Abstract
Background
Gastric cancer is the third leading cause of cancer-related death, while its molecular mechanism has not been fully clarified. This study aims to explore the role of Notch signaling in the pathogenesis of gastric cancer.
Material/Methods
A total of 64 patients with gastric cancer were enrolled. The expressions of NOTCH1 in tumor tissues and adjacent non-tumor tissues were detected by immunohistochemistry staining. The correlation between NOTCH1 expression and clinicopathological features of patients was analyzed. NOTCH1 was knocked down in gastric cancer cells. The effects of NOTCH1 blockade on cell proliferation, migration and cell cycle distribution were analyzed. The expressions of ERK1/2 and phospho-ERK1/2 (p-ERK1/2) were detected using western blotting.
Results
Gastric cancer tissues expressed higher level of NOTCH1 than adjacent non-tumor tissues (P<0.05). The high level of NOTCH1 was found to be correlated with gender (male) and lymph node metastasis. However, the expression level of NOTCH1 did not affect the overall survival of patients with gastric cancer. NOTCH1 knock-down repressed the migration and proliferation of gastric cancer cells. Moreover, the cell cycle was arrested at G0/G1 phase by NOTCH1 blockade. The expressions of ERK1/2 and p-ERK1/2 decreased with NOTCH1 knock-down. Further inhibition of ERK1/2 signaling by a MEK1/2 inhibitor U0126 reduced the proliferation of AGS cells, which aggravated the inhibition effect of NOTCH1 knock-down on cell proliferation.
Conclusions
NOTCH1 may play an oncogenic role in gastric cancer. Inhibition of NOTCH1 can efficiently attenuate gastric cancer cell progression, probably in part through cross-talking with ERK1/2 signaling pathway.
MeSH Keywords: Cell Proliferation; MAP Kinase Signaling System; Receptor, Notch1; Stomach Neoplasms
Background
Gastric cancer is prevalent worldwide; it ranks the third cause of death from cancer [1]. East Asia shows the highest incidence rate of gastric cancer. In China, gastric cancer ranks the second cause of cancer death among both men and women [2]. Surgical excision is currently the effective treatment for gastric cancer. However, the outcomes are poor in metastatic gastric cancer, with median survival being about 1 year [3]. Therefore, it is necessary to investigate the molecular mechanism of and to find novel therapeutic strategies for gastric cancer.
Notch signaling is evolutionarily conserved. It is involved in cell proliferation, migration, differentiation, apoptosis, determination of cell fate, and other cellular processes [4]. In mammals, Notch signaling has 4 receptors (Notch1/2/3/4) and 5 ligands (delta-like ligand (DLL) 1/3/4, and jagged 1/2). Notch signaling has been found to be involved in tumorigenesis and is highly context dependent. It can be an oncogene as well as a tumor suppressor gene, depending on cellular context [5]. For example, it plays an oncogenic role in colorectal cancer [6] and breast cancer [7], whereas it plays a tumor-suppressive role in squamous cell carcinoma [8].
Activation of Notch signaling was found to be critically involved in gastric cancer development [9,10]. Activated NOTCH1 was found to be a poor indicator of overall prognosis of gastric tumors [11]. High expression levels of NOTCH1-4 was correlated with an unfavorable rate of survival for 876 gastric cancer patients [12]. Also, elevated expression of NOTCH1 was correlated with Lauren classification, tumor invasion, lymph node metastasis, and differentiation of gastric cancer [13]. These findings suggested an oncogenic role of NOTCH1 in gastric cancer. However, other studies demonstrated a tumor-suppressive role of NOTCH1. Zhou et al. found that NOTCH1 had lower expression in gastric cancer tissues than in paired non-tumor tissues [14]. Bauer et al. demonstrated that increased NOTCH1 level was associated with a good prognosis of gastric cancer, indicating an anti-tumor role of NOTCH1 [15]. Thus, the function of Notch signaling in gastric cancer is still controversial and the regulatory mechanisms involved need to be further investigated.
