Abstract
Objective: It has been shown that JAK2/STAT3 is involved in the occurrence of various inflammatory diseases. The purpose of this study was to associate the expression of Janus kinase 2 (JAK2) and its receptor signal transducer and activator of transcription 3 (STAT3) and suppressors of cytokine signaling 3 (SOCS3), to periapical granuloma. Methods: Samples were collected from 40 patients who were divided into two groups, namely, healthy control (N=20) and periapical granuloma (N=20) groups in accordance with classified standards. The samples were prepared for histological analysis, immunohistochemistry, and double immunofluorescence staining. Results: Only slight inflammatory cell infiltration was observed in the tissues from the healthy control group. Extensive infiltration of inflammatory cells was observed in patients with chronic periapical disease. The periapical granuloma group had higher levels of JAK2, STAT3, p-JAK2, p-STAT3 and SOCS3 (all P<0.05) than the control group. Double immunofluorescence staining results showed the presence of JAK2-positive and STAT3-positive cells in the periapical lesion areas. Conclusions: This study demonstrated that JAK2, STAT3, and SOCS3 can be observed and may be associated with the inflammatory process in periapical lesions. The results of this study will provide new insights into the pathological mechanisms of human periapical granuloma.
Keywords: JAK2, inflammation, periapical granuloma, SOCS3, STAT3
Introduction
Janus kinase (JAK)/signal transducer and activator of transcription (STAT) plays an important role in regulating apoptosis, proliferation, differentiation, and inflammatory response [1]. JAK2/STAT3 participates in diverse inflammatory processes and in the development of bone destructive diseases, such as arthritis, Alzheimer’s disease, and osteosarcoma [2-4]. Juglanin represses the inflammatory response and apoptosis in rats with hepatitis induced by lipopolysaccharide (LPS) for fructose feeding via the JAK2/STAT3 signaling pathway [5]. JAK2 can be used by a substantial number of key class I cytokine receptors for signaling, such as growth hormone (GH) , erythropoietin, interleukin (IL)-3, IL-6, interferon-γ, and leptin [6-8]. GH can regulate bone growth and bone metabolism via the activation of JAK2 [9]. The lack of GH can affect the immune system and bone density [10]. Leptin can increase the amount of B cells expressing CD25 and human leukocyte antigen DR and activates the B cells to secrete cytokines by activating the JAK2/STAT3 pathway, which contributes to their immunoregulatory and inflammatory properties [11]. STATs are cytoplasmic transcription factors that play a critical role in cell fate [12]. STAT3 participates in inflammatory disease, such as periodontitis, head and neck cancer, and heatstroke [13-16]. STAT3 knock-out mice show an osteoporotic phenotype [17]. Through the JAK2/STAT3 pathway, leukemia inhibitory factor enhances fibroblast growth factor-stimulated IL-6 synthesis in osteoblast; thus, the JAK2/STAT3 pathway participates in the homeostasis of bone [17,18]. JAK2-STAT3 elicits a pro-inflammatory response in electromagnetic field (EMF)-stimulated microglia and may thus be a therapeutic target for reducing the pro-inflammatory response of EMF-activated microglial cells [19].
The protein called suppressor of cytokine signaling 3 (SOCS3) is the inhibitor of JAK2/STAT3 and can induce a loss of STAT3 activity. This process mainly occurs when SOCS3 accumulates and the binding of SOCS3 to JAK2 and TYK2 is reinforced and causes the ubiquitination and degradation of JAK2 and TYK2 [20].
STAT3 is an oncogene and can be used as a therapeutic target in many neoplastic diseases, and the inhibitor of JAK2/STAT3 can function as a potential therapeutic strategy for patients with osteosarcoma [3].
The above evidence suggests that the JAK2/STAT3 pathway is involved in the inflammatory development of various diseases and is an essential component of osteoclast activation in pathological bone resorption, and SOCS3 may disrupt some inflammatory processes.
