Abstract
Purpose: To determine the toxicity profile of transarterial chemoembolization (TACE) at 6 months and 1 year after treatment in patients with hepatocellular carcinoma (HCC) in a standardized oncology protocol so that TACE could be compared with systemic chemotherapeutic regimens for liver cancer.
Materials and Methods: The study was authorized by the institutional review board. Between January 2002 and January 2007, 190 patients (155 men, 35 women; median age, 65 years; age range, 18–84 years) with HCC who underwent TACE treatment were identified from a prospectively collected database. Clinical records of complete blood cell counts and chemical profiles at baseline and at 6 and 12 months after treatment were studied retrospectively. Toxicity was graded according to the common terminology criteria for adverse events (CTCAE). A transition (survival) analysis perspective was used to estimate the distribution of toxicity grades. Patient survival from the first TACE session was calculated with Kaplan-Meier analysis.
Results: Grade 3 or 4 toxicity 6 and 12 months, respectively, after treatment included leukocytopenia (7% and 19%); anemia (9% and 19%); thromobocytopenia (13% and 23%); prolonged activated partial thromboplastin time (8% and 18%); elevated aspartate aminotransferase (15% and 18%), alanine aminotransferase (10% and 18%), and alkaline phosphatase (8% and 18%) levels; hypoalbuminemia (10% and 19%); hyperbilirubinemia (10% and 22%); and alopecia (18%). The cumulative survival rate was 58% at 1 year, 39% at 2 years, and 29% at 3 years. These toxicity rates were considerably lower than those reported after treatment with currently used systemic chemotherapeutic agents.
Conclusion: Study results show that TACE has a favorable long-term toxicity profile in patients with HCC. Data clearly support the role of TACE in the treatment of patients with nonresectable HCC.
© RSNA, 2008
Keywords: CTCAE = Common Terminology Criteria for Adverse Events, HCC = hepatocellular carcinoma, TACE = transarterial chemoembolization
Hepatocellular carcinoma (HCC) is the fifth most common cancer in the world (1). The incidence of and mortality rate with HCC continue to rise steadily in North America and Europe, mainly owing to the concomitant increase in hepatitis C viral infections (2,3).
Surgical treatments, including hepatic resection, liver transplantation, and percutaneous ablation, are considered the only curative treatments for patients with early-stage HCC and yield 5-year survival rates of 50%–70% (4). However, fewer than 20% of patients with HCC can be treated surgically, and given the lack of a survival benefit from systemic chemotherapy, locoregional therapeutic options such as transarterial chemoembolization (TACE) have become the mainstay of therapy.
A key theoretic advantage of chemoembolization over systemic chemotherapy is that the chemotherapeutic agents used (doxorubicin, cisplatin, and others) are not intravenously infused throughout the systemic circulation; rather, they are administered locally through the hepatic artery. This is especially important in patients with HCC, who also have underlying liver dysfunction. One of the main concerns regarding systemic chemotherapy is the fact that patients who receive this form of treatment may experience side effects that include pain, nausea, vomiting, myelosuppresion, and alopecia and/or serious adverse events such as cardiac toxicity. These side effects are well described in the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0 (5), and represent one of the major disadvantages of using systemically delivered chemotherapy. Moreover, rates of response to single or combination chemotherapy are still very low—typically less than 20% (6).
The subjectively reported side effects of TACE are mild compared with those caused by systemic chemotherapy. These side effects are in large part attributed to postembolization syndrome and include nausea, vomiting, abdominal pain, fever, and loss of appetite (7,8). Because the focus of most studies is patient survival rather than toxicities caused by TACE, precise analysis of potential TACE-induced systemic toxicities is lacking (9,10).
Yet the potential lack of severe systemic toxicities after TACE compared with those after systemic chemotherapy is one of the most important advantages of using this locoregional therapeutic approach. Therefore, the goal of our study was to determine the toxicity profile of TACE at 6 months and 1 year after treatment in patients with HCC in a standardized oncology protocol (CTCAE, version 3.0) so that TACE could be compared with the use of the systemic chemotherapeutic agents most commonly used for liver cancer (ie, doxorubicin, cisplatin, and 5-fluorouracil).
