Abstract
Background:
Toxicant-associated steatohepatitis has been described in adults but less is known regarding the role of toxicants in liver disease of children. Perfluoroalkyl substances (PFAS) cause hepatic steatosis in rodents, but few previous studies have examined PFAS effects on severity of liver injury in children.
Objectives:
We aimed to examine the relationship of PFAS to histologic severity of nonalcoholic fatty liver disease (NAFLD) in children.
Methods:
Seventy-four children with physician-diagnosed NAFLD were recruited from Children’s Healthcare of Atlanta between 2007 and 2015. Biopsy-based liver histological features were scored for steatosis, lobular and portal inflammation, ballooning, and fibrosis. Plasma concentrations of perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonic acid (PFHxS), and untargeted plasma metabolomic profiling, were determined using liquid chromatography with high-resolution mass spectrometry. A metabolome-wide association study coupled with pathway enrichment analysis was performed to evaluate metabolic dysregulation associated with PFAS. A structural integrated analysis was applied to identify latent clusters of children with more severe form of NAFLD based on their PFAS levels and metabolite pattern.
Results:
Patients were 7-19 years old, mostly boys (71%), Hispanic (51%), and obese (85%). The odds of having nonalcoholic steatohepatitis (NASH), compared to children with steatosis alone, was significantly increased with each interquartile range (IQR) increase of PFOS (OR: 3.32, 95% CI: 1.40-7.87) and PFHxS (OR: 4.18, 95% CI: 1.64-10.7). Each IQR increase of PFHxS was associated with increased odds for liver fibrosis (OR: 4.44, 95% CI: 1.34-14.8), lobular inflammation (OR: 2.87, 95% CI: 1.12-7.31), and higher NAFLD activity score (β coefficient 0.46; 95% CI: 0.03, 0.89). A novel integrative analysis identified a cluster of children with NASH, characterized by increased PFAS levels and altered metabolite patterns including higher plasma levels of phosphoethanolamine, tyrosine, phenylalanine, aspartate and creatine, and decreased plasma levels of betaine.
Conclusions:
Higher PFAS exposure was associated with more severe disease in children with NAFLD. PFAS may be an important toxicant contributing to NAFLD progression; however larger, longitudinal studies are warranted to confirm these findings.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a wide range of disorders from uncomplicated steatosis to nonalcoholic steatohepatitis (NASH), a condition characterized by liver inflammation, ballooning degeneration, and/or fibrosis, which can ultimately progress to cirrhosis and hepatocellular carcinoma.(Cohen et al. 2011) NAFLD has become a burgeoning health problem worldwide; in the U.S. alone it is estimated to affect 75-100 million individuals.(Rinella 2015) Among children, the prevalence of NAFLD has almost tripled over the past two decades, currently affecting approximately 12% of the general pediatric population and over 30% of obese children.(Anderson et al. 2015; Welsh et al. 2013) One third of children diagnosed with NAFLD have NASH at the time of diagnosis and almost 75% have fibrosis;(Patton et al. 2008) the pathophysiologic drivers of this variation in severity is unknown. Obesogenic diet and sedentary lifestyle have major roles in NAFLD pathogenesis but do not completely explain the variation in severity of disease seen clinically. Experimental evidence indicates that exposure to some environmental pollutants disrupts lipid homeostasis in the liver and causes hepatic steatosis(Foulds et al. 2017; Heindel et al. 2017) and “toxicant-associated steatohepatitis. (TASH) (Al-Eryani et al. 2015) Thus, environmental pollutants may contribute to a toxicant associated increase in severity in children with NAFLD; however few studies have examined this.
Perfluoroalkyl substances (PFAS) are a group of synthetic chemicals widely used in industrial applications and consumer products such as protective coatings for cookware, food packaging and furniture.(Lindstrom et al. 2011) The strong carbon-fluorine bond of PFAS make them extremely resistant to degradation. They bioaccumulate in food chains and drinking water and they can have long half-lives in humans.(Fromme et al. 2009) Animal studies show that PFAS exposure to rodents causes liver enlargement, hepatocellular hypertrophy, elevated liver enzymes, and hepatic steatosis.(Das et al. 2017; Martin et al. 2007; Son et al. 2008; Wan et al. 2012; Wang, L. et al. 2013; Wu et al. 2017; Wu et al. 2018) Recent cross-sectional studies in adults showed that elevated serum concentrations of PFOA were associated with increased levels of alanine aminotransferase (ALT), a surrogate marker for NAFLD (Gleason et al. 2015; Lin et al. 2010), and cytokeratin 18, a marker for liver apoptosis (Bassler et al. 2019) Children are reported to have increased burden of PFAS exposure relative to body size (Morck et al. 2015); however, there are few studies investigating PFAS hepatotoxicity in children. The only study published to date found negative associations of prenatal and childhood plasma PFAS concentrations with ALT levels at age 8 years.(Mora et al. 2018)
The objective of this study was to examine the associations between plasma PFAS concentrations and histopathologic severity of liver disease in children with NAFLD. We also aimed to understand the underlying metabolic disturbances linking PFAS exposure to NAFLD severity by performing a high-resolution metabolomics analysis. We hypothesized that increased plasma PFAS concentrations would be associated with more advanced liver disease in children with NAFLD and with alterations in key metabolic pathways implicated in NAFLD pathophysiology.
2. Methods
2.1. Study Participants
This study utilized data and samples from the Emory University Pediatric Liver Biopsy Data Repository, a cross-sectional cohort of patients enrolled from Children’s Healthcare of Atlanta between 2007 and 2015, prior to undergoing a clinically-indicated liver biopsy for suspected liver disease or for monitoring of liver disease. Liver disease documented in this cohort included a full spectrum of liver disorders such as congenital disease (e.g., biliary atresia), viral hepatitis, autoimmune liver disease, and NAFLD/NASH. General exclusion criteria included fever in the past two weeks, renal disease and renal insufficiency (defined by creatinine > 2mg/dl ), or pregnancy. From the data biorepository, a total of 79 children (7-19 years) with physician-diagnosed NAFLD had both liver histopathologic assessment and archived blood samples available for PFAS measurement. Among these 79 patients, we excluded one participant who had type 2 diabetes (fasting plasma glucose greater than 200 mg/dl) and another participant who was missing data for BMI and portal inflammation score. Additionally, three participants were excluded due to implausible values for PFAS levels, resulting in a final sample size of 74 children and adolescents. There were no differences in sociodemographic characteristics between participants and non-participants in the current analysis (Supplementary Table 1). The study protocol was approved by the Emory University and University of Southern California (USC) Institutional Review Boards, and informed written consent (parental consent for participants <18 years) and assent (from all children ≥11 years) were obtained for each participant before initiation of the study.
