Syntheses and crystal structures of three metal complexes [M(acac)2(TMEDA)] [M = Mn (1), Fe (2) and Zn (3)] with acetylacetonate and N,N,N′,N′-tetramethylethylenediamine are discussed.
Keywords: crystal structure, acetylacetonate, tetramethylethylenediamine, transition metal complex
Abstract
The complexes bis(acetylacetonato-κ2 O,O′)(N,N,N′,N′-tetramethylethylenediamine-κ2 N,N′)manganese(II), [Mn(C5H7O2)2(C6H16N2)], bis(acetylacetonato-κ2 O,O′)(N,N,N′,N′-tetramethylethylenediamine-κ2 N,N′)iron(II), [Fe(C5H7O2)2(C6H16N2)], and bis(acetylacetonato-κ2 O,O′)(N,N,N′,N′-tetramethylethylenediamine-κ2 N,N′)zinc(II), [Zn(C5H7O2)2(C6H16N2)], were synthesized from the reaction of the corresponding metal acetylacetonates [M(acac)2(H2O)2] with N,N,N′,N′-tetramethylethylenediamine (TMEDA) in toluene. Each of the complexes displays a central metal atom which is nearly octahedrally surrounded by two chelating acac and one chelating TMEDA ligand, resulting in an N2O4 coordination set. Despite the chemical similarity of the complex units, the packing patterns for compounds 1–3 are different and thus the crystal structures are not isotypic.
Chemical context
Pentane-2,4-dionate (acac) and ethylenediamine derivatives are amongst the most widely used chelate ligands in transition metal chemistry. The crystal structures of mixed complexes [M(acac)2(TMEDA)] (TMEDA = N,N,N′,N′-tetramethylethylenediamine) containing both types of ligands have been reported for several divalent metals, e.g. M = V (Ma et al., 1999 ▸), Co (Pasko et al., 2004 ▸), Ni (Trimmel et al., 2002 ▸; Zeller et al., 2004 ▸) and Ru (Halbach et al., 2012 ▸). The synthesis of [Zn(acac)2(TMEDA)] was reported recently in conjunction with the Ru derivative but without crystal structure determination (Halbach et al., 2012 ▸). Typically, [M(acac)2(TMEDA)] complexes are used as valuable starting materials for the preparation of organometallic and coordination compounds (Kaschube et al. 1988 ▸; Nelkenbaum et al., 2005 ▸; Albrecht et al., 2019 ▸). Moreover, there is an increasing interest in [M(acac)2(TMEDA)] and related [M(hfa)2(TMEDA)] (hfa = 1,1,1,5,5,5-hexafluoropentane-2,4-dionate) complexes as precursor materials for CVD deposition of Co3O4 (Pasko et al., 2004 ▸), Fe2O3 (Barreca et al., 2012 ▸) and MnF2 (Malandrino et al., 2012 ▸).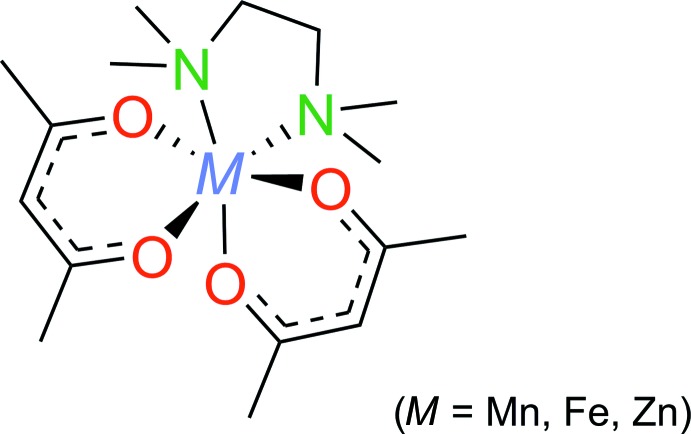
Typically, [M(acac)2(TMEDA)] complexes are synthesized from the reaction of the metal acetylacetonates with TMEDA. Following this procedure, we obtained the complexes [Mn(acac)2(TMEDA)] (1), [Fe(acac)2(TMEDA)] (2) and [Zn(acac)2(TMEDA)] (3) from the corresponding dihydrates [M(acac)2(H2O)2] and TMEDA in toluene as solvent. Recrystallization from n-hexane at 248 K afforded [Mn(acac)2(TMEDA)] (1) as yellow, [Fe(acac)2(TMEDA)] (2) as red–brown and [Zn(acac)2(TMEDA)] (3) as colorless products. Determination of the magnetic moments for [Mn(acac)2(TMEDA)] (5.7 B.M.) and [Fe(acac)2(TMEDA)] (5.1 B.M.) indicates a high-spin configuration in both cases.
Structural commentary
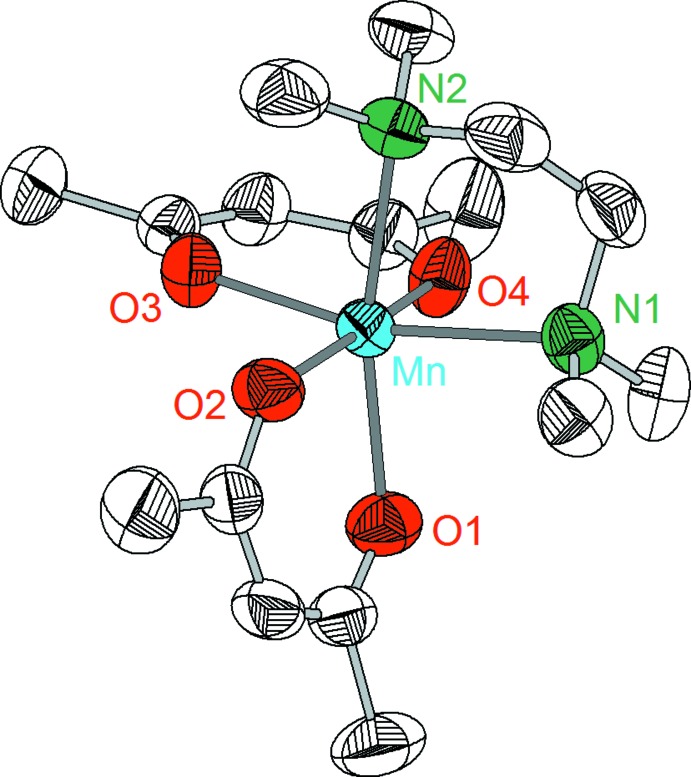
Compounds 1–3 crystallize in the monoclinic system, space group P21/n with Z = 4. However, despite the similarity of the lattice parameters and the analogous molecular structures, complexes 1–3 are not isotypic. The crystal structures consist of discrete complex molecules [M(acac)2TMEDA] in which the central metal atoms are coordinated nearly octahedrally by four oxygen atoms of two acac ligands and two nitrogen atoms of the TMEDA ligand (Figs. 1 ▸–3 ▸ ▸). Mn complex 1 exhibits Mn—O and Mn—N distances of 2.127 (1)–2.150 (1) Å and 2.356 (2)–2.364 (2) Å, respectively (Table 1 ▸). Similar geometric parameters have been reported for [Mn(acac)2(H2O)2] [Mn—O: 2.123 (8)–2.142 (8) Å; Montgomery & Lingafelter, 1968 ▸], [Mn(acac)2(1,10-phenanthroline)] [Mn—O: 2.116 (5)–2.152 (5) Å, Mn—N: 2.307 (5) Å; Stephens, 1977 ▸], [Mn(acac)2(2,2′-bipyridine)] [Mn—O: 2.148 (2)–2.158 (2) Å, Mn—N: 2.283 (2)–2.288 (3) Å; van Gorkum et al., 2005 ▸] or [Mn(hfa)2(TMEDA)] [Mn—O: 2.139 (4)–2.178 (4) Å, Mn—N: 2.299 (5)—2.307 (5) Å; Malandrino et al., 2012 ▸].
Figure 1.
Molecular structure of complex 1 showing the labeling scheme. Displacement ellipsoids drawn at 50% probability level, H atoms are omitted.
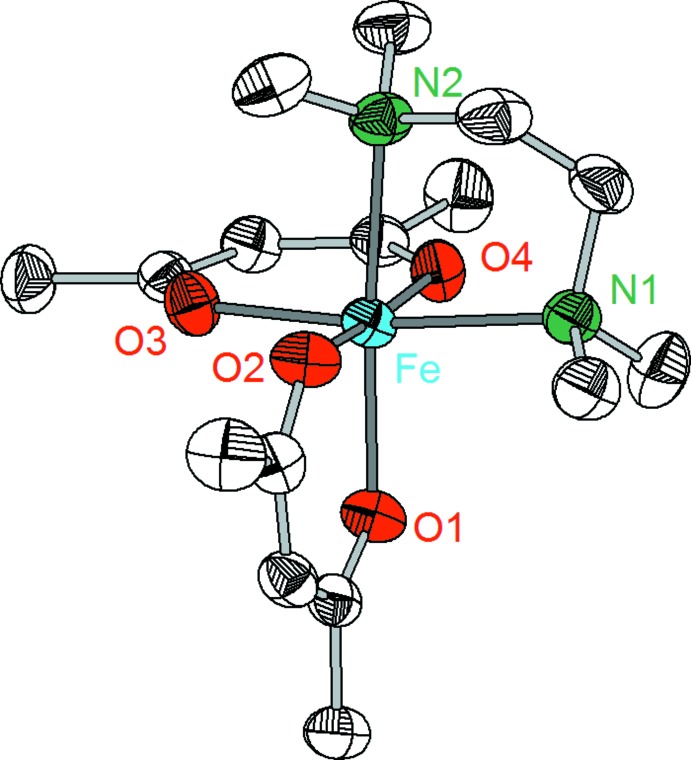
Figure 2.
Molecular structure of complex 2 showing the labeling scheme. Displacement ellipsoids drawn at 50% probability level, H atoms are omitted.
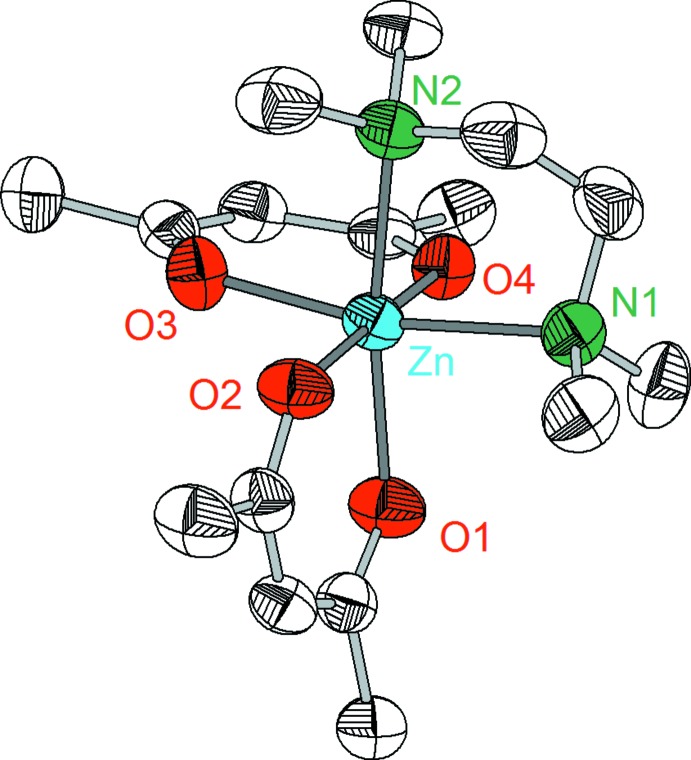
Figure 3.