MATERIALS AND METHODS
Patient Selection
The study was authorized by the institutional review board of Johns Hopkins Hospital. We retrospectively analyzed prospectively collected data on all patients with HCC who were evaluated at Johns Hopkins Hospital Liver Clinic for possible TACE between January 1, 2002, and January 1, 2007. For all of these patients, the diagnosis of HCC was based on either the findings in histologic specimens obtained with needle biopsy or the finding of a hypervascular lesion on cross-sectional magnetic resonance (MR) images in addition to an α-fetoprotein level higher than 400 U/L (400 μg/L). Only those patients who were not suitable for curative therapies such as resection, liver transplantation, or percutaneous intervention were considered for TACE. Patients were required to be at least 18 years old, have preserved liver function (Child-Pugh class A or B) without substantial liver decompensation, and have an Eastern Cooperative Oncology Group performance status score of 0–2. Encephalopathy, severe variceal bleeding, and/or either ascites, marked thrombocytopenia, prolonged impaired renal function, acute renal failure, or severe liver failure was considered an absolute contraindication to TACE. All patients provided written informed consent before undergoing any study-specific procedures. Only those patients whose baseline evaluation was performed at our institution were included. Baseline evaluation included complete blood cell count, a biochemical profile, and dynamic MR imaging.
Chemoembolization Technique
All chemoembolizations were performed by a single experienced interventional radiologist (J.F.H.G., K.H., C.S.G.) and by using the same technique. An 18-gauge single-wall needle was used with the Seldinger technique to access the right common femoral artery. A 5-F vascular sheath was placed in the right common femoral artery over a 0.035-inch guidewire (Terumo Medical, Somerset, NJ). With fluoroscopic guidance, a 5-F glide Simmons-1 catheter (Cordis, Miami, Fla) was advanced into the aortic arch and then used to select the celiac axis. The catheter was advanced over the guidewire and into the desired hepatic artery branch, depending on the tumor location. Selective catheterization was performed to achieve lobar or segmental embolization based on the targeted lesions. A solution containing 100 mg of cisplatin (Platinol; Bristol-Myers Squibb, Princeton, NJ), 50 mg of doxorubicin (Adriamycin; Pharmacia-Upjohn, Kalamazoo, Mich), and 10 mg of mitomycin C (Mutamycin C; Bedford Laboratories, Bedford, Ohio) in a 1:1 mixture with iodized oil was infused and followed by the infusion of either polyvinyl alcohol particles or gelatin-coated trisacryl microspheres (Embosphere particles; Biosphere Medical, Rockland, Mass) until stasis was achieved.
Collected Data and Follow-up
According to the protocol, patients underwent contrast material–enhanced and diffusion MR imaging 4–6 weeks after TACE for assessment of tumor response. Complete blood cell counts and biochemistry profiles were acquired to assess toxicity. Patients with nearly complete tumor necrosis were followed up with MR imaging, complete blood cell counts, and biochemistry profiles every 6–8 weeks. Patients with residual enhancement and a maintained clinical performance status underwent additional TACE treatment(s). Toxicity was assessed and graded according to the CTCAE, version 3.0, for toxicities. The CTCAE, version 3.0, are used to define grade 1–5 toxicities, with unique clinical descriptions of the severity for each adverse event (AE) based on the following general guidelines: Grade 1 indicates mild AE; grade 2, moderate AE; grade 3, severe AE; grade 4, life-threatening or disabling AE; and grade 5, death related to AE. At the time of analysis, the survival statuses of all patients were obtained from the Social Security Death Index. A decision was also made to exclude from the analysis any measurements that had been obtained within 3 weeks after TACE. This decision was based on the fact that transient transaminase elevation is a normal response to TACE (without clinical consequences) that is seen in nearly all patients who undergo this treatment.
Typically, up to three separate TACE treatments are performed in a treatment cycle, similar to systemic chemotherapy cycles. The decision to repeat treatment was based on residual enhancement seen at MR imaging. We chose two time points at which to analyze the data: 6 months after the first TACE for evaluation of short-term toxicity after a complete TACE cycle and 1 year after TACE for assessment of the long-term effect of TACE on systemic and liver-specific toxicities. These time points also allowed us to compare our results with those obtained with other liver chemotherapy regimens.
Statistical Analyses
The goal of our analysis was to estimate the distribution of toxicity grades at 6-month and 1-year follow-up after the first TACE. In the following paragraph, we discuss the methods that we used to estimate the toxicity distributions for one value at the 6 month follow-up, as these distributions are analogous for all toxicity substances and both follow-up times.
For the patients in whom toxicity measurements were obtained both before and after 6 months, the closest measurements were used to obtain interpolated grades of toxicity at 6 months. For patients who were alive but in whom measurements had stopped before 6 months had passed, interpolation was not possible. To estimate the goal under reasonable assumptions, we viewed the problem from a transition—that is, survival analysis—perspective. From this perspective, for each patient, i, there are six transition times—T0,i, T1,i, T2,i, T3,i, T4,i, and Tdeath,i—that start from the first TACE and end at the time (closest to 6 months) at which the patient transitions to toxicity grade 0, to grade 1, to grade 2, to grade 3, to grade 4, and to death, respectively. From this perspective, the early stopping of measurements before 6 months is expressed as a censoring of some transition times. For example, if the last toxicity measurement in a patient was obtained at 4 months and was grade 3, then times T4,i and Tdeath,i are censored at 4 months, whereas the times T3,i, T2,i, T1,i, and T0,i are uncensored and can be determined by tracing back the patient's measurements.
