Abstract
Liver metastases are a major cause of death from colorectal cancer. Intraarterial therapy options for colorectal liver metastases include chemoinfusion via a hepatic arterial pump or port, irinotecan-loaded drug-eluting beads, and radioembolization using 90Y microspheres. Intraarterial therapy allows the delivery of a high dose of chemotherapy or radiation into liver tumors while minimizing the impact on liver parenchyma and avoiding systemic effects. Specificity in intraarterial therapy can be achieved both through preferential arterial flow to the tumor and through selective catheter positioning. In this review, we discuss indications, contraindications, preprocedure evaluation, activity prescription, follow-up, outcomes, and complications of radioembolization of colorectal liver metastases. Methods for preventing off-target embolization, increasing the specificity of microsphere delivery, and reducing the lung-shunt fraction are discussed. There are 2 types of 90Y microspheres: resin and glass. Because glass microspheres have a higher activity per particle, they can deliver a particular radiation dose with fewer particles, likely reducing embolic effects. Glass microspheres thus may be more suitable when early stasis or reflux is a concern, in the setting of hepatocellular carcinoma with portal vein invasion, and for radiation segmentectomy. Because resin microspheres have a lower activity per particle, more particles are needed to deliver a particular radiation dose. Resin microspheres thus may be preferable for larger tumors and those with high arterial flow. In addition, resin microspheres have been approved by the U.S. Food and Drug Administration for colorectal liver metastases, whereas institutional review board approval is required before glass microspheres can be used under a compassionate-use or research protocol. Finally, radiation segmentectomy involves delivering a calculated lobar activity of 90Y microspheres selectively to treat a tumor involving 1 or 2 liver segments. This technique administers a very high radiation dose and effectively causes the ablation of tumors that are too large or are in a location considered unsafe for thermal ablation. The selective delivery spares surrounding normal liver, reducing the risk of liver failure.
Keywords: irinotecan-loaded drug-eluting beads, liver metastases, colorectal cancer, radioembolization, 90Y, microspheres
Colorectal cancer (CRC) often metastasizes to the liver, and liver metastases are a major cause of death (1). Chemotherapy options for metastatic CRC include folinic acid–5-fluorouracil–oxaliplatin (FOLFOX) and folinic acid–5-fluorouracil–irinotecan (FOLFIRI). Adding bevacizumab, a vascular endothelial growth factor inhibitor, to fluorouracil-based chemotherapy results in improved survival (2). Adding cetuximab, an epidermal growth factor receptor inhibitor, to FOLFIRI improves survival in patients with KRAS wild-type metastatic CRC (3).
CRC liver metastases in a small fraction (10%–20%) of patients are potentially resectable (4). Surgical resection of liver metastases results in long-term (>10-y) cure in 16% of patients (5). For patients with small-volume hepatic disease, thermal ablation is a less invasive alternative to surgery, and it has a comparable 5-y overall survival rate of about 50% (6–8). Ablation has been used extensively for patients with liver metastases smaller than 3–4 cm, especially patients who are not candidates for surgery, as well as for recurrences after hepatectomy (9) that can be ablated with margins larger than 5 mm (10,11). In addition, ablation of resectable liver metastases allows for a “test-of-time” approach: surgery can be avoided in 76% of patients, either because they were disease-free after ablation or because they developed new metastases that could not be resected (12). Importantly, no tumors became unresectable after ablation because of the growth of existing metastases.
