In each of two isostructural 3-(5-aryloxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-ones, the thiophene unit is disordered over two sets of atomic sites and a combination of C—H⋯N and C—H⋯O hydrogen bonds link the molecules into sheets.
Keywords: heterocyclic compounds, pyrazoles, crystal structure, disorder, molecular conformation, hydrogen bonding, supramolecular assembly
Abstract
Two new chalcones containing both pyrazole and thiophene substituents have been prepared and structurally characterized. 3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one, C23H18N2O2S (I), and 3-[3-methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one, C24H20N2O2S (II), are isomorphous as well as isostructural, and in each the thiophene substituent is disordered over two sets of atomic sites having occupancies 0.844 (3) and 0.156 (3) in (I), and 0.883 (2) and 0.117 (2) in (II). In each structure, the molecules are linked into sheets by a combination of C—H⋯N and C—H⋯O hydrogen bonds. Comparisons are made with some related compounds.
Chemical context
Pyrazole derivatives exhibit a wide range of pharmacological activity (Karrouchi et al., 2018 ▸), including analgesic (Vijesh et al., 2013 ▸), anticancer (Dawood et al., 2013 ▸; Koca et al., 2013 ▸), antidepressant (Mathew et al., 2014 ▸), antifungal (Zhang et al., 2017 ▸), anti-inflammatory (Badawey & El-Ashmawey, 1998 ▸) and antimicrobial (Vijesh et al., 2013 ▸) activities. In addition, a range of thiophene-based heterocyclic compounds have been shown to exhibit antimicrobial activity (Mabkhot et al., 2016 ▸).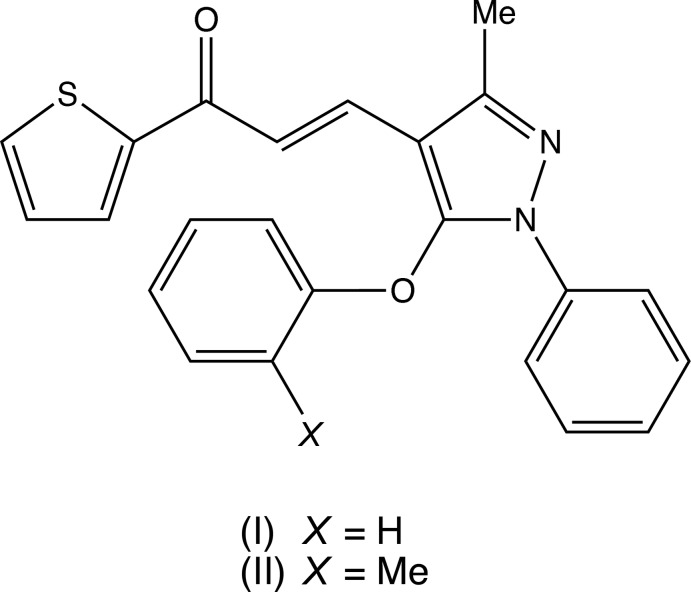
With these observations in mind, we have now synthesized two new chalcones containing both pyrazole and thiophene moieties, namely 3-(3-methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one, C23H18N2O2S (I) (Fig. 1 ▸), and 3-[3-methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one, C24H20N2O2S (II) (Fig. 2 ▸), and here we report their molecular and supramolecular structures.
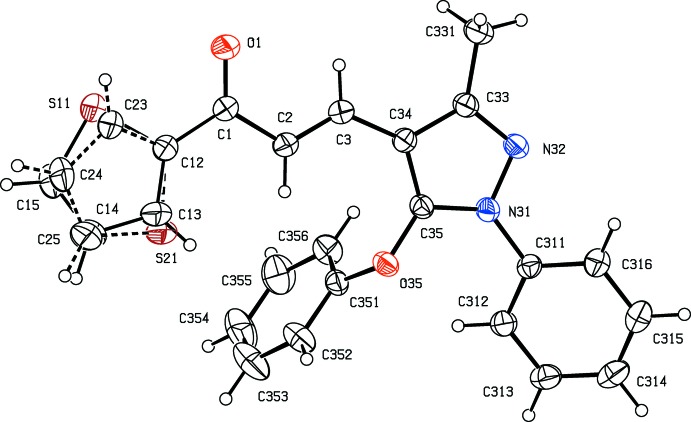
Figure 1.
The molecular structure of compound (I), showing the atom-labelling scheme, and the disorder in the thiophen-2-yl substituent, where the major disorder component has been drawn using full lines and the minor disorder component has been drawn using dashed lines.
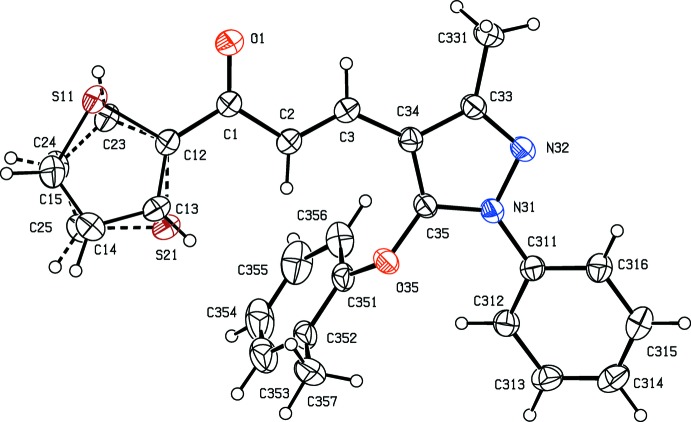
Figure 2.
The molecular structure of compound (II), showing the atom-labelling scheme, and the disorder in the thiophen-2-yl substituent, where the major disorder component has been drawn using full lines and the minor disorder component has been drawn using dashed lines.
Structural commentary
Compounds (I) and (II) are isomorphous with unit-cell volumes which differ by only ca 1% and, with appropriate adjustment of the substituent at atom C352 (H versus CH3), each structure can be smoothly refined using the atomic coordinates of the other as the starting point.
In each structure, the thienyl group is disordered over two sets of atomic sites having occupancies 0.844 (3) and 0.156 (3) in (I), and 0.883 (2) and 0.117 (2) in (II): in each case, the two disorder components are approximately related by a rotation of ca 180° about the C1—C12 bond (Figs. 1 ▸ and 2 ▸). It is by no means clear why the occupancies of the two disorder components in each compound are so different, particularly as the two disorder components form similar intermolecular hydrogen bonds (Section 3).
For both compounds, the central space unit between atoms C12 and C34, the pyrazole ring and the major disorder component of the thienyl ring are almost coplanar, and the r.m.s. deviations of the atoms from the mean planes through these units are only 0.055 Å in (I) and 0.102 Å in (II). By contrast, the two pendent aryl rings are markedly displaced from this plane: the dihedral angles between the pyrazole ring and the rings (C311–C316) and (C351–C356) are 29.99 (11) and 78.60 (6)°, respectively, in (I), and 27.90 (11) and 81.13 (6)° in (II). On the other hand, atom C35 is, in each structure, displaced from the plane (O35/C351–C356) by only 0.097 (3) Å in (I) and 0.017 (3) Å in (II). Associated with this near co-planarity, the two exocyclic C—C—O angles at atom C351 differ in each structure by ca 9°, as typically found in planar alkoxyarenes (Seip & Seip, 1973 ▸; Ferguson et al., 1996 ▸).
Supramolecular features
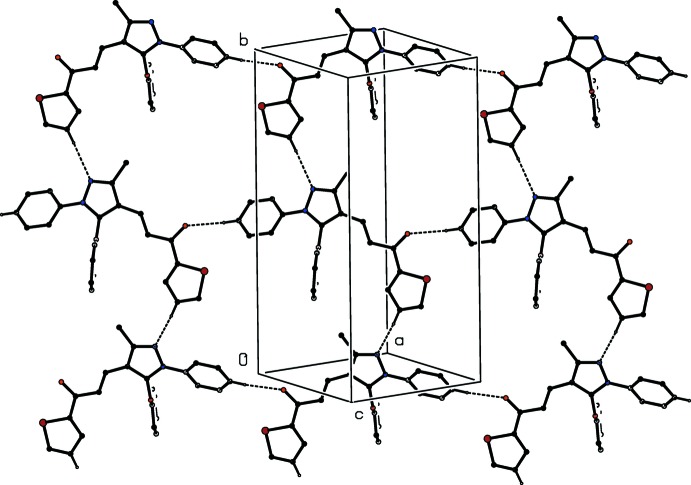
The supramolecular assembly of compound (I) depends upon just two hydrogen bonds, one each of C—H⋯N and C—H⋯O types (Table 1 ▸). The C—H⋯O hydrogen bonds links molecules which are related by translation to form a C(12) (Etter, 1990 ▸; Etter et al., 1990 ▸; Bernstein et al., 1995 ▸) chain running parallel to the [101] direction (Fig. 3 ▸). The C—H⋯N hydrogen bond links molecules which are related by the 21 screw axis along (0.5, y, 0.25) to form a C(10) chain running parallel to the [010] direction (Fig. 3 ▸). The chain formation along [010] is independent of the disorder, since both atom C14 in the major disorder component and atom C25 in the minor component (cf. Fig. 1 ▸) form similar C—H⋯N hydrogen bonds. The combination of these two chain motifs generates a sheet in the form of a (4,4) net (Batten & Robson, 1998 ▸) built from 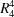 (35) rings and lying parallel to (10
(35) rings and lying parallel to (10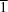 ). The supramolecular assembly of compound (II) is entirely similar to that in (I), although the C—H⋯N hydrogen bond formed by the minor disorder component is rather long (Table 2 ▸).