Details of the procedure used to transform the original data to such censored transition data are given in the Appendix. For each grade g, we estimated the fraction of patients (Pr) for whom the transition time to grade g, (Tg,i), was longer than 6 months, Pr(Tg,i > 6 m), by using survival analysis methods that account for censoring (see below). Finally, assuming that for the unobserved toxicity grades within a period close to 6 months or 1 year toxicity does not decrease, our goal—to determine the fraction of patients who were at a particular toxicity grade at 6 months, Pr(g6m)—could be achieved by using our estimates of Pr(Tg,i > 6 m) according to the following formula:
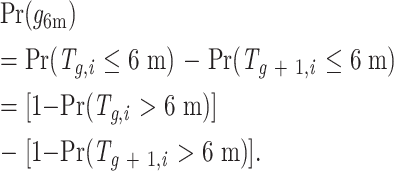 |
In other words, patients who were at toxicity grade g at 6 months are those patients who had transitioned to grade g on or before the sixth month (Tg,i ≤ 6 m) but had not transitioned to grade g + 1 on or before the sixth month (Tg+1,i ≤ 6 m). Because the baseline toxicity grade can be predictive of both the transition times and the censoring rates, we first estimated Pr(Tg,i > 6 m) within strata of baseline grades by using the within-strata Kaplan-Meier estimator (11). Then we reweighted these estimators according to the baseline grade distribution. We estimated baseline distributions of toxicity grades, transition distributions (see equation) within strata of baseline grades for 6 months and 1 year, and overall transition distributions.
RESULTS
Patient Characteristics
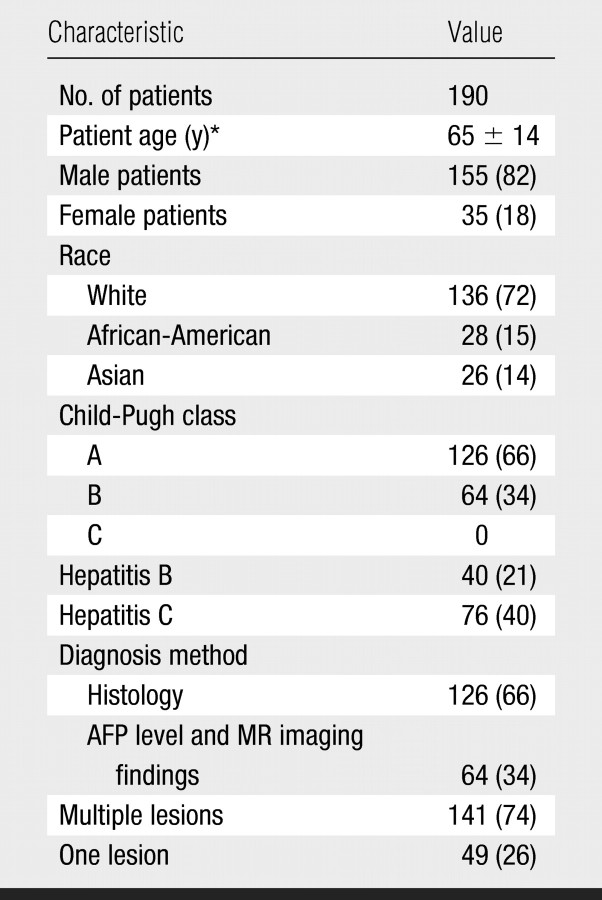
At analysis of the information in our database, we identified a total of 190 patients. The diagnosis of HCC was confirmed at histologic examination in 126 (66%) patients. The diagnosis of HCC in the remaining 64 patients was based on cross-sectional MR imaging findings and elevated serum α-fetoprotein levels. Patient characteristics are shown in the Table. There were 155 male and 35 female patients (mean age, 65 years; age range, 18–84 years), and most of them (72%) were white. Forty patients had chronic hepatitis B, and 76 had chronic hepatitis C. The majority of patients (66%) had Child-Pugh class A cirrhosis. One hundred forty-one patients had multiple liver tumors. The average number of TACE sessions performed per patient was 2.4 (range, 1–3).
Patient Characteristics
Note.—All except age data are numbers of patients, with percentages in parentheses. AFP = α-fetoprotein.
Mean age ± standard deviation.