2.2. Clnical Assessment and Liver Histology
Height (m) and weight (kg) were measured by research coordinators, and BMI percentile was determined using the Centers for Disease Control and Prevention (CDC) age- and sex-specific growth charts.(Kuczmarski et al. 2002) Obesity was defined as BMI percentile greater than the 95th percentile; overweight was defined as BMI percentile greater than 85th but less than 95th percentile. Prior to liver biopsy, fasting blood samples of patients were collected, placed immediately on ice, and processed within 1 hour. Plasma aliquots were frozen and stored for future research measurements.
Liver biopsies were performed using automated 16 guage liver biopsy needles and 2 cores were obtained and prepared for histological evaluation. Liver biopsy specimens were fixed in neutral buffered formalin and embedded in paraffin. Cut sections of tissue (3–4 μm) were stained with haematoxylin and eosin (H&E) for morphologic review and Masson’s trichrome for assessment of fibrosis. Histological features were scored by an experienced pediatric pathologist masked to clinical data, utilizing the Nonalcoholic Steatohepatitis Clinical Research Network recommended scoring system.(Kleiner et al. 2005) Briefly, the grade of steatosis was scored as: 0= <5%; 1=5-33%; 2= 34-66%; and 3= >66%. Lobular inflammation was scored into 4 categories: 0 = no foci; 1 = <2 foci/200x field; 2 = 2-4 foci/200x field; and 3 = >4 foci/200x field. Because pediatric NAFLD may present with a distinct histopathological pattern characterized mainly by the presence of portal-based disease,(Nobili et al. 2016) portal inflammation score was also assigned to each biopsy as: 0 = none or scattered rare lymphocytes in portal tracts; 1 = mild; and 2 = moderate to severe. Hepatocellular ballooning was graded as: 0 = none; 1 = few balloon cells; and 2 = many cells/prominent ballooning. Fibrosis stage was scored separately as: 0 = no fibrosis; 1A = mild, zone 3, perisinusoidal; 1B = moderate, zone 3, perisinusoidal; 1C = portal/periportal; 2 = perisinusoidal and portal/periportal; 3 = bridging fibrosis; and 4 = cirrhosis. NASH was defined as presence of steatosis, lobular inflammation and hepatocyte ballooning. The aggregate NAFLD activity score was also calculated as the sum of scores for steatosis grade (0-3), lobular inflammation (0-3), and hepatocellular ballooning (0-2).(Brunt et al. 2011)
2.3. High-resolution metabolomics
High-resolution metabolomic profiling was completed using previously standardized methods.(Soltow et al. 2013) Samples were prepared and analyzed in batches of 40; each batch included six replicate analyses of Children’s Health Exposure Analysis Resource (CHEAR) pooled plasma for quality control purposes and reference standardization. Aliquots were first treated with acetonitrile to precipitate proteins, and analyzed using dual column liquid chromatography and Fourier transform high-resolution MS (Dionex Ultimate 3000, Q-Exactive HF, Thermo Scientific). For each sample, 10 μL aliquots were analyzed in triplicate using hydrophilic interaction liquid chromatography (HILIC) with the electrospray ionization (ESI) source operated in positive mode for metabolomic profiling and reverse phase chromatography (RPC) with ESI in negative mode for quantification of PFAS.(Alderete et al. 2019) Previous comparison of these analytical modes has shown that the combination of HILIC with positive ESI and RPC with negative ESI provides complementary detection of compounds with limited overlap since different functional groups preferentially form adducts in one of the polarity modes.(Liu et al. 2016) No other chromatography modes or polarities were analyzed. The high-resolution mass spectrometer was operated at 120,000 resolution and mass-to-charge ratio (m/z) range from 85 to 1275. Raw data files were extracted and aligned using apLCMS(Yu, T. et al. 2013) with modifications by xMSanalyzer,(Uppal et al. 2013) which included batch correction using ComBat,(Johnson et al. 2007) and quality assessment and averaging of triplicates. Uniquely detected ions consisted of mass-to-charge ratio (m/z), retention time and ion abundance, referred to as metabolite features. Prior to data analysis, metabolite features were filtered to remove those with coefficient of variation (CV) ≥ 100% or greater than 30% non-detected values across all samples. Initially, 13,013 metabolite features were extracted from the HILIC column and 6,494 remained for statistical analysis.
2.4. Quantification of PFAS concentrations
Concentrations of PFOA, PFOS and PFHxS in plasma samples were quantified by reference standardization. (Go et al. 2015a) Using this approach, analyte identification was confirmed by matching MS2 ion dissociation patterns, precursor m/z and retention time to authentic reference standards. Concentrations of PFAS were determined in the CHEAR pooled plasma by comparison of PFAS peak intensity detected in CHEAR pleasma and NIST standard reference material 1950 (Metabolites in Frozen Human Plasma analyzed in the same batch). Reference concentrations of PFAS measured in NIST 1950 (Simon-Manso et al. 2013) were then used to estimate concentrations of PFOA, PFOS and PFHxS in CHEAR plasma. To calculate PFAS concentrations in study samples, six replicates of CHEAR plasma were analyzed within the batch, and the response factor for each analyte was determined using the M-H adduct. Plasma concentrations were calculated by single point calibration via response factors (calculated as the ratio between the known concentration of the compound being quantified and ion intensity in CHEAR plasma replicates analyzed in each batch). We have previously demonstrated reference standardization provides estimates of concentrations consistent with both endogenous and exogenous compounds,(Accardi et al. 2016; Go et al. 2015a; Walker et al. 2019) and high-correlation with PFAS measurements by validated, targeted approaches.(Kingsley et al. 2019)
Calculated limits of detection for PFOA, PFOS and PFHxS were 0.02, 0.1, and 0.03 ng/mL, respectively. PFOA, PFOS, and PFHxS were detected in 100%, 100%, and 94.6% of participants. For concentrations below the detection limits, a value equal to the detection limit divided by the square root of 2 was used for statistical analyses.(Lin et al. 2010)
Statistical analysis
We calculated mean and standard deviation (SD) for normally distributed continuous variables or median (interquartile range, IQR) for variables that were not normally distributed. Logistic regression models were applied to determine associations of plasma PFAS concentrations with the presence of NASH. Multinomial logistic regression models were used to analyze associations between PFAS concentrations and liver histology, including steatosis grade, lobular and portal inflammation, hepatocellular ballooning, categorized NAFLD Activity Score, and fibrosis (normal histology as the reference group). Odds ratios (ORs) and 95% confidence intervals (CIs), adjusted for age, sex, ethnicity (Hispanics or not), and BMI Z score, were calculated for liver histological outcomes compared to the reference group. Due to the moderate to high correlation of the PFAS (r from 0.25 to 0.66, Supplementary Table 2), we also performed a principal component (PC) analysis for the 3 PFAS (PFOA, PFOS, PFHxS), and we selected the first component (“PC1”), which explained 73.2% of the variance, as a composite variable representing PFAS burden. Prior studies have shown that PFAS effects may vary by sex(Halldorsson et al. 2012; Mora et al. 2017) and ethnicity,(Boronow et al. 2019; Park et al. 2019), therefore, effect modification by sex and ethnicity was examined with an interaction term and stratification analyses.
