Abstract
Few studies have examined systemic mitochondrial function in conjunction with brain imaging in human immunodeficiency virus (HIV) disease. Oxidative phosphorylation enzyme protein levels of peripheral blood mononuclear cells were measured in association with neuroimaging indices in 28 HIV+ individuals. T1-weighted magnetic resonance imaging yielded volumes of seven brain regions of interest; diffusion tensor imaging determined fractional anisotropy (FA) and mean diffusivity (MD) in the corpus callosum (CC). Higher nicotinamide adenine dinucleotide dehydrogenase levels correlated with lower volumes of thalamus (p = .005) and cerebral white matter (p = .049) and, in the CC, with lower FA (p = .011, body; p = .005, genu; p = .009, total CC) and higher MD (p = .023, body; p = .035, genu; p = .019, splenium; p = .014, total CC). Greater cytochrome c oxidase levels correlated with lower thalamic (p = .034) and cerebellar gray matter (p = .021) volumes. The results indicate that systemic mitochondrial cellular bioenergetics are associated with brain health in HIV.
Keywords: brain, HIV, mitochondria, oxidative phosphorylation, oxidative stress
Introduction
Approximately 40 million people worldwide are infected with the human immunodeficiency virus (HIV).1 Many HIV+ individuals demonstrate evidence of abnormal brain morphometry on magnetic resonance imaging (MRI), including thinning of the cerebral cortex and decreased volumes of cortical and subcortical gray matter regions.2–7 Diffuse microstructural changes in white matter have been revealed by diffusion tensor imaging (DTI).8–13 Suppressive combination antiretroviral therapy (cART) does not reverse these brain structural alterations,14–16 even when initiated during primary infection (<12 months after exposure).17 To devise strategies for patient care, research is needed to delineate the key mechanisms underlying the evolution of brain abnormalities in HIV.
Converging data implicate the impairment of brain mitochondrial dynamics in HIV neuropathogenesis. HIV proteins alter the physiology of mitochondria.18 For example, neuronal damage, dysfunction, and atrophy can be induced in vitro19,20 by the HIV-1 transactivator of transcription protein (Tat): Tat exposure perturbs mitochondrial oxidative phosphorylation (OXPHOS) enzyme activities,21,22 changes the morphology of cortical mitochondria,23 and contributes to altered neuronal synaptic transmission.22,24 OXPHOS is essential for mitochondrial respiration.25 Dysfunction of OXPHOS enzymes increases oxidative stress and generation of reactive oxygen species (ROS), activating the intrinsic apoptotic mitochondrial pathway.26–29 Failure to inhibit this cycle has been linked to neurodegenerative diseases29,30 and HIV-associated dementia.31 However, the possible role of mitochondrial OXPHOS in HIV-associated brain structural pathology has not been investigated.
The present study examined regional brain volumes and white matter microstructural integrity (assessed by multimodal neuroimaging) in relation to systemic mitochondrial parameters, that is, protein levels of mitochondrial OXPHOS complex I (nicotinamide adenine dinucleotide: ubiquinone oxidoreductase, CI) and complex IV (cytochrome c oxidase, CIV) in peripheral blood mononuclear cells (PBMCs). Brain volumetric analyses focused on regions of interest (ROIs) identified in prior studies as vulnerable to HIV and/or oxidative stress (i.e., pallidum, thalamus, caudate, hippocampus, cerebral white matter, cerebral subcortical gray matter, and cerebellar gray matter4,5,32–34). DTI was used to assess microstructural properties of the largest commissural pathway, the corpus callosum (CC), which has previously shown diffusion abnormalities associated with HIV infection.13,35,36 CI and CIV represent the initial and terminal aspects of the electron transport chain (ETC).37 HIV-infected individuals who are cART-naïve exhibit increased oxidative damage, mitochondrial DNA (mtDNA) depletion, and decreased activities of CI-CIV in PBMCs.38 We hypothesized that altered PBMC mitochondrial CI and CIV levels would be associated with lower brain volumes on MRI and with abnormal, DTI-based, white matter microstructural indices.
Methods
Study sample
Protein levels of OXPHOS CI and CIV were obtained at entry in a subset of participants from the Hawaii Aging with HIV Cohort–Cardiovascular Disease (HAHC-CVD) study,39 a longitudinal study of subclinical cerebro-CVD in HIV-infected individuals on cART. Regional brain volumes were obtained once, cross-sectionally, to correspond to an annual visit of this study: MRI was performed at entry for 19 participants, while scans for the remaining nine were acquired in association with annual visits conducted 1 or 2 years later. Recruitment was conducted through referrals from the Hawaii Center for AIDS, community physicians, community advisory board members, and AIDS service organizations. Inclusion criteria included (1) ≥ 40 years old; (2) documented history of HIV infection; (3) on stable cART ≥3 months; (4) English as their primary language; and (5) able to understand and provide informed consent. Exclusion criteria included (1) uncontrolled major affective disorder; (2) active psychosis; (3) recorded loss of consciousness >5 min; (4) pregnancy or breastfeeding; (5) factors precluding MRI (e.g., claustrophobia); and (6) any past or present condition [e.g., central nervous system (CNS) infection, traumatic brain injury, stroke, or substance abuse] that was determined by the evaluating physician to present confounding variables. All study participants underwent a general and focused HIV/neurological history and physical examination. Each participant provided written informed consent. The study was approved by the Institutional Review Board in the Office of Compliance at the University of Hawaii.