In the present study, we tried to reveal the exact role of Notch signaling on gastric cancer progression. We first detected the expression profile of NOTCH1 in gastric tumor tissues and contiguous non-tumor tissues; and then knocked down NOTCH1 in vitro to explore the effect of Notch signaling on the biological process of human gastric cancer cells.
Material and Methods
Patients and tissue specimens
Samples of both gastric cancer tissue and nearby normal tissue were collected from 64 patients with gastric cancer who received a gastrectomy between July 2015 and January 2016 at the Department of General Surgery, Shanxi Provincial People’s Hospital (Shanxi, China). The study included 47 males and 17 females with a mean age of 61.7±9.3 years. None of the patients had received chemotherapy or radiotherapy prior to the surgery. All tissue samples were formalin-fixed and paraffin-embedded (FFPE) and were confirmed by pathological diagnosis. Clinicopathological features of all patients were collected (Table 1). All patients were followed up until April 2019.
Table 1.
Clinicopathological features of patients with gastric cancer.
| Clinicopathological Features | NOTCH1 | P value | |
|---|---|---|---|
| Negative (%) | Positive (%) | ||
| Gender | |||
| Male | 15 (31.9) | 32 (68.1) | 0.000 |
| Female | 15 (88.2) | 2 (11.8) | |
| Age (years) | |||
| ≤60 | 15 (55.6) | 12 (44.4) | 0.235 |
| >60 | 15 (40.5) | 22 (59.5) | |
| Tumor invasion | |||
| T1–T2 | 4 (50.0) | 4 (50.0) | 0.850 |
| T3–T4 | 26 (46.4) | 30 (53.6) | |
| Lymph node metastasis | |||
| Negative | 11 (78.6) | 3 (21.4) | 0.007 |
| Positive | 19 (38.0) | 31 (62.0) | |
| Distant metastasis | |||
| Negative | 29 (48.3) | 31 (51.7) | 0.365 |
| Positive | 1 (25.0) | 3 (75.0) | |
| TNM staging | |||
| I–II | 10 (55.6) | 8 (44.4) | 0.384 |
| III–IV | 20 (43.5) | 26 (56.5) | |
| Differentiation | |||
| Poor | 13 (52.0) | 12 (48.0) | 0.360 |
| Moderate and well | 17 (45.9) | 20 (54.1) | |
| Other | 0 (0.0) | 2 (100.0) | |
This study was complied with the approved guidelines of the Human Research Ethics Committee of Shanxi Provincial People’s Hospital and informed consent was obtained from all patients.
Immunohistochemistry (IHC) staining
Sections were cut from the FFPE blocks and mounted on silanized slides. The slides were baked at 65°C for 2 hours, then the paraffin wax was removed with xylene before dehydration with graded ethanol. In order to retrieve the antigens, the slides were boiled in citric acid buffer for 20 minutes. After cooling and washing, the slides were blocked with 5% bovine serum albumin (BSA) for 10 minutes at room temperature. The slides were then incubated with rabbit anti-human NOTCH1 monoclonal antibody (1: 100; ab52627, Abcam, Cambridge, MA, USA) overnight at 4°C. Phosphate-buffered saline (PBS) instead of the primary antibody was used in the negative control. After washing with PBS, the slides were incubated with secondary antibody (Boster Biological Technology, Wuhan, China) for 1 hour and subsequently stained with DAB.
The expression of NOTCH1 was blindly assessed by 2 experienced pathologists using high-power light microscopy (200×). The pathologists scored both the staining intensity and the ratio of positive cells. Staining intensity was scored on a 0 to 3 scale: 0 (negative), 1 (weak), 2 (moderate), and 3 (intense). The percentage of positive cells was quantified as 0 was <5%), 1 was 5–25%, 2 was 26–50%, 3 was 51–75%, and 4 as 76–100%. Multiplying the intensity score by the percentage score resulted in the final staining score. The final scores were then graded into 4 groups: (−)=a final score of 0, (+) staining=final score between 1 and 4, (++) staining=final score between 5 and 8, (+++) staining=final score between 9 and 12. For Pearson’s χ2 analysis, the final score was grouped into negative (final score, 0) or positive (final score, 1–12).