Molecular structure of complex 3 showing the labeling scheme. Displacement ellipsoids drawn at 50% probability level, H atoms are omitted.
Table 1. Selected geometric parameters (Å, °) for 1 .
| Mn—O1 | 2.1271 (13) | Mn—O4 | 2.1365 (12) |
| Mn—O2 | 2.1500 (12) | Mn—N1 | 2.3643 (15) |
| Mn—O3 | 2.1375 (12) | Mn—N2 | 2.3560 (15) |
| O1—Mn—O2 | 83.61 (5) | O2—Mn—N2 | 90.36 (5) |
| O1—Mn—O3 | 107.00 (5) | O3—Mn—O4 | 83.78 (5) |
| O1—Mn—O4 | 93.25 (5) | O3—Mn—N1 | 165.43 (5) |
| O1—Mn—N1 | 86.01 (5) | O3—Mn—N2 | 90.61 (5) |
| O1—Mn—N2 | 161.29 (6) | O4—Mn—N1 | 89.07 (5) |
| O2—Mn—O3 | 89.71 (5) | O4—Mn—N2 | 94.95 (6) |
| O2—Mn—O4 | 171.63 (5) | N1—Mn—N2 | 77.34 (6) |
| O2—Mn—N1 | 98.41 (5) |
The Fe—O and Fe—N distances in compound 2 [2.050 (1)–2.097 (1) Å and 2.302 (1)–2.318 (1) Å, respectively; Table 2 ▸] are on average shorter than the corresponding Mn—O and Mn—N distances in complex 1. The Fe—O and Fe—N distances compare well with the data that have been observed in the compounds [Fe(acac)2(H2O)2] [Fe—O: 2.034–2.041 Å; Tsodikov et al., 1995 ▸], [Fe(hfa)2(picoline)2] [Fe—O: 2.057 (1) Å, Fe—N: 2.190 (3)–2.224 (3) Å; Novitchi et al., 2017 ▸] or [Fe(hfa)2(TMEDA)] [Fe—O: 2.064 (1)–2.094 (1), Fe—N: 2.229 (2) Å; Dickman et al., 1998 ▸].
Table 2. Selected geometric parameters (Å, °) for 2 .
| Fe—O1 | 2.0876 (10) | Fe—O4 | 2.0520 (9) |
| Fe—O2 | 2.0497 (10) | Fe—N1 | 2.3021 (12) |
| Fe—O3 | 2.0970 (10) | Fe—N2 | 2.3184 (12) |
| O1—Fe—O2 | 85.58 (4) | O2—Fe—N2 | 84.18 (4) |
| O1—Fe—O3 | 93.98 (4) | O3—Fe—O4 | 86.00 (4) |
| O1—Fe—O4 | 99.11 (4) | O3—Fe—N1 | 170.93 (4) |
| O1—Fe—N1 | 92.44 (4) | O3—Fe—N2 | 95.43 (4) |
| O1—Fe—N2 | 166.73 (4) | O4—Fe—N1 | 86.66 (4) |
| O2—Fe—O3 | 95.84 (4) | O4—Fe—N2 | 90.87 (4) |
| O2—Fe—O4 | 174.85 (4) | N1—Fe—N2 | 79.35 (4) |
| O2—Fe—N1 | 91.04 (5) |
[Zn(acac)2(TMEDA)] (3) displays Zn—O and Zn—N distances of 2.061 (1)–2.077 (1) and 2.253 (1)–2.272 (1) Å, respectively (Table 3 ▸). In comparison with the iron complex 2, the average metal–oxygen distances and metal–nitrogen distances are slightly shortened. On the whole, the Zn—O and Zn—N distances in compound 3 are similar to those observed in the related compounds [Zn(acac)2(H2O)2] [Zn—O: 2.032 (1)–2.049 (1) Å; Harbach et al., 2003 ▸], [Zn(acac)2(1,10-phenanthroline)] [Zn—O: 2.044 (1)–2.085 (1) Å, Zn—N: 2.196 (1) Å; Brahma et al., 2008 ▸], [Zn(acac)2(2,2′-bipyridine)] [Zn—O: 2.051 (1)–2.089 (1) Å, Zn—N: 2.197 (2)–2.208 (2) Å; Brahma et al., 2008 ▸] or [Zn(hfa)2(TMEDA)] [Zn—O: 2.103 (1)–2.126 (1) Å, Zn—N: 2.145 (1)–2.151 (1) Å; Ni et al., 2005 ▸].
Table 3. Selected geometric parameters (Å, °) for 3 .
| Zn—O1 | 2.0771 (12) | Zn—O4 | 2.0607 (10) |
| Zn—O2 | 2.0611 (11) | Zn—N1 | 2.2722 (13) |
| Zn—O3 | 2.0645 (11) | Zn—N2 | 2.2533 (13) |
| O1—Zn—O2 | 87.50 (4) | O2—Zn—N2 | 89.57 (5) |
| O1—Zn—O3 | 101.58 (5) | O3—Zn—O4 | 87.96 (4) |
| O1—Zn—O4 | 88.49 (4) | O3—Zn—N1 | 168.61 (5) |
| O1—Zn—N1 | 89.28 (5) | O3—Zn—N2 | 89.09 (5) |
| O1—Zn—N2 | 168.94 (5) | O4—Zn—N1 | 88.92 (5) |
| O2—Zn—O3 | 90.18 (5) | O4—Zn—N2 | 94.86 (5) |
| O2—Zn—O4 | 175.16 (4) | N1—Zn—N2 | 80.27 (5) |
| O2—Zn—N1 | 93.76 (5) |
In general, the above-mentioned [M(hfa)2(TMEDA)] (M = Mn, Fe, Zn) complexes exhibit shorter M—N distances than the corresponding [M(acac)2(TMEDA)] complexes. This effect is probably due to the electron-withdrawing effect of the CF3 groups of the hfa ligands.
The iron complex 2 displays a subtle elongation (0.041 Å) of the Fe—O bonds trans to the N atoms with respect to the Fe—O bonds trans to oxygen. A similar effect was observed for [Co(acac)2(TMEDA)] (Pasko et al., 2004 ▸). In the case of the Mn and Zn complexes 1 and 3, the trans influence is negligible as reported for [Ni(acac)2(TMEDA)] (Trimmel et al., 2002 ▸) and [Ru(acac)2(TMEDA)] (Halbach et al., 2012 ▸). A reverse effect with a shortening of the Zn—O bonds trans to nitrogen was detected for [Zn(acac)2(2,2′-bipyridine)] and [Zn(acac)2(1,10-phenanthroline)] (Brahma et al., 2008 ▸).
Each of the complexes 1–3 exhibits nearly planar six-membered acac-M chelate rings. The maximum deviation from planarity, as indicated by the dihedral angle between the M/O1/O2 (M/O3/O4) plane of the chelate ring and the best plane through O1/C2/C3/C4/O2 (O3/C7/C8/C9/O4), is 6.2 (1)° in the case of the zinc complex 3. PLATON (Spek, 2009 ▸) was used to calculate the dihedral angles. The five-membered M-TMEDA ring adopts a twist conformation with approximate C 2 symmetry. As a result of the centrosymmetric crystal structure, both types of the enantiomeric chelate rings with λ and δ conformations are present.
The MO4N2 coordination polyhedra in compounds 1–3 deviate moderately from a regular octahedron. The O—M—O angles are in the range 171.7 (1)° (complex 1) to 175.2 (1)° (complex 3) and the N—M–-O angles vary from 161.3 (1)° (complex 1) to 170.9 (1)° (complex 2). The smallest acac bite angle is observed in compound 1 [83.6 (1)°], the largest is found in compound 3 [88.0 (1)°]. In the case of the TMEDA ligands, the bite angles are marginally smaller with a range between 77.3 (1)° (compound 1) and 80.3 (1)° (compound 3). Overall, the distortion of the MO4N2 octahedra in compounds 1–3 is very similar to that observed in the analogous V, Ni and Co complexes [M(acac)2(TMEDA)].
Supramolecular features
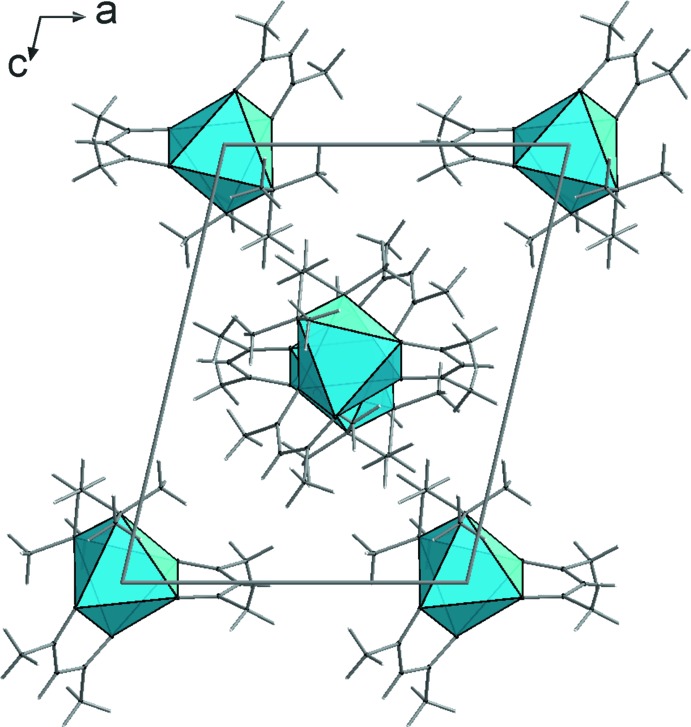
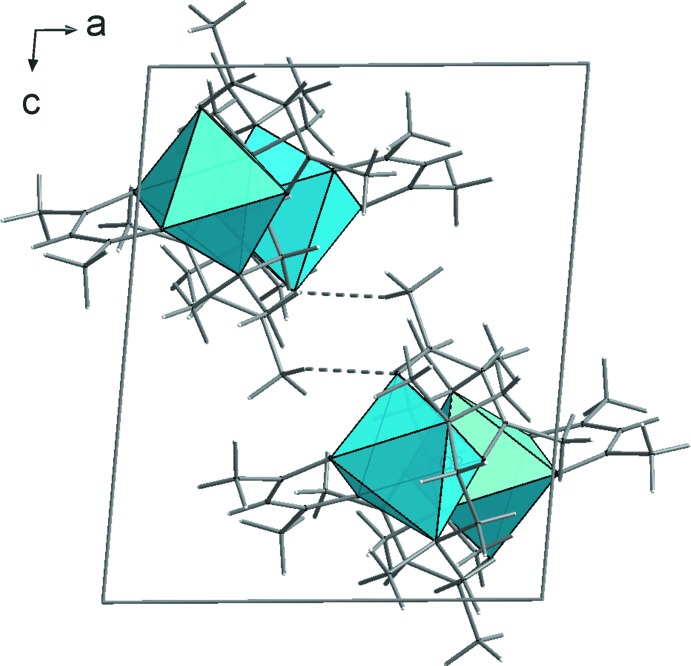
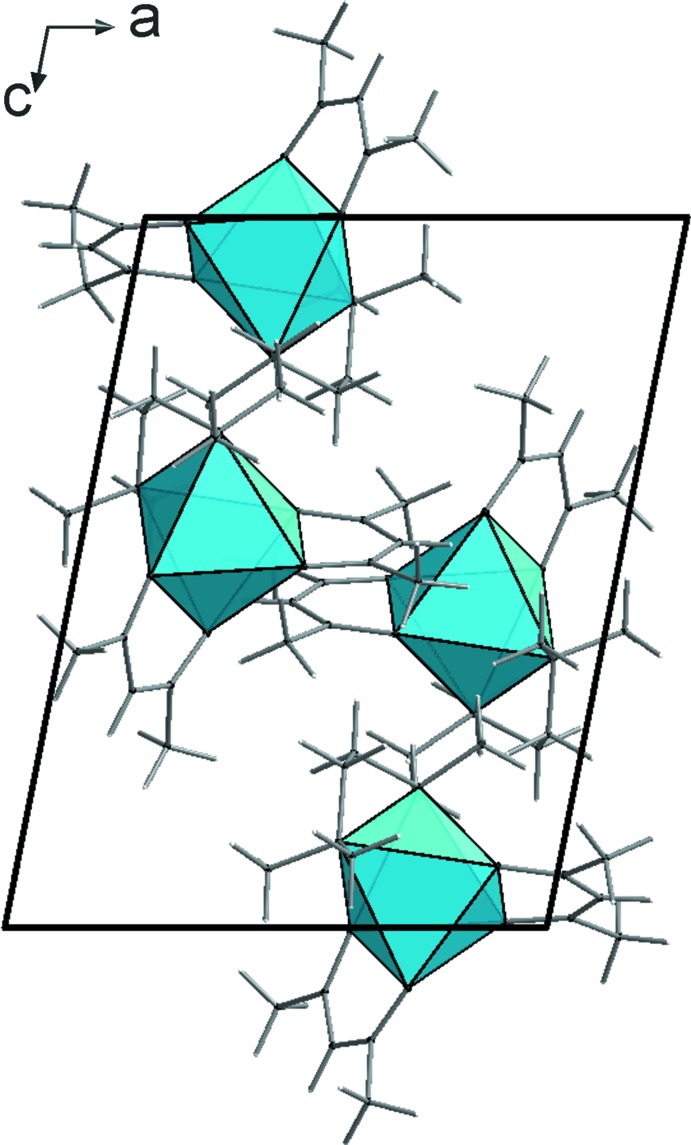
The packing of the [M(acac)2(TMEDA)] units is dominated by van der Waals interactions. The mutual arrangement of the complex units 1–3 is similar but not identical (Figs. 4 ▸–6 ▸ ▸). In the case of the iron compound 2 there is also a contribution from weak C—H⋯O hydrogen bridges (Table 4 ▸). As a result, the complexes are associated by R 2 2(8) type motifs, forming centrosymmetric dimers (Fig. 5 ▸).
