Abstract
Background
Low back pain (LBP) is regarded as a frequent disease that causes disability. We aimed to explore the effect of naringin on intervertebral disc degeneration (IDD) in IL-1β-induced human nucleus pulposus (NP) cells and its corresponding molecular mechanisms.
Material/Methods
Human NP cells were identified by toluidine blue and Safranin O staining. Cell viability was determined by MTT assay. The expression levels of matrix metalloproteinases (MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, collagen II, aggrecan), inflammatory genes (tumor necrosis factor [TNF]-α, interleukin [IL]-6), kappa B kinase α (IκBα), p65 and p53 were determined by quantitative real-time polymerase chain reaction (qPCR) and western blotting. Immunofluorescence study was performed to detect the position and expression of p65 protein in IL-1β-induced human NP cells.
Results
Human NP cells were successfully separated from intervertebral disc tissue. We found that naringin could significantly reduce the expressions of matrix metalloproteinases (MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5) and inflammatory genes in IL-1β-stimulated human NP cells, while collagen II and aggrecan were increased at mRNA and protein level. Immunofluorescence showed that naringin pretreatment decreased the p65 protein expression in the nucleus and suppressed the phosphorylation of IκBα and p65.
Conclusions
These results demonstrated that naringin could attenuate matrix metalloproteinase catabolism and inflammation in IL-1β-treated human nucleus pulposus cells via downregulating NF-κB pathway and p53 expression, suggesting that naringin has the potential to prevent and treat IDD.
MeSH Keywords: Cell Nucleus, Intervertebral Disc Degeneration, NF-kappa B
Background
Low back pain (LBP) is a complicated and frequent disease that causes disability [1]. Intervertebral disc degeneration (IDD) plays a significant role in the development of low back pain [2,3]. Though the physiopathology of LBP remains unclear, it has been reported that the nucleus pulposus (NP), the most important component of intervertebral disc, is the main region where synthetic barrier and degradation of extracellular matrix (ECM) in the NP cell occurs and IDD is induced [4]. ECM is vital in disc forming [5–7] and is related to the regulation of matrix metalloproteinases (MMPs) and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) [8,9]. Thus, to control the degradation of ECM in the NP cells is possibly an effective strategy to treat IDD.
Previous studies indicated that the inflammatory process in NP is responsible for the development of IDD [10]. Some cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor (TNF)-α are associated with inflammatory in NP cells [11–13]. IL-1β is a pro-inflammatory cytokine that decreases the most significantly the gene and protein level of MMPs and ADAMTS, thereby causing the degradation of ECM [14–16]. Decreased aggrecan (Agg) and type II collagen (Col II) in NP also contribute to IDD [17]. Studies have found that pro-inflammatory cytokine IL-1β could regulate the expression of ADAMTS-4 and Agg degradation. As a result, reducing the activity of IL-1β or IL-1β-stimulated inflammation in the NP cells can ameliorate the forming of IDD [18].
Nuclear factor-kappa B (NF-κB) is a regulatory pathway receptor to inflammatory responses and its activation promotes IDD and regulates the overexpression of matrix-degrading enzyme [19]. IL-1β can upregulate NF-κB pathway, resulting in the activation of relevant protein of NF-κB pathway, which mediates the inflammation in NP cells [20]. Studies showed that the inhibition of NF-κB signaling pathway reduces the mRNA and protein levels of inflammatory cytokines [21], therefore, anti-inflammatory drugs such as corticosteroids [22] are used for treating IDD, however, they increase the risk of cardiovascular events [23]. In this regard, developing drugs whose component is mainly derived from natural plants may be safer for the treatment of IDD.
Naringin is a flavonoid extracted from natural plant and has special pharmacological properties such as antiarthritic, anti-cancer, anti-oxidant and anti-inflammatory activities [24–27]. Moreover, previous studies have reported that naringin could exert anti-inflammatory and anti-oxidative effects through downregulating NF-κB signaling pathway [21]. Studies proved that naringin reduces the expressions of aggrecan, BMP-2, and Sox6 and it also inhibits the expressions of TNF-α and MMP3 in human NP cells [28]. However, whether naringin exerts anti-inflammatory effects on nucleus pulposus cells is still unknown [29–31]. Therefore, in this study, we investigate the effects of naringin on IL-1β-induced human nucleus pulposus (NP) cells and the corresponding molecular mechanisms.
Material and Methods
Ethics statement and specimen source
The experiments were approved and supervised by Shanxi Provincial People’s Hospital Review Board and written informed consents were obtained from patients. Human intervertebral disc tissues were collected from the patients (4 male and 4 female patients, aged between 28 and 60 years) who were diagnosed with III–IV grade lumbar disc degeneration between May 2018 and December 2018 in Shanxi Provincial People’s Hospital. The intervertebral disc degeneration classification was determined according to the Pfirrmann grading scale [32].