). The supramolecular assembly of compound (II) is entirely similar to that in (I), although the C—H⋯N hydrogen bond formed by the minor disorder component is rather long (Table 2 ▸).
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C14—H14⋯N32i | 0.93 | 2.62 | 3.462 (9) | 151 |
| C25—H25⋯N32i | 0.93 | 2.51 | 3.33 (5) | 148 |
| C314—H314⋯O1ii | 0.93 | 2.38 | 3.305 (3) | 175 |
Symmetry codes: (i) 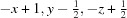 ; (ii)
; (ii) 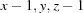 .
.
Figure 3.
Part of the crystal structure of compound (I) showing the formation of a hydrogen-bonded sheet lying parallel to (10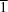 ). Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms which are not involved in the motifs shown have been omitted.
). Hydrogen bonds are drawn as dashed lines and, for the sake of clarity, the minor disorder component and the H atoms which are not involved in the motifs shown have been omitted.
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C14—H14⋯N32i | 0.93 | 2.55 | 3.483 (4) | 177 |
| C25—H25⋯N32i | 0.93 | 2.69 | 3.47 (2) | 142 |
| C314—H314⋯O1ii | 0.93 | 2.51 | 3.432 (3) | 171 |
Symmetry codes: (i) 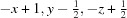 ; (ii)
; (ii) 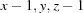 .
.
In view of the similarities in the hydrogen bonds formed by (I) and (II), and their similar molecular conformations (see Section 2), these isomorphous compounds can be described as isostructural, although it is not always the case that isomorphous pairs are strictly isostructural (Bowes et al., 2003 ▸; Acosta et al., 2009 ▸; Blanco et al., 2012 ▸).
Database survey
It is of interest to briefly compare the structures of compounds (I) and (II) reported here with those of some related compounds. 2,5-Bis[(3,5-dimethylpyrazol-1-yl)carbonyl]thiophene (III) crystallizes with Z′ = 2 in space group P21/m (Guzei et al., 2009 ▸): the two independent molecules are weakly linked by a C—H⋯O hydrogen bond but the only other direction-specific interactions between the molecules are π–π interactions involving inversion-related pairs of pyrazole rings. In contrast to the simplicity of the molecular constitution of (III) above, in most other structures containing both pyrazole and thiophene units, at least one of the rings is fused. In 3,6-dimethyl-1-phenyl-4-(thiophen-2-yl)-8-(thiophen-2-ylmethylene)-5,6,7,8- tetrahydro-1H-pyrazolo[3,4-b][1,6]naphthyridine (IV) (Peng et al., 2009 ▸), the molecules are linked into C(11) chains by means of C—H⋯N hydrogen bonds. The molecules of 2-(3,4-dimethyl-5,5-dioxo-2H,4H-pyrazolo[4,3-c][1,2]benzothiazin-2-yl)-N′-(thiophen-2-ylmethylidene)acetohydrazide (V) (Ahmad et al., 2010 ▸) are linked by a combination of N—H⋯O and C—H⋯N hydrogen bonds: although the resulting aggregation was described as consisting of dimers, the molecules are, in fact, linked into chains of rings, as clearly illustrated in the original report. A chain of rings, built from a combination of N—H⋯N and C—H⋯N hydrogen bonds is also found in the structure of (Z)-ethyl 2-cyano-2-{2-[5,6-dimethyl-4-(thiophen-2-yl)-1H-pyrazolo[3,4-b]pyridin-3-yl]hydrazinylidene}acetate (VI) (Fun et al., 2011 ▸).
In 9-(thiophen-2-yl)-8,9-dihydro-3H-pyrazolo[4,3-f]quinolin-7(6H)-one ethanol monosolvate (VII) (Peng & Jia, 2012 ▸), the thiophene ring is disordered over two sets of atomic sites having unequal occupancies, 0.692 (7) and 0.308 (7), much as found here for compounds (I) and (II). The molecular components in (VII) are linked by N—H⋯O and O—H⋯N hydrogen bonds to form a complex chain of rings. The thiophene ring in 5,6-dimethyl-4-(thiophen-2-yl)-1–pyrazolo[3,4-b]pyridin-3-amine (VIII) (Abdel-Aziz et al., 2012 ▸) is also disordered, with occupancies of 0.777 (4) and 0.223 (4), and the molecules are again linked into a chain of rings, this time by two independent N—H⋯N hydrogen bonds. Finally, we note that in [4-(2-methoxyphenyl)-3-methyl-1-phenyl-6-trifluoromethyl-1H-pyrazolo[3,4-b]pyridin-5-yl](thiophen-2-yl)methanone (IX) (Rajni Swamy et al., 2014 ▸), where the thiophene ring is fully ordered, there are no significant hydrogen bonds of any kind.
Synthesis and crystallization
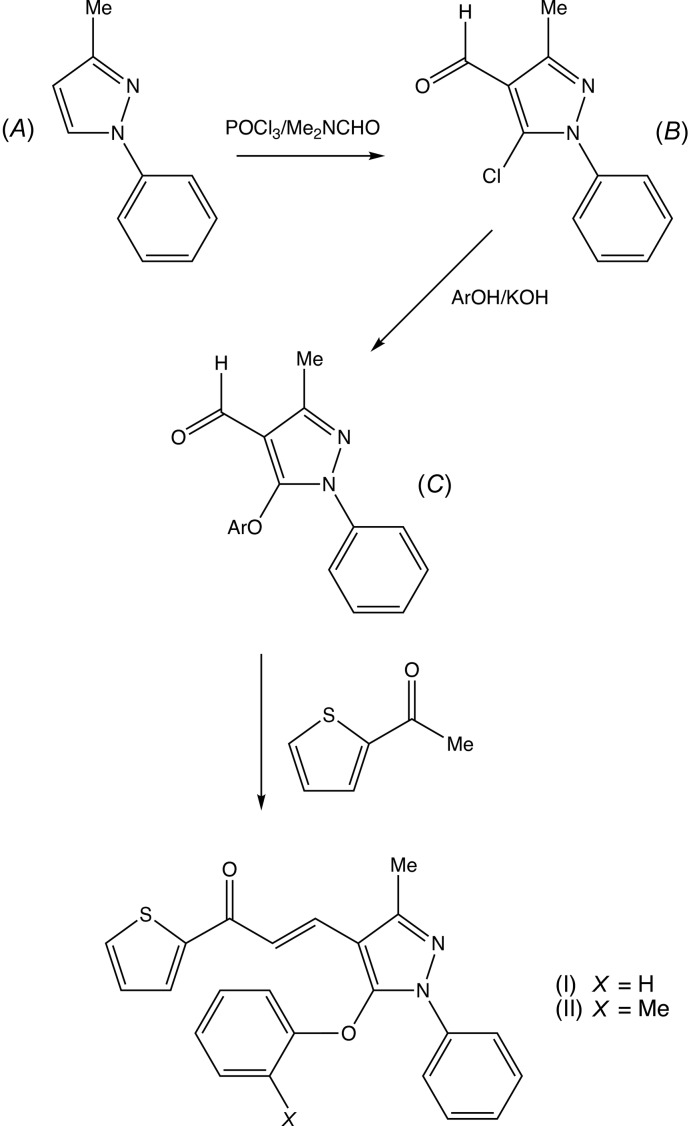
Compounds (I) and (II) were prepared using a three-step procedure, starting from the readily accessible 3-methyl-1-phenyl-1H-pyrazole (A) (see Fig. 4 ▸), which was converted to the corresponding 5-chloro-4-carbaldehyde (B) under Vilsmeier–Haack conditions, followed by nucleophilic substitution (Asma et al., 2017 ▸) to provide the 5-aryloxy intermediates (C). Condensation with 2-acetylethiophene then gave the products (I) and (II) in yields of 86% and 84%, respectively. Thus the appropriate 3-methyl-5-aryloxy-1-phenyl-1H-pyrazole 4-carbaldehydes (Asma et al., 2017 ▸) [1.7 mmol; 445 mg for (I), or 469 mg for (II)] and 2-acetyl thiophene (1.7 mmol, 214 mg) were dissolved in ethanol (20 ml) at 273 K; a solution of potassium hydroxide (2.1 mmol, 112 mg) in ethanol (5 ml) was then added dropwise, and the resulting mixtures were then stirred for 4 h. When the reactions were complete, as judged by thin-layer chromatography, the resulting solid products were collected by filtration, washed with water, dried in air and then recrystallized from ethanol–dimethylformamide (9:1, v/v), to give crystals suitable for single-crystal X-ray diffraction. Compound (I). Yield 86%, m.p. 425–427 K. IR (cm−1) 1667 (C=O), 1591 (C=N). Analysis: found C 71.5, H 4.7, N 7.2%: C23H18N2O2S requires C 71.5, H 4.7, N 7.3%. Compound (II). Yield 84%, m.p. 401–405 K. IR (cm−1) 1671 (C=O), 1564 (C=N). Analysis: found C 72.0, H 5.1, N 7.1%: C24H20N2O2S requires C 72.0, H 5.0, N 7.0%.