Apical periodontitis is a common oral disease that is mainly caused by the accumulation of bacteria in the pulp. If these bacteria remain untreated, they proliferate further, causing numerous inflammatory cells to infiltrate around the root apex and numerous osteoclasts to accumulate around the root apex, thereby leading to bone destruction and apical periodontitis development [21]. Diverse inflammatory mediators and osteoclastic factors, such as IL-27, the cytokine receptor activator of nuclear factor ĸB-ligand (RANKL), and the toll-like receptor (TLR)-4, are associated with periradicular lesions [22-24]. Despite increasing knowledge of the pathogenesis of periapical lesions, the exact regulatory cytokines associated with periapical potential pathological mechanisms during its development remain unclear. To our knowledge, no study on the role of JAK2/STAT3/SOCS3 in the development of apical periodontitis has been conducted. In this study, we hypothesized that JAK2/STAT3/SOCS3 is involved in the development of inflammation during the development of periapical granuloma. Hopefully, our experiments may shed new light on the treatment of apical lesions and can help improve the success rate of treatment for teeth with periapical lesion.
Materials and methods
Experimental design
40 patients (16 females and 24 males with ages ranging from 18 to 40) who had visited the dental clinic of Qingdao University Affiliated Hospital between June 2016 and February 2017 were enrolled in this study. Patients who were pregnant, who had a systematic or infectious disease, or who had taken antibiotic therapy in the last 6 months were excluded. There were no statistically significant differences in sex and age between the groups in this study. The experimental protocol was approved by the Ethical Committee on Human Rights Related to Human Experimentation, Qingdao University. The participants were informed of the risks and benefits and signed an approved informed consent document prior to enrollment.
The tissues were divided into two groups according to established criteria, such as X-ray results, operative exploration, and histopathological findings.
Samples from the healthy control group were extracted from the periodontal membranes of premolars extracted for orthodontics; the premolars exhibited healthy periapical tissues and complete root formation. The inclusion criteria were as follows: ① the presence of vital teeth; ② the absence of a low-density radiolucent shadow or a high-density radiolucent shadow in the X-ray examination results; ③ a smooth and continuous edge of the periodontal ligament, and the absence of injury in the extraction process; and ④ the absence of significant capillary hyperplasia and inflammatory cell infiltration under histological observation.
In the periapical granuloma group, samples were extracted from the periapical tissues of the patients with untreatable periapical diseases. The tissues had no retention value. The inclusion criteria were as follows: ① teeth without value; ② X-ray image showing the presence of an ovoid or round low-density radiolucent shadow in the teeth root apex; ③ the presence of periapical solid soft-tissue lesions during tooth extraction; and ④ histological observations revealing apical granuloma, capillary hyperplasia, and inflammatory cell infiltration.
Immunohistochemistry
Tissues were extracted from patients and then fixed in 10% buffered formalin for 24-48 h. The specimens were dehydrated and embedded in paraffin. Sections (4 μm) of fixed embedded specimens were cut, placed on glass slides, and deparaffinized. Rabbit polyclonal antibodies against JAK2 (elabscience, E-AB-15734), STAT3 (elabscience, E-AB-15734), p-JAK2 (elabscience, E-AB-21017), P-STAT3 (elabscience, E-AB-32981) and SOCS3 (Abcam, 16030) were used as primary antibodies at 4°C overnight. The sections were washed and stained with an SP kit (ZSGB-BIO) in accordance with the manufacturer’s manual. Adequate washing of the slides with fresh water was required for each step, and 3,30-diaminobenzidine (ZSGB-BIO) was used as chromogen. The slides were counterstained with hematoxylin. The stained specimen sections on the slides were analyzed under a microscope.