Figure 4.
Crystal structure of compound 1, viewed along the b axis.
Figure 5.
Crystal structure of compound 2, viewed along the b axis. The intermolecular C—H⋯O hydrogen bonds are shown as dashed lines.
Figure 6.
Crystal structure of compound 3, viewed along the b axis.
Table 4. Hydrogen-bond geometry (Å, °) for 2 .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C1—H2⋯O1i | 0.96 | 2.62 | 3.5269 (18) | 157 |
Symmetry code: (i) 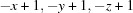 .
.
Database survey
A search in the Cambridge Structural Database (CSD, Version 5.40, February 2019 update; Groom et al., 2016 ▸) for complexes with a composition [M(acac)2(TMEDA)] analogous to 1–3 revealed the crystal structures for the M = V, Ni, Co and Ru derivatives (Ma et al., 1999 ▸; Pasko et al., 2004 ▸; Trimmel et al., 2002 ▸; Zeller et al., 2004 ▸; Halbach et al., 2012 ▸). However, none of these complexes is isotypic with the three title compounds. In the case of the related hfa derivatives, complexes of the type [M(hfa)2(TMEDA)] (hfa = 1,1,1,5,5,5-hexafluoropentane-2,4-dionate) with M = Mg, Mn, Fe, Co, Cu and Zn have been reported.
Synthesis and crystallization
TMEDA (7.5 ml, 5.8 g, 50 mmol) was added to a suspension of [M(acac)2(H2O)2] (25 mmol, M = Mn: 9.71 g, Fe: 9.73 g, Zn: 9.97 g) in toluene (30 ml). The suspension was stirred at 323 K for 2 h. After removal of the solvent under reduced pressure, n-hexane (25 ml) was added and insoluble parts were filtered off. The filtrates were kept at 248 K to obtain the products as yellow (1), red–brown (2) and colourless (3) crystalline solids in yields around 90%.
Characterization
[Mn(acac)2TMEDA] (1)
C16H30MnN2O4 calculated C 52.03, H 8.19, N 7.59%, found: C 51.71, H 8.13, N 7.14%; IR (ATR): ν = 3067 w, 2993 w, 2970 w, 2917 w, 2986 w, 2860 w, 2828 w, 2788 w, 2772 w, 1595 m, 1512 s, 1468 m, 1449 m, 1412 s, 1391 m, 1353 m, 1288 m, 1251 m, 1190 w, 1159 w, 1124 w, 1095 w, 1063 w, 1045 m, 1026 w, 1011 m, 950 m, 934 w, 913 m, 794 m, 771 w, 751 m, 650 w, 583 w, 526 m, 468 w, 448 w, 436 w, 400 s, 325 m, 212 s cm−1.
M.p.: 362 K.
[Fe(acac)2TMEDA] (2)
C16H30FeN2O4 calculated C 51.90, H 8.17, N 7.57%, found: C 51.75, H 8.08, N 7.23%; IR (ATR): ν = 3074 w, 3001 w, 2967 w, 2911 w, 2869 w, 2836 w, 2790 w, 1583 m, 1510 s, 1455 m, 1411 s, 1382 m, 1357 w, 1289 m, 1274 w, 1256 m, 1188 w, 1165 w, 1127 w, 1101 w, 1030 w 1012 m, 952 m, 917 m, 793 m, 762 s, 651 w, 583 w, 543 m, 475 w, 436 w, 404 w, 382 s, 296 w, 265 m, 227 s cm−1.
M.p.: 361 K.
[Zn(acac)2TMEDA] (3)
C16H30N2O4Zn calculated C 50.60, H 7.96, N 7.38%, found: C 50.33, H 8.13, N 7.23%; 1H-NMR (CDCl3, 399.962 MHz) δ = 5.15 [s, 2H, C(O)CHC(O)], 2.49 (s, 4H, Me2N-CH 2), 2.31 (s, 12H, (CH 3)2N), 1.85 [s, 12H, CH 3C(O)]; 13C-NMR (CDCl3,100.581 MHz) δ = 190.9 [C(O)], 98.4 [C(O)CHC(O)], 56.5 (NCH2), 46.6 [(CH3)2N], 28.3 (C(O)CH3) ppm; IR (ATR): ν = 3071 w, 3001 w, 2975 w, 2881 w, 2835 w, 2792 w, 1615 m, 1593 m, 1515 s, 1469 m, 1455 m, 1411 m, 1390 s, 1354 m, 1290 m, 1252 m, 1190 w, 1166 w, 1128 w, 1101 w, 1061 w, 1032 m, 1013 s, 953 m, 936 w, 918 m, 798 m, 770 m, 754 m, 649 w, 584 w, 543 m, 474 w, 440 m, 405 s, 382 w, 208 s cm−1.
M.p.: 362 K.
Refinement
Crystal data, data collection and structure refinement details are summarized in Table 5 ▸. All hydrogen atoms were positioned geometrically and refined using a riding model with U iso(H) = 1.2(CH and CH2) or 1.5(CH3) times U eq(C). Reflections with error/e.s.d. > 8 were omitted. Error/e.s.d. = (wD 2/<wD 2>)0.5 where D = F o 2 - F c 2.
Table 5. Experimental details.
| 1 | 2 | 3 | |
|---|---|---|---|
| Crystal data | |||
| Chemical formula | [Mn(C5H7O2)2(C6H16N2)] | [Fe(C5H7O2)2(C6H16N2)] | [Zn(C5H7O2)2(C6H16N2)] |
| M r | 369.36 | 370.27 | 379.79 |
| Crystal system, space group | Monoclinic, P21/n | Monoclinic, P21/n | Monoclinic, P21/n |
| Temperature (K) | 213 | 213 | 200 |
| a, b, c (Å) | 10.4234 (4), 14.3123 (5), 13.6047 (5) | 10.2021 (3), 15.4708 (4), 12.4881 (4) | 10.2335 (3), 14.2134 (6), 13.6738 (5) |
| β (°) | 103.154 (3) | 95.382 (3) | 101.208 (3) |
| V (Å3) | 1976.33 (13) | 1962.37 (10) | 1950.96 (12) |
| Z | 4 | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα | Mo Kα |
| μ (mm−1) | 0.69 | 0.79 | 1.28 |
| Crystal size (mm) | 0.35 × 0.25 × 0.20 | 0.26 × 0.25 × 0.23 | 0.45 × 0.39 × 0.33 |
| Data collection | |||
| Diffractometer | STOE IPDS 2 | STOE IPDS 2 | STOE IPDS 2T |
| Absorption correction | Numerical (X-AREA; Stoe & Cie, 2016 ▸) | Numerical (X-AREA; Stoe & Cie, 2016 ▸) | Numerical (X-AREA; Stoe & Cie, 2016 ▸) |
| T min, T max | 0.798, 0.912 | 0.814, 0.894 | 0.627, 0.779 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 12607, 4139, 3475 | 18586, 5276, 4425 | 22385, 4124, 3456 |
| R int | 0.030 | 0.037 | 0.047 |
| (sin θ/λ)max (Å−1) | 0.634 | 0.688 | 0.633 |
| Refinement | |||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.034, 0.099, 1.06 | 0.031, 0.086, 1.04 | 0.027, 0.076, 1.07 |
| No. of reflections | 4139 | 5276 | 4124 |
| No. of parameters | 216 | 216 | 216 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.22, −0.24 | 0.32, −0.22 | 0.37, −0.26 |
Supplementary Material
Crystal structure: contains datablock(s) 1, 2, 3. DOI: 10.1107/S2056989019016372/wm5524sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989019016372/wm55241sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989019016372/wm55242sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989019016372/wm55243sup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank A. Kiowski for technical support.