PBMC isolation
Blood was collected in EDTA vacutainer tubes. PBMCs were isolated over a Ficoll-Paque gradient and washed three times with phosphate-buffered saline (PBS). An aliquot of cells was counted using trypan blue and a hemocytometer. Cells were then viably cryopreserved at a concentration of 10 million/1 mL freezing media (10% fresh dimethyl sulfoxide/90% heated fetal bovine serum) in 0.5-mL aliquots (5 million cells each) in 1.5–2-mL O-RINGED screw-capped cryovials.
OXPHOS enzymes
CI and CIV protein levels were determined in duplicate by immunoassays, as described elsewhere.40 Vials of PBMCs were thawed and washed in 0.5 mL of PBS twice before addition of 0.5 mL of ice-cold extraction buffer [1.5% lauryl maltoside, 25 mM HEPES (pH 7.4), 100 mM NaCl, plus protease inhibitors (P-8340; Sigma)]. Samples were mixed gently, kept on ice for 20 min, and microcentrifuged at 16,400 rpm for 20 min at 4°C. Samples were loaded on the immunoassays with equal amounts of total cell protein following established guidelines.40 CI and CIV levels were quantified using densitometric scanning with a Hamamatsu ICA-1000 reader. Protein level was measured as optical density (OD)/μg of protein × 103.
Neuroimaging
MRI was performed on a 3.0 Tesla Philips Medical Systems Achieva scanner using an eight-channel head coil (InVision Imaging, Honolulu). High-resolution, MRI anatomical data were obtained for each subject using a sagittal, 3D, turbo field echo T1-weighted (3D TFE T1W) sequence [echo time (TE)/repetition time (TR) = 3.2 ms/6.9 ms; flip angle 8°; slice thickness 1.2 mm with no gaps between slices; in-plane resolution 1.0 mm2; field of view (FOV) 256 × 256 mm2]. Diffusion-weighted MRI scans were acquired using a single-shot, echo planar imaging (EPI) sequence: 24 cm FOV, TR/TE = 7,859 ms/80 ms, flip angle 90°, 3.0-mm-thick slices, 0-mm gap, SENSE factor = 3.1, maximum slew rate 120mT/m/ms, gradient amplitude 40 mT/m, 96 × 95 acquisition matrix, 2.5 × 2.5 mm2 in-plane resolution, and a variable number of slices determined by head size. One image without diffusion sensitization was obtained (i.e., a T2-weighted b0 image). Diffusion weighting was applied along 15 noncollinear directions evenly distributed over a sphere with a b-factor of 1,000 s/mm2 and four signal averages to increase the signal-to-noise ratio (SNR). Scan time was 8.6 min.
Regional brain volumes were obtained by processing T1-weighted MRI data with FreeSurfer (version 5.0, https://surfer.nmr.mgh.harvard.edu).41–44 The process includes skull stripping,45 intensity normalization,46 Talairach transformation, subcortical white matter and deep gray matter segmentation,42,43 and cortical gray/white matter boundary and pial surface reconstruction.41 Intracranial volume (ICV) was used to correct for differences in head size.47 Following visual quality control of the surfaces and segmentations, volumetric data were determined from the left and right hemispheres for seven ROIs known to be affected by HIV: pallidum, hippocampus, thalamus, caudate, cerebral subcortical gray matter, cerebellar gray matter, and cerebral white matter.4,13,34,48–52 Total regional volumes were computed by summing over the left and right brain hemispheres.
Diffusion data were processed using DTI protocols, as previously described.53 In brief, the FSL eddy_correct tool was used to correct for motion- and eddy current-induced distortions. To correct for EPI-induced susceptibility artifacts, each subject's b0 image was nonlinearly warped to the corresponding anatomical T1-weighted image. The dtifit command in FSL was used to estimate diffusion tensors from the preprocessed images and to obtain maps of the DTI scalar metrics, fractional anisotropy (FA) and mean diffusivity (MD). The FA image corresponding to the Johns Hopkins University (JHU) Eve atlas was registered to each individual FA scan. The warps were applied to the white matter labels that defined the ROIs in the CC. FA and MD were derived for the genu, body, and splenium of the CC. Each subregion and the total CC were included in the DTI analyses.
Statistical analyses
Descriptive statistics were computed for patient demographics, clinical parameters, PBMC CI and CIV protein levels, regional brain volumes, and DTI indices. Multiple linear regression examined associations between neuroimaging indices and CI and CIV levels (separately). Analyses of DTI metrics focused on FA and MD of the genu, body, splenium, and total CC.
Sensitivity analyses were conducted for both brain volume and DTI models to evaluate potential contributions from additional variables, including age, sex, ICV, CD4 nadir, years since HIV diagnosis, years on cART, substance use, and use of any of the three nucleoside reverse transcriptase inhibitors (NRTIs): azidothymidine (AZT), stavudine (d4T), or didanosine (ddI). Age was the only covariate retained in the final regression models that examined DTI metrics. ICV was utilized as a covariate in analyses of regional brain volumes. Significance was defined by p < .05, with 0.05 ≤ p < .1 considered indicative of a trend. Volumes and DTI measurements were assessed for normality through visual inspection of histograms and the Shapiro–Wilk test. Regression diagnostics for all models were examined for violations. SPSS 22 and SAS, v9.4 (SAS Institute, Inc., Cary, NC), were used for statistical analyses.