Human nucleus pulposus (NP) cell isolation and culture
Human nucleus pulposus (NP) cells were isolated from intervertebral disc tissue samples as previously described [28]. The isolated NP cells were suspended in DMEM/F12 (12500062, Gibco, USA) containing 10% fetal bovine serum (FBS, 16140071, Invitrogen, USA), 1% penicillin and streptomycin (15070063, Gibco, USA), NP cells were cultured at 37°C in 5% CO2 atmosphere, and the medium was changed every 3 days. After human NP cells attached to the wall, the cells were trypsinized by 0.25% trypsin-EDTA (Gibco, Invitrogen) and passaged, then, NP cells at passage 3 (P3) were used for further experimentation. Naringin was dissolved in dimethylsulfoxide (DMSO) as a stock solution at 2 mM, stored at −20°C, and the experiment was conducted before the temporary defrost preparation. The NP cells were stimulated with IL-1β (10 ng/mL; PHC0813, Gibco, USA) for 24 hours, and then treated by different concentrations of naringin (0.4, 0.8, 1.2, and 1.6 μM) (71662, Sigma-Aldrich, USA) for 24 hours.
Cell proliferation (MTT) assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, M6494, Invitrogen, USA) was dissolved in 10% phosphate-buffered saline (PBS; 20012043, Gibco, USA) and collected at 4°C for downstream applications. The NP cells were treated by different concentrations of naringin (0.4, 0.8, 1.2, and 1.6 μM), and then MTT assay was conducted to determine the cell viabilities. The NP cells were added into MTT solution (450 μM) and incubated at 37°C for 4 hours and then 10% sodium dodecyl sulfate (SDS, AM9823, Invitrogen, USA) were added. The microplate was cultured at 37°C for 24, 48, and 72 hours, and the absorbance was read at 570 nm.
RNA isolation and quantitative polymerase chain reaction (PCR)
Total RNA was extracted from human NP cells according to the protocol of TRIzol reagent (15596026, Invitrogen, USA) and RNA was reverse-transcribed using SuperScript cDNA synthesis kit (18080300, Invitrogen, USA). Forward and reverse primer sequences of MMP-3, 13, ADAMTS-4, 5, TNF-α, IL-6, AGG, and Col II are listed in Table 1. The polymerase chain reaction (PCR) amplification was carried out in a Bio-Rad detection system (185-5196, Bio-Rad, China) with SYBR dye-based master mix (AB1220A, Thermo Scientific, USA) under the following conditions: initial denaturation at 95˚C for 5 minutes, followed by 30 cycles at 95°C for 90 seconds and at 60°C for 45 seconds, and extended at 72°C for 90 seconds. GADPH served as endogenous control for the data and 2−ΔΔCT method was used to calculate the expressions of target genes.
Table 1.
Primers of targeted genes.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IL-6 | GCTGAGTACAAAAGTCCTGATCCA | CTGCAGCCACTGGTTCTGT |
| TNF-α | TCTCGAACCCCGAGTGACAA | TGAAGAGGACCTGGGAGTAG |
| MMP-3 | GCTGTTTTTGAAGAATTTGGGTTC | GCACAGGCAGGAGAAAACGA |
| MMP-13 | ATGCAGTCTTTCTTCGGCTTAG | ATGCCATCGTGAAGTCTGGT |
| ADAMTS-4 | ACTGGTGGTGGCAGATGACA | TCACTGTTAGCAGGTAGCGCTTT |
| ADAMTS-5 | GCTTCTATCGGGGCACAGT | CAGCAGTGGCTTTAGGGTGTAG |
| AGG | TGAAACCACCTCTGCATTCCA | GACGCCTCGCCTTCTTGAA |
| Col II | GTCACAGAAGACCTCACGCCTC | TCCACACCGAATTCCTGCTC |
| GADPH | CGGAGTCAACGGATTTGGTCGTAT | AGCCTTCTCCATGGTGGTGAAGAC |
Western blot analysis
Total protein was extracted from NP cells using radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). The protein concentration was measured using Pierce BCA Protein Assay Kit (23225, Thermo Scientific, USA). Antibodies used against the proteins were anti-MMP-3 antibody (ab53015, 1: 500, Abcam, UK), anti-MMP-13 antibody (ab39012, 1: 3000, Abcam, UK), anti-ADAMTS-4 antibody (ab185722, 1: 500, Abcam, UK), anti-ADAMTS-5 antibody (ab185722, 1: 500, Abcam, UK), anti-AGG antibody (ab36861, 1 μg/mL, Abcam, UK), anti-ColII antibody (ab34712, 1: 5000, Abcam, UK), anti-p53 antibody (ab32389, 2 μg/mL, Abcam, UK), anti-p-IκBα antibody (#5174, 1: 1000, CST, USA), anti-IκBα antibody (#9242, 1: 1000, CST, USA), anti-p-p65 antibody (#3039, 1: 1000, CST, USA), anti-p65 antibody (#8242, 1: 1000, CST, USA), and anti-GADPH antibody (#5174, 1: 1000, CST, USA). A number of NP cell proteins were separated on precast gel sodium lauryl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) 4–20% (ab119209, Abcam, UK) and transferred onto polyvinylidene difluoride (PVDF, ab133411, Abcam, UK) membranes, which were blocked by 5% non-fat milk at room temperature for 15 minutes and incubated with primary antibodies at 4°C and then with specific anti-IgG secondary antibodies (ab150077, Abcam, UK) at room temperature for 3 hours. The membranes were immersed in enhanced chemiluminescence solution (Millipore, WBKLS0100, USA) at room temperature in dark for 1 minute and visualized under a ChemiDicTM Imaging System (Bio-Rad, USA). GADPH were used as a standard for target protein bands.