Figure 4.
The synthetic route to compounds (I) and (II).
Refinement
Crystal data, data collection and structure refinement details are summarized In Table 3 ▸. In both compounds, the thienyl unit was disordered over two sets of atomic sites having unequal occupancies. In each case, the bonded distances and the 1,3 non-bonded distances in the minor disorder component were restrained to be the similar to the equivalent distances in the major disorder component, subject to s.u. values of 0.01 Å and 0.02° for bonds and angles, respectively, and the anisotropic displacement parameters for pairs of partial-occupancy atoms occupying essentially the same physical space were constrained to be equal. All H atoms, apart from those in the minor disorder components were located in difference maps, and then treated as riding atoms in geometrically idealized positions, with C—H distances of 0.93 Å (alkenyl, aromatic and thienyl) or 0.96 Å (methyl), and with U iso(H) = kU eq(C), where k = 1.5 for the methyl groups, which were permitted to rotate but not to tilt, and 1.2 for all other H atoms. The H atoms in the minor disorder components were included on the same basis. Subject to these conditions, the occupancies of the disorder components refined to 0.844 (3) and 0.156 (3) in (I), and 0.883 (2) and to 0.117 (2) in (II).
Table 3. Experimental details.
| (I) | (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C23H18N2O2S | C24H20N2O2S |
| M r | 386.45 | 400.48 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/c |
| Temperature (K) | 296 | 296 |
| a, b, c (Å) | 9.6158 (5), 19.8846 (11), 10.3773 (6) | 9.4336 (4), 20.6071 (9), 10.5866 (4) |
| β (°) | 93.712 (2) | 93.106 (2) |
| V (Å3) | 1980.04 (19) | 2055.00 (15) |
| Z | 4 | 4 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.18 | 0.18 |
| Crystal size (mm) | 0.20 × 0.20 × 0.15 | 0.30 × 0.20 × 0.15 |
| Data collection | ||
| Diffractometer | Bruker Kappa APEXII CCD | Bruker Kappa APEXII CCD |
| Absorption correction | Multi-scan (SADABS; Bruker, 2012 ▸) | Multi-scan (SADABS; Bruker, 2012 ▸) |
| T min, T max | 0.941, 0.973 | 0.926, 0.973 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 31970, 3725, 2446 | 35938, 4735, 2877 |
| R int | 0.043 | 0.040 |
| (sin θ/λ)max (Å−1) | 0.608 | 0.651 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.042, 0.117, 1.06 | 0.045, 0.142, 1.02 |
| No. of reflections | 3725 | 4735 |
| No. of parameters | 268 | 277 |
| No. of restraints | 10 | 10 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.20, −0.14 | 0.19, −0.23 |
Supplementary Material
Crystal structure: contains datablock(s) global, I, II. DOI: 10.1107/S205698901901658X/zl2765sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901901658X/zl2765Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S205698901901658X/zl2765IIsup3.hkl
Supporting information file. DOI: 10.1107/S205698901901658X/zl2765Isup4.cml
Supporting information file. DOI: 10.1107/S205698901901658X/zl2765IIsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
MAES thanks the University of Mysore for research facilities.
supplementary crystallographic information
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Crystal data
| C23H18N2O2S | F(000) = 808 |
| Mr = 386.45 | Dx = 1.296 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.6158 (5) Å | Cell parameters from 3725 reflections |
| b = 19.8846 (11) Å | θ = 2.1–25.6° |
| c = 10.3773 (6) Å | µ = 0.18 mm−1 |
| β = 93.712 (2)° | T = 296 K |
| V = 1980.04 (19) Å3 | Block, colourless |
| Z = 4 | 0.20 × 0.20 × 0.15 mm |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Data collection
| Bruker Kappa APEXII CCD diffractometer | 3725 independent reflections |
| Radiation source: fine-focus sealed tube | 2446 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.043 |
| Detector resolution: 7.3910 pixels mm-1 | θmax = 25.6°, θmin = 2.1° |
| φ and ω scans | h = −10→11 |
| Absorption correction: multi-scan (SADABS; Bruker, 2012) | k = −24→24 |
| Tmin = 0.941, Tmax = 0.973 | l = −12→12 |
| 31970 measured reflections |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.042 | w = 1/[σ2(Fo2) + (0.0467P)2 + 0.573P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.117 | (Δ/σ)max < 0.001 |
| S = 1.06 | Δρmax = 0.20 e Å−3 |
| 3725 reflections | Δρmin = −0.14 e Å−3 |
| 268 parameters | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 10 restraints | Extinction coefficient: 0.0059 (9) |
| Primary atom site location: difference Fourier map |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.6952 (2) | 0.38677 (11) | 0.5021 (2) | 0.0485 (5) | |
| O1 | 0.76942 (19) | 0.43097 (8) | 0.55125 (17) | 0.0798 (6) | |
| C2 | 0.5895 (2) | 0.40140 (11) | 0.3982 (2) | 0.0480 (5) | |
| H2 | 0.5376 | 0.3663 | 0.3604 | 0.058* | |
| C3 | 0.5660 (2) | 0.46399 (10) | 0.35662 (19) | 0.0451 (5) | |
| H3 | 0.6175 | 0.4976 | 0.4000 | 0.054* | |
| S11 | 0.82626 (11) | 0.29817 (5) | 0.67269 (8) | 0.0664 (3) | 0.844 (3) |
| C12 | 0.7111 (2) | 0.31696 (10) | 0.5460 (2) | 0.0484 (5) | 0.844 (3) |
| C13 | 0.6491 (14) | 0.2615 (5) | 0.5017 (13) | 0.0930 (17) | 0.844 (3) |
| H13 | 0.5837 | 0.2612 | 0.4316 | 0.112* | 0.844 (3) |
| C14 | 0.6904 (13) | 0.2030 (2) | 0.5695 (15) | 0.106 (3) | 0.844 (3) |
| H14 | 0.6547 | 0.1605 | 0.5506 | 0.128* | 0.844 (3) |
| C15 | 0.7865 (9) | 0.2158 (3) | 0.6639 (9) | 0.080 (2) | 0.844 (3) |
| H15 | 0.8270 | 0.1831 | 0.7185 | 0.096* | 0.844 (3) |
| S21 | 0.614 (2) | 0.2534 (7) | 0.484 (2) | 0.0930 (17) | 0.156 (3) |
| C22 | 0.7111 (2) | 0.31696 (10) | 0.5460 (2) | 0.0484 (5) | 0.156 (3) |