Results
PBMC CI and CIV levels and neuroimaging data were available for 28 HIV+ individuals who were all on stable cART and between the ages of 40 and 70 [mean age 52 ± 7 years; predominantly male (86%); mean duration of HIV infection = 20.5 ± 7.0 years; mean duration of cART = 18.1 ± 5.9 years]. Plasma HIV RNA was undetectable (<50 copies/mL) in 23 (82%) of the study participants and the remaining five subjects had a median HIV RNA count of 180 copies/mL (range: 53–6,280). CI values ranged from 15 to 56 OD/μg × 103, while the range of CIV was narrower (11–35 OD/μg × 103). The median (interquartile range) time from the blood draw to MRI was 2 (1–24) months. Demographic and clinical data are summarized in Table 1 and regional brain volumes and DTI measures (FA and MD) presented in Table 2. CI and CIV levels were not associated with substance use variables or with history of treatment with AZT, d4T, or ddI.
Table 1.
Study Sample: Demographics, Clinical Variables, and Peripheral Blood Mononuclear Cell Oxidative Phosphorylation Protein Levels
| Characteristics | |
|---|---|
| N | 28 |
| Age (years) | 52.5 ± 7.2 |
| Sex (male) | 24 (85.7%) |
| Race/ethnicity (Caucasian) | 13 (46.4%) |
| Years since HIV diagnosis | 20.5 ± 7.0 |
| Years on cART | 18.1 ± 5.9 |
| CD4 count (cells/mm3) | 501 ± 203 |
| Nadir CD4 count (cells/mm3) | 176 ± 144 |
| Undetectable plasma HIV RNA (<50 copies/mL) | 23 (82.1%) |
| PBMC OXPHOS Complex 1 protein level (OD/μg × 103) | 33.8 ± 9.4 |
| PBMC OXPHOS Complex 4 protein level (OD/μg × 103) | 27.6 ± 5.6 |
| Ever used AZT, d4T, or ddI | 18 (64.3%) |
| Ever used any druga | 25 (89.3%) |
| Marijuana use (lifetime frequency)b | |
| Never | 0 (0%) |
| 1–10 times | 7 (28%) |
| >10 times | 18 (72%) |
| Methamphetamine use (lifetime frequency)b | |
| Never | 15 (60%) |
| 1–10 times | 3 (12%) |
| >10 times | 7 (28%) |
| Use of stimulants (lifetime frequency)b | |
| Never | 20 (80%) |
| 1–10 times | 2 (8%) |
| >10 times | 3 (12%) |
| Alcohol usec | |
| Never | 11 (42.3%) |
| Sometimes (≤4 times/month) | 11 (42.3%) |
| Frequently (>2 times/week) | 4 (15.4%) |
Data are given as mean ± SD for continuous variables and n (%) for categorical variables.
Drugs include marijuana, cocaine, crack, stimulants, phencyclidine, methamphetamine, heroin, lysergic acid diethylamide, ecstasy, nitrates, glue, ketamine, methadone, barbiturates, painkillers, and sedatives.
n = 25.
n = 26.
AZT, azidothymidine; cART, combination antiretroviral therapy; d4T, stavudine; ddI, didanosine; OD, optical density; OXPHOS, oxidative phosphorylation; PBMCs, peripheral blood mononuclear cells; SD, standard deviation.
Table 2.
Regional Brain Volumes, Intracranial Volume, and Diffusion Tensor Imaging-Derived Fractional Anisotropy and Mean Diffusivity for the Corpus Callosum
| Brain region | Volume (mm3) |
|---|---|
| Pallidum | 3,319 ± 371 |
| Hippocampus | 7,970 ± 762 |
| Cerebral subcortical GM | 77,316 ± 5,986 |
| Cerebellar GM | 87,493 ± 8,737 |
| Thalamus | 12,913 ± 1,182 |
| Caudate | 7,188 ± 731 |
| Cerebral WM | 488,178 ± 54,007 |
| ICV (in 103 mm3) | 1,455.10 ± 277.09 |
| Brain region | FA |
| Genu of CC | 0.50 ± 0.04 |
| Body of CC | 0.47 ± 0.04 |
| Splenium of CC | 0.55 ± 0.03 |
| Total CC | 0.51 ± 0.03 |
| Brain region | MD |
| Genu of CC | 9.5 × 10−4 ± 9.2 × 10−5 |
| Body of CC | 1.1 × 10−3 ± 9.0 × 10−5 |
| Splenium of CC | 9.9 × 10−4 ± 7.2 × 10−5 |
| Total CC | 9.9 × 10−4 ± 7.6 × 10−5 |
Mean values ± SDs reported for all variables; n = 28.
CC, corpus callosum; FA, fractional anisotropy; GM, gray matter; ICV, intracranial volume; MD, mean diffusivity; WM, white matter.
