Abstract
Ferroptosis is a form of regulated cell death involving lethal peroxidation of phospholipids (Hirschhorn and Stockwell, 2018). Recent results reveal that ferroptosis mediates the tumor suppressive activity of interferon gamma secreted by CD8+ T cells in response to immune checkpoint blockade, suggesting the immune system may function in part through ferroptosis to prevent tumorigenesis (Wang et al., 2019).
Most anti-cancer therapies, both existing and envisioned, act ultimately by selectively inducing the death of cancer cells. Conventional chemotherapeutic agents generally induce apoptosis or a mixture of apoptosis and unregulated necrosis, in some cases involving mitotic catastrophe with moderate selectivity for tumor cells, but with significant toxicity to numerous normal tissues (Montero et al., 2015). Targeted agents, such as kinase inhibitors or antibodies, frequently take advantage of the addiction of cancer cells to specific oncogenic kinases, such as mutated BRAF or amplified EGFR, inducing apoptosis in sensitive cancers. New therapies, including engineered cell therapies such as CAR T cells, also are believed to trigger apoptosis or unregulated necrosis, but their selectivity derives from their selective recognition of tumor cells via presentation by tumor cells of peptide neoantigens not found in normal cells (Davenport et al., 2018). However, with the emergence of newly discovered forms of regulated cell death, it may be possible to induce tumor cell killing through new mechanisms with advantages over driving apoptosis or unregulated necrosis (Galluzzi et al., 2018).
Ferroptosis is a form of regulated cell death (Dixon et al., 2012), meaning that it can be attenuated or accelerated by specific genetic or pharmacological manipulations (Galluzzi et al., 2018). The activation of ferroptotic cell death requires three hallmarks that ultimately result in lethal lipid peroxidation (Dixon and Stockwell, 2019). First, ferroptosis requires the presence of phospholipids containing polyunsaturated fatty acyl tails, which are the substrates for peroxidation (Stockwell et al., 2017). Second, ferroptosis requires the presence of redox-active iron, most likely both in the labile iron pool and in the form of iron-dependent lipoxygenases, to drive the peroxidation process (Gao et al., 2015). Third, ferroptosis requires failure of the complex repair system that eliminates lipid peroxidation products under normal homeostatic conditions (Stockwell et al., 2017). When all these conditions are met, conditions are ripe for overwhelming peroxidation of unrepaired phospholipids that results in cell death.
Ferroptosis is involved in numerous diseases including neurodegeneration, ischemic organ injury, and cancer (Stockwell et al., 2017). Manipulation of ferroptosis has been explored as a potential therapeutic strategy. However, whether ferroptosis has a natural function in any physiological contexts remains relatively enigmatic. There is some evidence suggesting that ferroptosis may serve a normal tumor suppressive function, including the observation that the tumor suppressors p53, fumarase, and BAP1 can drive ferroptosis under some conditions, and that some negative regulators of ferroptosis, such as SLC7A11, GPX4 and NRF2 are overexpressed, amplified or otherwise activated in a variety of tumors (Dixon and Stockwell, 2019). Nonetheless, the question of whether normal tumor suppression in multicellular organisms requires ferroptosis remains an open question.
One of the most impactful classes of anti-cancer therapies developed in recent years are the immune checkpoint blockade therapies, which work by activating the natural tumor-selective killing activity of T cells, part of the adaptive arm of the immune system (Sanmamed and Chen, 2018). Recently, Wang et al. reported that CD8+ T cells, which represent the classic subtype of tumor-killing T cells, drive ferroptosis in tumor cells (Wang et al., 2019). As such, the remarkable clinical benefit of immunotherapies such as immune checkpoint blockade, may derive, at least in part, by driving ferroptosis in tumor cells. This study is important conceptually, as it not only lends further support to the notion that ferroptosis may serve a natural tumor suppressive function, but also unveils a novel mechanism in that the immune system can propel cancer cell ferroptosis to suppress tumorigenesis.
Wang et al. discovered that treatment of mice bearing ovarian or melanoma tumors with an immune checkpoint therapy involving PD-L1 blockade caused increased lipid peroxidation and production of downstream degradation products of lipid peroxides, two markers of ferroptosis, in tumor cells. Ferroptosis-resistant tumor cells did not respond to PD-L1 blockade, and suppression of ferroptosis prevented the benefit of PD-L1 blockade, confirming that ferroptosis induction in tumor cells was necessary for the efficacy of immunotherapy in these models.