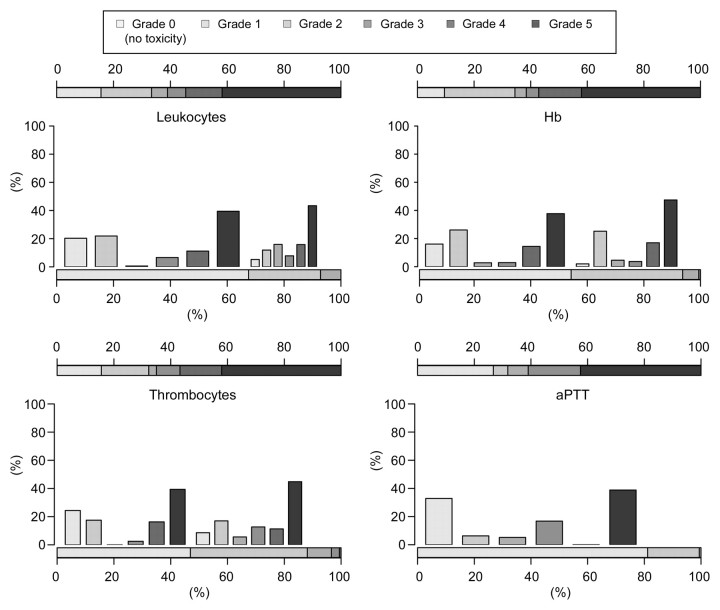
Hematologic Toxicity
The hematologic values observed in the 190 patients at baseline and 1 year after TACE were graded according to the CTCAE, version 3.0, and are presented in Figure 1. Leukocytopenia, anemia, thrombocytopenia, and prolonged activated partial thromboplastin time were the most common hematologic toxic effects. For most patients, the observed toxicity was mild (grade 1 or 2). Grade 3 and grade 4 leukocytopenia was detected in 6% and 1% of the patients, respectively, after 6 months and in 6% and 13% of the patients, respectively, after 1 year. Grade 3 and grade 4 anemia was detected in 6% and 3% of the patients, respectively, after 6 months and in 4% and 15% of the patients, respectively, after 1 year. Grade 3 and grade 4 thrombocytopenia was detected in 10% and 3% of the patients, respectively, after 6 months and in 8% and 15% of the patients, respectively, after 1 year. Grade 3 prolonged activated partial thromboplastin time was detected in 8% of the patients after 6 months and in 18% of the patients after 1 year.
Figure 1:
Hematologic toxicities. Histograms show estimated distributions at baseline and at 1 year after first TACE in all 190 patients. Horizontal bar scales convey the distributions of toxicity grades at baseline (bottom scale) and at 1 year (top scale). Vertical bars convey the possible associations between toxicity grades across the two time points. aPTT = activated partial thromboplastin time, Hb = hemoglobin.
To assess the assumption that within a period close to 6 months or 1 year after TACE toxicity generally does not decrease, for each toxicity type we assessed the data for all patients with measurements obtained both before and after 6 months—or before and after 1 year—and thus in whom the assumption could be tested. Among these patients, the percentages of patients with measurements that were monotonic around 6 months ranged from 87% (glucose) to 99% (creatinine); these proportions ranged from 83% (alanine aminotransferase) to 100% (creatinine) around 12 months, indicating that our assumption was predominantly valid for the data with which it could be assessed.
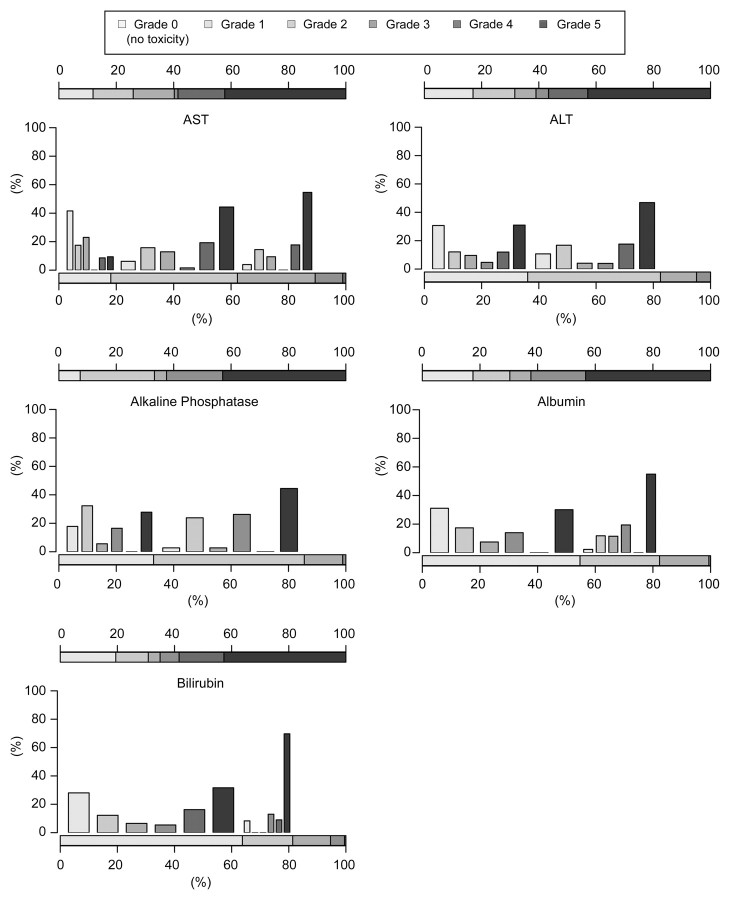
Nonhematologic Toxicity
Postembolization syndrome consisting of abdominal pain, fever, loss of appetite, and nausea developed in most patients. However, these symptoms were generally mild and transient. Alopecia developed in 34 (18%) patients. Elevated aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase levels; hypoalbuminemia; and hyperbilirubinemia were frequent nonhematologic adverse effects (Fig 2). Grade 3 or 4 toxicity manifested as elevated aspartate aminotransferase levels in 15% of the patients after 6 months and in 18% after 1 year. Grade 3 or 4 alanine aminotransferase level elevations were detected in 10% of the patients after 6 months and in 18% after 1 year. Grade 3 alkaline phosphatase level elevations were detected in 8% of the patients after 6 months and in 18% after 1 year. Grade 3 hypoalbuminemia was detected in 10% of the patients after 6 months and in 19% after 1 year. Grade 3 or 4 total bilirubin level elevation was detected in 10% of the patients after 6 months and in 22% after 1 year.