PBMC OXPHOS CI and CIV protein levels and regional brain volumes
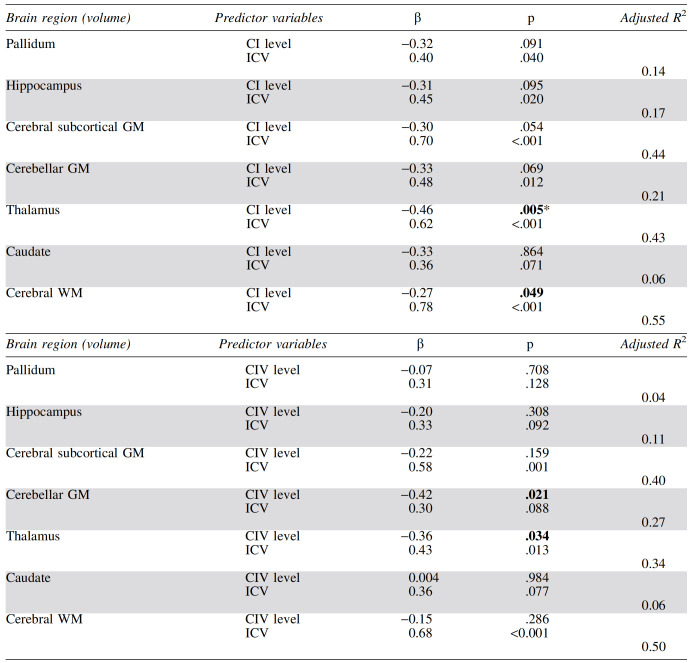
After controlling for ICV, we found associations (p < .05) between higher CI protein levels and smaller thalamic and cerebral white matter volumes (Table 3). Only the former relationship was significant after Bonferroni correction for multiple comparisons (p < .007 = .05/7). Volumes of cerebral subcortical gray matter, cerebellar gray matter, pallidum, and hippocampus showed trend-level inverse associations with CI protein levels (p < .10). Significant associations were observed between higher CIV levels and lower cerebellar gray matter and thalamic volumes independently of ICV, although neither survived multiple comparison correction.
Table 3.
Associations Between Regional Brain Volumes and Levels of Peripheral Blood Mononuclear Cell Complex I and Complex IV by Multiple Regression Controlling for Intracranial Volume
CI and CIV levels are measured as OD/μg of protein × 103. Volumes are in mm3. p-Values <.05 are shown in bold, and standardized β-values are presented along with the model's adjusted R2; n = 28.
Significant after Bonferroni correction for multiple comparisons.
CI, complex I; CIV, complex IV; ICV, intracranial volume; OD, optical density.
When regression analyses were restricted to the 23 participants with undetectable plasma HIV RNA, the CI associations with regional volumes became stronger and more significant (β = −0.51, p = .002 for thalamus; β = −0.32, p = .039 for cerebral white matter). Similarly, CIV was associated with thalamic volume (β = −0.39, p = .025), and the relationship between CIV and cerebellar gray matter was strong enough to survive Bonferroni correction (β = −0.55, p = .001).
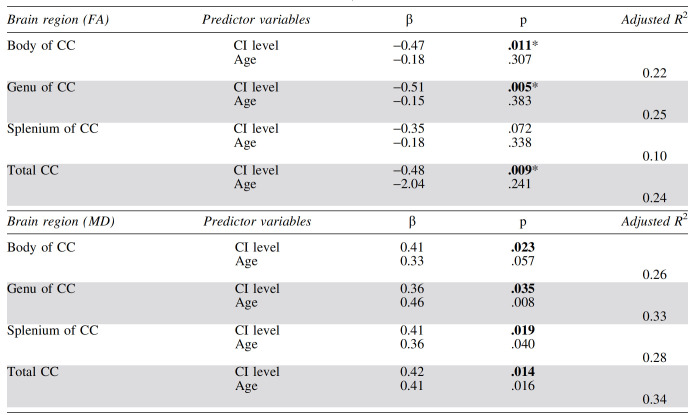
PBMC OXPHOS CI and CIV protein levels and DTI metrics
After adjustment for age, higher CI protein levels were significantly associated with lower FA in the total CC, genu, and body after Bonferroni correction (p < .013 = .05/4). A trend was observed between increased CI and decreased FA in the splenium (p < .10) (Table 4). Similarly, we found a trend-level association between higher CIV protein levels and lower FA in the splenium. The CIV level was not associated with FA in other callosal regions (Table 5).
Table 4.
Associations Between Diffusion Tensor Imaging−Derived Metrics for the Corpus Callosum (Fractional Anisotropy and Mean Diffusivity) and Peripheral Blood Mononuclear Cell Complex I Level by Multiple Regression, Controlling for Age
p-Values <.05 are shown in bold, and standardized β-values are presented along with the model's adjusted R2. CI level is measured as OD/μg of protein × 103; n = 28.
Significant after Bonferroni correction for multiple comparisons.
Table 5.
Associations Between Diffusion Tensor Imaging−Derived Metrics for the Corpus Callosum (Fractional Anisotropy and Mean Diffusivity) and Peripheral Blood Mononuclear Cell Complex IV Level by Multiple Regression, Controlling for Age
Standardized β-values are presented along with the model's adjusted R2. CIV level is measured as OD/μg of protein × 103; n = 28.
Higher levels of CI were linked to increased MD in the genu, body, splenium, and total CC (Table 4), although the associations were not significant when Bonferroni corrected. CIV levels did not relate to MD in the genu, body, or total CC. A trend toward a positive correlation between CIV and MD was noted in the splenium (Table 5).