Cell culture and stable cell lines establishment
An AGS cell line, derived from human gastric carcinoma, was obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The AGS cells were cultured in F-12K medium (Sigma-Aldrich, St Louis, USA) containing 10% fetal bovine serum (FBS) (Gibco, New York, USA) at 37°C in a 5% CO2 atmosphere.
Three GIPZ lentiviral vectors targeting human NOTCH1 (shN1-1, shN1-2, shN1-3), one GIPZ lentiviral control vector (Vec), packaging plasmid psPAX2, and envelope expressing plasmid pMD2.G were generously provided by Dr. Huangxuan Shen (Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China). Stable AGS cell lines with NOTCH1 knock-down were established using the lentivirus system. The GIPZ lentiviral vectors combined with psPAX2 and pMD2.G were transfected into 293T cells using the Effectene Transfection Reagent (Qiagen, Valencia, CA, USA). AGS cells were plated at 50–70% confluence in 6-well plates and were subsequently infected with 2 mL medium with a virus containing 8 μg/mL polybrene (Sigma-Aldrich, St Louis, MO, USA) for 24 hours. Finally, the AGS cells were screened using 1 μg/mL puromycin (Sigma-Aldrich, St Louis, MO, USA) for 48 hours.
Western blotting
Total protein was extracted from control cells and NOTCH1 knock-down cells. The western blotting procedures were as previously described [16]. The primary antibodies used were as following: rabbit anti-human NOTCH1 monoclonal antibody (1: 1000; ab52627, Abcam, Cambridge, MA, USA), rabbit anti-human ERK1/2 polyclonal antibody (1: 500; D151753, Sangon Biotech, Shanghai, China), and rabbit anti-human phospho-ERK1/2 polyclonal antibody (1: 500; D151384, Sangon Biotech, Shanghai, China). Mouse anti-human GAPDH monoclonal antibody (1: 500; BM1623, Boster Biological Technology Wuhan, China) was used as a loading control.
EdU assay and Cell Counting Kit-8 (CCK-8) assay
A Cell-Light EdU DNA cell proliferation kit (RiboBio, Guangzhou, China) was utilized to quantify proliferation of AGS cells. The cells were then incubated with grown medium supplemented with 10 μM EdU for 2 hours at 37°C. Following incubation, AGS cells were washed twice using PBS, and fixed in 4% paraformaldehyde (PFA). The cells were permeabilized with 0.5% Triton-X-100 followed by 30 minutes of incubation with Apollo® staining solution and then 30 minutes with Hoechst 33342. Photographs were captured with a fluorescent microscope (Nikon Corporation, Tokyo, Japan). The quantity of EdU positive cells and Hoeschst 33342 positive cells were determined by NIS-Elements BR Imaging Software (Nikon Corporation, Tokyo, Japan).
The proliferation of AGS cells was further studied by a CCK-8 assay. Briefly, plated the cells into 96-well plates and treated cells with none, DMSO, 10 μM U0126 (Medchem Express, Shanghai, China), 20 μM U0126 for 0 hours, 24 hours, and 48 hours. The proliferation rate was tested using a commercial CCK-8 kit (Dojindo, Shanghai, China). Cells were incubated with 10 μL of the CCK-8 reagent at 37°C for 2 hours. An absorbance reading of 450 nm was obtained using a Synergy™ 4 Multi–Mode Reader (BioTek, Winooski, VT, USA).
Scratch assay
AGS cells were plated in 6-well plates in growth medium to reach a confluence. At time 0, a 200 μL pipette tip was used to create a scratch wound, and then the cells were washed and cultured in medium with 2% FBS. Cells that migrated into the scratch area were observed and images were taken with a microscope (Nikon Corporation, Tokyo, Japan) after 24 hours and 48 hours. ImageJ software (National Institutes of Health, Bethesda, MD, USA) was used to quantify changes in scratch area.