Figure 2:
Nonhematologic toxicities. Histograms show estimated distributions at baseline and at 1 year after first TACE in all 190 patients. Horizontal bar scales convey the distributions of toxicity grades at baseline (bottom scale) and at 1 year (top scale). Vertical bars convey possible associations between toxicity grades across the two time points. ALT = alanine aminotransferase, AST = aspartate aminotransferase.
In our patient group, severe complications were rare. Acute liver failure occurred in five (2.6%) patients and led to death in three of them. One patient died of variceal bleeding within 1 month after TACE. An intrahepatic abscess that developed in one patient 4 weeks after TACE was managed with drainage. One patient had a myocardial infarction 2 days after TACE and was treated with coronary artery bypass graft surgery.
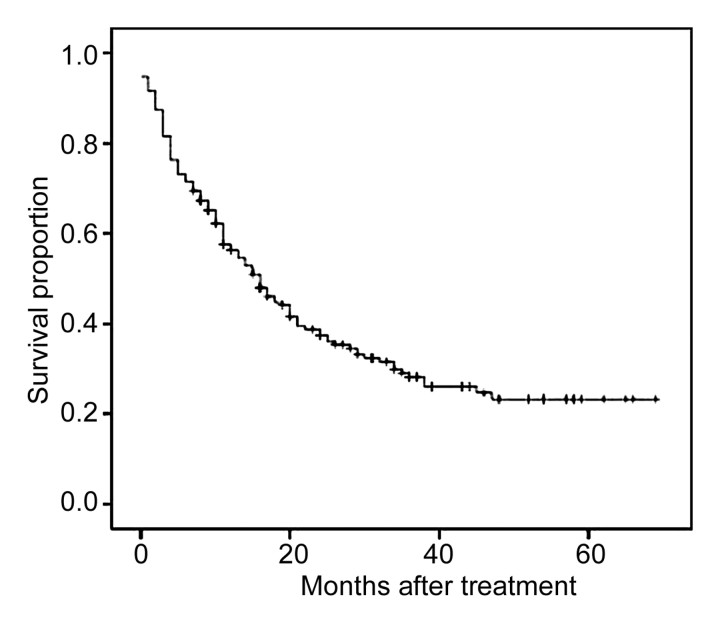
Survival
The data of all patients were included in the survival analysis. Mean and median survival times after diagnosis were 27 and 16 months, respectively. Cumulative survival rates were 58% at 1 year, 39% at 2 years, and 29% at 3 years (Fig 3). Survival analysis of data for the 126 patients with Child-Pugh class A cirrhosis revealed cumulative survival rates of 68% at 1 year, 44% at 2 years, and 31% at 3 years.
Figure 3:
Overall survival curve for all 190 patients with nonresectable HCC who were treated with TACE.
DISCUSSION
HCC is one of the most common fatal cancers in the world. The incidence of HCC in the United States is on the rise owing to increased exposure to the hepatitis C virus (12). The prognosis is invariably poor, with a mean survival time of 6 months (13). Unfortunately, only a selected percentage of patients (10%–15%) are candidates for curative therapies because of the advanced stage of their disease at the time of diagnosis or the presence of comorbidity (14). TACE has become the mainstay of treatment for patients with nonresectable HCC. The aim in performing TACE is to deliver a high concentration of chemotherapeutic agents followed by an embolic agent to the tumor. This embolization blocks the arterial inflow and thus limits the washout of drugs and reduces the systemic side effects (15). Although this is a widely accepted notion, to our knowledge, there are no studies in the literature to date in which the systemic toxicities after TACE have been fully described. Therefore, our aim was to determine the toxicity profile of TACE in patients at 6 months and 1 year after treatment for HCC.