| C23 | 0.813 (2) | 0.2935 (10) | 0.626 (2) | 0.0664 (3) | 0.156 (3) |
| H23 | 0.8837 | 0.3205 | 0.6635 | 0.080* | 0.156 (3) |
| C24 | 0.805 (6) | 0.2239 (12) | 0.647 (6) | 0.080 (2) | 0.156 (3) |
| H24 | 0.8581 | 0.2009 | 0.7111 | 0.096* | 0.156 (3) |
| C25 | 0.713 (8) | 0.1950 (8) | 0.564 (9) | 0.106 (3) | 0.156 (3) |
| H25 | 0.7042 | 0.1488 | 0.5511 | 0.128* | 0.156 (3) |
| N31 | 0.31385 (16) | 0.49026 (8) | 0.08717 (15) | 0.0417 (4) | |
| N32 | 0.36763 (17) | 0.55440 (8) | 0.09923 (17) | 0.0474 (4) | |
| C33 | 0.4603 (2) | 0.55130 (10) | 0.1990 (2) | 0.0444 (5) | |
| C34 | 0.4706 (2) | 0.48590 (10) | 0.25279 (19) | 0.0412 (5) | |
| C35 | 0.3759 (2) | 0.44920 (9) | 0.17696 (18) | 0.0397 (5) | |
| C311 | 0.2118 (2) | 0.47606 (10) | −0.01477 (19) | 0.0400 (5) | |
| C312 | 0.1122 (2) | 0.42704 (10) | −0.0019 (2) | 0.0480 (5) | |
| H312 | 0.1113 | 0.4020 | 0.0737 | 0.058* | |
| C313 | 0.0143 (2) | 0.41552 (11) | −0.1021 (2) | 0.0555 (6) | |
| H313 | −0.0532 | 0.3826 | −0.0937 | 0.067* | |
| C314 | 0.0151 (2) | 0.45223 (12) | −0.2146 (2) | 0.0609 (7) | |
| H314 | −0.0508 | 0.4439 | −0.2822 | 0.073* | |
| C315 | 0.1141 (3) | 0.50138 (13) | −0.2260 (2) | 0.0596 (6) | |
| H315 | 0.1146 | 0.5266 | −0.3015 | 0.071* | |
| C316 | 0.2127 (2) | 0.51353 (11) | −0.1264 (2) | 0.0499 (5) | |
| H316 | 0.2794 | 0.5468 | −0.1345 | 0.060* | |
| C331 | 0.5426 (2) | 0.61231 (11) | 0.2386 (2) | 0.0609 (6) | |
| H31A | 0.6353 | 0.6082 | 0.2102 | 0.091* | |
| H31B | 0.5468 | 0.6165 | 0.3309 | 0.091* | |
| H31C | 0.4987 | 0.6514 | 0.2001 | 0.091* | |
| O35 | 0.35064 (14) | 0.38210 (6) | 0.17345 (13) | 0.0458 (4) | |
| C351 | 0.2722 (2) | 0.35338 (10) | 0.2680 (2) | 0.0445 (5) | |
| C352 | 0.2634 (3) | 0.28476 (12) | 0.2623 (3) | 0.0687 (7) | |
| H352 | 0.3095 | 0.2609 | 0.2009 | 0.082* | |
| C353 | 0.1856 (3) | 0.25194 (15) | 0.3483 (4) | 0.0947 (10) | |
| H353 | 0.1786 | 0.2053 | 0.3453 | 0.114* | |
| C354 | 0.1183 (3) | 0.28709 (17) | 0.4383 (4) | 0.0972 (11) | |
| H354 | 0.0656 | 0.2644 | 0.4965 | 0.117* | |
| C355 | 0.1281 (3) | 0.35565 (16) | 0.4432 (3) | 0.0854 (9) | |
| H355 | 0.0820 | 0.3793 | 0.5049 | 0.102* | |
| C356 | 0.2065 (2) | 0.39028 (12) | 0.3568 (2) | 0.0603 (6) | |
| H356 | 0.2138 | 0.4369 | 0.3594 | 0.072* |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0522 (13) | 0.0469 (13) | 0.0450 (12) | −0.0042 (10) | −0.0060 (10) | 0.0003 (10) |
| O1 | 0.0919 (13) | 0.0535 (10) | 0.0872 (13) | −0.0176 (9) | −0.0475 (10) | 0.0094 (9) |
| C2 | 0.0493 (12) | 0.0441 (12) | 0.0491 (12) | −0.0035 (10) | −0.0085 (10) | −0.0002 (10) |
| C3 | 0.0465 (12) | 0.0443 (12) | 0.0437 (12) | −0.0051 (9) | −0.0022 (10) | −0.0015 (9) |
| S11 | 0.0811 (6) | 0.0537 (5) | 0.0608 (6) | −0.0027 (4) | −0.0243 (5) | 0.0092 (4) |
| C12 | 0.0550 (13) | 0.0451 (13) | 0.0442 (12) | 0.0012 (10) | −0.0038 (10) | −0.0002 (10) |
| C13 | 0.138 (7) | 0.048 (2) | 0.086 (4) | −0.017 (2) | −0.050 (3) | −0.004 (2) |
| C14 | 0.167 (6) | 0.0398 (17) | 0.104 (3) | −0.009 (3) | −0.050 (5) | 0.002 (3) |
| C15 | 0.113 (4) | 0.055 (2) | 0.070 (4) | 0.007 (2) | −0.016 (3) | 0.0186 (17) |
| S21 | 0.138 (7) | 0.048 (2) | 0.086 (4) | −0.017 (2) | −0.050 (3) | −0.004 (2) |
| C22 | 0.0550 (13) | 0.0451 (13) | 0.0442 (12) | 0.0012 (10) | −0.0038 (10) | −0.0002 (10) |
| C23 | 0.0811 (6) | 0.0537 (5) | 0.0608 (6) | −0.0027 (4) | −0.0243 (5) | 0.0092 (4) |
| C24 | 0.113 (4) | 0.055 (2) | 0.070 (4) | 0.007 (2) | −0.016 (3) | 0.0186 (17) |
| C25 | 0.167 (6) | 0.0398 (17) | 0.104 (3) | −0.009 (3) | −0.050 (5) | 0.002 (3) |
| N31 | 0.0402 (9) | 0.0369 (9) | 0.0473 (10) | 0.0007 (7) | −0.0034 (8) | 0.0033 (8) |
| N32 | 0.0489 (10) | 0.0350 (10) | 0.0571 (11) | −0.0031 (8) | −0.0054 (9) | 0.0051 (8) |
| C33 | 0.0428 (11) | 0.0390 (12) | 0.0509 (13) | −0.0024 (9) | −0.0002 (10) | 0.0011 (10) |
| C34 | 0.0393 (11) | 0.0401 (11) | 0.0437 (11) | −0.0002 (9) | −0.0002 (9) | 0.0016 (9) |
| C35 | 0.0393 (11) | 0.0345 (11) | 0.0450 (11) | 0.0012 (9) | 0.0016 (9) | 0.0022 (9) |
| C311 | 0.0372 (11) | 0.0397 (11) | 0.0425 (11) | 0.0050 (9) | −0.0028 (9) | −0.0025 (9) |
| C312 | 0.0471 (12) | 0.0425 (12) | 0.0534 (13) | 0.0040 (10) | −0.0045 (10) | −0.0020 (10) |
| C313 | 0.0485 (13) | 0.0508 (14) | 0.0655 (15) | 0.0010 (11) | −0.0082 (11) | −0.0108 (12) |
| C314 | 0.0554 (14) | 0.0677 (16) | 0.0570 (15) | 0.0127 (13) | −0.0149 (11) | −0.0184 (13) |
| C315 | 0.0631 (15) | 0.0706 (16) | 0.0441 (13) | 0.0090 (13) | −0.0037 (12) | 0.0026 (11) |
| C316 | 0.0473 (12) | 0.0552 (14) | 0.0470 (13) | 0.0025 (10) | 0.0016 (10) | 0.0025 (10) |
| C331 | 0.0622 (15) | 0.0451 (13) | 0.0738 (16) | −0.0077 (11) | −0.0082 (12) | 0.0016 (12) |
| O35 | 0.0525 (9) | 0.0332 (8) | 0.0515 (9) | −0.0010 (6) | 0.0036 (7) | 0.0004 (6) |
| C351 | 0.0411 (11) | 0.0398 (12) | 0.0515 (13) | −0.0025 (9) | −0.0047 (10) | 0.0105 (10) |
| C352 | 0.0714 (16) | 0.0420 (14) | 0.093 (2) | −0.0053 (12) | 0.0095 (15) | 0.0076 (13) |
| C353 | 0.094 (2) | 0.0543 (17) | 0.138 (3) | −0.0114 (16) | 0.023 (2) | 0.0266 (19) |
| C354 | 0.093 (2) | 0.087 (2) | 0.115 (3) | −0.0112 (18) | 0.027 (2) | 0.046 (2) |
| C355 | 0.086 (2) | 0.095 (2) | 0.0777 (19) | −0.0017 (17) | 0.0287 (16) | 0.0142 (17) |
| C356 | 0.0628 (15) | 0.0546 (14) | 0.0640 (15) | −0.0025 (12) | 0.0093 (12) | 0.0034 (12) |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Geometric parameters (Å, º)
| C1—O1 | 1.223 (2) | C35—O35 | 1.356 (2) |
| C1—C2 | 1.462 (3) | C311—C316 | 1.378 (3) |
| C1—C12 | 1.466 (3) | C311—C312 | 1.379 (3) |
| C2—C3 | 1.332 (3) | C312—C313 | 1.376 (3) |