Cell cycle analysis
Effect of NOTCH1 knock-down on the cell cycle progression was analyzed using Cell Cycle Detection Kit (KeyGEN BioTECH, Jiangsu, China) by flow cytometry. Control cells and NOTCH1 knock-down cells were fixed in 70% ethanol and incubated at 4°C for at least 4 hours. The cells were stained with propidium iodide (PI) containing RNase A after collection, then incubated for 60 minutes in the dark. The fraction of cells at different cell-cycle phases was determined using an FC500 flow cytometer (Coulter, Beckman, Palo Alto, CA, USA).
Statistical analyses
Three repetitions of each experiment were performed in order to improve accuracy of data. Statistical analyses were carried out using SPSS version 20.0 (Chicago, IL, USA). The data were described as mean±standard deviation. The correlation between NOTCH1 expression and clinicopathological features was determined by Pearson’s χ2 test. The significance of differences for comparisons between groups was determined using Student t-test. Overall survival curves stratified by NOTCH1 expression were estimated by Kaplan-Meier analyses, and the significance was calculated with log-rank test. A P value of <0.05 was considered statistically significant.
Results
The expression of NOTCH1 increased in gastric cancer
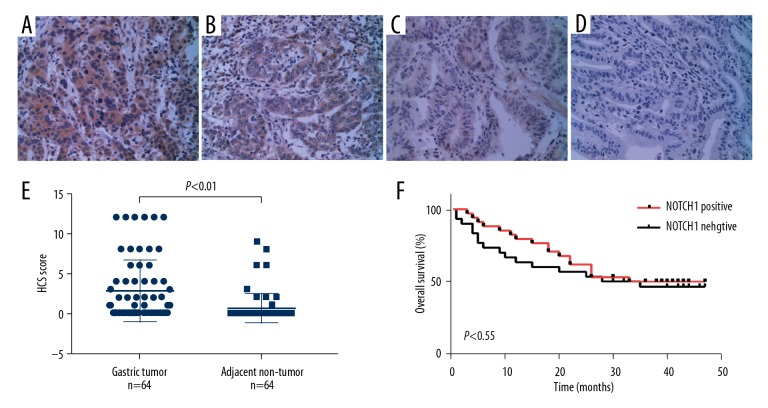
Immunohistochemistry (IHC) staining was utilized to detect NOTCH1 expression in gastric tumor and contiguous non-tumor tissues from patients with gastric cancer. The IHC staining levels of NOTCH1 was graded as (−), (+), (++), and (+++) according to the IHC scores (Figure 1A–1D). The proportion of NOTCH1-positive tissues (IHC+, ++, or +++) in gastric cancer tissues was 53.1% (n=34); which was strongly higher that in contiguous non-tumor tissues (14.1%, n=9). Additionally, comparison between the IHC score of the 2 groups showed a significant difference (P<0.01) (Figure 1E).
Figure 1.
Immunohistochemistry (IHC) staining of NOTCH1 in gastric cancer tissues. The NOTCH1 protein expression in gastric cancer tissues was examined by IHC staining. (A–D) Representative images of IHC staining, denoting (−), (+), (++), and (+++) staining level of NOTCH1 in gastric cancer tissues, respectively. Images were taken at magnification of 200×. (E) IHC score of NOTCH1 were compared between gastric tumor and adjacent non-tumor tissues. (F) Overall survival curves for patients with gastric cancer stratified by NOTCH1 expression.
The clinical significance of NOTCH1 expression in gastric cancer
In order to assess the clinical significance of NOTCH1 expression in gastric cancer, we explored the correlation between NOTCH1 expression and clinicopathological characteristics in patients with gastric cancer through Pearson’s χ2 test. The results showed that the elevated expression of NOTCH1 strongly correlated with gender (male, P=0.000) and lymph node metastasis (P=0.007) (Table 1). However, the high expression of NOTCH1 showed no correlation with depth of invasion, TNM staging, differentiation and other clinicopathological features. We further evaluated the prognostic value of NOTCH1 expression in gastric cancer by Kaplan-Meier analysis. No statistically significant difference in overall survival was observed between NOTCH1-positive and NOTCH1-negative groups (P=0.55) (Figure 1F).