supplementary crystallographic information
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Crystal data
| [Mn(C5H7O2)2(C6H16N2)] | F(000) = 788 |
| Mr = 369.36 | Dx = 1.241 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.4234 (4) Å | Cell parameters from 13227 reflections |
| b = 14.3123 (5) Å | θ = 1.4–27.2° |
| c = 13.6047 (5) Å | µ = 0.69 mm−1 |
| β = 103.154 (3)° | T = 213 K |
| V = 1976.33 (13) Å3 | Block, clear yellow |
| Z = 4 | 0.35 × 0.25 × 0.20 mm |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Data collection
| STOE IPDS 2 diffractometer | 4139 independent reflections |
| Radiation source: sealed X-ray tube, 12 x 0.4 mm long-fine focus, Incoatec Iµs | 3475 reflections with I > 2σ(I) |
| Plane graphite monochromator | Rint = 0.030 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 26.8°, θmin = 2.1° |
| rotation method scans | h = −13→13 |
| Absorption correction: numerical (X-AREA; Stoe & Cie, 2016) | k = −17→18 |
| Tmin = 0.798, Tmax = 0.912 | l = −17→16 |
| 12607 measured reflections |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H-atom parameters constrained |
| wR(F2) = 0.099 | w = 1/[σ2(Fo2) + (0.0447P)2 + 0.6571P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max = 0.001 |
| 4139 reflections | Δρmax = 0.22 e Å−3 |
| 216 parameters | Δρmin = −0.24 e Å−3 |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Mn | 0.50579 (2) | 0.74721 (2) | 0.49407 (2) | 0.04120 (11) | |
| O1 | 0.67069 (13) | 0.65614 (9) | 0.53426 (10) | 0.0602 (3) | |
| O2 | 0.64605 (12) | 0.84003 (9) | 0.45153 (11) | 0.0554 (3) | |
| O3 | 0.50538 (13) | 0.83274 (9) | 0.62280 (10) | 0.0546 (3) | |
| O4 | 0.38216 (14) | 0.65820 (9) | 0.55845 (10) | 0.0565 (3) | |
| N1 | 0.44810 (15) | 0.65991 (11) | 0.34289 (11) | 0.0523 (4) | |
| N2 | 0.33093 (14) | 0.83481 (11) | 0.39666 (12) | 0.0533 (4) | |
| C1 | 0.8801 (3) | 0.5868 (2) | 0.5617 (2) | 0.0943 (9) | |
| H1 | 0.8477 | 0.5484 | 0.6086 | 0.141* | |
| H3 | 0.9667 | 0.6089 | 0.5928 | 0.141* | |
| H2 | 0.8838 | 0.5506 | 0.5029 | 0.141* | |
| C2 | 0.7888 (2) | 0.66910 (16) | 0.53114 (14) | 0.0593 (5) | |
| C3 | 0.8405 (2) | 0.75159 (16) | 0.50286 (17) | 0.0664 (6) | |
| H4 | 0.9308 | 0.7532 | 0.5070 | 0.080* | |
| C4 | 0.76938 (18) | 0.83223 (14) | 0.46880 (15) | 0.0568 (5) | |
| C5 | 0.8448 (2) | 0.91826 (18) | 0.4505 (2) | 0.0885 (8) | |
| H5 | 0.9136 | 0.9006 | 0.4178 | 0.133* | |
| H6 | 0.8827 | 0.9477 | 0.5138 | 0.133* | |
| H7 | 0.7860 | 0.9611 | 0.4082 | 0.133* | |
| C6 | 0.4607 (2) | 0.89848 (16) | 0.77048 (18) | 0.0714 (6) | |
| H8 | 0.5521 | 0.9144 | 0.7936 | 0.107* | |
| H10 | 0.4263 | 0.8782 | 0.8267 | 0.107* | |
| H9 | 0.4125 | 0.9522 | 0.7400 | 0.107* | |
| C7 | 0.44674 (17) | 0.82045 (13) | 0.69347 (13) | 0.0498 (4) | |
| C8 | 0.3712 (2) | 0.74328 (13) | 0.70557 (16) | 0.0568 (5) | |
| H11 | 0.3367 | 0.7416 | 0.7629 | 0.068* | |
| C9 | 0.34284 (19) | 0.66822 (13) | 0.63927 (15) | 0.0558 (4) | |
| C10 | 0.2576 (3) | 0.59062 (18) | 0.6645 (2) | 0.0920 (9) | |
| H12 | 0.2171 | 0.6107 | 0.7176 | 0.138* | |
| H13 | 0.3109 | 0.5365 | 0.6861 | 0.138* | |
| H14 | 0.1904 | 0.5754 | 0.6057 | 0.138* | |
| C11 | 0.3189 (2) | 0.69516 (18) | 0.28808 (16) | 0.0693 (6) | |
| H16 | 0.2506 | 0.6684 | 0.3173 | 0.083* | |
| H15 | 0.3030 | 0.6753 | 0.2182 | 0.083* | |
| C12 | 0.3114 (2) | 0.79961 (18) | 0.29216 (16) | 0.0703 (6) | |
| H17 | 0.3781 | 0.8263 | 0.2612 | 0.084* | |
| H18 | 0.2260 | 0.8199 | 0.2535 | 0.084* | |
| C13 | 0.4397 (3) | 0.55956 (14) | 0.36410 (18) | 0.0728 (6) | |
| H20 | 0.3743 | 0.5496 | 0.4026 | 0.109* | |
| H21 | 0.5236 | 0.5378 | 0.4018 | 0.109* | |
| H19 | 0.4156 | 0.5258 | 0.3016 | 0.109* | |
| C14 | 0.5481 (2) | 0.67378 (17) | 0.28339 (15) | 0.0647 (5) | |
| H24 | 0.5227 | 0.6403 | 0.2208 | 0.097* | |
| H22 | 0.6316 | 0.6509 | 0.3207 | 0.097* | |
| H23 | 0.5553 | 0.7392 | 0.2698 | 0.097* | |
| C15 | 0.21051 (19) | 0.82208 (18) | 0.43395 (18) | 0.0711 (6) | |
| H25 | 0.1889 | 0.7568 | 0.4331 | 0.107* | |
| H27 | 0.1393 | 0.8557 | 0.3914 | 0.107* | |
| H26 | 0.2246 | 0.8454 | 0.5017 | 0.107* | |
| C16 | 0.3638 (2) | 0.93462 (15) | 0.4003 (2) | 0.0784 (7) | |
| H29 | 0.2945 | 0.9685 | 0.3560 | 0.118* | |
| H28 | 0.4448 | 0.9435 | 0.3793 | 0.118* | |
| H30 | 0.3736 | 0.9572 | 0.4680 | 0.118* |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Mn | 0.03895 (15) | 0.04541 (18) | 0.03899 (15) | −0.00103 (10) | 0.00838 (10) | 0.00066 (10) |
| O1 | 0.0612 (8) | 0.0613 (8) | 0.0561 (8) | 0.0159 (6) | 0.0090 (6) | 0.0076 (6) |
| O2 | 0.0433 (6) | 0.0523 (7) | 0.0725 (9) | −0.0029 (5) | 0.0168 (6) | 0.0043 (6) |
| O3 | 0.0591 (7) | 0.0537 (7) | 0.0526 (7) | −0.0101 (6) | 0.0160 (6) | −0.0103 (6) |
| O4 | 0.0700 (8) | 0.0495 (7) | 0.0567 (8) | −0.0136 (6) | 0.0282 (6) | −0.0085 (6) |
| N1 | 0.0538 (8) | 0.0612 (9) | 0.0426 (8) | −0.0076 (7) | 0.0124 (6) | −0.0048 (7) |
| N2 | 0.0431 (7) | 0.0595 (9) | 0.0546 (9) | 0.0040 (7) | 0.0054 (6) | 0.0063 (7) |
| C1 | 0.0924 (18) | 0.108 (2) | 0.0744 (15) | 0.0575 (16) | 0.0027 (13) | −0.0049 (14) |
| C2 | 0.0561 (11) | 0.0758 (14) | 0.0411 (9) | 0.0216 (10) | 0.0009 (8) | −0.0109 (9) |
| C3 | 0.0392 (9) | 0.0933 (17) | 0.0658 (13) | 0.0089 (10) | 0.0100 (9) | −0.0196 (11) |
| C4 | 0.0454 (9) | 0.0699 (12) | 0.0589 (11) | −0.0094 (9) | 0.0197 (8) | −0.0190 (9) |
| C5 | 0.0657 (14) | 0.0870 (17) | 0.124 (2) | −0.0267 (13) | 0.0450 (15) | −0.0239 (16) |
| C6 | 0.0770 (14) | 0.0710 (14) | 0.0676 (13) | 0.0026 (11) | 0.0191 (11) | −0.0237 (11) |
| C7 | 0.0473 (9) | 0.0556 (10) | 0.0450 (9) | 0.0070 (8) | 0.0076 (7) | −0.0062 (8) |
| C8 | 0.0628 (12) | 0.0614 (12) | 0.0525 (10) | −0.0014 (9) | 0.0262 (9) | −0.0058 (8) |
| C9 | 0.0581 (10) | 0.0560 (11) | 0.0590 (11) | −0.0039 (9) | 0.0253 (9) | −0.0014 (9) |
| C10 | 0.110 (2) | 0.0822 (17) | 0.104 (2) | −0.0364 (15) | 0.0658 (17) | −0.0179 (15) |
| C11 | 0.0544 (11) | 0.0974 (17) | 0.0505 (11) | −0.0062 (11) | 0.0004 (9) | −0.0157 (11) |
| C12 | 0.0594 (12) | 0.0975 (17) | 0.0483 (11) | 0.0137 (11) | 0.0000 (9) | 0.0114 (11) |
| C13 | 0.1012 (17) | 0.0554 (12) | 0.0660 (13) | −0.0177 (11) | 0.0276 (12) | −0.0174 (10) |
| C14 | 0.0666 (12) | 0.0849 (15) | 0.0466 (10) | −0.0047 (11) | 0.0210 (9) | −0.0051 (10) |
| C15 | 0.0432 (10) | 0.0931 (16) | 0.0760 (14) | 0.0085 (10) | 0.0118 (9) | 0.0033 (12) |
| C16 | 0.0653 (13) | 0.0614 (13) | 0.1024 (19) | 0.0125 (10) | 0.0063 (12) | 0.0203 (12) |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')manganese(II) (1) . Geometric parameters (Å, º)
| Mn—O1 | 2.1271 (13) | C6—H10 | 0.9600 |
| Mn—O2 | 2.1500 (12) | C6—H9 | 0.9600 |
| Mn—O3 | 2.1375 (12) | C6—C7 | 1.515 (3) |
| Mn—O4 | 2.1365 (12) | C7—C8 | 1.388 (3) |
| Mn—N1 | 2.3643 (15) | C8—H11 | 0.9300 |
| Mn—N2 | 2.3560 (15) | C8—C9 | 1.391 (3) |
| O1—C2 | 1.255 (2) | C9—C10 | 1.510 (3) |
| O2—C4 | 1.258 (2) | C10—H12 | 0.9600 |
| O3—C7 | 1.263 (2) | C10—H13 | 0.9600 |
| O4—C9 | 1.266 (2) | C10—H14 | 0.9600 |
| N1—C11 | 1.472 (3) | C11—H16 | 0.9700 |
| N1—C13 | 1.472 (3) | C11—H15 | 0.9700 |
| N1—C14 | 1.472 (2) | C11—C12 | 1.499 (4) |