Immunofluorescence
The NP cells were plated on slides and immersed in PBS (#12528, CST, USA) for 3 minutes, stimulated by IL-1β for 24 hours and then pretreated with naringin (1.6 μM) for 24 hours. Next, the NP cells were fixed by paraformaldehyde (4%) for 20 minutes and then washed by PBS. After that, the NP cells were permeabilized by 0.5% Triton X-100 for 20 minutes at room temperature, washed by PBS 3 times and incubated with anti-p65 antibody (1: 400) overnight at 4°C. Next, the NP cells incubated with anti-rabbit secondary antibodies (#7074, 1: 2000, CST, USA) for 1 hour at 37°C the next day. The NP cells nuclei were stained by DAPI (#4083, CST, USA) and the images were observed and captured by a fluorescence microscope (Nikon, Japan).
Statistical analysis
The data were shown as mean±standard error. Statistical analysis was performed using SPSS v.18.0 software. Comparison between quantitative variable were conducted using one-way ANOVA. P<0.05 was a considered statistically significant difference.
Results
Degenerated human NP cell culture
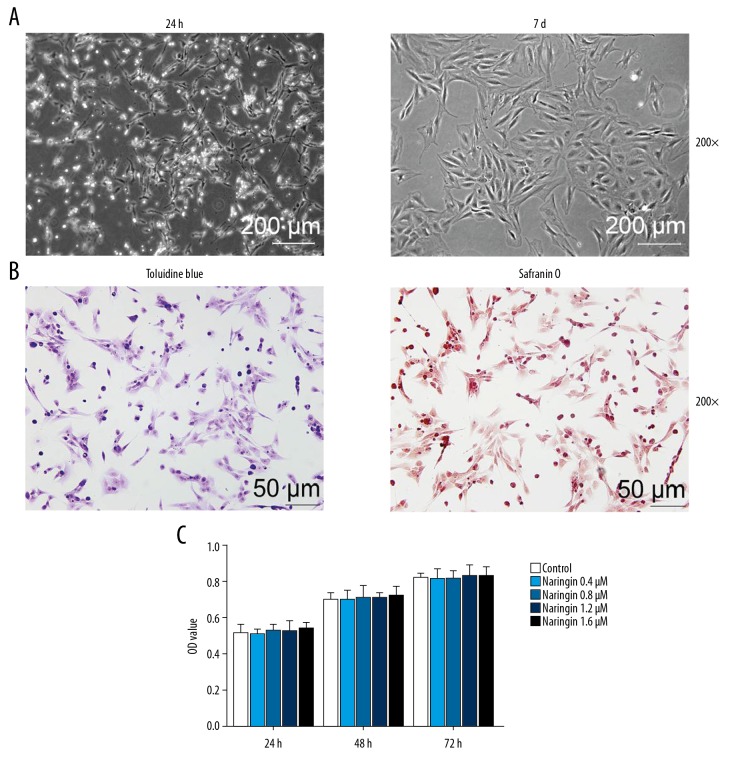
NP cells were isolated from IDD patients and cell morphology was observed using an inverted microscope, and the results showed that the NP cells had limited adherence to the plate after 24-hours cultivation, while the NP cell nuclei were big and round, short fusiform, with fluent cytoplasm, and had protrusion connection at the 7th of culture (Figure 1A). The results of Toluidine blue showed that the NP cell nuclei were stained blue and the cell matrixes were rather light, indicating that the NP cells could secrete glycosaminoglycans. The image of Safranin O stain indicated that the NP cells were stained red, oval or short fusiform, proving that NP cells produced proteoglycanase (Figure 1B). The results showed that degenerated human NP cells were successfully separated from NP tissues.
Figure 1.
The cultivation, identification and naringin treatment on the human degenerated nucleus pulposus (NP) cells (A) Left image is primary NP cells cultivated for 24 hours, while right image is degenerative nucleus pulposus cells cultivated for 7 days (magnification 200×). (B) Toluidine blue and Safrain O stain of cultured human NP cells (magnification 200×). (C) MTT assay for the detection of cytotoxic effects of human NP cells pretreated by naringin (0.4, 0.8, 1.2, and 1.6 μM).
Effect of naringin on the NP cells viability
Human NP cells were treated with different concentrations of naringin (0.4, 0.8, 1.2, and 1.6 μM) and cultured at 37°C for 24, 48, and 72 hours. Then, we detected the effects of naringin on cell viability, and found that the OD value of the NP cells increased from 0.5 to 0.8 after 72-hour culture, and there was no obvious effect on cell viability compared with negative control group at 48 and 72 hours of co-cultures (Figure 1C) with naringin at different concentrations. Therefore, naringin at a certain concentration range does not have toxic effect on the NP cells, and 1.6 μM naringin was selected for subsequent experiments.