| C2—H2 | 0.9300 | C312—H312 | 0.9300 |
| C3—C34 | 1.437 (3) | C313—C314 | 1.377 (3) |
| C3—H3 | 0.9300 | C313—H313 | 0.9300 |
| S11—C15 | 1.684 (5) | C314—C315 | 1.375 (3) |
| S11—C12 | 1.705 (2) | C314—H314 | 0.9300 |
| C12—C13 | 1.322 (7) | C315—C316 | 1.378 (3) |
| C13—C14 | 1.402 (10) | C315—H315 | 0.9300 |
| C13—H13 | 0.9300 | C316—H316 | 0.9300 |
| C14—C15 | 1.327 (5) | C331—H31A | 0.9600 |
| C14—H14 | 0.9300 | C331—H31B | 0.9600 |
| C15—H15 | 0.9300 | C331—H31C | 0.9600 |
| S21—C25 | 1.685 (12) | O35—C351 | 1.398 (2) |
| C23—C24 | 1.404 (14) | C351—C356 | 1.365 (3) |
| C23—H23 | 0.9300 | C351—C352 | 1.368 (3) |
| C24—C25 | 1.329 (9) | C352—C353 | 1.366 (4) |
| C24—H24 | 0.9300 | C352—H352 | 0.9300 |
| C25—H25 | 0.9300 | C353—C354 | 1.364 (4) |
| N31—C35 | 1.349 (2) | C353—H353 | 0.9300 |
| N31—N32 | 1.379 (2) | C354—C355 | 1.367 (4) |
| N31—C311 | 1.424 (2) | C354—H354 | 0.9300 |
| N32—C33 | 1.323 (3) | C355—C356 | 1.391 (3) |
| C33—C34 | 1.416 (3) | C355—H355 | 0.9300 |
| C33—C331 | 1.492 (3) | C356—H356 | 0.9300 |
| C34—C35 | 1.374 (3) | ||
| O1—C1—C2 | 121.57 (19) | C316—C311—N31 | 118.45 (18) |
| O1—C1—C12 | 120.34 (19) | C312—C311—N31 | 121.18 (18) |
| C2—C1—C12 | 118.09 (18) | C313—C312—C311 | 119.4 (2) |
| C3—C2—C1 | 121.35 (19) | C313—C312—H312 | 120.3 |
| C3—C2—H2 | 119.3 | C311—C312—H312 | 120.3 |
| C1—C2—H2 | 119.3 | C312—C313—C314 | 120.7 (2) |
| C2—C3—C34 | 127.83 (19) | C312—C313—H313 | 119.6 |
| C2—C3—H3 | 116.1 | C314—C313—H313 | 119.6 |
| C34—C3—H3 | 116.1 | C315—C314—C313 | 119.5 (2) |
| C15—S11—C12 | 92.15 (19) | C315—C314—H314 | 120.3 |
| C13—C12—C1 | 130.3 (5) | C313—C314—H314 | 120.3 |
| C13—C12—S11 | 110.0 (5) | C314—C315—C316 | 120.4 (2) |
| C1—C12—S11 | 119.77 (15) | C314—C315—H315 | 119.8 |
| C12—C13—C14 | 114.2 (6) | C316—C315—H315 | 119.8 |
| C12—C13—H13 | 122.9 | C315—C316—C311 | 119.7 (2) |
| C14—C13—H13 | 122.9 | C315—C316—H316 | 120.2 |
| C15—C14—C13 | 111.9 (4) | C311—C316—H316 | 120.2 |
| C15—C14—H14 | 124.0 | C33—C331—H31A | 109.5 |
| C13—C14—H14 | 124.0 | C33—C331—H31B | 109.5 |
| C14—C15—S11 | 111.7 (4) | H31A—C331—H31B | 109.5 |
| C14—C15—H15 | 124.1 | C33—C331—H31C | 109.5 |
| S11—C15—H15 | 124.1 | H31A—C331—H31C | 109.5 |
| C24—C23—H23 | 123.3 | H31B—C331—H31C | 109.5 |
| C25—C24—C23 | 111.4 (14) | C35—O35—C351 | 119.13 (15) |
| C25—C24—H24 | 124.3 | C356—C351—C352 | 122.3 (2) |
| C23—C24—H24 | 124.3 | C356—C351—O35 | 123.30 (19) |
| C24—C25—S21 | 110.8 (12) | C352—C351—O35 | 114.3 (2) |
| C24—C25—H25 | 124.6 | C353—C352—C351 | 118.9 (3) |
| S21—C25—H25 | 124.6 | C353—C352—H352 | 120.5 |
| C35—N31—N32 | 110.54 (15) | C351—C352—H352 | 120.5 |
| C35—N31—C311 | 130.24 (16) | C354—C353—C352 | 120.4 (3) |
| N32—N31—C311 | 119.16 (15) | C354—C353—H353 | 119.8 |
| C33—N32—N31 | 104.92 (15) | C352—C353—H353 | 119.8 |
| N32—C33—C34 | 112.18 (17) | C353—C354—C355 | 120.1 (3) |
| N32—C33—C331 | 119.74 (18) | C353—C354—H354 | 119.9 |
| C34—C33—C331 | 128.03 (19) | C355—C354—H354 | 119.9 |
| C35—C34—C33 | 103.60 (17) | C354—C355—C356 | 120.6 (3) |
| C35—C34—C3 | 129.46 (18) | C354—C355—H355 | 119.7 |
| C33—C34—C3 | 126.81 (18) | C356—C355—H355 | 119.7 |
| N31—C35—O35 | 120.45 (17) | C351—C356—C355 | 117.6 (2) |
| N31—C35—C34 | 108.73 (17) | C351—C356—H356 | 121.2 |
| O35—C35—C34 | 130.44 (17) | C355—C356—H356 | 121.2 |
| C316—C311—C312 | 120.35 (19) | ||
| O1—C1—C2—C3 | −2.8 (3) | C33—C34—C35—N31 | −0.8 (2) |
| C12—C1—C2—C3 | 177.6 (2) | C3—C34—C35—N31 | −176.83 (19) |
| C1—C2—C3—C34 | 177.2 (2) | C33—C34—C35—O35 | 172.0 (2) |
| O1—C1—C12—C13 | −175.3 (10) | C3—C34—C35—O35 | −4.1 (4) |
| C2—C1—C12—C13 | 4.3 (11) | C35—N31—C311—C316 | −148.8 (2) |
| O1—C1—C12—S11 | 4.0 (3) | N32—N31—C311—C316 | 27.9 (3) |
| C2—C1—C12—S11 | −176.40 (16) | C35—N31—C311—C312 | 32.6 (3) |
| C15—S11—C12—C13 | −0.6 (9) | N32—N31—C311—C312 | −150.69 (18) |
| C15—S11—C12—C1 | 180.0 (4) | C316—C311—C312—C313 | 0.5 (3) |
| C1—C12—C13—C14 | −179.5 (10) | N31—C311—C312—C313 | 179.09 (18) |
| S11—C12—C13—C14 | 1.1 (17) | C311—C312—C313—C314 | 0.2 (3) |
| C12—C13—C14—C15 | −1 (2) | C312—C313—C314—C315 | −0.7 (3) |
| C13—C14—C15—S11 | 0.7 (17) | C313—C314—C315—C316 | 0.6 (3) |
| C12—S11—C15—C14 | −0.1 (11) | C314—C315—C316—C311 | 0.1 (3) |
| C23—C24—C25—S21 | −12 (9) | C312—C311—C316—C315 | −0.6 (3) |
| C35—N31—N32—C33 | −1.3 (2) | N31—C311—C316—C315 | −179.25 (18) |
| C311—N31—N32—C33 | −178.64 (17) | N31—C35—O35—C351 | −109.5 (2) |
| N31—N32—C33—C34 | 0.8 (2) | C34—C35—O35—C351 | 78.5 (3) |
| N31—N32—C33—C331 | 178.55 (18) | C35—O35—C351—C356 | 7.1 (3) |
| N32—C33—C34—C35 | 0.0 (2) | C35—O35—C351—C352 | −174.51 (18) |
| C331—C33—C34—C35 | −177.6 (2) | C356—C351—C352—C353 | 0.2 (4) |
| N32—C33—C34—C3 | 176.16 (19) | O35—C351—C352—C353 | −178.2 (2) |
| C331—C33—C34—C3 | −1.4 (4) | C351—C352—C353—C354 | −0.1 (5) |
| C2—C3—C34—C35 | 4.2 (4) | C352—C353—C354—C355 | 0.0 (5) |
| C2—C3—C34—C33 | −171.0 (2) | C353—C354—C355—C356 | 0.0 (5) |
| N32—N31—C35—O35 | −172.30 (16) | C352—C351—C356—C355 | −0.2 (4) |
| C311—N31—C35—O35 | 4.7 (3) | O35—C351—C356—C355 | 178.1 (2) |
| N32—N31—C35—C34 | 1.3 (2) | C354—C355—C356—C351 | 0.0 (4) |
| C311—N31—C35—C34 | 178.29 (18) |
3-(3-Methyl-5-phenoxy-1-phenyl-1H-pyrazol-4-yl)-1-(thiophen-2-yl)prop-2-en-1-one (I) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C14—H14···N32i | 0.93 | 2.62 | 3.462 (9) | 151 |
| C25—H25···N32i | 0.93 | 2.51 | 3.33 (5) | 148 |
| C314—H314···O1ii | 0.93 | 2.38 | 3.305 (3) | 175 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x−1, y, z−1.