| N2—C12 | 1.478 (3) | C12—H17 | 0.9700 |
| N2—C15 | 1.468 (2) | C12—H18 | 0.9700 |
| N2—C16 | 1.467 (3) | C13—H20 | 0.9600 |
| C1—H1 | 0.9600 | C13—H21 | 0.9600 |
| C1—H3 | 0.9600 | C13—H19 | 0.9600 |
| C1—H2 | 0.9600 | C14—H24 | 0.9600 |
| C1—C2 | 1.512 (3) | C14—H22 | 0.9600 |
| C2—C3 | 1.388 (3) | C14—H23 | 0.9600 |
| C3—H4 | 0.9300 | C15—H25 | 0.9600 |
| C3—C4 | 1.393 (3) | C15—H27 | 0.9600 |
| C4—C5 | 1.512 (3) | C15—H26 | 0.9600 |
| C5—H5 | 0.9600 | C16—H29 | 0.9600 |
| C5—H6 | 0.9600 | C16—H28 | 0.9600 |
| C5—H7 | 0.9600 | C16—H30 | 0.9600 |
| C6—H8 | 0.9600 | ||
| O1—Mn—O2 | 83.61 (5) | C7—C6—H8 | 109.5 |
| O1—Mn—O3 | 107.00 (5) | C7—C6—H10 | 109.5 |
| O1—Mn—O4 | 93.25 (5) | C7—C6—H9 | 109.5 |
| O1—Mn—N1 | 86.01 (5) | O3—C7—C6 | 115.89 (17) |
| O1—Mn—N2 | 161.29 (6) | O3—C7—C8 | 125.92 (17) |
| O2—Mn—O3 | 89.71 (5) | C8—C7—C6 | 118.18 (17) |
| O2—Mn—O4 | 171.63 (5) | C7—C8—H11 | 117.2 |
| O2—Mn—N1 | 98.41 (5) | C7—C8—C9 | 125.50 (18) |
| O2—Mn—N2 | 90.36 (5) | C9—C8—H11 | 117.2 |
| O3—Mn—O4 | 83.78 (5) | O4—C9—C8 | 125.99 (17) |
| O3—Mn—N1 | 165.43 (5) | O4—C9—C10 | 115.94 (18) |
| O3—Mn—N2 | 90.61 (5) | C8—C9—C10 | 118.07 (18) |
| O4—Mn—N1 | 89.07 (5) | C9—C10—H12 | 109.5 |
| O4—Mn—N2 | 94.95 (6) | C9—C10—H13 | 109.5 |
| N1—Mn—N2 | 77.34 (6) | C9—C10—H14 | 109.5 |
| C2—O1—Mn | 129.95 (14) | H12—C10—H13 | 109.5 |
| C4—O2—Mn | 128.56 (13) | H12—C10—H14 | 109.5 |
| C7—O3—Mn | 129.44 (12) | H13—C10—H14 | 109.5 |
| C9—O4—Mn | 129.29 (12) | N1—C11—H16 | 109.2 |
| C11—N1—Mn | 106.37 (12) | N1—C11—H15 | 109.2 |
| C13—N1—Mn | 111.10 (12) | N1—C11—C12 | 111.88 (17) |
| C13—N1—C11 | 110.20 (18) | H16—C11—H15 | 107.9 |
| C13—N1—C14 | 108.71 (17) | C12—C11—H16 | 109.2 |
| C14—N1—Mn | 109.60 (12) | C12—C11—H15 | 109.2 |
| C14—N1—C11 | 110.86 (16) | N2—C12—C11 | 112.25 (17) |
| C12—N2—Mn | 106.22 (12) | N2—C12—H17 | 109.2 |
| C15—N2—Mn | 110.63 (12) | N2—C12—H18 | 109.2 |
| C15—N2—C12 | 110.40 (17) | C11—C12—H17 | 109.2 |
| C16—N2—Mn | 110.75 (12) | C11—C12—H18 | 109.2 |
| C16—N2—C12 | 110.14 (18) | H17—C12—H18 | 107.9 |
| C16—N2—C15 | 108.69 (17) | N1—C13—H20 | 109.5 |
| H1—C1—H3 | 109.5 | N1—C13—H21 | 109.5 |
| H1—C1—H2 | 109.5 | N1—C13—H19 | 109.5 |
| H3—C1—H2 | 109.5 | H20—C13—H21 | 109.5 |
| C2—C1—H1 | 109.5 | H20—C13—H19 | 109.5 |
| C2—C1—H3 | 109.5 | H21—C13—H19 | 109.5 |
| C2—C1—H2 | 109.5 | N1—C14—H24 | 109.5 |
| O1—C2—C1 | 115.9 (2) | N1—C14—H22 | 109.5 |
| O1—C2—C3 | 125.51 (18) | N1—C14—H23 | 109.5 |
| C3—C2—C1 | 118.6 (2) | H24—C14—H22 | 109.5 |
| C2—C3—H4 | 117.1 | H24—C14—H23 | 109.5 |
| C2—C3—C4 | 125.86 (18) | H22—C14—H23 | 109.5 |
| C4—C3—H4 | 117.1 | N2—C15—H25 | 109.5 |
| O2—C4—C3 | 125.44 (19) | N2—C15—H27 | 109.5 |
| O2—C4—C5 | 116.4 (2) | N2—C15—H26 | 109.5 |
| C3—C4—C5 | 118.17 (19) | H25—C15—H27 | 109.5 |
| C4—C5—H5 | 109.5 | H25—C15—H26 | 109.5 |
| C4—C5—H6 | 109.5 | H27—C15—H26 | 109.5 |
| C4—C5—H7 | 109.5 | N2—C16—H29 | 109.5 |
| H5—C5—H6 | 109.5 | N2—C16—H28 | 109.5 |
| H5—C5—H7 | 109.5 | N2—C16—H30 | 109.5 |
| H6—C5—H7 | 109.5 | H29—C16—H28 | 109.5 |
| H8—C6—H10 | 109.5 | H29—C16—H30 | 109.5 |
| H8—C6—H9 | 109.5 | H28—C16—H30 | 109.5 |
| H10—C6—H9 | 109.5 | ||
| Mn—O1—C2—C1 | −177.50 (14) | N1—C11—C12—N2 | −60.6 (2) |
| Mn—O1—C2—C3 | 2.8 (3) | C1—C2—C3—C4 | 177.1 (2) |
| Mn—O2—C4—C3 | 13.4 (3) | C2—C3—C4—O2 | −5.6 (3) |
| Mn—O2—C4—C5 | −166.67 (16) | C2—C3—C4—C5 | 174.5 (2) |
| Mn—O3—C7—C6 | −176.26 (13) | C6—C7—C8—C9 | 176.5 (2) |
| Mn—O3—C7—C8 | 3.2 (3) | C7—C8—C9—O4 | 0.7 (4) |
| Mn—O4—C9—C8 | 1.1 (3) | C7—C8—C9—C10 | −179.4 (2) |
| Mn—O4—C9—C10 | −178.82 (17) | C13—N1—C11—C12 | 162.74 (17) |
| Mn—N1—C11—C12 | 42.22 (19) | C14—N1—C11—C12 | −76.9 (2) |
| Mn—N2—C12—C11 | 42.41 (19) | C15—N2—C12—C11 | −77.6 (2) |
| O1—C2—C3—C4 | −3.2 (3) | C16—N2—C12—C11 | 162.39 (17) |
| O3—C7—C8—C9 | −3.0 (3) |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Crystal data
| [Fe(C5H7O2)2(C6H16N2)] | F(000) = 792 |
| Mr = 370.27 | Dx = 1.253 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.2021 (3) Å | Cell parameters from 16780 reflections |
| b = 15.4708 (4) Å | θ = 1.6–29.6° |
| c = 12.4881 (4) Å | µ = 0.79 mm−1 |
| β = 95.382 (3)° | T = 213 K |
| V = 1962.37 (10) Å3 | Block, clear reddish brown |
| Z = 4 | 0.26 × 0.25 × 0.23 mm |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Data collection
| STOE IPDS 2 diffractometer | 5276 independent reflections |
| Radiation source: sealed X-ray tube, 12 x 0.4 mm long-fine focus, Incoatec Iµs | 4425 reflections with I > 2σ(I) |
| Plane graphite monochromator | Rint = 0.037 |
| Detector resolution: 6.67 pixels mm-1 | θmax = 29.3°, θmin = 2.1° |
| rotation method scans | h = −13→13 |
| Absorption correction: numerical (X-AREA; Stoe & Cie, 2016) | k = −21→20 |
| Tmin = 0.814, Tmax = 0.894 | l = −17→17 |
| 18586 measured reflections |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.031 | H-atom parameters constrained |
| wR(F2) = 0.086 | w = 1/[σ2(Fo2) + (0.0464P)2 + 0.3681P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max = 0.002 |
| 5276 reflections | Δρmax = 0.32 e Å−3 |
| 216 parameters | Δρmin = −0.22 e Å−3 |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.63613 (15) | 0.53037 (11) | 0.42322 (12) | 0.0444 (3) | |
| H2 | 0.5847 | 0.4797 | 0.4338 | 0.067* | |
| H3 | 0.7061 | 0.5160 | 0.3800 | 0.067* | |
| H1 | 0.5810 | 0.5739 | 0.3874 | 0.067* | |
| C2 | 0.69359 (13) | 0.56425 (9) | 0.53071 (11) | 0.0352 (3) | |
| C3 | 0.81769 (14) | 0.53357 (9) | 0.57243 (12) | 0.0396 (3) | |
| H4 | 0.8603 | 0.4948 | 0.5305 | 0.047* | |
| C4 | 0.88149 (13) | 0.55660 (9) | 0.67116 (12) | 0.0388 (3) | |
| C5 | 1.01582 (16) | 0.51923 (13) | 0.70525 (16) | 0.0584 (4) | |
| H7 | 1.0343 | 0.4733 | 0.6572 | 0.088* | |
| H5 | 1.0172 | 0.4970 | 0.7771 | 0.088* | |
| H6 | 1.0813 | 0.5636 | 0.7031 | 0.088* | |
| C6 | 0.39240 (17) | 0.48770 (10) | 0.87153 (14) | 0.0496 (4) | |
| H10 | 0.3000 | 0.4817 | 0.8499 | 0.074* | |
| H9 | 0.4064 | 0.4888 | 0.9486 | 0.074* | |
| H8 | 0.4391 | 0.4397 | 0.8446 | 0.074* | |
| C7 | 0.44227 (13) | 0.57093 (9) | 0.82668 (11) | 0.0359 (3) | |
| C8 | 0.35444 (13) | 0.64029 (10) | 0.81410 (12) | 0.0379 (3) | |
| H11 | 0.2707 | 0.6321 | 0.8359 | 0.046* | |
| C9 | 0.38200 (13) | 0.72026 (9) | 0.77178 (11) | 0.0350 (3) | |
| C10 | 0.27918 (16) | 0.79023 (12) | 0.77050 (16) | 0.0542 (4) | |
| H14 | 0.2694 | 0.8171 | 0.7009 | 0.081* | |
| H13 | 0.3059 | 0.8328 | 0.8241 | 0.081* | |
| H12 | 0.1967 | 0.7654 | 0.7857 | 0.081* | |
| C11 | 0.78186 (19) | 0.85071 (11) | 0.76164 (14) | 0.0537 (4) | |
| H16 | 0.7020 | 0.8793 | 0.7792 | 0.064* | |
| H15 | 0.8440 | 0.8949 | 0.7446 | 0.064* | |
| C12 | 0.83901 (17) | 0.80004 (13) | 0.85721 (14) | 0.0545 (4) | |
| H17 | 0.9192 | 0.7718 | 0.8398 | 0.065* | |
| H18 | 0.8617 | 0.8393 | 0.9167 | 0.065* | |
| C13 | 0.6596 (2) | 0.83936 (13) | 0.58754 (16) | 0.0593 (4) | |
| H19 | 0.6381 | 0.8022 | 0.5269 | 0.089* | |
| H21 | 0.7000 | 0.8912 | 0.5641 | 0.089* | |
| H20 | 0.5807 | 0.8539 | 0.6198 | 0.089* | |
| C14 | 0.87086 (16) | 0.77473 (12) | 0.61406 (14) | 0.0519 (4) | |