A metabolome-wide association study (MWAS) was performed to identify changes of plasma metabolite features associated with PFAS concentrations, including PFOA, PFOS and PFHxS. In this analysis, multivariate linear regression models were fit to examine associations between log2 transformed metabolite features (m/z) and plasma concentrations of each PFAS after adjusting for age, sex, ethnicity (Hispanic or not) and BMI Z-score. Metabolite features were depicted by Manhattan plots as a function of the m/z. False discovery rate (FDR) was computed using Benjamini-Hochberg method.(Benjamini and Hochberg 1995) As a second step, a pathway enrichment analysis was performed by including all metabolite features significantly correlated with PFAS (p<0.05) in Mummichog (10,000 permutations), a set of algorithms specifically designed for high-throughput metabolomics data that can bypass conventional metabolite identification and directly map metabolites to known metabolic networks to predict functional activity.(Li et al. 2013) For discovery of metabolic alterations associated with plasma PFAS concentrations, we did not focus on the results of the MWAS as it utilizes strict multiple comparison procedures that assume independence of the variables of interest (metabolites) that could lead to type II statistical error. Instead, we focused on the pathway enrichment approach which uses a random sampling framework to identify a null distribution of metabolites across a metabolic network. The comparison of the null distribution to annotated metabolites from signals with p<0.05 removes false positives, which would be randomly distributed across the metabolic map and allows isolation of true biological effects. (Go et al. 2015b; Li et al. 2013; Uppal et al. 2016)
We lastly performed an integrated latent variable analysis to identify latent clusters of children with NASH, based on their PFAS exposure and metabolite data, using the LUCIDus R package (https://CRAN.R-project.org/package=LUCIDus).(Peng et al. 2019) For the estimation of the number of latent clusters, we used Bayesian Information Criteria. We used the PFAS composite variable based on the principal component analysis to represent PFOA, PFOS and PFHxS exposure. Metabolites were selected from the 5 most significantly altered metabolic pathways (based on p value and the numbers of significant metabolite features) associated with the plasma PFAS in the Mummichog analysis. The LUCIDus package provides effect estimates for the association of each estimated latent cluster with NASH and the association of the PFAS composite variable with the estimated latent cluster It also provides a posterior probability of each child’s membership in each identified latent cluster.(Peng et al. 2019)
All statistical tests were performed using SAS, version 9.4 (SAS Institute, Cary, NC) and the R statistical environment version 3.5.0.
Results
Demographics and clinical characteristics of study population
Mean age of participants was 14.0 years (SD 2.81). The majority were boys (70.3%), Hispanic (52.7%), and obese (85.1%) (Table 1). Thirty-eight children (51.4%) had NASH. Nearly 70% had lobular inflammation and 43.2% had portal inflammation. Hepatocellular ballooning was seen in 40% of these children, but few had prominent ballooning (6.8%). Fibrosis was observed in 70% of the patients (51% stage 1, 19% stage 2 or 3). None had cirrhosis (Table 1).
Table 1.
Clinical characteristics and liver histology in children with NAFLD
| Demographics | |
|---|---|
| Age, years, mean (SD) | 14.0 (2.81) |
| Male, n (%) | 52 (70.3) |
| Ethnicity, n (%) | |
| Caucasian | 26 (35.1) |
| Hispanic | 39 (52.7) |
| African American | 7 (9.46) |
| Asian | 1 (1.35) |
| No answer | 1 (1.35) |
| BMI percentile, median (IQR) | 98.4 (2.30) |
| Normal weight, n (%) | 4 (5.4) |
| Overweight, n (%) | 7 (9.5) |
| Obesity, n (%) | 63 (85.1) |
| Liver histology | |
| Presence of NASH, n (%) | 38 (51.4) |
| NAFLD Activity Score, mean (SD) | 3.24 (1.79) |
| Grade of steatosis, n (%) | |
| 0 (<5%) | 7 (9.5) |
| 1 (5-33%) | 20 (27.0) |
| 2 (34-66%) | 13 (17.6) |
| 3 (>66%) | 34 (45.9) |
| Lobular inflammation, n (%) | |
| 0 (no foci) | 24 (32.4) |
| 1 (<2 foci per 200x field) | 43 (58.1) |
| 2 (2-4 foci per 200x field) | 7 (9.5) |
| 3 (>4 foci per 200x field) | 0 (0) |
| Portal inflammation, n (%) | |
| 0 (none) | 42 (56.8) |
| 1 (mild) | 24 (32.4) |
| 2 (moderate to severe) | 8 (10.8) |
| Hepatocellular Ballooning, n (%) | |
| 0 (none) | 44 (59.5) |
| 1 (few balloon cells) | 25 (33.8) |
| 2 (many cells/prominent ballooning) | 5 (6.76) |
| Fibrosis stage, n (%) | |
| 0 (none) | 22 (29.7) |
| 1 (Perisinusoidal or periportal) | 38 (51.4) |
| 2 (Perisinusoidal and portal/periportal) | 8 (10.8) |
| 3 (Bridging) | 6 (8.1) |
| 4 (Cirrhosis) | 0 (0) |
Note: BMI, body mass index; IQR, interquartile range; NAS, NAFLD Activity Score; SD, standard deviation.