CI and CIV associations with DTI metrics increased in strength and significance when only the participants with undetectable plasma HIV RNA were considered. CI was significantly related to FA in the total CC (β = −0.70, p < .001) and all subregions (e.g., β = −0.63, p = .002, for splenium) after correction for multiple comparisons. Notably, all associations between CI and MD survived Bonferroni correction (e.g., β = 0.62, p < .001, for total CC). CIV showed a trend relationship to FA in the genu (β = −0.38, p = .074) and was linked to MD in the splenium (β = 0.44, p = .021) and total CC (β = 0.34, p = .068).
Discussion
Results from this study provide the first examination of systemic OXPHOS CI and CIV levels and neuroimaging metrics in HIV+ individuals. A higher PBMC CI level was significantly associated with lower thalamic volume after correction for multiple comparisons. CI was also inversely related to cerebral white matter volume and showed trends toward similar relationships with volumes of cerebral subcortical gray matter, cerebellar gray matter, hippocampus, and pallidum. A higher CIV level was associated with lower volumes of cerebellar gray matter and thalamus. These results are supported by well-known differences in brain regional sensitivity to oxidative stress. The thalamus is selectively vulnerable to neurodegeneration induced by impaired oxidative metabolism.54,55 Neurons in the cerebellar granule layer (unlike those in the cerebral cortex) are highly susceptible to oxidative stress and consequent cell death, as are hippocampal CA1 neurons.33 In earlier work, we identified strong associations between increased PBMC levels of the ROS-induced lesion 8-oxo-2′-deoxyguanosine (8-oxo-dG), a marker of mtDNA oxidative damage,56 and reduced brain volumes in an HIV+ sample that included all participants of the current study.32 HIV alters mitochondrial morphology,23 physiology,18 and respiratory dynamics.57 Our study suggests that such mitochondrial changes exert an adverse impact on brain structure.
Mitochondrial dysfunction may underlie multiple aging-related and neurodegenerative pathologies58 such as Alzheimer's disease,59 Parkinson's disease,60 and HIV-associated neurocognitive disorders (HANDs).61 Examination of frontal cortex autopsy tissue from patients with HANDs has revealed significant mitochondrial abnormalities: increased mtDNA 8-oxo-dG damage,62 accumulated mtDNA mutations and deletions,62 and dysregulated mitochondrial fission and fusion.63 Reduced mitochondrial biogenesis and increased neuroinflammation were recently identified in frontal cortices of cART-treated HAND donors.64 ROS-induced oxidative DNA damage may be crucial in HIV-related neurodegenerative processes.62
The etiology of HIV-related mitochondrial dysfunction is likely to be multifactorial. Prior studies implicate nucleoside NRTIs65–68; however, no association was observed in the present study between treatment history with AZT, d4T, or ddI and mitochondrial complex protein levels. Other work has focused on deleterious effects of substance use on mitochondrial function in HIV. Methamphetamine is especially disruptive,69,70 particularly in the setting of HIV infection.71 The lack of correlation between substance use histories and CI and CIV levels in the current study suggests that mitochondrial function may be more strongly linked to the HIV disease process. HIV proteins and disease dynamics disrupt mitochondrial integrity and potentiate the apoptotic pathway.72–75
Also identified in our study were relationships between increased PBMC complex levels and reduced microstructural integrity of brain white matter. Higher CI levels corresponded to significantly reduced FA and increased MD within the genu, body, and entire CC and at trend level in the splenium. Trend associations of higher CIV levels with decreased FA and increased MD were noted in the splenium. Relationships between DTI measures and CIV (unlike CI) may have failed to reach significance because of the more restricted range of CIV levels. Progressively diminishing mitochondrial respiratory chain dysfunction has been reported with the movement of electrons down the ETC: a study of cART-naïve HIV-infected patients found that the activities of respiratory chain complexes II, III, and IV were reduced by 41%, 38%, and 19%, respectively, compared with HIV-negative controls.38
DTI studies of HIV+ adults have reported compromised microstructural integrity of the CC; for example, FA reductions in the splenium (relative to HIV-negative controls), which were associated with diminished neurocognitive functioning.13 Anterior callosal thinning has been detected in HIV+ individuals and linked to T cell decline.76 Results of the current investigation are consistent with published HIV research; moreover, this is the first study to link PBMC CI and CIV protein levels to FA and MD in the CC. The observed connection between PBMC complex levels and callosal DTI metrics constitutes evidence that systemic mitochondrial dysfunction may contribute to reduced microstructural brain integrity in HIV.
It is worth noting that our participants were chronically infected and on cART for an average of 18 years. PBMC CI and CIV levels corresponded to neuroimaging measures independently of cART regimen or age. Interestingly, these associations were stronger in individuals who were virally suppressed: long-term damage and oxidative disruption may be more clearly delineated when not masked by variability due to inclusion of viremic participants in the analyses. As ours was a cross-sectional study, we could not determine whether disrupted OXPHOS was secondary to mitochondrial damage during acute HIV infection or represents an ongoing process that continues despite potent cART. Our PBMC CI and CIV data are in accordance with decreased PBMC mtDNA and functional disruption along the ETC observed in antiretroviral-naïve HIV+ individuals.38
Higher levels of CI and CIV can be expected to lead to increased generation of ROS and to cellular injury, including mitochondrial damage. The results presented here are consistent with our previous report that greater mtDNA damage as measured by PBMC mtDNA 8-oxo-dG is associated with regional brain atrophy.32 Indeed, in our cohort, we see a positive correlation between CI levels and mtDNA 8-oxo-dG levels (Gangcuangco LMA et al., in review).