Double immunofluorescence labeling
The sections were rehydrated and then treated with 0.3% Triton X-100 (Sigma-Aldrich) for 5 min at room temperature. Bovine serum albumin (1%) was used for preventing unspecific staining for 1.5 h. The sections were then incubated at 4°C overnight with JAK2 rabbit polyclonal antibody, and then subjected to Alexa Fluor 555 labeling of donkey against rabbit lgG (H+L) (Beyotime Biotechnology, Shanghai, China).The process was repeated for the mouse polyclonal antibodies against human STAT3 for 1 h at 37°C, and then Alexa fluor 488-labeled goat anti mouse IgG (H+L) (Beyotime Biotechnology, Shanghai, China) was added for 1 h at room temperature. Subsequently, the nuclei were revealed after counterstaining 40,6-diamidino-2-phenylindole. A fluorescent microscope with a camera was then used for obtaining figures. The tissue sections were observed by two blinded researchers using a fluorescence microscope with a camera (Leica, Wetzlar, Germany).
Cell counting and statistical analysis
In each specimen, JAK2-, STAT3-, p-JAK2-, p-STAT3-, and SCOS3- positive cells were counted under high-power magnification (400×). Counting was performed by two blinded observers randomly picking up 5 visual fields in the areas. The data of this study were presented as the mean±standard deviation (SD) and analyzed using SPSS16. The significance level was set to two sided. Values with P<0.05 at α=0.05 were considered significant. A t-test for equality of means was applied in the comparison of the average densities of the JAK2-, STAT3-, p-JAK2-, p-STAT3-, and SCOS3-positive cells. A correlation analysis was performed between JAK2 and STAT3, P-JAK2 and P-STAT3 in periapical granuloma tissues, respectively.
Results
Histological observation
Only slight inflammatory cell infiltration was observed in the healthy tissues (Figure 1A), but thin-walled capillaries and hyperplastic fibroblasts and extensive inflammatory cell infiltration were observed in the granulomatous tissues (Figure 1B).
Figure 1.
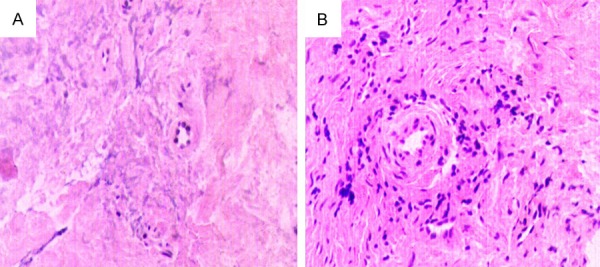
Histological observations of tissues both from the healthy and periapical granuloma groups using H E staining. A. A small number of infiltrating inflammatory cells were observed in the healthy group specimens. B. Numerous infiltrating inflammatory cells, capillaries, and fibroblasts were observed in the periapical granuloma specimens. (original magnification, ×400).
Immunohistochemical observation
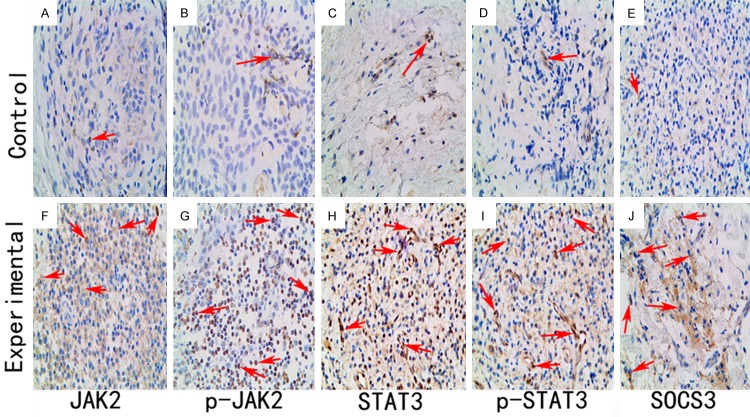
The JAK2-, STAT3-, p-JAK2-, p-STAT3-, and SCOS3-positive cells were stained brown via immunohistochemical staining. The JAK2-, STAT3-, p-JAK2-, p-STAT3-, and SCOS3-positive cells could be observed in both healthy (Figure 2A-E) and periapical (Figure 2F-I) tissues. The number of JAK2-, STAT3-, p-JAK2-, p-STAT3- and SOCS3-positive cells was significantly increased in the lesion tissues. Statistically significant differences are listed in Table 1. A correlation analysis shows that there is a significant correlation between JAK2 and STAT3 (R=0.912) and between p-JAK2 and p-STAT3 (R=0.917), as shown in Figure 3A, 3B.