The results of several nonrandomized trials have demonstrated the positive effect of TACE in terms of increased tumor necrosis, as well as the improvements in patient survivals (16,17). However, few controlled randomized studies have been published. Early randomized clinical trials revealed no survival benefit for patients with HCC who were treated with TACE (18,19). This can be explained by the fact that in these early trials, either the enrolled patients or the methods used for TACE were heterogeneous. The two most recent prospective randomized trials, however, revealed markedly longer survivals after chemoembolization (9,10).
HCC is especially difficult to treat with systemic chemotherapy, and although multiple clinical trials have been performed to test many single- and combined-agent chemotherapies, to our knowledge, no regimen has facilitated a substantial tumor response or survival benefit. Furthermore, systemic toxicity is a well known disadvantage of chemotherapy. In fact, systemic toxicity is the limiting factor in establishing the dose of systemic chemotherapy, and in patients with HCC, who are already compromised owing to underlying liver disease, such toxicity can be extremely dangerous (20,21). Sorafenib, an oral multikinase inhibitor, has induced partial tumor response; however, clinical trials are still underway and an extensive toxicity profile has yet to be determined (22). We believe that locoregional therapy, such as TACE, is unique because it delivers highly concentrated doses of chemotherapy to the tumor in a specific manner while preserving the nontumorous healthy liver tissue. In theory, this should prevent the occurrence of major systemic side effects.
The single chemotherapeutic agents that have facilitated a consistent tumor response rate of more than 10% are doxorubicin, 5-fluorouracil, and cisplatin. These agents reportedly have induced hematologic toxicities in as high as 22% of patients (grade 3 or 4 anemia), 28% of patients (grade 3 or 4 thrombocytopenia), and 67% of patients (grade 3 or 4 leukocytopenia) (23). In comparison, our results confirm the advantage of using locoregional treatment: Grade 3 or 4 hematologic toxicity manifesting as anemia, thrombocytopenia, or leukocytopenia was detected in only 9%, 13%, and 7% of the patients 6 months after TACE, respectively. The reported median survival time after systemic chemotherapy for HCC is less than 6 months (range, 6–20 weeks) (23). Our results indicate a median survival time of 16 months, and hematologic toxicities manifesting as leukocytopenia, anemia, thrombocytopenia, and prolonged activated partial thromboplastin time were detected in only 18%–23% of the patients after 1 year. Such results are even more remarkable given that all of these patients also had evidence of cirrhosis and thus were even more susceptible to the harmful effects from chemotherapy. In the patients with hematologic toxicities, we found no treatment-related mortalities, whereas treatment-related death rates as high as 25% have been reported in patients treated with doxorubicin (24).
Alopecia is considered to be one of the most distressing side effects of cancer therapy (25). This common side effect of systemic chemotherapy usually occurs 2–3 weeks after the first cycle of treatment. The likelihood of alopecia is related to the type of drugs used and the schedule of administration (26). Single-drug treatment with systemically administered anthracyclines leads to total alopecia in approximately 90% of treated patients (27). In our study, however, alopecia occurred in only 18% of the patients treated with TACE. This suggests that a substantial portion of the locally delivered chemotherapeutic agent in TACE stays in the tumor region. At the very least, it appears that TACE causes markedly lower rates of this traumatic side effect than does systemic chemotherapy.
Rarely, TACE induces hepatic failure that results in increased serum levels of aminotransferases and bilirubin, ascites, or hepatic encephalopathy (28). These adverse effects usually are transient, with liver function returning to baseline levels within 3 weeks after TACE—even in patients with advanced HCC (but with Child-Pugh class A or B cirrhosis). These effects are reportedly independent of patient age, embolization site, and number of treatments (29). In our patient population, grade 3 or 4 toxicities manifested as elevated aminotransferase and bilirubin serum levels in 18%–22% of the patients after 1 year. However, we cannot state conclusively whether this was a result of TACE or a part of the natural progression of liver disease. Acute liver failure occurred within 1 month after TACE in 2.6% of the patients and led to death in 1.6% of them.