Plasma PFAS concentrations and associations with NAFLD severity
Median concentrations (IQRs) of plasma PFOA, PFOS, and PFHxS were 3.42 (1.65), 3.59 (4.46), and 1.53 (3.17) ng/ml, respectively (Supplementary Table 2). Table 2 shows the odds ratios (ORs) and 95% confidence intervals (CIs) for liver histological features in relation to plasma PFAS concentrations with adjustment for age, sex, ethnicity, and BMI z-score. The odds of having NASH significantly increased in association with each IQR increase of plasma concentrations of PFOS (OR: 3.32, 95% CI: 1.40-7.87), PFHxS (OR: 4.18, 95% CI: 1.64-10.7), and PFAS composite variable (OR: 4.89, 95% CI: 1.86-12.8). The adjusted ORs of having lobular inflammation (2-4 foci per 200x field), compared to children with no lobular inflammation, were 2.87 (95% CI: 1.12-7.31) and 3.44 (95% CI: 1.11-10.7) per IQR increase of PFHxS and PFAS composite variable, respectively. The odds of having mild portal inflammation was also significantly increased with each IQR increase of PFHxS (OR: 2.87, 95% CI: 1.27-5.67) and PFAS composite variable (OR: 2.71, 95% CI: 1.18-6.25). In addition, each IQR increase of PFHxS plasma levels was associated with increased risk of mild (OR: 3.43, 95% CI: 1.09-10.8) and moderate fibrosis (OR: 4.44, 95%CI: 1.34-14.8), and higher NAFLD activity score (b coefficient 0.46; 95% CI: 0.03, 0.89) (Table 4). We showed no associations of PFAS exposures with grade of steatosis. Also, we showed no significant interactions between PFAS and sex for ethnicity or any liver histologic features, but these estimates were imprecise due to small sample size.
Table 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for liver histology in relation to PFAS (per IQR increase)
| PFOA | PFOS | PFHxS | PFAS scorea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) |
OR (95% CI) |
Median (IQR) |
OR (95% CI) |
Median (IQR) |
OR (95% CI) |
OR (95% CI) |
||||
| Presence of NASH | ||||||||||
| No | 3.35 (1.78) | 1 (reference) | 3.06 (2.12) | 1 (reference) | 0.77 (1.40) | 1 (reference) | 1 (reference) | |||
| Yes | 3.61 (1.80) | 1.21 (0.67, 2.18) | 5.64 (4.05) | 3.32 (1.40, 7.87) | 2.37 (5.06) | 4.18 (1.64, 10.7) | 4.89 (1.86, 12.8) | |||
| Grade of steatosis | ||||||||||
| <5-33% | 3.42 (1.76) | 1 (reference) | 3.66 (4.98) | 1 (reference) | 1.11 (1.67) | 1 (reference) | 1 (reference) | |||
| 34-66% | 3.51 (1.13) | 1.41 (0.61-3.23) | 3.22 (3.88) | 1.37 (0.54-3.51) | 1.78 (2.81) | 1.87 (0.81-4.34) | 1.69 (0.68-4.19) | |||
| >66% | 3.31 (1.93) | 1.21 (0.60-2.47) | 3.60 (4.07) | 0.88 (0.39-1.97) | 1.86 (3.83) | 1.86 (0.90-3.87) | 1.37 (0.63-2.95) | |||
| Lobular inflammation | ||||||||||
| None | 3.42 (1.90) | 1 (reference) | 3.88 (4.56) | 1 (reference) | 1.10 (2.08) | 1 (reference) | 1 (reference) | |||
| <2 foci per 200 × field | 3.30 (1.61) | 0.90 (0.45, 1.81) | 3.47 (3.49) | 0.50 (0.21, 1.22) | 1.79 (3.55) | 1.47 (0.72, 3.00) | 0.91 (0.41, 2.02) | |||
| 2-4 foci per 200 × field | 3.64 (2.39) | 1.32 (0.52, 3.39) | 6.97 (7.96) | 2.92 (0.92, 9.23) | 1.58 (10.2) | 2.87 (1.12, 7.31) | 3.44 (1.11, 10.7) | |||
| Portal inflammation | ||||||||||
| None | 3.46 (1.95) | 1 (reference) | 3.19 (3.30) | 1 (reference) | 0.94 (1.63) | 1 (reference) | 1 (reference) | |||
| Mild | 3.41 (1.66) | 1.26 (0.65, 2.43) | 4.94 (4.42) | 1.85 (0.82, 4.21) | 2.51 (3.86) | 2.69 (1.27, 5.67) | 2.71 (1.18, 6.25) | |||
| Moderate-to-severe | 3.22 (0.72) | 0.65 (0.18, 2.39) | 6.00 (5.09) | 2.26 (0.75, 6.79) | 3.08 (5.43) | 2.34 (0.94, 5.82) | 2.65 (0.91, 7.79) | |||
| Ballooning | ||||||||||
| None | 3.50 (1.65) | 1 (reference) | 3.19 (4.63) | 1 (reference) | 1.10 (1.73) | 1 (reference) | 1 (reference) | |||
| Few balloon cells | 3.14 (2.07) | 0.99 (0.52, 1.86) | 4.10 (3.48) | 1.11 (0.52, 2.37) | 1.96 (2.70) | 1.19 (0.69, 2.03) | 1.19 (0.60, 2.34) | |||
| Many cells/prominent ballooning | 2.96 (0.59) | 0.42 (0.07, 2.60) | 3.52 (5.69) | 1.12 (0.26, 4.95) | 2.35 (4.61) | 2.08 (0.72, 5.96) | 1.93 (0.45, 8.32) | |||
| Liver fibrosis | ||||||||||
| None (stage 0) | 3.10 (1.62) | 1 (reference) | 3.14 (4.85) | 1 (reference) | 0.61 (1.43) | 1 (reference) | 1 (reference) | |||
| Mild (stage 1) | 3.77 (1.96) | 1.68 (0.75, 3.73) | 3.67 (4.04) | 1.71 (0.73, 4.03) | 1.86 (2.87) | 3.43 (1.09, 10.8) | 2.59 (0.96, 6.96) | |||
| Significant (stage 2-4) | 3.21 (1.18) | 0.97 (0.33, 2.82) | 3.84 (3.91) | 1.51 (0.53, 4.35) | 1.77 (5.83) | 4.44 (1.34, 14.8) | 3.02 (0.99, 9.19) | |||
| beta (95% CI) | beta (95% CI) | beta (95% CI) | beta (95% CI) | |||||||
| NAS | 0.1 (−0.43, 0.62) | 0.1 (−0.49, 0.73) | 0.46 (0.03, 0.89) | 0.38 (−0.17, 0.93) | ||||||
Note: IQR, interquartile range; NAS, NAFLD Activity Score; OR, odds ratio; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFHxS, perfluorohexane sulfonic acid; PFAS, perfluoroalkyl substances.