CD8+ T cells release cytokines, including tumor necrosis factor and interferon gamma (IFNγ), to drive killing of tumor cells. Wang et al found that IFNγ was the mediator of CD8+ T cell ferroptosis-driving activity, in that blocking this cytokine abolished the ferroptosis-inducing activity of these T cells. They further found that IFNγ downregulated the expression of system xc-, a crucial regulator of ferroptosis (see Figure 1). Furthermore, they found that expression of system xc- correlates with patient outcomes in response to PD-1 blockade.
Figure 1.
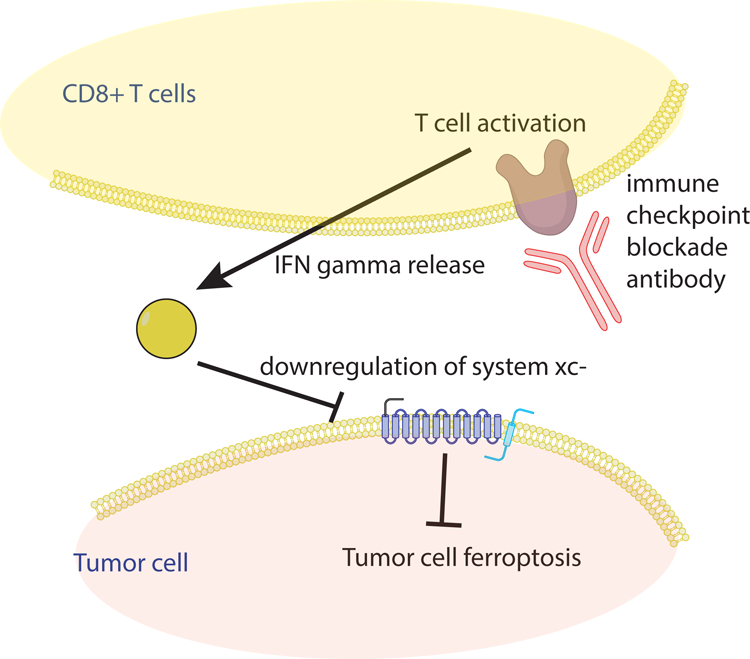
Overview of the Mechanism by which Immune Checkpoint Blockade Drives Ferroptosis in Tumor Cells
These findings suggest a few exciting avenues for future investigations. First, other anti-tumor immune functions and immunotherapies may act by promoting tumor cell ferroptosis, either through system xc- downregulation or through numerous other ferroptosis-triggering mechanisms that have been described. Second, some oncogenes and tumor suppressors may function by interrupting the pro-ferroptotic tumor suppressive function of the immune system. Third, it is possible that the immune system triggers ferroptosis in normal tissues in some pathological contexts, resulting in degenerative diseases. Furthermore, a variety of environmental influences may affect the pro-ferroptotic tumor suppressive activity of the immune system. For example, dietary polyunsaturated fatty acid (PUFA) or iron intake might influence the susceptibility of tumor cells to CD8+-T-cell-driven ferroptosis. Indeed, dietary PUFA intake has been shown to correlate with reduced incidence of a variety of tumors. Together, these new findings provide a wealth of future possibilities for leveraging the pro-ferroptotic activity of the immune system to reduce cancer morbidity and mortality.
Acknowledgements
We thank the National Cancer Institute for research support related to ferroptosis (R35CA209896 and P01CA087497 to B.R.S; R01CA204232 to X.J.).
Footnotes
Declaration of interests
BRS holds equity in and serves as a consultant to Inzen Therapeutics, consults with GLG and Guidepoint Global, and is an inventor on patents and patent applications related to ferroptosis. XJ is an inventor on patent applications related to ferroptosis.
References:
- Davenport AJ, Cross RS, Watson KA, Liao Y, Shi W, Prince HM, Beavis PA, Trapani JA, Kershaw MH, Ritchie DS, et al. (2018). Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proc Natl Acad Sci U S A 115, E2068–E2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon SJ, and Stockwell BR (2019). The Hallmarks of Ferroptosis. Annual Review of Cancer Biology 3, 35–54. [Google Scholar]
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, et al. (2018). Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 25, 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Monian P, Quadri N, Ramasamy R, and Jiang X (2015). Glutaminolysis and Transferrin Regulate Ferroptosis. Mol Cell 59, 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn T, and Stockwell BR (2018). The Development of the Concept of Ferroptosis. Free Radic Biol Med [DOI] [PMC free article] [PubMed]
- Montero J, Sarosiek KA, DeAngelo JD, Maertens O, Ryan J, Ercan D, Piao H, Horowitz NS, Berkowitz RS, Matulonis U, et al. (2015). Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell 160, 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmamed MF, and Chen L (2018). A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 175, 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, et al. (2017). Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 171, 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Green M, Choi JE, Gijon M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. (2019). CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]