Reported survival rates after TACE in patients with HCC vary between 60% and 88% at 1 year, between 30% and 60% at 2 years, and between 18% and 50% at 3 years, depending on several risk factors, such as Child-Pugh class, α-fetoprotein level, and presence or absence of portal vein thrombosis (30–32). In the present study, survival rates were 58% at 1 year, 39% at 2 years, and 29% at 3 years; however, we did not stratify the patients for potential risk factors. Portal vein thrombosis traditionally has been considered one of the main contraindications to performing TACE (33). However, we previously reported that TACE can be performed safely in patients with this condition (34). Therefore, patients with portal vein thrombosis were not excluded from this study. Moreover, 64 patients had baseline Child-Pugh class B cirrhosis. The relative risk factors for Child-Pugh class B cirrhosis (compared with Child-Pugh class A disease) and portal vein thrombosis are reported to be 1.72 and 1.58, respectively, and therefore may influence survival rates (35). The survival analysis of data for the patients with Child-Pugh class A cirrhosis in our study revealed cumulative survival rates of 68% at 1 year, 44% at 2 years, and 31% at 3 years.
This study had several limitations: First, the study design was retrospective and not controlled. The uncontrolled nature of the study limited our ability to compare our study outcomes with those of other studies. However, our endpoints were objective and were obtained from a standardized source. Second, for obvious ethical reasons, not all patients underwent biopsy to confirm the diagnosis of HCC. We believe that the use of imaging features combined with elevated α-fetoprotein levels is a well-established alternative for confirming the diagnosis. Last, because it is difficult to differentiate procedural toxicity from progressive cirrhosis, our results represent findings in the worst-case scenario.
In conclusion, our results show that TACE has a favorable toxicity profile in patients with HCC, with minimal long-term toxicities. These data clearly support the role of TACE in the treatment of patients with nonresectable HCC. Our results give clinicians a good overview of the toxicities that can be expected after TACE and thereby will be helpful for optimizing treatment strategies.
APPENDIX
Below we describe in detail the procedure used to transform the original data to censored transition data. We describe the procedure used to obtain the transition data relevant for estimating the proportion of patients whose toxicity grade at D months is larger than grade g, Pr(Tg,i > D). There are two main cases:
Case 1
In case 1, the patient has measurements obtained both before and after the date D. In this case, we define the measurement grade on the left or right of the interval, which includes date D as level.left or level.right. Accordingly, time.left and time.right are defined as the times when these measurements are made. There are two subcases. In subcase 1, the toxicity grade, g, increases around date D: (a) If level.right is less than g, then the transition data for the time to cross toxicity grade g (ie, time of follow-up and observation status) are defined as (date D + 1, 0). (b) If level.left is less than g and level.right is greater than or equal to g, then the transition data for the time to cross toxicity grade g are defined as (crossing time, 1), where crossing time is the interpolated time between time.left and time.right for crossing g. (c) If both level.left and level.right are greater than or equal to g, then the transition data for the time to cross toxicity grade g are defined as (track back, 1), where track back is the first time the grade crossed g.
In subcase 2, the toxicity grade either decreases or does not change around date D: (a) If that grade is less than g, then the transition data for the time to cross toxicity grade g are (date D + 1, 0). (b) Otherwise, the transition data for the time to cross toxicity grade g are (track back, 1).
Case 2
In case 2, there is no measurement after date D. In this case, level.left denotes the second to the last measurement and level.right denotes the last measurement. Accordingly, time.left and time.right are defined. There are three conditions: (a) If level.right is less than g, then the transition data are (time.right, 0). (b) If level.left is less than g and level.right is greater than or equal to g, then the transition data are (crossing time, 1). (c) If both level.left and level.right are greater than or equal to g, then the transition data are (track back, 1).
We provide an example: A patient had a measurement of glucose toxicity of grade 0 at 153 days, and the next measurement was obtained at grade 2 at 226 days. This patient is predicted to have crossed grade 2 at day 202; thus, this patient's contribution to estimating the proportion of patients whose toxicity grade at 180 days has crossed grade 2, Pr(T2,i > 180 days), belongs in category 1(1)b (case 1, subcase 1, condition b) and the transition data are (202, 1).
Acknowledgments
The authors are grateful to R. Torrance Andrews, MD, University of Washington, Seattle, Wash, for his assistance.
Our results show that transarterial chemoembolization has a favorable toxicity profile in patients with hepatocellular carcinoma, with minimal long-term toxicities.
Footnotes
Authors stated no financial relationship to disclose.