Median and IQR of PFAS are in ng/ml. ORs were adjusted for age, sex, ethnicity, and BMI z-score
PFAS score is the composite variable, based on the principal component analysis, which explains 73.2% of variance of all three PFAS
Metabolome-wide association study and Mummichog pathway analysis
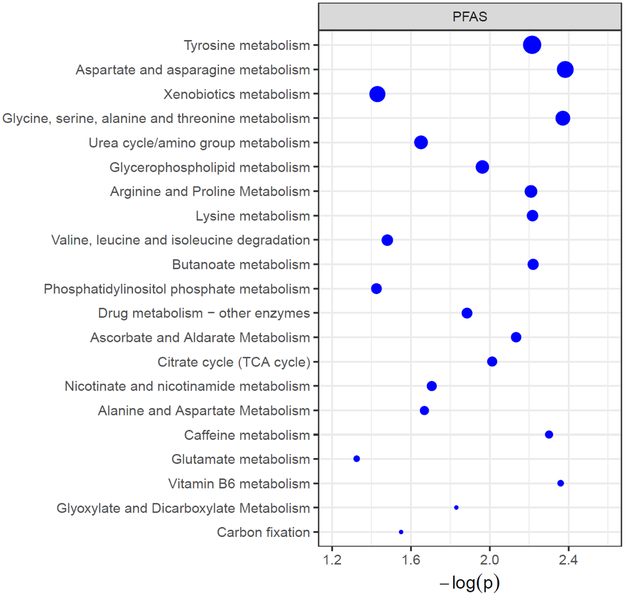
To identify metabolic alterations associated with PFAS, the 6,494 metabolite features were tested for association with exposure using a metabolome-wide association study (MWAS) framework. The MWAS identified 348, 349, and 662 features associated with PFOA, PFOS, and PFHxS, respectively, at p<0.05 (Supplementary Figure 1). We next performed Mummichog pathway enrichment analysis using detected metabolite features associated with PFAS exposure. Pathway enrichment identified 21 metabolic pathways associated with plasma concentrations of PFAS (Figure 1). The most affected pathways (having the greatest numbers of significantly altered metabolic features) included tyrosine metabolism, aspartate and asparagine metabolism, glycine, serine, alanine and threonine metabolism, urea cycle metabolism, and glycerophospholipid metabolism (Figure 1). Similar amino-acid (tyrosine, aspartate and asparagine, lysine, glycine, serine, alanine, and threonine) and lipid pathways (glycerophospholipid) were significantly associated with the outcome (NASH) (data not shown).
Figure 1. Dysregulated metabolic pathways associated with plasma PFAS (including PFOA, PFOS and PFHxS) in children with NAFLD.
The vertical axis represents the pathways (blue circles) with radius representing the numbers of associated metabolite features. The horizontal axis represents the negative log10 (P-value) of each pathway. Only pathways with >5 hits of significant metabolites (overlapping size) are shown.
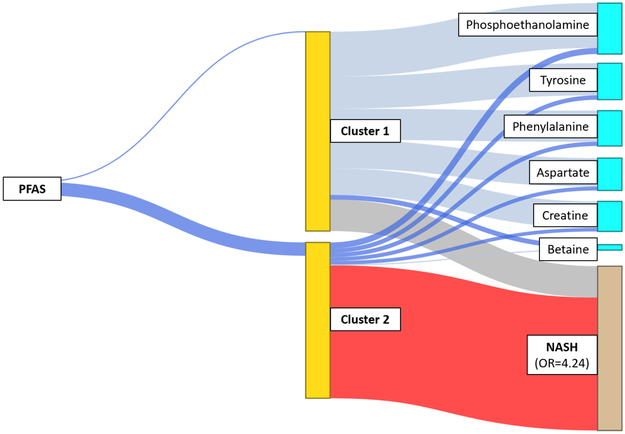
Identification of latent clusters with more severe NAFLD
Two latent clusters were identified as shown in Figure 2. Cluster 2 was associated with increased odds of having NASH (Figure 2). This high risk cluster was also positively associated with the PFAS composite variable and with an altered plasma metabolites pattern, including increased plasma levels of phosphoethanolamine (metabolite in glycerophospholipid metabolism), tyrosine and phenylalanine (tyrosine metabolism), aspartate (aspartate and asparagine metabolism) and creatine (urea cycle metabolism), and decreased plasma levels of betaine (glycine, serine, alanine and threonine metabolism) (Figure 2 and Supplementary Table 3). In order to characterize these clusters qualitatively, we assigned each child to one of the two clusters based on an estimated probability greater than 0.5 for membership within a cluster. Children assigned to the high risk cluster had higher PFAS concentrations, associations reflective of the metabolites characterizing cluster 2 in Figure 2, and a substantially larger proportion of children with inflammation and fibrosis than children assigned to cluster 1 (Supplementary Table 4).
Figure 2. Structural integrated analysis of PFAS composite variable and plasma metabolites that identify a cluster of children with increased odds of having NASH.
Blue lines represent positive effects and grey lines indicate negative effects, with the width proportional to the effect size. The thick blue line connecting PFAS to “Cluster 2” indicates an association with higher levels of PFAS as compared to lower levels with “Cluster 1” (indicated by a thin blue line). The blue lines connecting clusters to metabolites indicate positive associations, while the grey lines connecting clusters to metabolites indicate negative associations. The red line indicates an increased risk for NASH associated with “Cluster 2” (OR=4.24), compared to those in “Cluster 1” (OR=1, reference).
Discussion
This is the first study to investigate environmental chemical exposures and NAFLD severity in children based on biopsy-based liver histological features. We found that higher plasma PFAS concentrations were associated with increased risk of NASH in children diagnosed with NAFLD. We also combined plasma PFAS chemical analysis and untargeted metabolomics analyses showing that PFAS exposure may be associated with changes in key amino-acids and lipids pathways underlying NAFLD pathophysiology.