In the same manuscript under review, we also demonstrate that CI levels in our HIV-infected participants were lower (not higher) compared with HIV-negative control participants of similar age and gender. Lower CD4 count and higher levels of circulating proinflammatory cytokines are known risk factors for HIV-related CNS pathology and correlated with lower CI and CIV protein levels within the HIV+ group. We hypothesize that these results, which at first glance appear inconsistent with our present study findings, may be secondary to the measurement of protein levels and not the functional capacity of the OXPHOS system. It is possible that HIV is associated initially with impaired OXPHOS function before decreases in protein levels can be detected. A compensatory increase in OXPHOS may lead to enhanced ROS levels, which damage DNA, lipids, proteins, and membrane permeability within mitochondria.77 CI, in particular, has been identified as a common site of superoxide generation.77,78 Increased ROS production can be a consequence of functional alterations such as stoichiometric mismatches in the ETC complexes. Such mismatches result in longer residence time of electrons on sites of complexes that mediate electron reduction of O2−, resulting in increased production of H202 and superoxide.27
Important limitations of the present work merit discussion. Our study involved cross-sectional examination of PBMC OXPHOS levels rather than mitochondrial assessments in the CNS, and the CI/CIV protein level, but not activity, was measured. It is possible that inclusion of CNS markers or enzymatic activities would yield additional associations. CI/CIV protein and activity levels are highly correlated,79 and our results indicate that mitochondrial OXPHOS protein levels in PBMCs account for considerable variance in neuroimaging indices. Still, the adjusted R2 values (only moderately large even for models showing significant associations) suggest the influence of unmeasured factors. Additional research must elucidate the causal relationship between systemic mitochondrial PBMC dysfunction and brain alterations, with consideration given to other possible mechanisms by which HIV may affect the brain.
While regional brain volumetric decreases and callosal degradation may promote neurocognitive decline, their impact on functional outcomes was beyond the scope of this paper. Larger comprehensive studies are required to determine the relationships between mitochondrial function, brain structure, and cognitive/behavioral performance in HIV-infected individuals. The interval between blood collection and MRI varied across our participants, and mitochondrial assessments were done in bulk PBMCs and not specifically within lymphocytes or monocytes and macrophages. Given that much of the CNS pathology in HIV infection is believed to be monocyte/macrophage-mediated, it will be important to conduct separate mitochondrial assessments to determine the contributions of each cell type. Finally, our sample size was restricted, although the study was sufficiently powered to identify significant relationships among variables of interest.
In summary, the present study revealed significant associations between PBMC mitochondrial CI and CIV levels and brain imaging markers in chronically infected HIV+ individuals, independently of cART regimen or age. Further research is needed to define the role of mitochondrial dysfunction in development of brain abnormalities in HIV.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by grants from the NIH: R01 HL095135; U54 MD007584 (Shikuma); U54 EB020403 (Thompson); R01 AG059874 (Jahanshad); P20 GM113134 and U54 MD007601 (Gerschenson); and 1P20GM125526.
References
- 1. Tanne JH: Nearly 40 million people worldwide are infected with HIV. BMJ 2006;332:1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thompson PM, Dutton RA, Hayashi KM, et al. : 3D mapping of ventricular and corpus callosum abnormalities in HIV/AIDS. Neuroimage 2006;31:12–23 [DOI] [PubMed] [Google Scholar]
- 3. Thompson PM, Dutton RA, Hayashi KM, et al. : Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci U S A 2005;102:15647–15652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker JT, Sanders J, Madsen SK, et al. : Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011 [DOI] [PMC free article] [PubMed]
- 5. Kallianpur KJ, Shikuma C, Kirk GR, et al. : Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology 2013;80:1792–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kallianpur KJ, Valcour VG, Lerdlum S, et al. : HIV DNA in CD14+ reservoirs is associated with regional brain atrophy in patients naive to combination antiretroviral therapy. AIDS 2014;28:1619–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nir TM, Jahanshad N, Ching CRK, et al. : Progressive brain atrophy in chronically infected and treated HIV+ individuals. J Neurovirol 2019;25:342–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gongvatana A, Cohen RA, Correia S, et al. : Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 2011;17:477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gongvatana A, Schweinsburg BC, Taylor MJ, et al. : White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J Neurovirol 2009;15:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO: White matter abnormalities in HIV-1 infection: A diffusion tensor imaging study. Psychiatry Res 2001;106:15–24 [DOI] [PubMed] [Google Scholar]
- 11. Paul RH, Ernst T, Brickman AM, et al. : Relative sensitivity of magnetic resonance spectroscopy and quantitative magnetic resonance imaging to cognitive function among nondemented individuals infected with HIV. J Int Neuropsychol Soc 2008;14:725–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ragin AB, Wu Y, Storey P, Cohen BA, Edelman RR, Epstein LG: Diffusion tensor imaging of subcortical brain injury in patients infected with human immunodeficiency virus. J Neurovirol 2005;11:292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB: Diffusion alterations in corpus callosum of patients with HIV. AJNR Am J Neuroradiol 2006;27:656–660 [PMC free article] [PubMed] [Google Scholar]