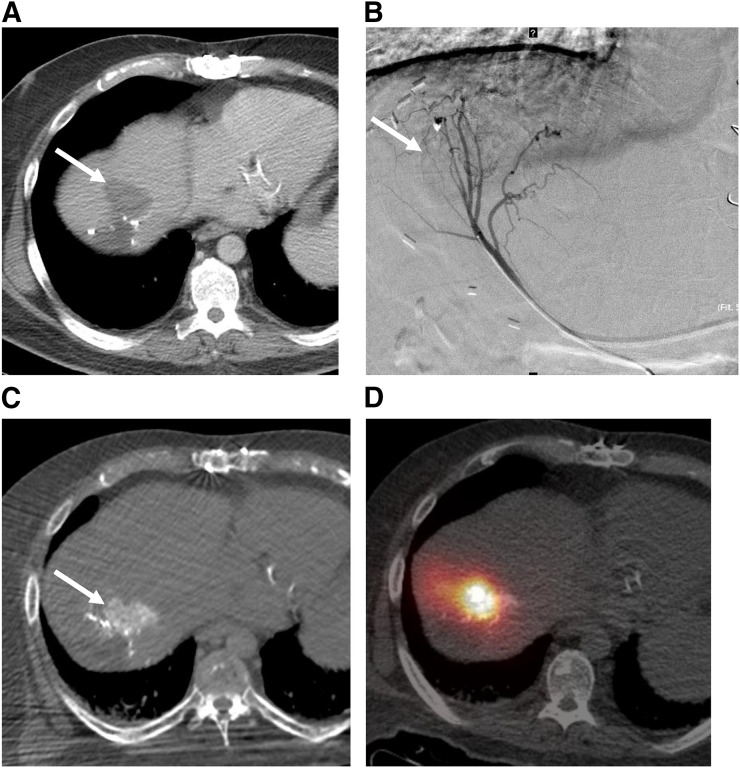
Patients who have liver-dominant metastases and who are not candidates for resection or ablation can be treated by intraarterial infusion of chemotherapy, drug-eluting beads, or radioactive particles into the hepatic artery. The rationale for intraarterial therapy is that a high dose of chemotherapy or radiation can be delivered through a catheter selectively into the tumor arterioles while minimizing liver toxicity and avoiding systemic effects. The normal liver is supplied mostly by the portal vein, whereas hepatocellular carcinoma (HCC) and hypervascular metastases (such as neuroendocrine tumors) are supplied mostly by the hepatic artery (13). Hypovascular metastases (such as colorectal cancer) also have greater hepatic artery supply than the liver parenchyma (14). Specificity in intraarterial therapy can be achieved both through preferential arterial flow to the tumor and through selective catheter positioning (Fig. 1).
FIGURE 1.
CRC liver metastases can be hypervascular on angiography, allowing selective intraarterial delivery of particles into tumor. (A) CRC liver metastases (arrow) are typically considered hypovascular because they do not enhance as much as background liver on contrast-enhanced CT. (B) However, on catheter angiogram (segment 4), which shows only arterial blood supply, this liver metastasis is actually hypervascular (greater hepatic artery supply than normal liver). (C) Hypervascularity is confirmed by enhancement of lesion on helical CT during injection of contrast material into segment 4 artery. (D) Bremsstrahlung SPECT/CT after injection of 90Y microspheres into same artery shows preferential flow of microspheres into tumor.
Intraarterial chemotherapy can be delivered via a hepatic arterial infusion pump (15), which can be implanted surgically, or percutaneously (16), although percutaneous ports are currently available only in Europe and Japan. Liver resection followed by systemic and hepatic arterial infusion chemotherapy resulted in an impressive 10-y survival of 61% (15). This approach, applied to selected patients with liver-only metastases, resulted in the prolongation of survival in comparison to historical survival rates.
Compared with FOLFIRI, the infusion of irinotecan-loaded drug-eluting beads into the hepatic artery resulted in a 7-mo improvement in overall survival as well as improved quality of life in a small randomized trial (17). In another small randomized trial, intraarterial irinotecan-loaded drug-eluting beads added to FOLFOX resulted in improved progression-free survival (18).
When used as an adjuvant to first-line chemotherapy, intraarterial infusion of 90Y microspheres (radioembolization) resulted in an 8-mo improvement in progression-free survival in the liver (19). Outcomes after radioembolization are reviewed in greater detail later in the article. Radioembolization is also known as transarterial radioembolization, selective internal radiation therapy, or intraarterial brachytherapy. The 2 brands of 90Y microspheres, SIR-Spheres (resin; Sirtex) and TheraSphere (glass; BTG), are compared later.
In this article, we review indications for radioembolization, preprocedure evaluation, radioembolization procedure, follow-up, and outcomes. Our primary focus is the radioembolization of colorectal liver metastases.
INDICATIONS FOR RADIOEMBOLIZATION
According to guidelines from the National Comprehensive Cancer Network, radioembolization is indicated for liver-dominant CRC and neuroendocrine tumor metastases that cannot be resected or ablated, as well as for HCC. National Comprehensive Cancer Network (20) and European Society for Medical Oncology (21) guidelines recommend considering radioembolization for chemoresistant colorectal liver metastases (salvage setting), as there currently is insufficient evidence to allow panel consensus on the use of radioembolization as a first-line therapy. In the setting of limited extrahepatic disease (liver-dominant disease), liver metastases are often the cause of death (1), so radioembolization may still be a reasonable treatment option. However, recent randomized trials suggested that an improved response in the liver after radioembolization does not necessarily translate into better survival, perhaps because of the progression of extrahepatic disease (19,22).