We found that NASH and more advanced stages of fibrosis were associated with higher plasma concentrations of PFAS in children. Further, increased plasma PFAS concentrations were associated with changes in key metabolic pathways (e.g., glycerophospholipids and tyrosine) that may be contributing to increased NAFLD severity. Given that prevalence of NAFLD in children is rapidly increasing and children may have higher PFAS exposure relative to their body size, these results have potential implications for public health and prevention policy.
PFAS have been in commercial production for over 60 years and have been widely used in many household items such as non-stick cookware, shampoo, and packaging of fast food, resulting in ubiquitous contamination to both the environment and in humans. For example, PFAS have been found to be released from nonstick cookware during typical cooking conditions and to migrate from food-contact packaging into foods.(Begley et al. 2008; Sinclair et al. 2007) A recent analysis indicated that PFAS contaminate over 70% of drinking water sources in the US and that the conventional water treatment system is not effective for removing all PFAS, especially shorter chain PFAS.(Appleman et al. 2014) Therefore, not surprisingly, PFAS compounds, such as PFOA, PFOS and PFHxS, are detected in the blood samples of more than 95% of the general US population.(Kato et al. 2011) In the current study, the plasma PFOS and PFHxS concentrations are comparable to the average serum concentrations reported in NHANES between 2007-2014 for children age 12-19 years.(CDC 2019) Median PFOA concentration in these pediatric NAFLD patients was slightly higher than that reported in the general US population of children of similar age.(CDC 2019)
The findings in this study are consistent with previous studies showing strong associations of serum PFOA and PFOS concentrations with elevated levels of ALT in populations drinking PFAS-contaminated water (Darrow et al. 2016; Gallo et al. 2012) and in U.S. adults in NHANES.(Gleason et al. 2015; Lin et al. 2010) Results from other epidemiologic studies have not been uniformly consistent. A recent pregnancy cohort study in the U.S. found that children with higher PFAS exposures had lower ALT levels.(Mora et al. 2018) In studies examining other markers of liver injury, positive associations were reported between PFAS (including PFOA and PFHxS) and cytokeratin 18, a marker for liver apoptosis, but inverse relationships were reported between PFAS and serum pro-inflammatory cytokines in adults.(Bassler et al. 2019)
There have been no previous studies examining PFAS exposure and histopathologic features of NAFLD in children. We showed that PFAS exposure and in particular PFHxS was associated with increased odds of NASH, liver fibrosis, lobular and portal inflammation, and increased NAFLD activity score. These results are supported by animal studies showing a dose-dependent increase in hepatocellular hypertrophy, and liver weight in response to PFHxS exposure.(Butenhoff et al. 2009; Das et al. 2017) However, a human study in bariatric surgery patients with NAFLD showed that PFHxS serum concentrations were inversely associated with lobular inflammation (Rantakokko et al., 2015). The reasons for the lack of consistency in animal and epidemiologic results merit further study. In human studies, there are no clear patterns of differences between adults and children or between populations with low and high PFAS exposure levels. In addition, comparisons between studies are limited by the heterogeneity of liver outcomes. Most of these studies have also been cross sectional and the possibility of reverse causation, that is, that NAFLD increases PFAS, cannot be excluded. For example, PFAS are excreted by the kidneys, and NAFLD reduces glomerular filtration rate, so the elevated PFAS could be a result of more severe NAFLD rather than vice versa.(Musso et al. 2014; Pacifico et al. 2016)
The exact mechanisms underlying the association between PFAS and NAFLD remain largely unknown. Increased plasma PFAS concentrations were associated with metabolic perturbation in numerous amino acids and phospholipids, key metabolic pathways previously found also to be altered in NAFLD/NASH.(Puri et al. 2009; Sookoian and Pirola 2014) The structural integrated analysis identified a latent cluster characterized by high PFAS levels, dysregulation of glycerophospholipids and amino acids, and more severe fatty liver disease, characterized by NASH. These results are also generally consistent with previous studies in animal models, in which exposure to PFOA induced alteration of lipids (e.g., glycerophospholipids) and amino acids (e.g., tyrosine, phenylalanine, aspartate and asparagine, glycine, serine, alanine and threonine) metabolism in liver tissues.(Yu, N. et al. 2016) Other human studies showed plasma PFAS concentrations were also found to be associated with dysregulation of fatty acid metabolism in elderly U.S. adults (Salihovic et al. 2018) and in Chinese adult males.(Wang, X. et al. 2017) Two studies in children also showed that PFAS levels in blood were associated with significant alterations in glycophospholipid metabolism and several amino acid pathways such as tyrosine, aspartate and asparagine.(Alderete et al. 2019; Kingsley et al. 2019)
These PFAS dysregulated pathways are similar to the metabolic aberrations that have been well documented in patients with NAFLD not only in our but also in other studies. Abnormal bioactive lipids can lead to organelle dysfuction, cell injury and chronic inflammation, the hallmark of NASH.(Musso et al. 2018) For example, altered glycerophospholipids, such as increased phosphoethanolamine levels, were reported to be predictive biomarkers of NAFLD progression.(Gorden et al. 2015) Apart from alterations in lipid metabolism, the imbalance of amino acid metabolism might be another biological mechanism linking PFAS exposure to liver damage. Emerging evidence indicates that the phenotypic switching from hepatic steatosis to NASH entails a reprogramming of amino acid metabolism to fit a stressful metabolic environment in liver.(Sookoian and Pirola 2014) We showed metabolic derangement of a series of amino acids in association with PFAS exposure, with tyrosine metabolism being the most affected pathway. Dysregulated tyrosine metabolism has been shown previously to be associated with increased severity of hepatic steotosis (Jin et al. 2016), and increased tyrosine levels were associated with hepatocellular inflammation and ballooning.(Gaggini et al. 2018)
Strengths of the study included the assessment of NAFLD stages and severity based on liver biopsy, the use of high resolution metabolomics to gain a broad, untargeted view of the associations with PFAS and the application of the novel integrated analysis to identify subgroups of children with increased likelihood for NASH based on plasma PFAS levels and metabolomics profile. There are also some limitations. We acknowledge the uncertainty of the effect estimates as represented by some wide 95% confidence intervals, which could be due to the small sample size. However, this is a unique cohort of pediatric patients with liver biopsy documented NAFLD. In our study, we have excluded children with chronic renal disease and renal insufficiency (creatinine serum levels > 2mg/dl), however we cannot exclude the possibility of reverse causation due to impaired kidney function. Additionally, although we adjusted for sex, ethnicity and BMI, which may affect NAFLD severity,(Lim and Bernstein 2018) the latter is depended on multiple exposures; thus we cannot exclude the possibility of confounding from unmeasured variables such as pubertal status, diet and physical activity.