Figure 2.
Immunohistochemical observations of tissues from both the healthy and periapical granuloma groups. A. Almost no JAK2-positive cells were stained in specimens from the healthy group. B. Slight immunoreactions for p-JAK2 occurred in the healthy group specimens. C. A small number of STAT3-positive cells were observed in healthy group specimens. D. A few p-STAT3-positive cells were stained in the healthy specimens. E. A small number of SCOS3-positive cells were stained in the healthy group specimens. F. Numerous JAK2-positive cells were stained in specimens from the periapical granuloma group. G. Intense immunoreactions for p-JAK2 occurred in the periapical granuloma group. H. A large number of STAT3-positive cells were stained in the periapical granuloma specimens. I. Many p-STAT3-positive cells were observed in periapical granuloma specimens. J. Numerous SCOS3 -positive cells were stained in the periapical granuloma specimens. (original magnification, ×400).
Table 1.
Number of JAK2, P-JAK2, STAT3, P-STAT3 and SOCS3-positive Cells per 100 cells (×400) Each Group (Mean±Standard Deviation)
| Group | N | JAK2 cells/hpf | P-JAK2 cells/hpf | STAT3 cells/hpf | P-STAT3 cells/hpf | SOCS3 cells/hpf |
|---|---|---|---|---|---|---|
| Control | 20 | 10.95±2.58* | 11.6±3.60* | 21.95±4.51* | 8.40±3.08* | 3.30±1.53* |
| Experimental | 20 | 68.3±7.80* | 69.55±6.87* | 56.75±7.79* | 56.90±5.01* | 45.9±5.33* |
Statistical analysis was performed by using a t-test.
Means P<0.05, Control group versus the Experimental group.
Figure 3.
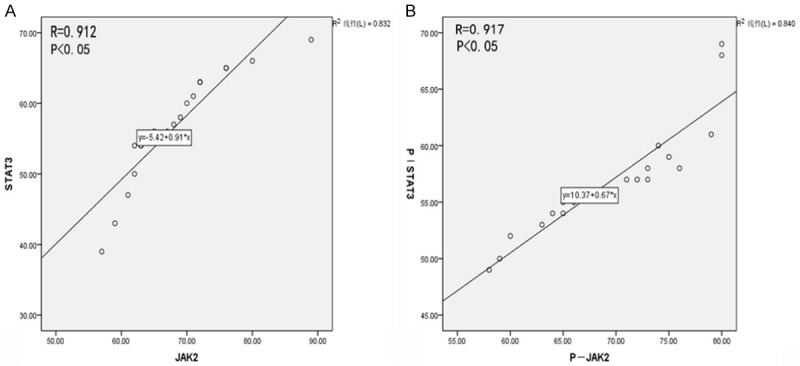
Correlation analysis between JAK2 and STAT3 (A, B).
Double immunofluorescence labeling
Double immunofluorescence staining resulted in JAK2-positive (Red) and STAT3-positive (Green) cells in the healthy group (Figure 4A-D) and the periapical granuloma group (Figure 4E-H). The merged image indicates that some of the JAK2-positive cells were positive for STAT3.
Figure 4.