| H24 | 0.9042 | 0.8268 | 0.5847 | 0.078* | |
| H22 | 0.8499 | 0.7338 | 0.5572 | 0.078* | |
| H23 | 0.9363 | 0.7506 | 0.6658 | 0.078* | |
| C15 | 0.64471 (18) | 0.77489 (13) | 0.94987 (14) | 0.0549 (4) | |
| H26 | 0.5825 | 0.7319 | 0.9677 | 0.082* | |
| H25 | 0.6001 | 0.8188 | 0.9060 | 0.082* | |
| H27 | 0.6853 | 0.8005 | 1.0147 | 0.082* | |
| C16 | 0.8174 (2) | 0.67090 (14) | 0.96194 (14) | 0.0619 (5) | |
| H29 | 0.8834 | 0.6428 | 0.9244 | 0.093* | |
| H28 | 0.7566 | 0.6286 | 0.9840 | 0.093* | |
| H30 | 0.8587 | 0.7000 | 1.0242 | 0.093* | |
| Fe | 0.65967 (2) | 0.67126 (2) | 0.73081 (2) | 0.03242 (7) | |
| N1 | 0.75139 (12) | 0.79468 (8) | 0.66685 (10) | 0.0407 (3) | |
| N2 | 0.74634 (12) | 0.73421 (9) | 0.89046 (10) | 0.0418 (3) | |
| O1 | 0.62568 (9) | 0.61851 (7) | 0.57684 (8) | 0.0395 (2) | |
| O2 | 0.83743 (9) | 0.60880 (7) | 0.73746 (9) | 0.0426 (2) | |
| O3 | 0.55981 (10) | 0.57212 (6) | 0.80388 (9) | 0.0414 (2) | |
| O4 | 0.48936 (9) | 0.74134 (6) | 0.73429 (8) | 0.0384 (2) |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0435 (7) | 0.0512 (8) | 0.0393 (7) | −0.0086 (6) | 0.0082 (6) | −0.0108 (6) |
| C2 | 0.0360 (6) | 0.0341 (6) | 0.0368 (7) | −0.0081 (5) | 0.0096 (5) | −0.0039 (5) |
| C3 | 0.0380 (7) | 0.0368 (7) | 0.0453 (8) | 0.0022 (5) | 0.0111 (6) | −0.0064 (6) |
| C4 | 0.0301 (6) | 0.0407 (7) | 0.0465 (8) | 0.0000 (5) | 0.0089 (5) | 0.0000 (6) |
| C5 | 0.0374 (8) | 0.0703 (12) | 0.0670 (11) | 0.0127 (8) | 0.0019 (7) | −0.0056 (9) |
| C6 | 0.0539 (9) | 0.0434 (8) | 0.0515 (9) | −0.0121 (7) | 0.0050 (7) | 0.0076 (7) |
| C7 | 0.0380 (6) | 0.0369 (7) | 0.0328 (6) | −0.0079 (5) | 0.0028 (5) | −0.0029 (5) |
| C8 | 0.0303 (6) | 0.0435 (7) | 0.0413 (7) | −0.0049 (5) | 0.0102 (5) | −0.0045 (6) |
| C9 | 0.0315 (6) | 0.0393 (7) | 0.0349 (6) | 0.0017 (5) | 0.0056 (5) | −0.0047 (5) |
| C10 | 0.0439 (8) | 0.0539 (9) | 0.0670 (11) | 0.0149 (7) | 0.0163 (7) | 0.0022 (8) |
| C11 | 0.0674 (11) | 0.0428 (8) | 0.0540 (10) | −0.0207 (8) | 0.0222 (8) | −0.0125 (7) |
| C12 | 0.0508 (9) | 0.0685 (11) | 0.0451 (9) | −0.0275 (8) | 0.0092 (7) | −0.0158 (8) |
| C13 | 0.0626 (11) | 0.0614 (11) | 0.0559 (10) | −0.0011 (8) | 0.0154 (8) | 0.0167 (8) |
| C14 | 0.0496 (8) | 0.0561 (9) | 0.0537 (9) | −0.0149 (7) | 0.0247 (7) | −0.0073 (7) |
| C15 | 0.0550 (9) | 0.0690 (11) | 0.0429 (8) | −0.0094 (8) | 0.0167 (7) | −0.0164 (8) |
| C16 | 0.0650 (11) | 0.0792 (13) | 0.0401 (9) | 0.0011 (9) | −0.0027 (8) | −0.0002 (8) |
| Fe | 0.02688 (10) | 0.03490 (11) | 0.03626 (11) | −0.00268 (7) | 0.00714 (7) | −0.00567 (7) |
| N1 | 0.0432 (6) | 0.0415 (6) | 0.0395 (6) | −0.0086 (5) | 0.0147 (5) | −0.0039 (5) |
| N2 | 0.0395 (6) | 0.0513 (7) | 0.0354 (6) | −0.0101 (5) | 0.0077 (5) | −0.0055 (5) |
| O1 | 0.0329 (5) | 0.0440 (5) | 0.0416 (5) | 0.0005 (4) | 0.0028 (4) | −0.0112 (4) |
| O2 | 0.0313 (5) | 0.0535 (6) | 0.0429 (5) | 0.0021 (4) | 0.0031 (4) | −0.0100 (5) |
| O3 | 0.0352 (5) | 0.0352 (5) | 0.0543 (6) | −0.0009 (4) | 0.0075 (4) | 0.0014 (4) |
| O4 | 0.0349 (5) | 0.0348 (5) | 0.0471 (5) | 0.0018 (4) | 0.0124 (4) | 0.0023 (4) |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Geometric parameters (Å, º)
| C1—H2 | 0.9600 | C11—C12 | 1.499 (3) |
| C1—H3 | 0.9600 | C11—N1 | 1.477 (2) |
| C1—H1 | 0.9600 | C12—H17 | 0.9700 |
| C1—C2 | 1.5076 (19) | C12—H18 | 0.9700 |
| C2—C3 | 1.406 (2) | C12—N2 | 1.475 (2) |
| C2—O1 | 1.2615 (16) | C13—H19 | 0.9600 |
| C3—H4 | 0.9300 | C13—H21 | 0.9600 |
| C3—C4 | 1.386 (2) | C13—H20 | 0.9600 |
| C4—C5 | 1.511 (2) | C13—N1 | 1.471 (2) |
| C4—O2 | 1.2687 (17) | C14—H24 | 0.9600 |
| C5—H7 | 0.9600 | C14—H22 | 0.9600 |
| C5—H5 | 0.9600 | C14—H23 | 0.9600 |
| C5—H6 | 0.9600 | C14—N1 | 1.4718 (19) |
| C6—H10 | 0.9600 | C15—H26 | 0.9600 |
| C6—H9 | 0.9600 | C15—H25 | 0.9600 |
| C6—H8 | 0.9600 | C15—H27 | 0.9600 |
| C6—C7 | 1.511 (2) | C15—N2 | 1.472 (2) |
| C7—C8 | 1.397 (2) | C16—H29 | 0.9600 |
| C7—O3 | 1.2583 (17) | C16—H28 | 0.9600 |
| C8—H11 | 0.9300 | C16—H30 | 0.9600 |
| C8—C9 | 1.385 (2) | C16—N2 | 1.470 (2) |
| C9—C10 | 1.506 (2) | Fe—O1 | 2.0876 (10) |
| C9—O4 | 1.2734 (16) | Fe—O2 | 2.0497 (10) |
| C10—H14 | 0.9600 | Fe—O3 | 2.0970 (10) |
| C10—H13 | 0.9600 | Fe—O4 | 2.0520 (9) |
| C10—H12 | 0.9600 | Fe—N1 | 2.3021 (12) |
| C11—H16 | 0.9700 | Fe—N2 | 2.3184 (12) |
| C11—H15 | 0.9700 | ||
| H2—C1—H3 | 109.5 | H19—C13—H20 | 109.5 |
| H2—C1—H1 | 109.5 | H21—C13—H20 | 109.5 |
| H3—C1—H1 | 109.5 | N1—C13—H19 | 109.5 |
| C2—C1—H2 | 109.5 | N1—C13—H21 | 109.5 |
| C2—C1—H3 | 109.5 | N1—C13—H20 | 109.5 |
| C2—C1—H1 | 109.5 | H24—C14—H22 | 109.5 |
| C3—C2—C1 | 118.30 (12) | H24—C14—H23 | 109.5 |
| O1—C2—C1 | 116.98 (13) | H22—C14—H23 | 109.5 |
| O1—C2—C3 | 124.72 (13) | N1—C14—H24 | 109.5 |
| C2—C3—H4 | 117.5 | N1—C14—H22 | 109.5 |
| C4—C3—C2 | 125.07 (13) | N1—C14—H23 | 109.5 |
| C4—C3—H4 | 117.5 | H26—C15—H25 | 109.5 |
| C3—C4—C5 | 119.35 (14) | H26—C15—H27 | 109.5 |
| O2—C4—C3 | 125.36 (13) | H25—C15—H27 | 109.5 |
| O2—C4—C5 | 115.28 (14) | N2—C15—H26 | 109.5 |
| C4—C5—H7 | 109.5 | N2—C15—H25 | 109.5 |
| C4—C5—H5 | 109.5 | N2—C15—H27 | 109.5 |
| C4—C5—H6 | 109.5 | H29—C16—H28 | 109.5 |
| H7—C5—H5 | 109.5 | H29—C16—H30 | 109.5 |
| H7—C5—H6 | 109.5 | H28—C16—H30 | 109.5 |
| H5—C5—H6 | 109.5 | N2—C16—H29 | 109.5 |
| H10—C6—H9 | 109.5 | N2—C16—H28 | 109.5 |
| H10—C6—H8 | 109.5 | N2—C16—H30 | 109.5 |
| H9—C6—H8 | 109.5 | O1—Fe—O2 | 85.58 (4) |
| C7—C6—H10 | 109.5 | O1—Fe—O3 | 93.98 (4) |
| C7—C6—H9 | 109.5 | O1—Fe—O4 | 99.11 (4) |
| C7—C6—H8 | 109.5 | O1—Fe—N1 | 92.44 (4) |
| C8—C7—C6 | 117.47 (13) | O1—Fe—N2 | 166.73 (4) |
| O3—C7—C6 | 117.22 (13) | O2—Fe—O3 | 95.84 (4) |
| O3—C7—C8 | 125.31 (13) | O2—Fe—O4 | 174.85 (4) |
| C7—C8—H11 | 117.3 | O2—Fe—N1 | 91.04 (5) |
| C9—C8—C7 | 125.32 (12) | O2—Fe—N2 | 84.18 (4) |
| C9—C8—H11 | 117.3 | O3—Fe—O4 | 86.00 (4) |
| C8—C9—C10 | 118.69 (13) | O3—Fe—N1 | 170.93 (4) |
| O4—C9—C8 | 125.57 (12) | O3—Fe—N2 | 95.43 (4) |
| O4—C9—C10 | 115.74 (13) | O4—Fe—N1 | 86.66 (4) |
| C9—C10—H14 | 109.5 | O4—Fe—N2 | 90.87 (4) |
| C9—C10—H13 | 109.5 | N1—Fe—N2 | 79.35 (4) |
| C9—C10—H12 | 109.5 | C11—N1—Fe | 105.70 (9) |
| H14—C10—H13 | 109.5 | C13—N1—C11 | 109.69 (14) |
| H14—C10—H12 | 109.5 | C13—N1—C14 | 107.39 (13) |
| H13—C10—H12 | 109.5 | C13—N1—Fe | 111.69 (10) |
| H16—C11—H15 | 108.0 | C14—N1—C11 | 111.18 (13) |
| C12—C11—H16 | 109.3 | C14—N1—Fe | 111.24 (10) |
| C12—C11—H15 | 109.3 | C12—N2—Fe | 104.52 (9) |
| N1—C11—H16 | 109.3 | C15—N2—C12 | 110.31 (14) |
| N1—C11—H15 | 109.3 | C15—N2—Fe | 112.54 (10) |
| N1—C11—C12 | 111.60 (14) | C16—N2—C12 | 109.77 (14) |
| C11—C12—H17 | 109.2 | C16—N2—C15 | 108.03 (14) |
| C11—C12—H18 | 109.2 | C16—N2—Fe | 111.65 (10) |
| H17—C12—H18 | 107.9 | C2—O1—Fe | 129.04 (9) |
| N2—C12—C11 | 111.91 (13) | C4—O2—Fe | 129.79 (9) |
| N2—C12—H17 | 109.2 | C7—O3—Fe | 128.42 (10) |
| N2—C12—H18 | 109.2 | C9—O4—Fe | 129.18 (9) |
| H19—C13—H21 | 109.5 |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')iron(II) (2) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C1—H2···O1i | 0.96 | 2.62 | 3.5269 (18) | 157 |
Symmetry code: (i) −x+1, −y+1, −z+1.