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Crystal data
| C24H20N2O2S | F(000) = 840 |
| Mr = 400.48 | Dx = 1.294 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.4336 (4) Å | Cell parameters from 5216 reflections |
| b = 20.6071 (9) Å | θ = 2.0–28.6° |
| c = 10.5866 (4) Å | µ = 0.18 mm−1 |
| β = 93.106 (2)° | T = 296 K |
| V = 2055.00 (15) Å3 | Block, colourless |
| Z = 4 | 0.30 × 0.20 × 0.15 mm |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Data collection
| Bruker Kappa APEXII CCD diffractometer | 4735 independent reflections |
| Radiation source: fine-focus sealed tube | 2877 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.040 |
| Detector resolution: 7.3910 pixels mm-1 | θmax = 27.6°, θmin = 2.0° |
| φ and ω scans | h = −12→11 |
| Absorption correction: multi-scan (SADABS; Bruker, 2012) | k = −26→26 |
| Tmin = 0.926, Tmax = 0.973 | l = −13→11 |
| 35938 measured reflections |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Refinement
| Refinement on F2 | Primary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.142 | w = 1/[σ2(Fo2) + (0.0624P)2 + 0.5761P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 4735 reflections | Δρmax = 0.19 e Å−3 |
| 277 parameters | Δρmin = −0.23 e Å−3 |
| 10 restraints |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.6834 (2) | 0.38495 (10) | 0.49061 (18) | 0.0484 (5) | |
| O1 | 0.75006 (19) | 0.42752 (8) | 0.54834 (16) | 0.0747 (5) | |
| C2 | 0.5798 (2) | 0.39981 (10) | 0.38620 (18) | 0.0484 (5) | |
| H2 | 0.5302 | 0.3661 | 0.3455 | 0.058* | |
| C3 | 0.5555 (2) | 0.46061 (10) | 0.34871 (18) | 0.0464 (5) | |
| H3 | 0.6047 | 0.4926 | 0.3949 | 0.056* | |
| S11 | 0.81442 (11) | 0.29803 (4) | 0.65506 (8) | 0.0669 (3) | 0.883 (2) |
| C12 | 0.7062 (2) | 0.31666 (10) | 0.52477 (17) | 0.0456 (5) | 0.883 (2) |
| C13 | 0.6609 (8) | 0.2617 (3) | 0.4682 (5) | 0.0603 (9) | 0.883 (2) |
| H13 | 0.6001 | 0.2616 | 0.3961 | 0.072* | 0.883 (2) |
| C14 | 0.7118 (5) | 0.20428 (17) | 0.5259 (4) | 0.0643 (12) | 0.883 (2) |
| H14 | 0.6912 | 0.1627 | 0.4961 | 0.077* | 0.883 (2) |
| C15 | 0.7941 (9) | 0.21758 (17) | 0.6295 (6) | 0.0671 (16) | 0.883 (2) |
| H15 | 0.8357 | 0.1859 | 0.6819 | 0.080* | 0.883 (2) |
| S21 | 0.6339 (18) | 0.2553 (6) | 0.4380 (13) | 0.0603 (9) | 0.117 (2) |
| C22 | 0.7062 (2) | 0.31666 (10) | 0.52477 (17) | 0.0456 (5) | 0.117 (2) |
| C23 | 0.784 (3) | 0.2933 (10) | 0.623 (2) | 0.0669 (3) | 0.117 (2) |
| H23 | 0.8396 | 0.3195 | 0.6777 | 0.080* | 0.117 (2) |
| C24 | 0.775 (8) | 0.2255 (11) | 0.638 (5) | 0.0671 (16) | 0.117 (2) |
| H24 | 0.8252 | 0.2018 | 0.7011 | 0.080* | 0.117 (2) |
| C25 | 0.685 (5) | 0.2001 (8) | 0.550 (3) | 0.0643 (12) | 0.117 (2) |
| H25 | 0.6544 | 0.1573 | 0.5494 | 0.077* | 0.117 (2) |
| N31 | 0.30777 (16) | 0.48865 (7) | 0.08120 (15) | 0.0431 (4) | |
| N32 | 0.36119 (18) | 0.55036 (8) | 0.09699 (16) | 0.0492 (4) | |
| C33 | 0.4529 (2) | 0.54660 (9) | 0.19571 (19) | 0.0469 (5) | |
| C34 | 0.4627 (2) | 0.48304 (9) | 0.24567 (18) | 0.0430 (4) | |
| C35 | 0.36867 (19) | 0.44824 (9) | 0.16871 (17) | 0.0405 (4) | |
| C311 | 0.20793 (19) | 0.47603 (9) | −0.02174 (18) | 0.0419 (4) | |
| C312 | 0.1090 (2) | 0.42664 (10) | −0.0158 (2) | 0.0495 (5) | |
| H312 | 0.1062 | 0.4009 | 0.0562 | 0.059* | |
| C313 | 0.0145 (2) | 0.41597 (11) | −0.1180 (2) | 0.0580 (6) | |
| H313 | −0.0515 | 0.3826 | −0.1147 | 0.070* | |
| C314 | 0.0169 (2) | 0.45393 (12) | −0.2241 (2) | 0.0624 (6) | |
| H314 | −0.0469 | 0.4462 | −0.2926 | 0.075* | |
| C315 | 0.1140 (2) | 0.50340 (13) | −0.2289 (2) | 0.0621 (6) | |
| H315 | 0.1154 | 0.5294 | −0.3006 | 0.075* | |
| C316 | 0.2098 (2) | 0.51483 (11) | −0.12766 (19) | 0.0506 (5) | |
| H316 | 0.2751 | 0.5485 | −0.1311 | 0.061* | |
| C331 | 0.5357 (3) | 0.60493 (10) | 0.2386 (2) | 0.0625 (6) | |
| H31A | 0.6318 | 0.6008 | 0.2142 | 0.094* | |
| H31B | 0.5349 | 0.6085 | 0.3290 | 0.094* | |
| H31C | 0.4936 | 0.6430 | 0.2003 | 0.094* | |
| O35 | 0.34553 (14) | 0.38351 (6) | 0.16246 (12) | 0.0470 (3) | |
| C351 | 0.2552 (2) | 0.35450 (10) | 0.24627 (19) | 0.0488 (5) | |
| C352 | 0.2429 (2) | 0.28766 (11) | 0.2301 (2) | 0.0614 (6) | |
| C353 | 0.1516 (3) | 0.25613 (15) | 0.3056 (3) | 0.0874 (10) | |
| H353 | 0.1404 | 0.2114 | 0.2977 | 0.105* | |
| C354 | 0.0766 (3) | 0.28876 (19) | 0.3921 (3) | 0.0949 (11) | |
| H354 | 0.0143 | 0.2661 | 0.4410 | 0.114* | |
| C355 | 0.0918 (3) | 0.35466 (17) | 0.4081 (3) | 0.0862 (9) | |
| H355 | 0.0411 | 0.3763 | 0.4681 | 0.103* | |
| C356 | 0.1842 (2) | 0.38895 (13) | 0.3332 (2) | 0.0656 (6) | |
| H356 | 0.1968 | 0.4335 | 0.3424 | 0.079* | |
| C357 | 0.3278 (3) | 0.25326 (12) | 0.1355 (3) | 0.0832 (9) | |
| H35A | 0.4265 | 0.2546 | 0.1625 | 0.125* | |
| H35B | 0.3141 | 0.2742 | 0.0547 | 0.125* | |
| H35C | 0.2971 | 0.2089 | 0.1285 | 0.125* |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0519 (12) | 0.0483 (12) | 0.0442 (11) | −0.0032 (10) | −0.0045 (9) | 0.0023 (9) |
| O1 | 0.0920 (12) | 0.0541 (9) | 0.0734 (10) | −0.0092 (9) | −0.0381 (9) | 0.0023 (8) |
| C2 | 0.0481 (11) | 0.0482 (12) | 0.0476 (11) | −0.0023 (9) | −0.0087 (9) | 0.0021 (9) |
| C3 | 0.0468 (11) | 0.0481 (12) | 0.0437 (11) | −0.0023 (9) | −0.0039 (9) | −0.0007 (9) |
| S11 | 0.0885 (6) | 0.0555 (4) | 0.0533 (5) | 0.0004 (4) | −0.0283 (3) | 0.0057 (3) |
| C12 | 0.0471 (11) | 0.0492 (11) | 0.0399 (10) | 0.0002 (9) | −0.0036 (8) | 0.0023 (9) |
| C13 | 0.071 (3) | 0.0556 (17) | 0.051 (3) | −0.0026 (16) | −0.0199 (18) | −0.0051 (17) |
| C14 | 0.079 (3) | 0.0449 (13) | 0.068 (2) | −0.0014 (13) | −0.004 (2) | −0.0002 (12) |
| C15 | 0.081 (3) | 0.0560 (16) | 0.0624 (19) | 0.0084 (19) | −0.008 (2) | 0.0112 (16) |
| S21 | 0.071 (3) | 0.0556 (17) | 0.051 (3) | −0.0026 (16) | −0.0199 (18) | −0.0051 (17) |
| C22 | 0.0471 (11) | 0.0492 (11) | 0.0399 (10) | 0.0002 (9) | −0.0036 (8) | 0.0023 (9) |
| C23 | 0.0885 (6) | 0.0555 (4) | 0.0533 (5) | 0.0004 (4) | −0.0283 (3) | 0.0057 (3) |