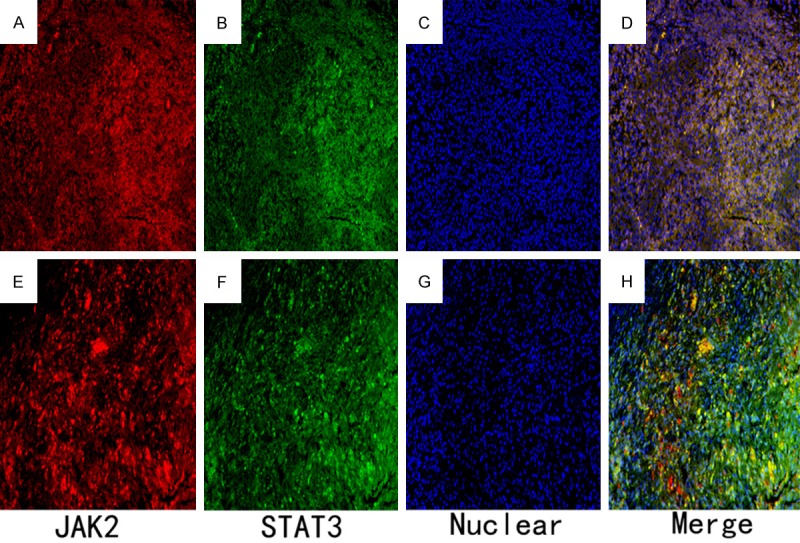
Double immunofluorescence staining observations for the healthy group and the periapical granuloma group. Immunofluorescence staining for the JAK2- and STAT3-positive cells was conducted followed by Alexa Fluor 555 labeling of donkey against rabbit lgG (H+L) (red) and Alexa Fluor 488-labeled goat anti mouse IgG (H+L) (green). Nuclear counterstaining was performed using 40,6-diamidino-2-phenylindole (blue). A. Slightly JAK2-positive cells were observed in the healthy control group. B. Minimal intense immunoreactions for STAT3-positive cells were also found in healthy control group. C. Nuclears were stained for the healthy control group. D. The merged image indicates that some JAK2-positive cells are positive for STAT3. E. Intense immunoreactions for JAK2-positive cells were observed in periapical granuloma group. F. Numerous STAT3-positive cells were also found in periapical granuloma group. G. Nuclears were stained for granuloma group. H. The merged image suggests that most JAK2-positive cells are also positive for STAT3 (×200).
Discussion
Apical periodontal diseases involve the inflammation and destruction of periapical tissues, and inflammatory responses occur as a consequence of endodontic pathogens. Bacteria and bacterial toxin lead to the infiltration of inflammatory cells and the production of a proinflammatory cytokine reaction, ultimately stimulating the formation of granulomas and cysts and bone destruction [25]. Many inflammatory cytokines and osteoclastic factors participate in the inflammatory response of periapical disease, such as IL-27 and RANKL [22,23]. In the current study, we speculated that JAK2/STAT3 plays an important role in the pathogenesis of apical periodontitis.
The JAK2/STAT3 signal transduction pathway is involved in the development of inflammation and the osteoclast activity of cells [3,19]. Western blot results of EMF-treated N9 microglial cells show a significant increase in p-JAK2 and p-STAT3, suggesting that the JAK2-STAT3 pathway promotes pro-inflammatory responses in EMF-stimulated microglial cells [19]. As an anti-proinflammatory propound, swertiamarin helps reduce the inflammation of the experimental adjuvant arthritis animal model by the inhibition of JAK2/STAT3 and NF-κB/IκB [2]. Hydroxy-safflor yellow A protects the induced Alzheimer’s disease model by inhibiting the inflammatory response, and this process may involve the JAK2/STAT3/NF-κB pathway [26].
In the immunohistochemical observation in the current study, numerous JAK2-, p-JAK2-, STAT3-, and p-STAT3-positive cells were observed in the periapical lesions. The double immunofluorescence labeling result also showed numerous JAK2- and STAT3-positive cells in periapical disease. Only a few JAK2-, p-JAK2-, STAT3-, and p-STAT3-positive cells could be found in healthy tissues. These results may indicate that JAK2 and STAT3 work together to participate in the inflammatory process of periapical lesions. A connection between JAK2/STAT3 and the periapical lesion inflammatory process exists.
PCR and Western blot results showed that by JAK2/STAT3 activation, leptin can stimulate human B cells to secrete inflammatory cytokines and participate in an inflammatory response, but the ability is lost when the JAK2/STAT3 inhibitor is added [11]. We found that the expression of SOCS3 was higher in the experimental group. The increased expression of SOCS3 may be the result of the increased expression of STAT3. The increase of SOCS3 may reduce the activation of STAT3 and thus reduce inflammation, which may explain the inhibitory effect of SOCS3 on the JAK2/STAT3 pathway and the protective role of SOCS3 on inflammation.
