelling. Nucleic Acids Res. 2016;25:6188–96. doi: 10.1093/nar/gkw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 37.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 38.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 Regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 2007;133:647–658. doi: 10.1053/j.gastro.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wu Z, Cheng F, Li W, Liu G, Tang Y. Computational prediction of microRNA networks incorporating environmental toxicity and disease etiology. Sci Rep. 2014;4:5576. doi: 10.1038/srep05576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 42.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.