Author contributions: Guarantors of integrity of entire study, M.B., J.A.V., J.F.H.G.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; literature research, M.B., J.A.V., K.H., C.S.G., E.L.; clinical studies, M.B., J.A.V., K.H., C.S.G., J.F.H.G.; statistical analysis, M.B., J.A.V., C.F., C.S.G., Y.C.; and manuscript editing, M.B., J.A.V., K.H., C.S.G., E.L., J.F.H.G.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001;94:153–156. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 1999;340:745–750. [DOI] [PubMed] [Google Scholar]
- 3.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:1198–1203. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002;35:519–524. [DOI] [PubMed] [Google Scholar]
- 5.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. [DOI] [PubMed] [Google Scholar]
- 6.Carr BI Hepatocellular carcinoma: current management and future trends. Gastroenterology 2004;127(5 suppl 1):S218–S224. [DOI] [PubMed] [Google Scholar]
- 7.Chung JW, Park JH, Han JK, et al. Hepatic tumors: predisposing factors for complications of transcatheter oily chemoembolization. Radiology 1996;198:33–40. [DOI] [PubMed] [Google Scholar]
- 8.Xia J, Ren Z, Ye S, et al. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol 2006;59:407–412. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–1739. [DOI] [PubMed] [Google Scholar]
- 10.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–1171. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 12.El-Serag HB Epidemiology of hepatocellular carcinoma in USA. Hepatol Res 2007;37(suppl 2):S88–S94. [DOI] [PubMed] [Google Scholar]
- 13.Capocaccia R, Sant M, Berrino F, Simonetti A, Santi V, Trevisani F. Hepatocellular carcinoma: trends of incidence and survival in Europe and the United States at the end of the 20th century. Am J Gastroenterol 2007;102:1661–1670. [DOI] [PubMed] [Google Scholar]
- 14.Stuart KE, Anand AJ, Jenkins RL. Hepatocellular carcinoma in the United States: prognostic features, treatment outcome, and survival. Cancer 1996;77:2217–2222. [DOI] [PubMed] [Google Scholar]
- 15.Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 16.Barone M, Ettorre GC, Ladisa R, et al. Transcatheter arterial chemoembolization (TACE) in treatment of hepatocellular carcinoma. Hepatogastroenterology 2003;50:183–187. [PubMed] [Google Scholar]
- 17.Bronowicki JP, Vetter D, Dumas F, et al. Transcatheter oily chemoembolization for hepatocellular carcinoma: a 4-year study of 127 French patients. Cancer 1994;74:16–24. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier G, Roche A, Ink O, et al. A randomized trial of hepatic arterial chemoembolization in patients with unresectable hepatocellular carcinoma. J Hepatol 1990;11:181–184. [DOI] [PubMed] [Google Scholar]
- 19.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology 1998;27:1578–1583. [DOI] [PubMed] [Google Scholar]
- 20.Aguayo A, Patt YZ. Nonsurgical treatment of hepatocellular carcinoma. Semin Oncol 2001;28:503–513. [DOI] [PubMed] [Google Scholar]
- 21.Di Maio M, De Maio E, Perrone F, Pignata S, Daniele B. Hepatocellular carcinoma: systemic treatments. J Clin Gastroenterol 2002;35(5 suppl 2):S109–S114. [DOI] [PubMed]
- 22.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293–4300. [DOI] [PubMed] [Google Scholar]
- 23.Simonetti RG, Liberati A, Angiolini C, Pagliaro L. Treatment of hepatocellular carcinoma: a systematic review of randomized controlled trials. Ann Oncol 1997;8:117–136. [DOI] [PubMed] [Google Scholar]
- 24.Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma: a prospective randomized trial. Cancer 1988;62:479–483. [DOI] [PubMed] [Google Scholar]
- 25.Gunnars B, Nygren P, Glimelius B. Assessment of quality of life during chemotherapy. Acta Oncol 2001;40:175–184. [DOI] [PubMed] [Google Scholar]
- 26.Ridderheim M, Bjurberg M, Gustavsson A. Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer 2003;11:371–377. [DOI] [PubMed] [Google Scholar]
- 27.Fischer DS, Knobf MT, Durivage HJ. The cancer chemotherapy handbook. 4th ed. St. Louis, Mo: Mosby, 1993.
- 28.Kiely JM, Rilling WS, Touzios JG, et al. Chemoembolization in patients at high risk: results and complications. J Vasc Interv Radiol 2006;17:47–53. [DOI] [PubMed] [Google Scholar]
- 29.Caturelli E, Siena DA, Fusilli S, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue—long-term prospective study. Radiology 2000;215:123–128. [DOI] [PubMed] [Google Scholar]
- 30.Geschwind JF, Ramsey DE, Choti MA, Thuluvath PJ, Huncharek MS. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol 2003;26:344–349. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda M, Okada S, Yamamoto S, et al. Prognostic factors in patients with hepatocellular carcinoma treated by transcatheter arterial embolization. Jpn J Clin Oncol 2002;32:455–460. [DOI] [PubMed] [Google Scholar]
- 32.Llado L, Virgili J, Figueras J, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer 2000;88:50–57. [DOI] [PubMed] [Google Scholar]
- 33.Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47–54. [DOI] [PubMed] [Google Scholar]
- 34.Georgiades CS, Hong K, D'Angelo M, Geschwind JF. Safety and efficacy of transarterial chemoembolization in patients with unresectable hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2005;16:1653–1659. [DOI] [PubMed] [Google Scholar]
- 35.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 1998;28:751–755. [DOI] [PubMed] [Google Scholar]