The observations reported in this paper demonstrate the increased expression of JAK2 and STAT3 in chronic periapical disease. Although some significant results were obtained, some limitations in the present study must be addressed. This study mainly detected the expression of JAK2, STAT3, and SOCS3 and described their role in chronic periapical disease. However, the specific mechanisms involved in the processes remain elusive, and further investigations are still necessary. Further studies on the pathogenic mechanism of periapical periodontitis will be summarized in our next study.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81500834).
Disclosure of conflict of interest
None.
References
- 1.Chatterjee-Kishore M, van den Akker F, Stark GR. Association of STATs with relatives and friends. Trends Cell Biol. 2000;10:106–111. doi: 10.1016/s0962-8924(99)01709-2. [DOI] [PubMed] [Google Scholar]
- 2.Saravanan S, Islam VI, Babu NP, Pandikumar P, Thirugnanasambantham K, Chellappandian M, Raj CS, Paulraj MG, Ignacimuthu S. Swertiamarin attenuates inflammation mediators via modulating NF-kappaB/I kappaB and JAK2/STAT3 transcription factors in adjuvant induced arthritis. Eur J Pharm Sci. 2014;56:70–86. doi: 10.1016/j.ejps.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Yan J, Wang Q, Zou K, Wang L, Schwartz EB, Fuchs JR, Zheng Z, Wu J. Inhibition of the JAK2/STAT3 signaling pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep. 2015;12:498–502. doi: 10.3892/mmr.2015.3439. [DOI] [PubMed] [Google Scholar]
- 4.Chiba T, Yamada M, Aiso S. Targeting the JAK2/STAT3 axis in Alzheimer’s disease. Expert Opin Ther Targets. 2009;13:1155–1167. doi: 10.1517/14728220903213426. [DOI] [PubMed] [Google Scholar]
- 5.Zhou GY, Yi YX, Jin LX, Lin W, Fang PP, Lin XZ, Zheng Y, Pan CW. The protective effect of juglanin on fructose-induced hepatitis by inhibiting inflammation and apoptosis through TLR4 and JAK2/STAT3 signaling pathways in fructose-fed rats. Biomed Pharmacother. 2016;81:318–328. doi: 10.1016/j.biopha.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Watling D, Guschin D, Müller M, Silvennoinen O, Witthuhn BA, Quelle FW, Rogers NC, Schindler C, Stark GR, Ihle JN, et al. Complementation by the protein tyrosine kinase JAK2 of a mutant cell line defective in the interferon-gamma signal transduction pathway. Nature. 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 7.Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 8.Waters MJ, Brooks AJ. JAK2 activation by growth hormone and other cytokines. Biochem J. 2015;466:1–11. doi: 10.1042/BJ20141293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morales O, Faulds MH, Lindgren UJ, Haldosén LA. 1Alpha,25-dihydroxyvitamin D3 inhibits GH-induced expression of SOCS-3 and CIS and prolongs growth hormone signaling via the Janus kinase (JAK2)/signal transducers and activators of transcription (STAT5) system in osteoblast-like cells. J Biol Chem. 2002;277:34879–34884. doi: 10.1074/jbc.M204819200. [DOI] [PubMed] [Google Scholar]
- 10.Slootweg MC. Growth hormone and bone. Horm Metab Res. 1993;25:335–343. doi: 10.1055/s-2007-1002115. [DOI] [PubMed] [Google Scholar]