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Crystal data
| [Zn(C5H7O2)2(C6H16N2)] | F(000) = 808 |
| Mr = 379.79 | Dx = 1.293 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.2335 (3) Å | Cell parameters from 19126 reflections |
| b = 14.2134 (6) Å | θ = 2.1–27.2° |
| c = 13.6738 (5) Å | µ = 1.28 mm−1 |
| β = 101.208 (3)° | T = 200 K |
| V = 1950.96 (12) Å3 | Block, clear colourless |
| Z = 4 | 0.45 × 0.39 × 0.33 mm |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Data collection
| STOE IPDS 2T diffractometer | 3456 reflections with I > 2σ(I) |
| Detector resolution: 6.67 pixels mm-1 | Rint = 0.047 |
| rotation method, ω scans | θmax = 26.7°, θmin = 2.1° |
| Absorption correction: numerical (X-AREA; Stoe & Cie, 2016) | h = −12→12 |
| Tmin = 0.627, Tmax = 0.779 | k = −17→16 |
| 22385 measured reflections | l = −17→17 |
| 4124 independent reflections |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.027 | H-atom parameters constrained |
| wR(F2) = 0.076 | w = 1/[σ2(Fo2) + (0.0494P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.001 |
| 4124 reflections | Δρmax = 0.37 e Å−3 |
| 216 parameters | Δρmin = −0.26 e Å−3 |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Zn | 0.74026 (2) | 0.26429 (2) | 0.54676 (2) | 0.03991 (8) | |
| O1 | 0.73445 (12) | 0.33774 (8) | 0.41466 (9) | 0.0534 (3) | |
| O2 | 0.85986 (11) | 0.16705 (8) | 0.49492 (8) | 0.0507 (3) | |
| O3 | 0.57869 (11) | 0.17554 (8) | 0.50853 (9) | 0.0529 (3) | |
| O4 | 0.61242 (9) | 0.36299 (8) | 0.58630 (8) | 0.0441 (2) | |
| N1 | 0.91461 (12) | 0.35505 (11) | 0.62041 (10) | 0.0499 (3) | |
| N2 | 0.78674 (12) | 0.19573 (11) | 0.69813 (10) | 0.0459 (3) | |
| C1 | 0.76595 (19) | 0.38103 (13) | 0.25509 (13) | 0.0575 (4) | |
| H1 | 0.6725 | 0.4006 | 0.2373 | 0.086* | |
| H2 | 0.7922 | 0.3504 | 0.1977 | 0.086* | |
| H3 | 0.8222 | 0.4363 | 0.2743 | 0.086* | |
| C2 | 0.78276 (15) | 0.31277 (12) | 0.34146 (11) | 0.0450 (4) | |
| C3 | 0.85137 (17) | 0.22957 (12) | 0.33344 (13) | 0.0492 (4) | |
| H4 | 0.8764 | 0.2169 | 0.2714 | 0.059* | |
| C4 | 0.88628 (15) | 0.16331 (12) | 0.40893 (12) | 0.0462 (4) | |
| C5 | 0.9672 (2) | 0.07864 (15) | 0.38836 (14) | 0.0672 (5) | |
| H5 | 1.0493 | 0.0752 | 0.4389 | 0.101* | |
| H6 | 0.9896 | 0.0849 | 0.3222 | 0.101* | |
| H7 | 0.9149 | 0.0212 | 0.3907 | 0.101* | |
| C6 | 0.3632 (2) | 0.11210 (15) | 0.48710 (15) | 0.0691 (5) | |
| H8 | 0.3797 | 0.0646 | 0.5401 | 0.104* | |
| H9 | 0.3758 | 0.0838 | 0.4242 | 0.104* | |
| H10 | 0.2717 | 0.1354 | 0.4796 | 0.104* | |
| C7 | 0.45930 (16) | 0.19273 (13) | 0.51387 (11) | 0.0476 (4) | |
| C8 | 0.41127 (15) | 0.27812 (12) | 0.54200 (12) | 0.0475 (4) | |
| H11 | 0.3180 | 0.2826 | 0.5391 | 0.057* | |
| C9 | 0.48712 (14) | 0.35764 (11) | 0.57395 (11) | 0.0412 (3) | |
| C10 | 0.41684 (16) | 0.44556 (13) | 0.59729 (13) | 0.0554 (4) | |
| H12 | 0.3393 | 0.4283 | 0.6257 | 0.083* | |
| H13 | 0.3875 | 0.4817 | 0.5359 | 0.083* | |
| H14 | 0.4780 | 0.4838 | 0.6454 | 0.083* | |
| C11 | 0.93211 (19) | 0.33443 (17) | 0.72727 (14) | 0.0676 (5) | |
| H15 | 0.8644 | 0.3692 | 0.7556 | 0.081* | |
| H16 | 1.0212 | 0.3563 | 0.7615 | 0.081* | |
| C12 | 0.91917 (19) | 0.23151 (16) | 0.74583 (15) | 0.0651 (5) | |
| H17 | 0.9887 | 0.1969 | 0.7194 | 0.078* | |
| H18 | 0.9335 | 0.2200 | 0.8185 | 0.078* | |
| C13 | 0.88618 (18) | 0.45547 (14) | 0.60157 (16) | 0.0671 (5) | |
| H19 | 0.9629 | 0.4929 | 0.6341 | 0.101* | |
| H20 | 0.8076 | 0.4732 | 0.6285 | 0.101* | |
| H21 | 0.8691 | 0.4673 | 0.5296 | 0.101* | |
| C14 | 1.03600 (16) | 0.33196 (16) | 0.58256 (16) | 0.0651 (5) | |
| H22 | 1.0212 | 0.3449 | 0.5108 | 0.098* | |
| H23 | 1.0573 | 0.2652 | 0.5944 | 0.098* | |
| H24 | 1.1102 | 0.3704 | 0.6172 | 0.098* | |
| C15 | 0.68709 (18) | 0.21961 (14) | 0.75840 (13) | 0.0560 (4) | |
| H25 | 0.6835 | 0.2881 | 0.7660 | 0.084* | |
| H26 | 0.7118 | 0.1902 | 0.8243 | 0.084* | |
| H27 | 0.5995 | 0.1964 | 0.7251 | 0.084* | |
| C16 | 0.7902 (2) | 0.09286 (14) | 0.68935 (15) | 0.0680 (5) | |
| H28 | 0.7024 | 0.0700 | 0.6562 | 0.102* | |
| H29 | 0.8137 | 0.0649 | 0.7560 | 0.102* | |
| H30 | 0.8568 | 0.0750 | 0.6500 | 0.102* |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Zn | 0.03590 (11) | 0.04475 (13) | 0.04087 (12) | 0.00666 (7) | 0.01186 (8) | −0.00208 (7) |
| O1 | 0.0628 (7) | 0.0534 (7) | 0.0470 (6) | 0.0178 (6) | 0.0187 (5) | 0.0051 (5) |
| O2 | 0.0555 (6) | 0.0545 (7) | 0.0465 (6) | 0.0158 (5) | 0.0204 (5) | 0.0024 (5) |
| O3 | 0.0488 (6) | 0.0496 (7) | 0.0592 (7) | −0.0007 (5) | 0.0078 (5) | −0.0096 (5) |
| O4 | 0.0335 (5) | 0.0470 (6) | 0.0538 (6) | 0.0028 (4) | 0.0136 (4) | −0.0040 (5) |
| N1 | 0.0353 (6) | 0.0602 (9) | 0.0557 (8) | −0.0041 (6) | 0.0125 (6) | −0.0053 (7) |
| N2 | 0.0431 (7) | 0.0537 (8) | 0.0422 (7) | 0.0079 (6) | 0.0114 (5) | 0.0024 (6) |
| C1 | 0.0653 (11) | 0.0582 (11) | 0.0492 (10) | −0.0021 (9) | 0.0114 (8) | 0.0046 (8) |
| C2 | 0.0406 (8) | 0.0525 (10) | 0.0417 (8) | −0.0032 (7) | 0.0077 (6) | −0.0012 (7) |
| C3 | 0.0529 (9) | 0.0559 (10) | 0.0417 (9) | 0.0035 (7) | 0.0163 (7) | −0.0050 (7) |
| C4 | 0.0444 (8) | 0.0481 (9) | 0.0485 (9) | 0.0042 (7) | 0.0149 (7) | −0.0079 (7) |
| C5 | 0.0817 (13) | 0.0620 (12) | 0.0632 (11) | 0.0230 (10) | 0.0271 (10) | −0.0048 (9) |
| C6 | 0.0680 (12) | 0.0741 (14) | 0.0632 (12) | −0.0250 (10) | 0.0074 (9) | −0.0086 (10) |
| C7 | 0.0474 (8) | 0.0576 (10) | 0.0356 (8) | −0.0083 (8) | 0.0028 (6) | 0.0009 (7) |
| C8 | 0.0323 (7) | 0.0653 (11) | 0.0451 (9) | −0.0004 (7) | 0.0081 (6) | 0.0028 (7) |
| C9 | 0.0368 (7) | 0.0515 (9) | 0.0370 (8) | 0.0066 (6) | 0.0113 (6) | 0.0068 (6) |
| C10 | 0.0443 (8) | 0.0596 (11) | 0.0674 (11) | 0.0127 (7) | 0.0233 (8) | 0.0043 (8) |
| C11 | 0.0517 (10) | 0.0963 (16) | 0.0527 (11) | −0.0182 (10) | 0.0053 (8) | −0.0141 (10) |
| C12 | 0.0449 (9) | 0.0990 (16) | 0.0484 (10) | 0.0056 (9) | 0.0017 (8) | 0.0119 (10) |
| C13 | 0.0529 (10) | 0.0562 (11) | 0.0935 (14) | −0.0131 (9) | 0.0175 (10) | −0.0120 (10) |
| C14 | 0.0375 (8) | 0.0825 (14) | 0.0785 (13) | −0.0044 (8) | 0.0194 (8) | −0.0032 (11) |
| C15 | 0.0540 (10) | 0.0724 (12) | 0.0452 (9) | 0.0086 (8) | 0.0183 (8) | 0.0043 (8) |
| C16 | 0.0905 (14) | 0.0552 (11) | 0.0627 (11) | 0.0198 (10) | 0.0256 (10) | 0.0137 (9) |
Bis(acetylacetonato-κ2O,O')(N,N,N',N'-tetramethylethylenediamine-κ2N,N')zinc(II) (3) . Geometric parameters (Å, º)
| Zn—O1 | 2.0771 (12) | C6—H9 | 0.9800 |
| Zn—O2 | 2.0611 (11) | C6—H10 | 0.9800 |
| Zn—O3 | 2.0645 (11) | C6—C7 | 1.508 (2) |
| Zn—O4 | 2.0607 (10) | C7—C8 | 1.391 (3) |
| Zn—N1 | 2.2722 (13) | C8—H11 | 0.9500 |
| Zn—N2 | 2.2533 (13) | C8—C9 | 1.393 (2) |