Effect of naringin on the NP cells matrix metalloproteinase
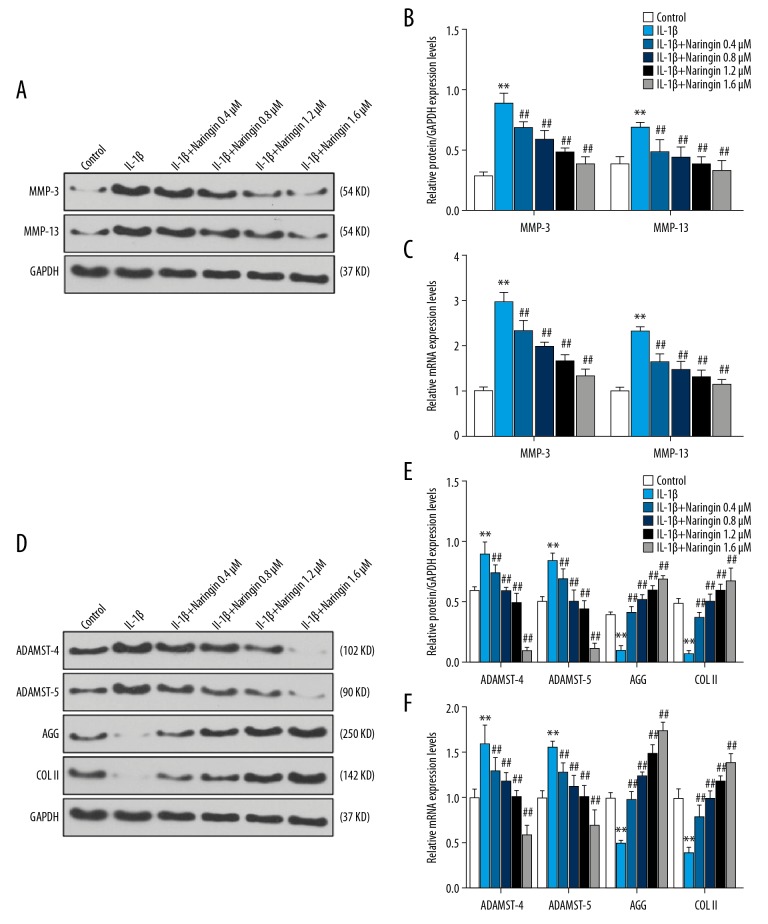
To explore the effects of different naringin concentrations (0.4, 0.8, 1.2, and 1.6 μM) on the NP cells induced by IL-1β (10 ng/mL) for 24 hours, the expressions of matrix metalloproteinases (MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, Coll II, and Agg) were determined at mRNA and protein levels by qPCR and western blotting analysis, respectively. We found that the IL-1β significantly upregulated MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 at mRNA and protein levels compared to the control group (P<0.01), whereas the levels of collagen II and aggrecan were downregulated (P<0.01, Figure 2). Compared with the IL-1β treatment group, naringin significantly decreased MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 but increased collagen II and aggrecan at mRNA and protein levels (P<0.01, Figure 2). Moreover, under increased concentration of naringin treatment, we observed that high concentration of naringin (1.6 μM) exerted an effect more strongly on matrix metalloproteinases than low concentration of naringin (0.4 μM). The result showed that IL-1β could upregulate the expression of matrix metalloproteinases (MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5), while NP cells pretreated by naringin had opposite results.
Figure 2.
Effect of naringin on the nucleus pulposus (NP) cells matrix metalloproteinases (MMP-3, 13, ADAMTS-4, 5, Agg, and Col II). (A, D) The picture of western blots showing protein expressions of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, Agg, and Col II treated by IL-1β and different concentrations of naringin (0.4, 0.8, 1.2, and 1.6 μM) for 24 hours in NP cells. (B, E) Western blot analysis on the protein levels of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, Agg, and Col II in NP cells stimulated by IL-1β and treated naringin for 24 hours, respectively. * Versus control, * P<0.05, ** P<0.01; # versus IL-1β, # P<0.05, ## P<0.01. (C, F) qPCR result showed the mRNA level of MMP-3, MMP-13, ADAMTS-4, ADAMTS-5, Agg, and Col II in NP cells stimulated with IL-1β and treated naringin for 24 hours, respectively. * Versus control, * P<0.05, ** P<0.01; # versus IL-1β, # P<0.05, ## P<0.01.
Effect of naringin on TNF-α and IL-6 in NP cells
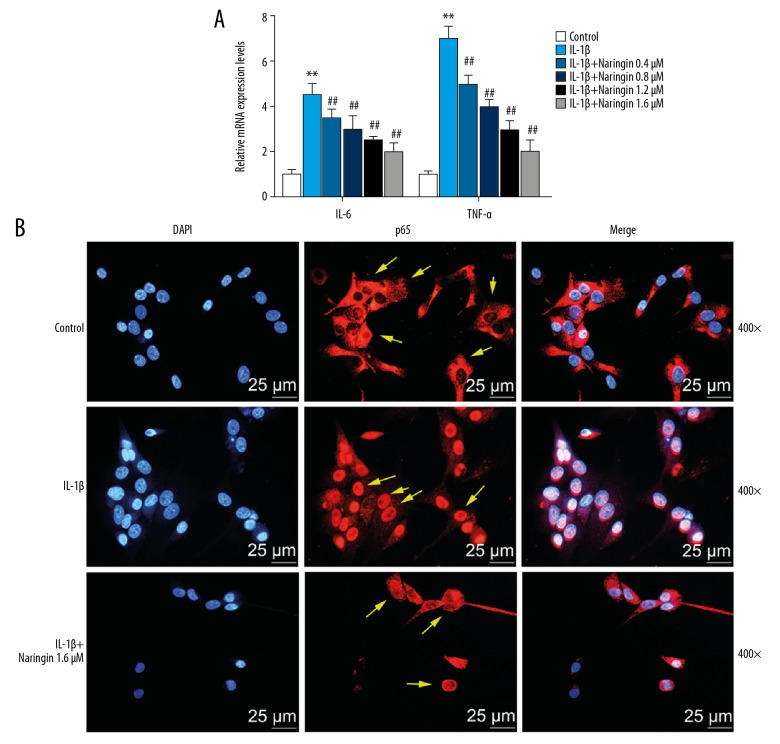
We determined whether naringin regulates the mRNA level of pro-inflammatory genes (TNF-α and IL-6) in the IL-1β-induced NP cells by qPCR. The data showed that IL-1β stimulation increased the mRNA expressions of TNF-α and IL-6, while naringin inhibited such effects (P<0.01, Figure 3A).