NOTCH1 knock-down suppressed cell proliferation
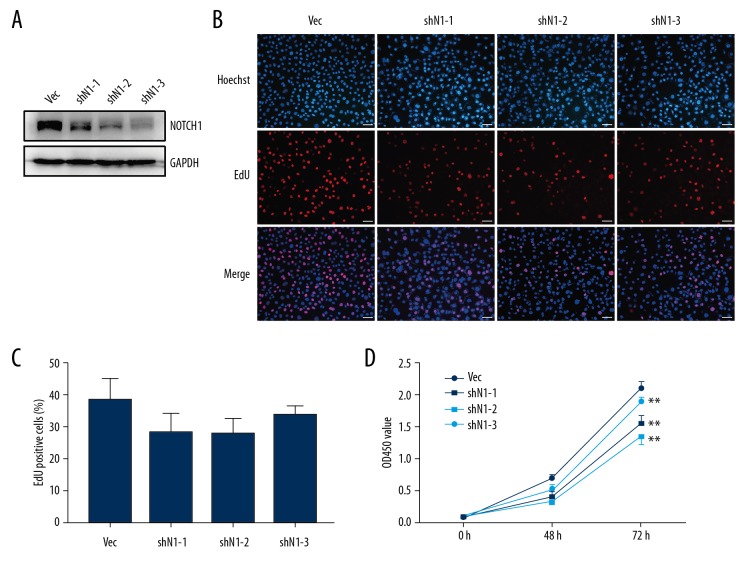
We established stable AGS cell lines with NOTCH1 knock-down using 3 shRNAs targeting NOTCH1 (shN1-1, shN1-2, and shN1-3). Western blot analysis was utilized to determine the knock-down efficiency of NOTCH1 (Figure 2A). The shN1-1 showed a minimal inhibition rate, while shN1-3 showed the highest inhibition rate.
Figure 2.
NOTCH1 knock-down suppresses the proliferation of AGS cells. (A) Stable NOTCH1 knock-down AGS cell lines were established. The knock-down efficiency was determined by western blot analysis. GAPDH was used as an internal control. (B) Representative images of EdU assay in AGS cells with or without NOTCH1 knock-down. Images were taken at magnification of 200×. Bar scale 50 μm. (C) Quantification analysis of B. EdU incorporation rate was shown as ratio of EdU positive cells relative to Hoechst 33342 positive cells. Vec representative AGS cells transfected with control vector, shN1-1/2/3 representative NOTCH1 knock-down cells which was transfected with shNOTCH1-1/2/3. (D) CCK-8 assay showed that AGS cell growth was significantly repressed by NOTCH1 knock-down. ** P<0.01 versus control group (Vec).
EdU assay was utilized to detect the effect of NOTCH1 knock-down on AGS cell growth. The proportion of EdU positive cells in the NOTCH1 knock-down cells, especially in shN1-2 cells, was less than that in the control cells (Vec group); however, this difference showed no statistically significance (Figure 2B, 2C). Notably, the shN1-3 cells, which showed the highest NOTCH1 inhibition rate, did not show the least EdU positive cells.
CCK-8 assay was utilized to further detect the function of NOTCH1 knock-down on cell growth. The results showed that NOTCH1 knock-down (shN1-1, shN1-2, shN1-3) suppressed cell growth significantly in AGS cells (P<0.01) (Figure 2D). At 72 hours, the proliferation rate of shN1-2 cells was inhibited at utmost. However, shN1-3 showed the lowest inhibition rate. These results indicated that NOTCH1 knock-down inhibited cell proliferation in gastric cancer in vitro, which was not in a dose-dependent manner.
NOTCH1 knock-down inhibited cell migration
Scratch assay was conducted to determine the effect of NOTCH1 knock-down on AGS cell migration (Figure 3A). The control cells demonstrated high capability of migration considering the scratch wound had reached almost full recovery after 48 hours of incubation. At 24 hours, significantly fewer cells in the shN1-2 and shN1-3 groups compared to the control cells had migrated into the scratch area. At 48 hours, a statistically significant decrease in scratch area reduction was found in all of the 3 groups with NOTCH1 knock-down (P<0.01) (Figure 3B). The data indicated that downregulation of NOTCH1 effectively inhibited the migratory ability of AGS cells.