- 14. Heaton RK, Clifford DB, Franklin DR Jr., et al. : HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuper M, Rabe K, Esser S, et al. : Structural gray and white matter changes in patients with HIV. J Neurol 2011;258:1066–1075 [DOI] [PubMed] [Google Scholar]
- 16. Valcour V, Sithinamsuwan P, Letendre S, Ances B: Pathogenesis of HIV in the central nervous system. Curr HIV/AIDS Rep 2011;8:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanford R, Ances BM, Meyerhoff DJ, et al. : Longitudinal trajectories of brain volume and cortical thickness in treated and untreated primary human immunodeficiency virus infection. Clin Infect Dis 2018;67:1697–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gerschenson M, Brinkman K: Mitochondrial dysfunction in AIDS and its treatment. Mitochondrion 2004;4:763–777 [DOI] [PubMed] [Google Scholar]
- 19. Kruman II, Nath A, Mattson MP: HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol 1998;154:276–288 [DOI] [PubMed] [Google Scholar]
- 20. Li W, Li G, Steiner J, Nath A: Role of Tat protein in HIV neuropathogenesis. Neurotox Res 2009;16:205–220 [DOI] [PubMed] [Google Scholar]
- 21. Lecoeur H, Borgne-Sanchez A, Chaloin O, et al. : HIV-1 Tat protein directly induces mitochondrial membrane permeabilization and inactivates cytochrome c oxidase. Cell Death Dis 2012;3:e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Norman JP, Perry SW, Kasischke KA, Volsky DJ, Gelbard HA: HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J Immunol 2007;178:869–876 [DOI] [PubMed] [Google Scholar]
- 23. Rozzi SJ, Avdoshina V, Fields JA, Mocchetti I: Human immunodeficiency virus Tat impairs mitochondrial fission in neurons. Cell Death Discov 2018;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norman JP, Perry SW, Reynolds HM, et al. : HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS One 2008;3:e3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huttemann M, Lee I, Samavati L, Yu H, Doan JW: Regulation of mitochondrial oxidative phosphorylation through cell signaling. Biochim Biophys Acta 2007;1773:1701–1720 [DOI] [PubMed] [Google Scholar]
- 26. Adam-Vizi V: Production of reactive oxygen species in brain mitochondria: Contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 2005;7:1140–1149 [DOI] [PubMed] [Google Scholar]
- 27. Murphy MP: How mitochondria produce reactive oxygen species. Biochem J 2009;417:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turrens JF: Mitochondrial formation of reactive oxygen species. J Physiol 2003;552(Pt 2):335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang JQ, Chen Q, Wang X, et al. : Dysregulation of mitochondrial calcium signaling and superoxide flashes cause mitochondrial genomic DNA damage in Huntington disease. J Biol Chem 2013;288:3070–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lin MT, Beal MF: Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787–795 [DOI] [PubMed] [Google Scholar]
- 31. Mattson MP, Haughey NJ, Nath A: Cell death in HIV dementia. Cell Death Differ 2005;12 Suppl 1:893–904 [DOI] [PubMed] [Google Scholar]
- 32. Kallianpur KJ, Gerschenson M, Mitchell BI, et al. : Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion 2016;28:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Michaelis EK: Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2010;2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chiang MC, Dutton RA, Hayashi KM, et al. : 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage 2007;34:44–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leite SC, Correa DG, Doring TM, et al. : Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J Magn Reson Imaging 2013;38:1488–1493 [DOI] [PubMed] [Google Scholar]
- 36. Nir TM, Jahanshad N, Busovaca E, et al. : Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 2014;35:975–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wallace DC, Fan W, Procaccio V: Mitochondrial energetics and therapeutics. Annu Rev Pathol 2010;5:297–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miro O, Lopez S, Martinez E, et al. : Mitochondrial effects of HIV infection on the peripheral blood mononuclear cells of HIV-infected patients who were never treated with antiretrovirals. Clin Infect Dis 2004;39:710–716 [DOI] [PubMed] [Google Scholar]
- 39. Shikuma CM, Seto T, Liang CY, et al. : Vitamin D levels and markers of arterial dysfunction in HIV. AIDS Res Hum Retroviruses 2012;28:793–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shikuma CM, Gerschenson M, Chow D, et al. : Mitochondrial oxidative phosphorylation protein levels in peripheral blood mononuclear cells correlate with levels in subcutaneous adipose tissue within samples differing by HIV and lipoatrophy status. AIDS Res Hum Retroviruses 2008;24:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dale AM, Fischl B, Sereno MI: Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–194 [DOI] [PubMed] [Google Scholar]
- 42. Fischl B, Salat DH, Busa E, et al. : Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355 [DOI] [PubMed] [Google Scholar]
- 43. Fischl B, van der Kouwe A, Destrieux C, et al. : Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22 [DOI] [PubMed] [Google Scholar]
- 44. Fischl B, Sereno MI, Dale AM: Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9:195–207 [DOI] [PubMed] [Google Scholar]
- 45. Segonne F, Dale AM, Busa E, et al. : A hybrid approach to the skull stripping problem in MRI. Neuroimage 2004;22:1060–1075 [DOI] [PubMed] [Google Scholar]
- 46. Sled JG, Zijdenbos AP, Evans AC: A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 1998;17:87–97 [DOI] [PubMed] [Google Scholar]
- 47. Buckner RL, Head D, Parker J, et al. : A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004;23:724–738 [DOI] [PubMed] [Google Scholar]