| C24 | 0.081 (3) | 0.0560 (16) | 0.0624 (19) | 0.0084 (19) | −0.008 (2) | 0.0112 (16) |
| C25 | 0.079 (3) | 0.0449 (13) | 0.068 (2) | −0.0014 (13) | −0.004 (2) | −0.0002 (12) |
| N31 | 0.0448 (9) | 0.0350 (8) | 0.0485 (9) | −0.0001 (7) | −0.0054 (7) | 0.0033 (7) |
| N32 | 0.0528 (10) | 0.0345 (9) | 0.0591 (10) | −0.0033 (7) | −0.0083 (8) | 0.0040 (8) |
| C33 | 0.0475 (11) | 0.0377 (10) | 0.0548 (12) | −0.0009 (9) | −0.0022 (9) | 0.0005 (9) |
| C34 | 0.0422 (10) | 0.0387 (10) | 0.0477 (11) | 0.0013 (8) | −0.0024 (8) | 0.0012 (8) |
| C35 | 0.0413 (10) | 0.0335 (10) | 0.0467 (11) | 0.0003 (8) | 0.0008 (8) | 0.0030 (8) |
| C311 | 0.0391 (10) | 0.0402 (10) | 0.0458 (11) | 0.0067 (8) | −0.0027 (8) | −0.0022 (8) |
| C312 | 0.0479 (11) | 0.0452 (11) | 0.0546 (12) | 0.0027 (9) | −0.0042 (9) | 0.0008 (9) |
| C313 | 0.0506 (12) | 0.0577 (13) | 0.0643 (14) | 0.0000 (11) | −0.0091 (10) | −0.0109 (11) |
| C314 | 0.0555 (13) | 0.0760 (16) | 0.0541 (14) | 0.0087 (12) | −0.0130 (10) | −0.0127 (12) |
| C315 | 0.0601 (14) | 0.0776 (16) | 0.0479 (12) | 0.0094 (12) | −0.0039 (10) | 0.0072 (11) |
| C316 | 0.0470 (11) | 0.0542 (13) | 0.0505 (12) | 0.0032 (10) | 0.0008 (9) | 0.0051 (10) |
| C331 | 0.0679 (15) | 0.0413 (12) | 0.0766 (15) | −0.0052 (10) | −0.0124 (12) | −0.0050 (11) |
| O35 | 0.0492 (8) | 0.0340 (7) | 0.0575 (8) | −0.0018 (6) | 0.0006 (6) | 0.0031 (6) |
| C351 | 0.0394 (10) | 0.0485 (12) | 0.0570 (12) | −0.0034 (9) | −0.0099 (9) | 0.0174 (10) |
| C352 | 0.0555 (13) | 0.0484 (13) | 0.0770 (15) | −0.0111 (11) | −0.0283 (12) | 0.0223 (12) |
| C353 | 0.0746 (18) | 0.0729 (19) | 0.111 (2) | −0.0286 (15) | −0.0290 (18) | 0.0405 (18) |
| C354 | 0.0654 (18) | 0.108 (3) | 0.111 (3) | −0.0234 (18) | −0.0064 (17) | 0.059 (2) |
| C355 | 0.0606 (16) | 0.116 (3) | 0.0831 (19) | 0.0065 (16) | 0.0130 (14) | 0.0311 (18) |
| C356 | 0.0562 (14) | 0.0687 (16) | 0.0725 (15) | 0.0046 (12) | 0.0073 (12) | 0.0193 (13) |
| C357 | 0.108 (2) | 0.0412 (13) | 0.097 (2) | −0.0010 (14) | −0.0277 (18) | −0.0037 (13) |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Geometric parameters (Å, º)
| C1—O1 | 1.223 (2) | C311—C316 | 1.378 (3) |
| C1—C12 | 1.466 (3) | C311—C312 | 1.385 (3) |
| C1—C2 | 1.468 (3) | C312—C313 | 1.382 (3) |
| C2—C3 | 1.330 (3) | C312—H312 | 0.9300 |
| C2—H2 | 0.9300 | C313—C314 | 1.371 (3) |
| C3—C34 | 1.437 (3) | C313—H313 | 0.9300 |
| C3—H3 | 0.9300 | C314—C315 | 1.373 (3) |
| S11—C15 | 1.689 (4) | C314—H314 | 0.9300 |
| S11—C12 | 1.7146 (19) | C315—C316 | 1.384 (3) |
| C12—C13 | 1.340 (5) | C315—H315 | 0.9300 |
| C13—C14 | 1.404 (6) | C316—H316 | 0.9300 |
| C13—H13 | 0.9300 | C331—H31A | 0.9600 |
| C14—C15 | 1.337 (3) | C331—H31B | 0.9600 |
| C14—H14 | 0.9300 | C331—H31C | 0.9600 |
| C15—H15 | 0.9300 | O35—C351 | 1.397 (2) |
| S21—C25 | 1.692 (11) | C351—C356 | 1.367 (3) |
| C23—C24 | 1.408 (11) | C351—C352 | 1.392 (3) |
| C23—H23 | 0.9300 | C352—C353 | 1.370 (4) |
| C24—C25 | 1.340 (9) | C352—C357 | 1.495 (4) |
| C24—H24 | 0.9300 | C353—C354 | 1.364 (5) |
| C25—H25 | 0.9300 | C353—H353 | 0.9300 |
| N31—C35 | 1.351 (2) | C354—C355 | 1.375 (4) |
| N31—N32 | 1.375 (2) | C354—H354 | 0.9300 |
| N31—C311 | 1.425 (2) | C355—C356 | 1.400 (3) |
| N32—C33 | 1.322 (2) | C355—H355 | 0.9300 |
| C33—C34 | 1.414 (3) | C356—H356 | 0.9300 |
| C33—C331 | 1.491 (3) | C357—H35A | 0.9600 |
| C34—C35 | 1.373 (3) | C357—H35B | 0.9600 |
| C35—O35 | 1.353 (2) | C357—H35C | 0.9600 |
| O1—C1—C12 | 120.05 (18) | C313—C312—H312 | 120.4 |
| O1—C1—C2 | 121.99 (19) | C311—C312—H312 | 120.4 |
| C12—C1—C2 | 117.96 (17) | C314—C313—C312 | 120.9 (2) |
| C3—C2—C1 | 121.21 (18) | C314—C313—H313 | 119.6 |
| C3—C2—H2 | 119.4 | C312—C313—H313 | 119.6 |
| C1—C2—H2 | 119.4 | C313—C314—C315 | 119.6 (2) |
| C2—C3—C34 | 128.12 (19) | C313—C314—H314 | 120.2 |
| C2—C3—H3 | 115.9 | C315—C314—H314 | 120.2 |
| C34—C3—H3 | 115.9 | C314—C315—C316 | 120.4 (2) |
| C15—S11—C12 | 91.91 (14) | C314—C315—H315 | 119.8 |
| C13—C12—C1 | 131.5 (3) | C316—C315—H315 | 119.8 |
| C13—C12—S11 | 109.4 (3) | C311—C316—C315 | 119.7 (2) |
| C1—C12—S11 | 119.10 (15) | C311—C316—H316 | 120.2 |
| C12—C13—C14 | 115.1 (3) | C315—C316—H316 | 120.2 |
| C12—C13—H13 | 122.4 | C33—C331—H31A | 109.5 |
| C14—C13—H13 | 122.4 | C33—C331—H31B | 109.5 |
| C15—C14—C13 | 110.7 (3) | H31A—C331—H31B | 109.5 |
| C15—C14—H14 | 124.6 | C33—C331—H31C | 109.5 |
| C13—C14—H14 | 124.6 | H31A—C331—H31C | 109.5 |
| C14—C15—S11 | 112.9 (3) | H31B—C331—H31C | 109.5 |
| C14—C15—H15 | 123.6 | C35—O35—C351 | 119.55 (16) |
| S11—C15—H15 | 123.6 | C356—C351—C352 | 123.8 (2) |
| C24—C23—H23 | 122.8 | C356—C351—O35 | 122.94 (19) |
| C25—C24—C23 | 110.3 (12) | C352—C351—O35 | 113.3 (2) |
| C25—C24—H24 | 124.9 | C353—C352—C351 | 116.7 (3) |
| C23—C24—H24 | 124.9 | C353—C352—C357 | 122.8 (3) |
| C24—C25—S21 | 112.0 (11) | C351—C352—C357 | 120.5 (2) |
| C24—C25—H25 | 124.0 | C354—C353—C352 | 121.5 (3) |
| S21—C25—H25 | 124.0 | C354—C353—H353 | 119.2 |
| C35—N31—N32 | 110.33 (15) | C352—C353—H353 | 119.2 |
| C35—N31—C311 | 130.62 (16) | C353—C354—C355 | 121.0 (3) |
| N32—N31—C311 | 118.98 (15) | C353—C354—H354 | 119.5 |
| C33—N32—N31 | 105.17 (15) | C355—C354—H354 | 119.5 |
| N32—C33—C34 | 112.14 (17) | C354—C355—C356 | 119.5 (3) |
| N32—C33—C331 | 120.20 (18) | C354—C355—H355 | 120.2 |
| C34—C33—C331 | 127.61 (18) | C356—C355—H355 | 120.2 |
| C35—C34—C33 | 103.57 (16) | C351—C356—C355 | 117.5 (3) |
| C35—C34—C3 | 129.00 (18) | C351—C356—H356 | 121.2 |
| C33—C34—C3 | 127.36 (18) | C355—C356—H356 | 121.2 |
| N31—C35—O35 | 120.86 (16) | C352—C357—H35A | 109.5 |
| N31—C35—C34 | 108.79 (16) | C352—C357—H35B | 109.5 |
| O35—C35—C34 | 129.88 (17) | H35A—C357—H35B | 109.5 |
| C316—C311—C312 | 120.13 (18) | C352—C357—H35C | 109.5 |
| C316—C311—N31 | 118.65 (18) | H35A—C357—H35C | 109.5 |
| C312—C311—N31 | 121.21 (17) | H35B—C357—H35C | 109.5 |