Figure 3.
NOTCH1 knock-down inhibits AGS cell migration. (A) The effect of NOTCH1 knock-down on cell migration was assessed by a scratch assay. At time 0, a scratch wound was made. After migrating for 24 hours and 48 hours, cells were then photographed to observe the remaining wound area. (B) Quantitative analysis of reduction in scratch area in graph A. ** P<0.01.
Inhibition of NOTCH1 caused G0/G1 cell cycle arrest
Since inhibition of NOTCH1 suppressed AGS cell growth, we further investigated the cell cycle distribution of AGS cells with or without NOTCH1 knock-down using flow cytometry. We found that AGS cells accumulated in the G0/G1 phase when NOTCH1 was knocked down (Figures 4A–4D). The percentage of cells in G0/G1 phase in Vec cells was 49.94±8.72%, while that in shN1-2 and shN1-3 cells were 72.47±3.82% and 70.72±3.27%, respectively (P<0.05) (Figure 4E).
Figure 4.
NOTCH1 knock-down arrests cell cycle at the G0/G1 phase. Cell cycle was determined by propidium iodide (PI) staining followed by flow cytometric analysis. (A–D) The cell cycles of AGS control cells (Vec) and cells with NOTCH1 knock-down (shN1-1, shN1-2, shN1-3) were tested by flow cytometry analysis. (E) Cell cycle distributions of AGS cells with or without NOTCH1 knock-down. * P<0.05.
ERK1/2 signaling participates in the NOTCH1 knock-down mediated repression of cell proliferation
Due to the famous role of ERK1/2 signaling in tumor progression, especially in cell cycle transition from G1 phase to S phase [17], we further measured whether NOTCH1 knock-down had an effect on activation of ERK1/2. The result indicated that expression of both ERK1/2 and p-ERK1/2 were reduced by NOTCH1 knock-down in a dose-dependent manner (Figure 5A). We next repressed the activation of ERK1/2 using a MEK1/2 inhibitor U0126 to test its effect on NOTCH1-mediated repression of cell proliferation. The proliferation of AGS control cells was slightly inhibited by U0126 at 10 μM, and significantly at 20 μM (Figure 5B). For AGS cells with NOTCH1 knock-down, the proliferation was significantly inhibited by U0126 at 20 μM to a larger extent (Figure 5C). Inhibition of ERK1/2 reduced the cell proliferation and aggravated the NOTCH1-mediated repression of cell proliferation.
Figure 5.
ERK1/2 is involved in NOTCH1 knock-down mediated repression of cell proliferation. (A) The expressions of ERK1/2 and p-ERK1/2 in control cells and NOTCH1 knock-down cells were detected by western blotting. GAPDH was used as an internal control. (B, C) AGS control cells (Vec) as well as cells with NOTCH1 knock-down (shN1-3) were treated with DMSO or MEK1/2 inhibitor U0126 at different concentration (10 μM and 20 μM). The effects of U0126 on cell growth were determined by CCK-8 assays.
Discussion
Notch signaling is still a controversial topic regarding its function in gastric cancer; some studies have claimed it to be an oncogene whereas others have claimed it to act as a tumor suppressor. In this study, we discovered an abundance of NOTCH1 expression in gastric cancer. Both the primary study and meta-analysis showed elevated expression of NOTCH1 in gastric cancer tissues [18]. Studies had reported that high level of NOTCH1 was correlated with an advanced tumor stage, tumor metastasis, depth of invasion and vessel invasion [11]. However, our study showed that high level of NOTCH1 was associated with gender (male) and lymph node metastasis, but not associated with tumor stage, depth of invasion and other pathological features. High expression of NOTCH1 in men may be responsible for the significant higher incidence of gastric cancer among men than that among women [1]. The abundance of NOTCH1 in gastric cancer with lymph node metastasis implied the unfavorable prognostic of gastric cancer. Studies have reported that the elevated expression of NOTCH1 was associated with poor overall survival in patients with gastric cancer [13]. However, our statistical analysis indicated no significant correlation between NOTCH1 expression level and overall survival in patients with gastric cancer. Therefore, NOTCH1 may play an oncogenic role in gastric cancer, but whether it is a diagnostic or a prognostic molecular biomarker for gastric cancer needs more evidences.