Conclusions
In summary, this study showed that PFAS exposure was associated with increased risk of NASH and fibrosis in children diagnosed with NAFLD. We also found that PFAS exposure was associated with dysregulation of several lipid and amino acid pathways that have been linked with NAFLD pathogenesis. Results suggest that PFAS may play a role in NAFLD severity in children; however, larger and longitudinal studies are needed to understand better the role of PFAS in NAFLD in children and the mechanisms underlying these associations.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health (NIH): NIH NIEHS P30ES007048, R21ES029681 (Chatzi, McConnell); NIH NIEHS P01ES022845 (Chatzi, McConnell, Jin), NIH NIEHS 1R01 ES029944 (Chatzi), NIH NIEHS F32ES029828 (Jin), R01-MH107205 (Jones and Walker), P30ES019776 (Jones, Miller, Vos, and Walker), U2CES026560 (Jones, Miller, Vos, and Walker), T32ES012870 (Walker), S10OD018006 (Jones), P01CA196569 (Conti and Peng), R01CA140561 (Conti and Peng), and by Environmental Protection Agency RD-83544101 (Chatzi, McConnell).
Footnotes
Competing Financial Interests
The authors declare they have no actual or potential competing financial interests.
References
- Accardi CJ, Walker DI, Uppal K et al. 2016. High-resolution metabolomics for nutrition and health assessment of armed forces personnel. J Occup Environ Med. 58,S80–88. [DOI] [PubMed] [Google Scholar]
- Al-Eryani L, Wahlang B, Falkner KC et al. 2015. Identification of environmental chemicals associated with the development of toxicant-associated fatty liver disease in rodents. Toxicologic pathology. 43,482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, Jin R, Walker DI et al. 2019. Perfluoroalkyl substances, metabolomic profiling, and alterations in glucose homeostasis among overweight and obese hispanic children: A proof-of-concept analysis. Environ Int. 126,445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EL, Howe LD, Jones HE et al. 2015. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PLoS One. 10,e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleman TD, Higgins CP, Quinones O et al. 2014. Treatment of poly- and perfluoroalkyl substances in u.S. Full-scale water treatment systems. Water Res. 51,246–255. [DOI] [PubMed] [Google Scholar]
- Bassler J, Ducatman A, Elliott M et al. 2019. Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines. Environ Pollut. 247,1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley TH, Hsu W, Noonan G et al. 2008. Migration of fluorochemical paper additives from food-contact paper into foods and food simulants. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 25,384–390. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological). 57,289–300. [Google Scholar]
- Boronow KE, Brody JG, Schaider LA et al. 2019. Serum concentrations of pfass and exposure-related behaviors in african american and non-hispanic white women. Journal of Exposure Science & Environmental Epidemiology. 29,206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt EM, Kleiner DE, Wilson LA et al. 2011. Nonalcoholic fatty liver disease (nafld) activity score and the histopathologic diagnosis in nafld: Distinct clinicopathologic meanings. Hepatology (Baltimore, Md). 53,810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Chang SC, Ehresman DJ et al. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in sprague dawley rats. Reprod Toxicol. 27,331–341. [DOI] [PubMed] [Google Scholar]
- CDC. 2019. National report on human exposure to environmental chemicals. https://www.cdc.gov/exposurereport/index.html (accessed 27 August 2019)
- Cohen JC, Horton JD, Hobbs HH 2011. Human fatty liver disease: Old questions and new insights. Science. 332,1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow LA, Groth AC, Winquist A et al. 2016. Modeled perfluorooctanoic acid (pfoa) exposure and liver function in a mid-ohio valley community. Environ Health Perspect. 124,1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KP, Wood CR, Lin MT et al. 2017. Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology. 378,37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds CE, Trevino LS, York B et al. 2017. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol. 13,445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Tittlemier SA, Volkel W et al. 2009. Perfluorinated compounds--exposure assessment for the general population in western countries. Int J Hyg Environ Health. 212,239–270. [DOI] [PubMed] [Google Scholar]
- Gaggini M, Carli F, Rosso C et al. 2018. Altered amino acid concentrations in nafld: Impact of obesity and insulin resistance. Hepatology. 67,145–158. [DOI] [PubMed] [Google Scholar]
- Gallo V, Leonardi G, Genser B et al. 2012. Serum perfluorooctanoate (pfoa) and perfluorooctane sulfonate (pfos) concentrations and liver function biomarkers in a population with elevated pfoa exposure. Environ Health Perspect. 120,655–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason JA, Post GB, Fagliano JA 2015. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the us population (nhanes), 2007-2010. Environ Res. 136,8–14. [DOI] [PubMed] [Google Scholar]
- Go YM, Walker DI, Liang Y et al. 2015a. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicol Sci. 148,531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Walker DI, Soltow QA et al. 2015b. Metabolome-wide association study of phenylalanine in plasma of common marmosets. Amino Acids. 47,589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorden DL, Myers DS, Ivanova PT et al. 2015. Biomarkers of nafld progression: A lipidomics approach to an epidemic. Journal of lipid research. 56,722–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halldorsson TI, Rytter D, Haug LS et al. 2012. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: A prospective cohort study. Environ Health Perspect. 120,668–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M et al. 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 68,3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Banton S, Tran VT et al. 2016. Amino acid metabolism is altered in adolescents with nonalcoholic fatty liver disease-an untargeted, high resolution metabolomics study. J Pediatr. 172,14–19.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A 2007. Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics. 8,118–127. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT et al. 2011. Trends in exposure to polyfluoroalkyl chemicals in the u.S. Population: 1999-2008. Environ Sci Technol. 45,8037–8045. [DOI] [PubMed] [Google Scholar]
- Kingsley SL, Walker DI, Calafat AM et al. 2019. Metabolomics of childhood exposure to perfluoroalkyl substances: A cross-sectional study. Metabolomics. 15,95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M et al. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41,1313–1321. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS et al. 2002. 2000 cdc growth charts for the united states: Methods and development. Vital Health Stat 111–190. [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S et al. 2013. Predicting network activity from high throughput metabolomics. PLoS Comput Biol. 9,e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Bernstein DE 2018. Risk factors for the development of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including genetics. Clin Liver Dis. 22,39–57. [DOI] [PubMed] [Google Scholar]