Figure 3.
Naringin decreases the mRNA expressions of TNF-α and IL-6 in the nucleus pulposus (NP) cells. (A) The mRNA expression of TNF-α and IL-6 was analyzed by qPCR. * Versus control, * P<0.05, ** P<0.01; # versus IL-1β, # P<0.05, ## P<0.01. (B) Immunofluorescence staining of nucleus (blue) and P65 proteins (red) was labeled with DAPI.
Effect of naringin on the p65 expression in NP cells
The localization and expression of p65 in NP cells were detected by immunofluorescence, and the result showed that in IL-1β treatment group, p65 protein was mainly expressed in the nuclei of the NP cells, while p65 protein was shown in cytoplasm in the control group. When cells were treated by 1.6 μM naringin, IL-1β, p65 protein was high-expressed in the cytoplasm of NP cells but was low-expressed in the nuclei (Figure 3B).
Effect of naringin on NF-κB pathway and p53 expression in NP cells
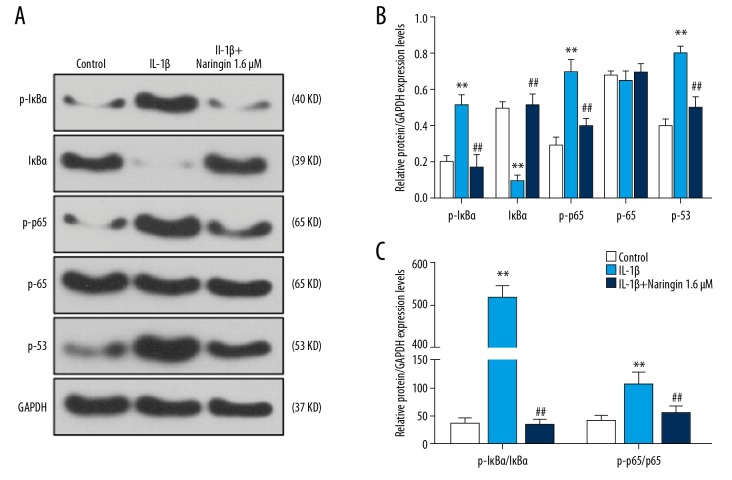
We explored the effect of naringin on NF-κB pathway and p53 expression by western blot, compared with the control group, IL-1β treatment significantly enhanced the phosphorylation of IκBα and p65 and upregulated the expression level of p53 (P<0.01, Figure 4A, 4B). The phosphorylation of IκBα and p65 was significantly lower in the IL-1β+naringin group than in IL-1β group, and p53 expression was found decreased. The protein ratio of p-IκBa/IκBa to p-p65/p65 was higher in IL-1β-treated NP cells (P<0.01, Figure 4C), while under naringin treatment, the protein ratio was consistent to the control group (P<0.01), suggesting that naringin could limit the effect of NF-κB signaling pathway-related protein phosphorylation. The results indicate that naringin significantly inhibits the activation of NF-κB signaling pathway.
Figure 4.
Naringin inhibits NF-κB pathway and p53 expression. (A) The picture of western blot showed that phosphorylation of IκBα, p65 and IκBα, p65, p53 protein expressions after IL-1β treatment for 24 hours in nucleus pulposus (NP) cells. (B) Western blot analysis demonstrated the effects of naringin on the phosphorylation of IκBα, p65 and IκBα, p65, and p53 protein expression by IL-1β treatment NP cells. (C) The intensity ratios of phosphorylation of IκBα to IκBα and phosphorylation of p65 relative to p65. * Versus control, * P<0.05, ** P<0.01; # versus IL-1β, # P<0.05, ## P<0.01.
Discussion
In this study, we investigated the effects of naringin on IL-1β-induced human nucleus pulposus (NP) cells and found its corresponding molecular mechanisms. The results suggest that naringin could attenuate the effect of IL-1β on NP cells by downregulating NF-κB pathway and p53 expression, meanwhile, causing significant decreases of matrix metalloproteinases and inflammatory genes.
Intervertebral disc degeneration (IDD) is a main cause to the development of LBP [33] and nucleus pulposus (NP) is responsible for the IDD [34]. Our studies found that certain dose of naringin (0.4, 0.8, 1.2, and 1.6 μM) did not produce -toxicity to NP cells viability, whereas previous studies focused on naringin at a concentration range between 1 to 100 μg/mL and cells may die when treated by naringin over 200 μg/mL [35]. In in vivo research, feeding rat models with 200 mg/kg naringin daily or even 400 mg/kg for 20 to 30 days through oral gavage could ameliorate the bone repair [36,37]. Thus, the function of naringin in animal is realized in dose-and time-dependent manners. Though the effects of naringin concentration and its mechanism still remain unknown, further research can focus on the optimization of naringin concentration and diet treatment. In addition, it remains to be investigated whether higher doses of naringin have more beneficial effects on NP cells.