Tumor cell proliferation and migration are important cellular processes involved in cancer progression. Tumor cells usually exhibit enhanced proliferation and migration abilities. The depletion of NOTCH1 by siRNA led to an inhibition of gastric cancer cell growth [19]. Downregulation of NOTCH1 inhibits migration, invasion, and proliferation, as well as epithelial-mesenchymal transition (EMT) in gastric cancer cell lines [20]. Similarly, we discovered that NOTCH1 blockade by shRNAs was able to repress the migration and proliferation of AGS cells. Therefore, blockage of NOTCH1 could attenuate gastric cancer progression.
The promoting effect of NOTCH1 on cancer progression may be realized by regulating the cell cycle. It has been reported that through regulating the cell cycle, NOTCH1 promoted cell proliferation [21]. Depletion of NOTCH1 induced cell cycle arrest at G0/G1 phase in glioma cancer U251 cells [22]. This study demonstrated that NOTCH1 blockade induced G0/G1 cell cycle arrest, and downregulated the expression of ERK1/2, as well as its activated form p-ERK1/2, in a dose-dependent manner. ERK1 (MAPK3) and ERK2 (MAPK1) are MAP kinase family members. Participating in the Ras/MAPK signaling cascade, they regulate cell proliferation, differentiation, cell cycle progression and other cellular processes. The interactions between Notch and Ras/MAPK signaling are complicated, which have not been fully clarified. A recent study reported that ERK1/2 signaling was hyper-activated by Notch-1-mediated inhibition of PTEN, which is necessary for cell proliferation and breast cancer stem cell survival [23]. However, another study indicated that Notch signaling was an important downstream target of Ras signaling in neoplastic transformation [24]. Thus, the roles of Ras and Notch are context dependent. Each pathway is able to qualitatively and/or quantitatively influence the other [25]. This study showed that the expression of ERK1/2 was reduced by NOTCH1 blockade. Inhibition of ERK1/2 alone could repress the cell proliferation. But the repression effect was aggravated to a larger extent when combined with NOTCH1 knock-down and ERK1/2 inhibition. Thus, it is probable that Notch signaling and ERK1/2 signaling contribute to the regulation of gastric cancer cell proliferation and tumor cell progression, through cross-talking with each other. Further investigation is necessary in order to reveal the exact interaction network between ERK1/2 and Notch signaling pathways.
Conclusions
The elevated expression of NOTCH1 in gastric cancer tissues and the linkage between NOTCH1 expression and lymph node metastasis of gastric cancer indicates an oncogenic role of NOTCH1 in gastric cancer. Knocking down NOTCH1 in gastric cancer cell line could attenuate tumor cell proliferation, migration, and arrest cell cycle, at least in part, through cross-talking with ERK1/2 signaling pathway. However, deeper understanding of molecular mechanisms and the functional collaboration of pathways in gastric cancer are needed for early-diagnosis and target therapy of gastric cancer.
Acknowledgements
We thank Dr. Huangxuan Shen (Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China) for kindly providing the vectors.
Footnotes
Conflicts of interest
None.