- Lin CY, Lin LY, Chiang CK et al. 2010. Investigation of the associations between low-dose serum perfluorinated chemicals and liver enzymes in us adults. Am J Gastroenterol. 105,1354–1363. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL 2011. Polyfluorinated compounds: Past, present, and future. Environ Sci Technol. 45,7954–7961. [DOI] [PubMed] [Google Scholar]
- Liu KH, Walker DI, Uppal K et al. 2016. High-resolution metabolomics assessment of military personnel: Evaluating analytical strategies for chemical detection. J Occup Environ Med. 58,S53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MT, Brennan RJ, Hu W et al. 2007. Toxicogenomic study of triazole fungicides and perfluoroalkyl acids in rat livers predicts toxicity and categorizes chemicals based on mechanisms of toxicity. Toxicol Sci. 97,595–613. [DOI] [PubMed] [Google Scholar]
- Mora AM, Oken E, Rifas-Shiman SL et al. 2017. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect. 125,467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora AM, Fleisch AF, Rifas-Shiman SL et al. 2018. Early life exposure to per- and polyfluoroalkyl substances and mid-childhood lipid and alanine aminotransferase levels. Environ Int. 111,1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morck TA, Nielsen F, Nielsen JK et al. 2015. Pfas concentrations in plasma samples from danish school children and their mothers. Chemosphere. 129,203–209. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, Tabibian JH et al. 2014. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 11,e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso G, Cassader M, Paschetta E et al. 2018. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 155,282–302.e288. [DOI] [PubMed] [Google Scholar]
- Nobili V, Alisi A, Newton KP et al. 2016. Comparison of the phenotype and approach to pediatric vs adult patients with nonalcoholic fatty liver disease. Gastroenterology. 150,1798–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifico L, Bonci E, Andreoli GM et al. 2016. The impact of nonalcoholic fatty liver disease on renal function in children with overweight/obesity. Int J Mol Sci. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Peng Q, Ding N et al. 2019. Determinants of per- and polyfluoroalkyl substances (pfas) in midlife women: Evidence of racial/ethnic and geographic differences in pfas exposure. Environ Res. 175,186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton HM, Lavine JE, Van Natta ML et al. 2008. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 135,1961–1971.e1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Wang J, Asante I, et al. 2019. A latent unknown clustering intergrating multi-omics data (lucid) with phenotypic traits. Bioinformatics. btz667. 10.1093/bioinformatics/btz667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri P, Wiest MM, Cheung O et al. 2009. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology (Baltimore, Md). 50,1827–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella ME 2015. Nonalcoholic fatty liver disease: A systematic review. Jama. 313,2263–2273. [DOI] [PubMed] [Google Scholar]
- Salihovic S, Fall T, Ganna A et al. 2018. Identification of metabolic profiles associated with human exposure to perfluoroalkyl substances. J Expo Sci Environ Epidemiol. [DOI] [PubMed] [Google Scholar]
- Simon-Manso Y, Lowenthal MS, Kilpatrick LE et al. 2013. Metabolite profiling of a nist standard reference material for human plasma (srm 1950): Gc-ms, lc-ms, nmr, and clinical laboratory analyses, libraries, and web-based resources. Anal Chem. 85,11725–11731. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Kim SK, Akinleye HB et al. 2007. Quantitation of gas-phase perfluoroalkyl surfactants and fluorotelomer alcohols released from nonstick cookware and microwave popcorn bags. Environmental Science & Technology. 41,1180–1185. [DOI] [PubMed] [Google Scholar]
- Soltow QA, Strobel FH, Mansfield KG et al. 2013. High-performance metabolic profiling with dual chromatography-fourier-transform mass spectrometry (dc-ftms) for study of the exposome. Metabolomics. 9,S132–s143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son HY, Kim SH, Shin HI et al. 2008. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch Toxicol. 82,239–246. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Pirola CJ 2014. Nafld. Metabolic make-up of nash: From fat and sugar to amino acids. Nat Rev Gastroenterol Hepatol. 11,205–207. [DOI] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH et al. 2013. Xmsanalyzer: Automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 14,15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Liu K et al. 2016. Computational metabolomics: A framework for the million metabolome. Chem Res Toxicol. 29,1956–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DI, Lane KJ, Liu K et al. 2019. Metabolomic assessment of exposure to near-highway ultrafine particles. J Expo Sci Environ Epidemiol. 29,469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HT, Zhao YG, Wei X et al. 2012. Pfos-induced hepatic steatosis, the mechanistic actions on beta-oxidation and lipid transport. Biochim Biophys Acta. 1820,1092–1101. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Liang Y et al. 2013. Specific accumulation of lipid droplets in hepatocyte nuclei of pfoa-exposed balb/c mice. Sci Rep. 3,2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Liu L, Zhang W et al. 2017. Serum metabolome biomarkers associate low-level environmental perfluorinated compound exposure with oxidative /nitrosative stress in humans. Environ Pollut. 229,168–176. [DOI] [PubMed] [Google Scholar]
- Welsh JA, Karpen S, Vos MB 2013. Increasing prevalence of nonalcoholic fatty liver disease among united states adolescents, 1988-1994 to 2007-2010. J Pediatr. 162,496–500.e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Liang M, Yang Z et al. 2017. Effect of acute exposure to pfoa on mouse liver cells in vivo and in vitro. Environ Sci Pollut Res Int. 24,24201–24206. [DOI] [PubMed] [Google Scholar]
- Wu X, Xie G, Xu X et al. 2018. Adverse bioeffect of perfluorooctanoic acid on liver metabolic function in mice. Environ Sci Pollut Res Int. 25,4787–4793. [DOI] [PubMed] [Google Scholar]
- Yu N, Wei S, Li M et al. 2016. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci Rep. 6,23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Li S et al. 2013. Hybrid feature detection and information accumulation using high-resolution lc-ms metabolomics data. J Proteome Res. 12,1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.