- 11.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abroun S, Saki N, Ahmadvand M, Asghari F, Salari F, Rahim F. STATs: an old story, yet mesmerizing. Cell J. 2015;17:395–411. doi: 10.22074/cellj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao Z, Cheng M, Wang SC, Lv W, Hu HQ, Li CF, Cao BZ. JAK2/STAT3 pathway mediating inflammatory responses in heatstroke-induced rats. Int J Clin Exp Pathol. 2015;8:6732–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Geiger JL, Grandis JR, Bauman JE. The STAT3 pathway as a therapeutic target in head and neck cancer: barriers and innovations. Oral Oncol. 2016;56:84–92. doi: 10.1016/j.oraloncology.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Souza JA, Nogueira AV, de Souza PP, Cirelli JA, Garlet GP, Rossa C Jr. Expression of suppressor of cytokine signaling 1 and 3 in ligature-induced periodontitis in rats. Arch Oral Biol. 2011;56:1120–8. doi: 10.1016/j.archoralbio.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 16.Leong PL, Andrews GA, Johnson DE, Dyer KF, Xi S, Mai JC, Robbins PD, Gadiparthi S, Burke NA, Watkins SF, Grandis JR. Targeted inhibition of stat3 with a decoy oligonucleotide abrogates head and neck cancer cell growth. Proc Natl Acad Sci U S A. 2003;100:4138–4143. doi: 10.1073/pnas.0534764100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh S, Udagawa N, Takahashi N, Yoshitake F, Narita H, Ebisu S, Ishihara K. A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone. 2006;39:505–512. doi: 10.1016/j.bone.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 18.Kozawa O, Otsuka T, Uematsu T. Leukemia inhibitory factor enhances bFGF-induced IL-6 synthesis in osteoblasts: involvement of JAK2/STAT3. Cell Signal. 2002;14:311–315. doi: 10.1016/s0898-6568(01)00248-0. [DOI] [PubMed] [Google Scholar]
- 19.Yang X, He G, Hao Y, Chen C, Li M, Wang Y, Zhang G, Yu Z. The role of the JAK2-STAT3 pathway in pro-inflammatory responses of EMF-stimulated N9 microglial cells. J Neuroinflammation. 2010;7:54. doi: 10.1186/1742-2094-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng HY, Cheng YC, Hsu YM, Wu GH, Kuo CC, Liou JP, Chang JY, Jin SL, Shiah SG. MPT0B098, a microtubule inhibitor, suppresses JAK2/STAT3 signaling pathway through modulation of SOCS3 stability in oral squamous cell carcinoma. PLoS One. 2016;11:e0158440. doi: 10.1371/journal.pone.0158440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segura-Egea JJ, Castellanos-Cosano L, Machuca G, López-López J, Martín-González J, Velasco-Ortega E, Sánchez-Domínguez B, López-Frías FJ. Diabetes mellitus, periapical inflammation and endodontic treatment outcome. Med Oral Patol Oral Cir Bucal. 2012;17:e356–361. doi: 10.4317/medoral.17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vernal R, Dezerega A, Dutzan N, Chaparro A, León R, Chandía S, Silva A, Gamonal J. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006;12:283–289. doi: 10.1111/j.1601-0825.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Wang R, Huang SG. Immunomodulatory activity of interleukin-27 in human chronic periapical diseases. Am J Transl Res. 2017;9:1460–1470. [PMC free article] [PubMed] [Google Scholar]
- 24.Leonardi R, Perrotta RE, Loreto C, Musumeci G, Crimi S, Dos Santos JN, Rusu MC, Bufo P, Barbato E, Pannone G. Toll-like receptor 4 expression in the epithelium of inflammatory periapical lesions. An immunohistochemical study. Eur J Histochem. 2015;59:2547. doi: 10.4081/ejh.2015.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stashenko P, Teles R, D’Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998;9:498–521. doi: 10.1177/10454411980090040701. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZH, Yu LJ, Hui XC, Wu ZZ, Yin KL, Yang H, Xu Y. Hydroxy-safflor yellow a attenuates abeta(1)(-)(4)(2)-induced inflammation by modulating the JAK2/STAT3/NF-kappaB pathway. Brain Res. 2014;1563:72–80. doi: 10.1016/j.brainres.2014.03.036. [DOI] [PubMed] [Google Scholar]