Source of support: This study was funded by Research Project Supported by Shanxi Scholarship Council of China (2015-108)
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388:2654–64. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 4.Bigas A, Espinosa L. The multiple usages of Notch signaling in development, cell differentiation and cancer. Curr Opin Cell Biol. 2018;55:1–7. doi: 10.1016/j.ceb.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Nowell CS, Radtke F. Notch as a tumour suppressor. Nat Rev Cancer. 2017;17:145–59. doi: 10.1038/nrc.2016.145. [DOI] [PubMed] [Google Scholar]
- 6.Li G, Zhou Z, Zhou H, et al. The expression profile and clinicopathological significance of Notch1 in patients with colorectal cancer: A meta-analysis. Future Oncol. 2017;13:2103–18. doi: 10.2217/fon-2017-0178. [DOI] [PubMed] [Google Scholar]
- 7.Bolos V, Mira E, Martinez-Poveda B, et al. Notch activation stimulates migration of breast cancer cells and promotes tumor growth. Breast Cancer Res. 2013;15:R54. doi: 10.1186/bcr3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. 2017;12:245–75. doi: 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SJ, Lee HW, Baek JH, et al. Activation of nuclear PTEN by inhibition of Notch signaling induces G2/M cell cycle arrest in gastric cancer. Oncogene. 2016;35:251–60. doi: 10.1038/onc.2015.80. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Chen H, Sun Z, et al. A systematic analysis of the association between Notch1 expression and the patients with digestive tract cancers. Biomark Med. 2018;12:1049–62. doi: 10.2217/bmm-2017-0429. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Wang X, Xu J, Sun Y. Notch1 activation is a poor prognostic factor in patients with gastric cancer. Br J Cancer. 2014;110:2283–90. doi: 10.1038/bjc.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Liu W, Tang D, et al. Prognostic values of four Notch receptor mRNA expression in gastric cancer. Sci Rep. 2016;6:28044. doi: 10.1038/srep28044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo DH, Zhou Q, Hu SK, et al. Differential expression of Notch1 intracellular domain and p21 proteins, and their clinical significance in gastric cancer. Oncol Lett. 2014;7:471–78. doi: 10.3892/ol.2013.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou W, Fu XQ, Zhang LL, et al. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013;4:e847. doi: 10.1038/cddis.2013.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauer L, Takacs A, Slotta-Huspenina J, et al. Clinical significance of NOTCH1 and NOTCH2 expression in gastric carcinomas: An immunohistochemical study. Front Oncol. 2015;5:94. doi: 10.3389/fonc.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin G, Long C, Liu W, et al. Identification and characterization of novel alternative splice variants of human SAMD11. Gene. 2013;530:215–21. doi: 10.1016/j.gene.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Meloche S, Pouyssegur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene. 2007;26:3227–39. doi: 10.1038/sj.onc.1210414. [DOI] [PubMed] [Google Scholar]
- 18.Du X, Cheng Z, Wang YH, et al. Role of Notch signaling pathway in gastric cancer: A meta-analysis of the literature. World J Gastroenterol. 2014;20:9191–99. doi: 10.3748/wjg.v20.i27.9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei G, Chang Y, Zheng J, et al. Notch1 silencing inhibits proliferation and invasion in SGC7901 gastric cancer cells. Mol Med Rep. 2014;9:1153–58. doi: 10.3892/mmr.2014.1920. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Peng Y, Liu YM, et al. Gastric cancer cell growth and epithelial-mesenchymal transition are inhibited by gamma-secretase inhibitor DAPT. Oncol Lett. 2014;7:2160–64. doi: 10.3892/ol.2014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiao Y, Huang B, Chen Y, et al. Integrated analyses reveal overexpressed Notch1 promoting porcine satellite cells’ proliferation through regulating the cell cycle. Int J Mol Sci. 2018;19(1) doi: 10.3390/ijms19010271. pii: E271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao J, Zheng K, Li C, et al. Interference of Notch1 inhibits the growth of glioma cancer cells by inducing cell autophagy and down-regulation of Notch1-Hes-1 signaling pathway. Med Oncol. 2015;32:610. doi: 10.1007/s12032-015-0610-2. [DOI] [PubMed] [Google Scholar]
- 23.Baker A, Wyatt D, Bocchetta M, et al. Notch-1-PTEN-ERK1/2 signaling axis promotes HER2+ breast cancer cell proliferation and stem cell survival. Oncogene. 2018;37:4489–504. doi: 10.1038/s41388-018-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weijzen S, Rizzo P, Braid M, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–86. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 25.Sundaram MV. The love-hate relationship between Ras and Notch. Genes Dev. 2005;19:1825–39. doi: 10.1101/gad.1330605. [DOI] [PubMed] [Google Scholar]