| O1—C2 | 1.2509 (19) | C9—C10 | 1.507 (2) |
| O2—C4 | 1.2578 (19) | C10—H12 | 0.9800 |
| O3—C7 | 1.2619 (19) | C10—H13 | 0.9800 |
| O4—C9 | 1.2626 (17) | C10—H14 | 0.9800 |
| N1—C11 | 1.467 (2) | C11—H15 | 0.9900 |
| N1—C13 | 1.469 (2) | C11—H16 | 0.9900 |
| N1—C14 | 1.473 (2) | C11—C12 | 1.495 (3) |
| N2—C12 | 1.476 (2) | C12—H17 | 0.9900 |
| N2—C15 | 1.470 (2) | C12—H18 | 0.9900 |
| N2—C16 | 1.468 (2) | C13—H19 | 0.9800 |
| C1—H1 | 0.9800 | C13—H20 | 0.9800 |
| C1—H2 | 0.9800 | C13—H21 | 0.9800 |
| C1—H3 | 0.9800 | C14—H22 | 0.9800 |
| C1—C2 | 1.512 (2) | C14—H23 | 0.9800 |
| C2—C3 | 1.390 (2) | C14—H24 | 0.9800 |
| C3—H4 | 0.9500 | C15—H25 | 0.9800 |
| C3—C4 | 1.392 (2) | C15—H26 | 0.9800 |
| C4—C5 | 1.518 (2) | C15—H27 | 0.9800 |
| C5—H5 | 0.9800 | C16—H28 | 0.9800 |
| C5—H6 | 0.9800 | C16—H29 | 0.9800 |
| C5—H7 | 0.9800 | C16—H30 | 0.9800 |
| C6—H8 | 0.9800 | ||
| O1—Zn—O2 | 87.50 (4) | C7—C6—H8 | 109.5 |
| O1—Zn—O3 | 101.58 (5) | C7—C6—H9 | 109.5 |
| O1—Zn—O4 | 88.49 (4) | C7—C6—H10 | 109.5 |
| O1—Zn—N1 | 89.28 (5) | O3—C7—C6 | 115.59 (16) |
| O1—Zn—N2 | 168.94 (5) | O3—C7—C8 | 125.65 (15) |
| O2—Zn—O3 | 90.18 (5) | C8—C7—C6 | 118.76 (16) |
| O2—Zn—O4 | 175.16 (4) | C7—C8—H11 | 116.9 |
| O2—Zn—N1 | 93.76 (5) | C7—C8—C9 | 126.12 (15) |
| O2—Zn—N2 | 89.57 (5) | C9—C8—H11 | 116.9 |
| O3—Zn—O4 | 87.96 (4) | O4—C9—C8 | 125.48 (15) |
| O3—Zn—N1 | 168.61 (5) | O4—C9—C10 | 115.84 (15) |
| O3—Zn—N2 | 89.09 (5) | C8—C9—C10 | 118.67 (13) |
| O4—Zn—N1 | 88.92 (5) | C9—C10—H12 | 109.5 |
| O4—Zn—N2 | 94.86 (5) | C9—C10—H13 | 109.5 |
| N1—Zn—N2 | 80.27 (5) | C9—C10—H14 | 109.5 |
| C2—O1—Zn | 127.18 (11) | H12—C10—H13 | 109.5 |
| C4—O2—Zn | 126.86 (11) | H12—C10—H14 | 109.5 |
| C7—O3—Zn | 127.15 (11) | H13—C10—H14 | 109.5 |
| C9—O4—Zn | 127.15 (10) | N1—C11—H15 | 109.3 |
| C11—N1—Zn | 105.16 (10) | N1—C11—H16 | 109.3 |
| C11—N1—C13 | 110.51 (16) | N1—C11—C12 | 111.53 (16) |
| C11—N1—C14 | 110.95 (15) | H15—C11—H16 | 108.0 |
| C13—N1—Zn | 111.23 (10) | C12—C11—H15 | 109.3 |
| C13—N1—C14 | 107.86 (15) | C12—C11—H16 | 109.3 |
| C14—N1—Zn | 111.17 (11) | N2—C12—C11 | 111.49 (15) |
| C12—N2—Zn | 105.59 (11) | N2—C12—H17 | 109.3 |
| C15—N2—Zn | 111.69 (10) | N2—C12—H18 | 109.3 |
| C15—N2—C12 | 110.50 (15) | C11—C12—H17 | 109.3 |
| C16—N2—Zn | 111.08 (11) | C11—C12—H18 | 109.3 |
| C16—N2—C12 | 110.16 (14) | H17—C12—H18 | 108.0 |
| C16—N2—C15 | 107.84 (15) | N1—C13—H19 | 109.5 |
| H1—C1—H2 | 109.5 | N1—C13—H20 | 109.5 |
| H1—C1—H3 | 109.5 | N1—C13—H21 | 109.5 |
| H2—C1—H3 | 109.5 | H19—C13—H20 | 109.5 |
| C2—C1—H1 | 109.5 | H19—C13—H21 | 109.5 |
| C2—C1—H2 | 109.5 | H20—C13—H21 | 109.5 |
| C2—C1—H3 | 109.5 | N1—C14—H22 | 109.5 |
| O1—C2—C1 | 116.12 (15) | N1—C14—H23 | 109.5 |
| O1—C2—C3 | 126.06 (16) | N1—C14—H24 | 109.5 |
| C3—C2—C1 | 117.82 (15) | H22—C14—H23 | 109.5 |
| C2—C3—H4 | 117.4 | H22—C14—H24 | 109.5 |
| C2—C3—C4 | 125.30 (15) | H23—C14—H24 | 109.5 |
| C4—C3—H4 | 117.4 | N2—C15—H25 | 109.5 |
| O2—C4—C3 | 126.43 (15) | N2—C15—H26 | 109.5 |
| O2—C4—C5 | 115.53 (15) | N2—C15—H27 | 109.5 |
| C3—C4—C5 | 118.02 (15) | H25—C15—H26 | 109.5 |
| C4—C5—H5 | 109.5 | H25—C15—H27 | 109.5 |
| C4—C5—H6 | 109.5 | H26—C15—H27 | 109.5 |
| C4—C5—H7 | 109.5 | N2—C16—H28 | 109.5 |
| H5—C5—H6 | 109.5 | N2—C16—H29 | 109.5 |
| H5—C5—H7 | 109.5 | N2—C16—H30 | 109.5 |
| H6—C5—H7 | 109.5 | H28—C16—H29 | 109.5 |
| H8—C6—H9 | 109.5 | H28—C16—H30 | 109.5 |
| H8—C6—H10 | 109.5 | H29—C16—H30 | 109.5 |
| H9—C6—H10 | 109.5 |
Funding Statement
This work was funded by Deutsche Forschungsgemeinschaft grant Open Access Publishing.
References
- Albrecht, R., Liebing, P., Morgenstern, U., Wagner, C. & Merzweiler, K. (2019). Z. Naturforsch. Teil B, 74, 233–240.
- Barreca, D., Carraro, G., Devi, A., Fois, E., Gasparotto, A., Seraglia, R., Maccato, C., Sada, C., Tabacchi, G., Tondello, E., Venzo, A. & Winter, M. (2012). Dalton Trans. 41, 149–155. [DOI] [PubMed]
- Brahma, S., Sachin, H. P., Shivashankar, S. A., Narasimhamurthy, T. & Rathore, R. S. (2008). Acta Cryst. C64, m140–m143. [DOI] [PubMed]
- Brandenburg, K. (2019). DIAMOND. Crystal Impact GbR, Bonn, Germany.
- Dickman, M. H. (1998). Acta Cryst. C54 IUC9800048.
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
- Gorkum, R. van, Buda, F., Kooijman, H., Spek, A. L., Bouwman, E. & Reedijk, J. (2005). Eur. J. Inorg. Chem. pp. 2255–2261.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Halbach, R. L., Nocton, G. & Andersen, R. A. (2012). Dalton Trans. 41, 8809–8812. [DOI] [PubMed]
- Harbach, P., Lerner, H.-W. & Bolte, M. (2003). Acta Cryst. E59, m724–m725.
- Kaschube, W., Pörschke, K. R. & Wilke, G. J. (1988). J. Organomet. Chem. 355, 525–532.
- Ma, Y. M., Reardon, D., Gambarotta, S., Yap, G., Zahalka, H. & Lemay, C. (1999). Organometallics, 18, 2773–2781.
- Malandrino, G., Toro, R. G., Catalano, M. R., Fragalà, M. E., Rossi, P. & Paoli, P. (2012). Eur. J. Inorg. Chem. pp.1021–1024.
- Montgomery, H. & Lingafelter, E. C. (1968). Acta Cryst. B24, 1127–1128.
- Nelkenbaum, E., Kapon, M. & Eisen, M. S. (2005). Organometallics, 24, 2645–2659.
- Ni, J., Yan, H., Wang, A., Yang, Y., Stern, C. L., Metz, A. W., Jin, S., Wang, L., Marks, T. J., Ireland, J. R. & Kannewurf, C. R. (2005). J. Am. Chem. Soc. 127, 5613–5624. [DOI] [PubMed]
- Novitchi, G., Jiang, S., Shova, S., Rida, F., Hlavička, I., Orlita, M., Wernsdorfer, W., Hamze, R., Martins, C., Suaud, N., Guihéry, N., Barra, A.-L. & Train, C. (2017). Inorg. Chem. 56, 14809–14822. [DOI] [PubMed]
- Pasko, S., Hubert-Pfalzgraf, L. G., Abrutis, A. & Vaissermann, J. (2004). Polyhedron, 23, 735–741.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Stephens, F. S. (1977). Acta Cryst. B33, 3492–3495.
- Stoe & Cie (2016). X-AREA. Stoe & Cie, Darmstadt, Germany.
- Trimmel, G., Lembacher, C., Kickelbick, G. & Schubert, U. (2002). New J. Chem. 26, 759–765.
- Tsodikov, M. V., Bukhtenko, O. V., Ellert, O. G., Petrunenko, I. A., Antsyshkina, A. S., Sadikov, G. G., Maksimov, Y. V., Titov, Y. V. & Novotortsev, V. M. (1995). Russ. Chem. Bull. 44, 1396–1400.
- Zeller, A., Herdtweck, E. & Strassner, Th. (2004). Inorg. Chem. Commun. 7, 296–301.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) 1, 2, 3. DOI: 10.1107/S2056989019016372/wm5524sup1.cif
Structure factors: contains datablock(s) 1. DOI: 10.1107/S2056989019016372/wm55241sup2.hkl
Structure factors: contains datablock(s) 2. DOI: 10.1107/S2056989019016372/wm55242sup3.hkl
Structure factors: contains datablock(s) 3. DOI: 10.1107/S2056989019016372/wm55243sup4.hkl
Additional supporting information: crystallographic information; 3D view; checkCIF report