- 48. Ances BM, Ortega M, Vaida F, Heaps J, Paul R: Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012;59:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heaps JM, Joska J, Hoare J, et al. : Neuroimaging markers of human immunodeficiency virus infection in South Africa. J Neurovirol 2012;18:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen RA, Harezlak J, Schifitto G, et al. : Effects of nadir CD4 count and duration of human immunodeficiency virus infection on brain volumes in the highly active antiretroviral therapy era. J Neurovirol 2010;16:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jernigan TL, Archibald S, Hesselink JR, et al. : Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Arch Neurol 1993;50:250–255 [DOI] [PubMed] [Google Scholar]
- 52. Moore DJ, Masliah E, Rippeth JD, et al. : Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 2006;20:879–887 [DOI] [PubMed] [Google Scholar]
- 53. Nir TM, Jahanshad N, Villalon-Reina JE, et al. : Effectiveness of regional DTI measures in distinguishing Alzheimer's disease, MCI, and normal aging. Neuroimage Clin 2013;3:180–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE: Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol 1999;58:946–958 [DOI] [PubMed] [Google Scholar]
- 55. Ke ZJ, Gibson GE: Selective response of various brain cell types during neurodegeneration induced by mild impairment of oxidative metabolism. Neurochem Int 2004;45:361–369 [DOI] [PubMed] [Google Scholar]
- 56. Valavanidis A, Vlachogianni T, Fiotakis C: 8-hydroxy-2’ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 2009;27:120–139 [DOI] [PubMed] [Google Scholar]
- 57. Tripathy MK, Mitra D: Differential modulation of mitochondrial OXPHOS system during HIV-1 induced T-cell apoptosis: Up regulation of Complex-IV subunit COX-II and its possible implications. Apoptosis 2010;15:28–40 [DOI] [PubMed] [Google Scholar]
- 58. Albers DS, Beal MF: Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 2000;59:133–154 [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Su B, Lee HG, et al. : Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. J Neurosci 2009;29:9090–9103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dawson TM, Dawson VL: Molecular pathways of neurodegeneration in Parkinson's disease. Science 2003;302:819–822 [DOI] [PubMed] [Google Scholar]
- 61. Shah A, Kumar A: HIV-1 gp120-mediated mitochondrial dysfunction and HIV-associated neurological disorders. Neurotox Res 2016;30:135–137 [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Wang M, Li H, et al. : Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Res 2012;1458:1–11 [DOI] [PubMed] [Google Scholar]
- 63. Fields JA, Serger E, Campos S, et al. : HIV alters neuronal mitochondrial fission/fusion in the brain during HIV-associated neurocognitive disorders. Neurobiol Dis 2016;86:154–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Swinton MK, Carson A, Telese F, et al. : Mitochondrial biogenesis is altered in HIV+ brains exposed to ART: Implications for therapeutic targeting of astroglia. Neurobiol Dis 2019:104502. [DOI] [PMC free article] [PubMed]
- 65. Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP: Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS 1998;12:1735–1744 [DOI] [PubMed] [Google Scholar]
- 66. Brinkman K, Smeitink JA, Romijn JA, Reiss P: Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 1999;354:1112–1115 [DOI] [PubMed] [Google Scholar]
- 67. Nolan D, Hammond E, Martin A, et al. : Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 2003;17:1329–1338 [DOI] [PubMed] [Google Scholar]
- 68. Walker UA, Setzer B, Venhoff N: Increased long-term mitochondrial toxicity in combinations of nucleoside analogue reverse-transcriptase inhibitors. AIDS 2002;16:2165–2173 [DOI] [PubMed] [Google Scholar]
- 69. Ramirez SH, Potula R, Fan S, et al. : Methamphetamine disrupts blood-brain barrier function by induction of oxidative stress in brain endothelial cells. J Cereb Blood Flow Metab 2009;29:1933–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wu CW, Ping YH, Yen JC, et al. : Enhanced oxidative stress and aberrant mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells during methamphetamine induced apoptosis. Toxicol Appl Pharmacol 2007;220:243–251 [DOI] [PubMed] [Google Scholar]
- 71. Banerjee A, Zhang X, Manda KR, Banks WA, Ercal N: HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: Potential role of the thiol antioxidant N-acetylcysteine amide. Free Radic Biol Med 2010;48:1388–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shedlock DJ, Hwang D, Choo AY, Chung CW, Muthumani K, Weiner DB: HIV-1 viral genes and mitochondrial apoptosis. Apoptosis 2008;13:1088–1099 [DOI] [PubMed] [Google Scholar]
- 73. Villeneuve LM, Purnell PR, Stauch KL, Callen SE, Buch SJ, Fox HS: HIV-1 transgenic rats display mitochondrial abnormalities consistent with abnormal energy generation and distribution. J Neurovirol 2016;22:564–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Berth S, Caicedo HH, Sarma T, Morfini G, Brady ST: Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro 2015;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G: gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem 2000;74:2373–2379 [DOI] [PubMed] [Google Scholar]
- 76. Hofer S, Frahm J: Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 2006;32:989–994 [DOI] [PubMed] [Google Scholar]
- 77. Guo C, Sun L, Chen X, Zhang D: Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res 2013;8:2003–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD: Mitochondrial proton and electron leaks. Essays Biochem 2010;47:53–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bentlage H, de Coo R, ter Laak H, et al. : Human diseases with defects in oxidative phosphorylation. 1. Decreased amounts of assembled oxidative phosphorylation complexes in mitochondrial encephalomyopathies. Eur J Biochem 1995;227:909–915 [DOI] [PubMed] [Google Scholar]