IL-1β is a highly inflammatory cytokine involves in disk matrix degradation and proteinases (aggrecan and collagen II), and it upregulates the expressions of matrix metalloproteinases [38,39]. In the current study, IL-1β improved the matrix metalloproteinases in the gene and protein and upregulated the expressions of inflammatory cytokines IL-6 and TNF-α in NP cells, which was consistent with the previous research [18,20]. The expressions of IL-1β and its receptor inhibition were proved to promote ECM repair and prevent IDD [40]. IL-1β has the ability to affect MMP-3, ECM at the gene level and in catabolic mediators [41]. Therefore, inhibiting the action of IL-1β or its receptor antagonist may be effective in preventing or even reversing the intervertebral disc degeneration.
The degradation and reduction of ECM forms intervertebral disc degeneration, in which the loss of proteoglycan (mainly aggrecan) and collagen components occur [42]. Aggrecan and collagen maintain water and contain a network for NP, while a series of stressors such as inflammatory reaction, oxidative, and environmental factors could promote the reduction of metalloproteinase-mediated ECM, resulting the progression of IDD. Maintaining matrix homeostasis in balance through improving anabolism or reducing catabolism is a more promising strategy to ameliorate the loss of the ECM in the NP cells, and some previous studies reported that inhibiting the expressions of MMPs and ADAMTS could be a novel strategy to alleviate the progression of intervertebral disc degeneration [43]. Our research demonstrated that naringin could upregulate the levels of aggrecan and collagen components by decreasing ECM relative enzyme MMPs and ADAMTS, showing that the IDD could be alleviated by naringin [8]. IL-6 and TNF-α are inflammatory cytokines that appeared in IDD [44] and the reduction in matrix synthesis leads to the inability to maintain intervertebral disc hydration, causing an increase of inflammatory cytokine level during disc degeneration [45]. In our research, the mRNA levels of IL-6 and TNF-α were downregulated by approximately 2 times after naringin treatment compared with stimulus in the NP cells. Therefore, we speculated that naringin could reduce the reduction of ECM in which enzymes including MMPs, ADAMTS, and proinflammatory cytokines were involved.
Nuclear factor-kappa B (NF-κB) is a common regulatory pathway receptor that plays a major role in cell injury and inflammation [46] and remains inactive state in cell cytoplasm, however, it will be translocated into cell nucleus under certain stimulation to control the transcription [47]. Previous studies reported that NF-κB is the main regulatory factor in the progression of IDD [48,49]. In our study, we found that stimulation such as IL-1β could translocate the NF-κB into the nucleus of the NP cells, meanwhile, phosphorylation of IκBα and p65 and p53 expression were significantly increased, while naringin reversed the effects on the protein compared with control group, suggesting that the activation of NF-κB signaling pathway was alleviated by naringin treatment. However, the role of NF-κB pathway in IDD remained unknown. Therefore, our research suggests that naringin could be used as a therapeutic target for treating IDD. Previous studies have found that p53 regulated MMP-14 promoter activity by competitively interacting with the transcription factor Sp1 at the MMP-14 promoter [50]. In the current study, the mechanism of p53 regulating matrix metalloproteinases and inflammatory genes remains to be further investigated.
Naringin belongs to the flavonoids can be used as an effective pharmaceutical component to treat IDD as it has the ability to inhibit the expressions of matrix metalloproteinases and its effective anti-inflammatory ability has also been proved [29,51]. Our observation of naringin treatment decreasing matrix metalloproteinase and inflammatory genes (TNF-α and IL-6) is consistent with a previous study [21,34]. Though NF-κB signaling pathway was reported to inhibit IL-1β-Induced NP cells in a previous report by some plant extract including celastrol [20], mori folium [8], and baicalein [18], emphasis was still laid on its anti-inflammatory activity. However, the exact mechanisms of plant extract naringin regulating NF-κB signaling pathway are still unknown. Further studies are required to explore the role and mechanism of naringin in disc nucleus pulposus degeneration.
Conclusions
In this study, we demonstrated that naringin attenuated matrix metalloproteinase catabolism and inflammation in IL-1β-treated human nucleus pulposus cells through the downregulation of NF-κB pathway and p53 expression. Naringin may be an effective therapeutic agent for treatment of IDD.