| C313—C312—C311 | 119.3 (2) | ||
| O1—C1—C2—C3 | 1.0 (3) | C3—C34—C35—N31 | −177.55 (18) |
| C12—C1—C2—C3 | −178.86 (19) | C33—C34—C35—O35 | 171.48 (19) |
| C1—C2—C3—C34 | 177.23 (19) | C3—C34—C35—O35 | −5.6 (3) |
| O1—C1—C12—C13 | −170.9 (5) | C35—N31—C311—C316 | −150.6 (2) |
| C2—C1—C12—C13 | 9.0 (6) | N32—N31—C311—C316 | 25.9 (3) |
| O1—C1—C12—S11 | 6.0 (3) | C35—N31—C311—C312 | 30.5 (3) |
| C2—C1—C12—S11 | −174.22 (15) | N32—N31—C311—C312 | −153.05 (18) |
| C15—S11—C12—C13 | −0.3 (5) | C316—C311—C312—C313 | 1.3 (3) |
| C15—S11—C12—C1 | −177.8 (4) | N31—C311—C312—C313 | −179.78 (18) |
| C1—C12—C13—C14 | 176.4 (4) | C311—C312—C313—C314 | −0.6 (3) |
| S11—C12—C13—C14 | −0.6 (7) | C312—C313—C314—C315 | −0.3 (3) |
| C12—C13—C14—C15 | 1.6 (9) | C313—C314—C315—C316 | 0.4 (3) |
| C13—C14—C15—S11 | −1.8 (9) | C312—C311—C316—C315 | −1.2 (3) |
| C12—S11—C15—C14 | 1.3 (7) | N31—C311—C316—C315 | 179.88 (18) |
| C23—C24—C25—S21 | 9 (8) | C314—C315—C316—C311 | 0.3 (3) |
| C35—N31—N32—C33 | −0.9 (2) | N31—C35—O35—C351 | −105.1 (2) |
| C311—N31—N32—C33 | −178.00 (16) | C34—C35—O35—C351 | 83.7 (2) |
| N31—N32—C33—C34 | 0.6 (2) | C35—O35—C351—C356 | 2.2 (3) |
| N31—N32—C33—C331 | 178.19 (19) | C35—O35—C351—C352 | −179.18 (16) |
| N32—C33—C34—C35 | 0.0 (2) | C356—C351—C352—C353 | 1.2 (3) |
| C331—C33—C34—C35 | −177.5 (2) | O35—C351—C352—C353 | −177.45 (18) |
| N32—C33—C34—C3 | 177.07 (18) | C356—C351—C352—C357 | −178.1 (2) |
| C331—C33—C34—C3 | −0.4 (4) | O35—C351—C352—C357 | 3.3 (3) |
| C2—C3—C34—C35 | 6.2 (4) | C351—C352—C353—C354 | 0.0 (4) |
| C2—C3—C34—C33 | −170.2 (2) | C357—C352—C353—C354 | 179.3 (2) |
| N32—N31—C35—O35 | −171.96 (16) | C352—C353—C354—C355 | −1.0 (4) |
| C311—N31—C35—O35 | 4.7 (3) | C353—C354—C355—C356 | 0.9 (4) |
| N32—N31—C35—C34 | 0.9 (2) | C352—C351—C356—C355 | −1.3 (3) |
| C311—N31—C35—C34 | 177.56 (18) | O35—C351—C356—C355 | 177.17 (19) |
| C33—C34—C35—N31 | −0.5 (2) | C354—C355—C356—C351 | 0.3 (4) |
3-[3-Methyl-5-(2-methylphenoxy)-1-phenyl-1H-pyrazol-4-yl]-1-(thiophen-2-yl)prop-2-en-1-one (II) . Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C14—H14···N32i | 0.93 | 2.55 | 3.483 (4) | 177 |
| C25—H25···N32i | 0.93 | 2.69 | 3.47 (2) | 142 |
| C314—H314···O1ii | 0.93 | 2.51 | 3.432 (3) | 171 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x−1, y, z−1.
Funding Statement
This work was funded by University Grants Commission grant BSR Faculty Fellowship to H. S. Yathirajan.
References
- Abdel-Aziz, H. A., Al-Rashood, K. A., Ghabbour, H. A., Chantrapromma, S. & Fun, H.-K. (2012). Acta Cryst. E68, o612–o613. [DOI] [PMC free article] [PubMed]
- Acosta, L. M., Bahsas, A., Palma, A., Cobo, J., Hursthouse, M. B. & Glidewell, C. (2009). Acta Cryst. C65, o92–o96. [DOI] [PubMed]
- Ahmad, M., Siddiqui, H. L., Khan, A. H. & Parvez, M. (2010). Acta Cryst. E66, o1265–o1266. [DOI] [PMC free article] [PubMed]
- Asma, Kalluraya, B. & Manju, N. (2017). Pharma Chem 9, 50–54.
- Badawey, E. A. M. & El-Ashmawey, I. M. (1998). Eur. J. Med. Chem. 33, 349–361.
- Batten, S. R. & Robson, R. (1998). Angew. Chem. Int. Ed. 37, 1460–1494. [DOI] [PubMed]
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl. 34, 1555–1573.
- Blanco, M. C., Palma, A., Cobo, J. & Glidewell, C. (2012). Acta Cryst. C68, o195–o198. [DOI] [PubMed]
- Bowes, K. F., Glidewell, C., Low, J. N., Melguizo, M. & Quesada, A. (2003). Acta Cryst. C59, o4–o8. [DOI] [PubMed]
- Bruker (2012). APEX2 and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2017). SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Dawood, K. M., Eldebss, T. M. A., El-Zahabi, H. S. A., Yousef, M. H. & Metz, P. (2013). Eur. J. Med. Chem. 70, 740–749. [DOI] [PubMed]
- Etter, M. C. (1990). Acc. Chem. Res. 23, 120–126.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Ferguson, G., Glidewell, C. & Patterson, I. L. J. (1996). Acta Cryst. C52, 420–423.
- Fun, H.-K., Hemamalini, M., Abdel-Aziz, H. A. & Aboul-Fadl, T. (2011). Acta Cryst. E67, o2145–o2146. [DOI] [PMC free article] [PubMed]
- Guzei, I. A., Spencer, L. C., Tshivashe, M. G. & Darkwa, J. (2009). Acta Cryst. E65, o2743. [DOI] [PMC free article] [PubMed]
- Karrouchi, K., Radi, S., Ramli, Y., Taoufik, J., Mabkhot, Y. N., Al-aizari, F. A. & Ansar, M. (2018). Molecules, 23, 134. doi: 10.3390/molecules23010134. [DOI] [PMC free article] [PubMed]
- Koca, I., Özgür, A., Coşkun, K. A. & Tutar, Y. (2013). Bioorg. Med. Chem. 21, 3859–3865. [DOI] [PubMed]
- Mabkhot, Y. N., Alatibi, F., El-Sayed, N. N. E., Kheder, N. A. & Al-Showiman, S. S. (2016). Molecules, 21, 1036. doi: 10.3390/molecules21081036. [DOI] [PMC free article] [PubMed]
- Mathew, B., Suresh, J. & Anbazhagan, S. (2014). EXCLI J, 13, 437–445. [PMC free article] [PubMed]
- Peng, J., Han, Z., Ma, N. & Tu, S. (2009). Acta Cryst. E65, o1109–o1110. [DOI] [PMC free article] [PubMed]
- Peng, J. & Jia, R. (2012). Acta Cryst. E68, o2608. [DOI] [PMC free article] [PubMed]
- Rajni Swamy, V., Gunasekaran, P., Krishnakumar, R. V., Srinivasan, N. & Müller, P. (2014). Acta Cryst. E70, o974–o975. [DOI] [PMC free article] [PubMed]
- Seip, H. M. & Seip, R. (1973). Acta Chem. Scand. 27, 4024–4027.
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Vijesh, A. M., Isloor, A. M., Shetty, P., Sundershan, S. & Fun, H.-K. (2013). Eur. J. Med. Chem. 62, 410–415. [DOI] [PubMed]
- Zhang, J., Tan, D.-J., Wang, T., Jing, S.-S., Kang, Y. & Zhang, Z.-T. (2017). J. Mol. Struct. 1149, 235–242.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I, II. DOI: 10.1107/S205698901901658X/zl2765sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901901658X/zl2765Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S205698901901658X/zl2765IIsup3.hkl
Supporting information file. DOI: 10.1107/S205698901901658X/zl2765Isup4.cml
Supporting information file. DOI: 10.1107/S205698901901658X/zl2765IIsup5.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report