Footnotes
Source of support: This work was supported by the Shanxi Provincial Health Commission Research Project under Grant [2018029]
Conflict of unterest
None.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang B, Guo W, Sun C, et al. Dysregulated MiR-3150a-3p promotes lumbar intervertebral disc degeneration by targeting aggrecan. Cell Physiol Biochem. 2018;45(6):2506–15. doi: 10.1159/000488269. [DOI] [PubMed] [Google Scholar]
- 3.Park EH, Moon SW, Suh HR, et al. Disc degeneration induces a mechano-sensitization of disc afferent nerve fibers that associates with low back pain. Osteoarthritis Cartilage. :2019. doi: 10.1016/j.joca.2019.07.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Kepler CK, Ponnappan RK, Tannoury CA, et al. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13(3):318–30. doi: 10.1016/j.spinee.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 6.Wu B, Meng C, Wang H, et al. Changes of proteoglycan and collagen II of the adjacent intervertebral disc in the cervical instability models. Biomed Pharmacother. 2016;84:754–58. doi: 10.1016/j.biopha.2016.09.077. [DOI] [PubMed] [Google Scholar]
- 7.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31(18):2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 8.Jeong JW, Lee HH, Lee KW, et al. Mori folium inhibits interleukin-1beta-induced expression of matrix metalloproteinases and inflammatory mediators by suppressing the activation of NF-kappaB and p38 MAPK in SW1353 human chondrocytes. Int J Mol Med. 2016;37(2):452–60. doi: 10.3892/ijmm.2015.2443. [DOI] [PubMed] [Google Scholar]
- 9.Li K, Li Y, Ma Z, Zhao J. Crocin exerts anti-inflammatory and anti-catabolic effects on rat intervertebral discs by suppressing the activation of JNK. Int J Mol Med. 2015;36(5):1291–99. doi: 10.3892/ijmm.2015.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. doi: 10.1038/nrrheum.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, Meng Q, Qiu C, et al. Potential therapeutic role of Co-Q10 in alleviating intervertebral disc degeneration and suppressing IL-1beta-mediated inflammatory reaction in NP cells. Int Immunopharmacol. 2018;64:424–31. doi: 10.1016/j.intimp.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 12.Hua W, Zhang Y, Wu X, et al. Icariin attenuates interleukin-1beta-induced inflammatory response in human nucleus pulposus cells. Curr Pharm Des. 2018;23(39):6071–78. doi: 10.2174/1381612823666170615112158. [DOI] [PubMed] [Google Scholar]
- 13.Jin H, Wang Q, Wu J, et al. Baicalein inhibits the IL-1beta-induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration in vivo. Inflammation. 2019;42(3):1032–44. doi: 10.1007/s10753-019-00965-8. [DOI] [PubMed] [Google Scholar]
- 14.Phillips KL, Cullen K, Chiverton N, et al. Potential roles of cytokines and chemokines in human intervertebral disc degeneration: Interleukin-1 is a master regulator of catabolic processes. Osteoarthritis Cartilage. 2015;23(7):1165–77. doi: 10.1016/j.joca.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Yang SD, Yang DL, Sun YP, et al. 17beta-estradiol protects against apoptosis induced by interleukin-1beta in rat nucleus pulposus cells by down-regulating MMP-3 and MMP-13. Apoptosis. 2015;20(3):348–57. doi: 10.1007/s10495-015-1086-4. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Chen Q, Wang H, et al. Andrographolide mitigates IL-1-beta-induced human nucleus pulposus cells degeneration through the TLR4/MyD88/NFkappaB signaling pathway. Mol Med Rep. 2018;18(6):5427–36. doi: 10.3892/mmr.2018.9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13(3):331–41. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin H, Wang Q, Wu J, et al. Baicalein inhibits the IL-1-beta-induced inflammatory response in nucleus pulposus cells and attenuates disc degeneration in vivo. Inflammation. 2019:42. doi: 10.1007/s10753-019-00965-8. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Yin Z, Liu C, Tian J. The changes in the expression of NF-κB in a degenerative human intervertebral disc model. Cell Biochem Biophys. 2015;72(1):115–22. doi: 10.1007/s12013-014-0417-3. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Xuan J, Gu YT, et al. Celastrol reduces IL-1beta induced matrix catabolism, oxidative stress and inflammation in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration in vivo. Biomed Pharmacother. 2017;91:208–19. doi: 10.1016/j.biopha.2017.04.093. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Wang C, Peng J, et al. Naringin inhibits TNF-alpha induced oxidative stress and inflammatory response in HUVECs via Nox4/NF-kappa B and PI3K/Akt pathways. Curr Pharm Biotechnol. 2014;15(12):1173–82. doi: 10.2174/1389201015666141111114442. [DOI] [PubMed] [Google Scholar]
- 22.Dilke TF, Burry HC, Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. Br Med J. 1973;2(5867):635–37. doi: 10.1136/bmj.2.5867.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farkouh ME, Greenberg JD, Jeger RV, et al. Cardiovascular outcomes in high risk patients with osteoarthritis treated with ibuprofen, naproxen or lumiracoxib. Ann Rheum Dis. 2007;66(6):764–70. doi: 10.1136/ard.2006.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caglayan C, Temel Y, Kandemir FM, et al. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ Sci Pollut Res Int. 2018;25(21):20968–84. doi: 10.1007/s11356-018-2242-5. [DOI] [PubMed] [Google Scholar]
- 25.Cao H, Liu J, Shen P, et al. Protective effect of naringin on DSS-induced ulcerative colitis in mice. J Agric Food Chem. 2018;66(50):13133–40. doi: 10.1021/acs.jafc.8b03942. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad SF, Attia SM, Bakheet SA, et al. Naringin attenuates the development of carrageenan-induced acute lung inflammation through inhibition of NF-kappab, STAT3 and pro-inflammatory mediators and enhancement of IkappaBalpha and anti-inflammatory cytokines. Inflammation. 2015;38(2):846–57. doi: 10.1007/s10753-014-9994-y. [DOI] [PubMed] [Google Scholar]
- 27.Chen M, Peng W, Hu S, Deng J. miR-126/VCAM-1 regulation by naringin suppresses cell growth of human non-small cell lung cancer. Oncol Lett. 2018;16(4):4754–60. doi: 10.3892/ol.2018.9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li N, Whitaker C, Xu Z, et al. Therapeutic effects of naringin on degenerative human nucleus pulposus cells for discogenic low back pain. Spine J. 2016;16(10):1231–37. doi: 10.1016/j.spinee.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Chen R, Qi QL, Wang MT, Li QY. Therapeutic potential of naringin: An overview. Pharm Biol. 2016;54(12):3203–10. doi: 10.1080/13880209.2016.1216131. [DOI] [PubMed] [Google Scholar]
- 30.Raja Kumar S, Mohd Ramli ES, Abdul Nasir NA, et al. Preventive effect of naringin on metabolic syndrome and its mechanism of action: A systematic review. Evid Based Complement Alternat Med. 2019;2019 doi: 10.1155/2019/9752826. 9752826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goupille P, Jayson MI, Valat JP, Freemont AJ. The role of inflammation in disk herniation-associated radiculopathy. Sem Arthritis Rheum. 1998;28(1):60–71. doi: 10.1016/s0049-0172(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 32.Pfirrmann CW, Metzdorf A, Zanetti M, et al. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–78. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 33.Marcu KB, Otero M, Olivotto E, et al. NF-kappaB signaling: Multiple angles to target OA. Curr Drug Targets. 2010;11(5):599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Z, Wang C, Lin J, et al. Therapeutic potential of naringin for intervertebral disc degeneration: involvement of autophagy against oxidative stress-induced apoptosis in nucleus pulposus cells. Am J Chin Med. :2018. doi: 10.1142/S0192415X18500805. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Dai KR, Yan SG, et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. Eur J Pharmacol. 2009;607(1–3):1–5. doi: 10.1016/j.ejphar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y, Wang L, Watts DC, et al. Controlled-release naringin nanoscaffold for osteoporotic bone healing. Dent Mater. 2014;30(11):1263–73. doi: 10.1016/j.dental.2014.08.381. [DOI] [PubMed] [Google Scholar]
- 37.Chen LL, Lei LH, Ding PH, et al. Osteogenic effect of drynariae rhizoma extracts and naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch Oral Biol. 2011;56(12):1655–62. doi: 10.1016/j.archoralbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Grang L, Gaudin P, Trocme C, et al. Intervertebral disk degeneration and herniation: the role of metalloproteinases and cytokines. Joint Bone Spine. 2001;68(6):547–53. doi: 10.1016/s1297-319x(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 39.Seguin CA, Pilliar RM, Madri JA, Kandel RA. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine. 2008;33(4):356–65. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- 40.Maidhof R, Jacobsen T, Papatheodorou A, Chahine NO. Inflammation induces irreversible biophysical changes in isolated nucleus pulposus cells. PLoS One. 2014;9(6):e99621. doi: 10.1371/journal.pone.0099621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kepczynska MA, Zaibi MS, Alomar SY, Trayhurn P. PCR arrays indicate that the expression of extracellular matrix and cell adhesion genes in human adipocytes is regulated by IL-1beta (interleukin-1beta) Arch Physiol Biochem. 2017;123(1):61–67. doi: 10.1080/13813455.2016.1248979. [DOI] [PubMed] [Google Scholar]
- 42.Le Maitre CL, Pockert A, Buttle DJ, et al. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soci Trans. 2007;35(Pt 4):652–55. doi: 10.1042/BST0350652. [DOI] [PubMed] [Google Scholar]
- 43.Wu X, Song Y, Liu W, et al. IAPP modulates cellular autophagy, apoptosis, and extracellular matrix metabolism in human intervertebral disc cells. Cell Death Discov. 2017;3:16107. doi: 10.1038/cddiscovery.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang H, Yang X, Liu C, et al. Effect of NF-κB signaling pathway on the expression of MIF, TNF-alpha, IL-6 in the regulation of intervertebral disc degeneration. J Musculoskeletal Neuronal Interact. 2018;18(4):551–56. [PMC free article] [PubMed] [Google Scholar]
- 45.Park JY, Kuh SU, Park HS, Kim KS. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: Analysis of living human nucleus pulposus. J Spinal Disord Tech. 2011;24(6):352–57. doi: 10.1097/BSD.0b013e3181fee4df. [DOI] [PubMed] [Google Scholar]
- 46.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Lee JI, Burckart GJ. Nuclear factor kappa B: Important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38(11):981–93. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Liu C, Sun Z, et al. IL-1beta increases asporin expression via the NF-kappaB p65 pathway in nucleus pulposus cells during intervertebral disc degeneration. Sci Rep. 2017;7(1):4112. doi: 10.1038/s41598-017-04384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang L, Hu J, Weng Y, et al. Sirtuin 6 prevents matrix degradation through inhibition of the NF-kappaB pathway in intervertebral disc degeneration. Exp Cell Res. 2017;352(2):322–32. doi: 10.1016/j.yexcr.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 50.Cathcart JM, Banach A, Liu A, et al. Interleukin-6 increases matrix metalloproteinase-14 (MMP-14) levels via down-regulation of p53 to drive cancer progression. Oncotarget. 2016;7(38):61107–20. doi: 10.18632/oncotarget.11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam MA, Subhan N, Rahman MM, et al. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5(4):404–17. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]