Contraindications for radioembolization include poor liver function (bilirubin, >2 mg/dL; albumin, <3 g/dL; uncontrolled ascites) and poor performance status (Eastern Cooperative Oncology Group performance status, >2).
PREPROCEDURE EVALUATION
Patients with colorectal liver metastases should undergo preprocedure CT of the chest, abdomen, and pelvis with contrast material to evaluate liver metastases and extrahepatic disease. 18F-FDG PET/CT is also recommended because a metabolic response can be seen 4–6 wk after radioembolization (23,24), whereas a response on CT or MRI may take 2–3 mo to evaluate. Follow-up imaging recommendations are discussed later.
A comprehensive metabolic panel, complete blood count, and international normalized ratio should be checked at the preprocedure clinic visit. Bilirubin should be rechecked 1 wk before each treatment to ensure adequate liver function. In patients with rising or borderline bilirubin, checking bilirubin again 24 h before radioembolization should be considered.
Many chemotherapeutic agents, including 5-fluorouracil, irinotecan, and oxaliplatin, are radiosensitizers (25) and thus may have a synergistic effect when combined with radioembolization. Combining systemic chemotherapy and radioembolization appears to improve response rates (19,26). These data suggest that chemotherapy should be continued during radioembolization, although dose reduction may be necessary to reduce toxicity (19).
Bevacizumab is typically withheld for at least 2 and ideally 4 wk before mapping angiogram and radioembolization procedures, although the optimal timing is unknown. Bevacizumab interferes with wound healing (27), may result in hepatic artery dissection (28), and increases the risk of stasis being reached—resulting in an inability to deliver the entire dose as well as possible reflux and gastroduodenal ulceration (29).
RESIN VERSUS GLASS 90Y MICROSPHERES
TheraSphere consists of 20- to 30-μm glass microspheres with embedded 90Y. It has received a Humanitarian Device Exemption from the U.S. Food and Drug Administration for the treatment of HCC, meaning that local institutional review board approval is required for use in the United States. The institutional review board protocol determines whether TheraSphere can be used only for HCC or for other pathologies as well.
SIR-Spheres consist of 20- to 60-μm (median, 33) resin microspheres with 90Y attached to the surface. They have been approved by the U.S. Food and Drug Administration for the treatment of CRC liver metastases but frequently are used off-label for other pathologies (30,31).
A key difference between glass and resin microspheres is the activity per particle: 40–80 Bq/particle for resin microspheres and 2,500 Bq/particle (at the time of calibration) for glass microspheres (32). Thus, for a given desired activity, resin microspheres have more particles and a greater embolic effect than glass microspheres (33). The practical implication is that early stasis has been seen in approximately 20% of patients in whom resin microspheres have been used (34). Early stasis has been seen even more frequently (38%) in patients who have received multiple prior lines of chemotherapy, including hepatic arterial infusion pump chemotherapy (35). The probability of early stasis can be reduced by delivering the microspheres using 5% dextrose (36) or 50% contrast material plus 50% saline (37) instead of sterile water. Early stasis results in the inability to deliver the entire dose and can result in the reflux of particles into off-target arteries. Thus, if there is concern about early stasis or reflux, glass microspheres may be more appropriate as less embolic agents that are less likely to result in reflux. Glass microspheres also may be preferable in the setting of HCC with portal vein invasion, where they were associated with lower toxicity and improved overall survival in a small single-institution retrospective study (38).
More particles (which can be obtained using resin microspheres or extended–shelf life glass microspheres) can result in more uniform particle distribution in a tumor (39). This may be important for larger tumor volumes or more hypervascular tumors. At present, there is no strong evidence suggesting an overall difference in outcomes between resin and glass microspheres. In studies on the radioembolization of colorectal liver metastases, similar survival rates were reported using either resin (40) or glass (41) microspheres.
TECHNIQUE
Before radioembolization, a mapping angiogram is obtained to identify the vessel(s) supplying the tumor, evaluate extrahepatic perfusion, and determine the lung-shunt fraction (LSF). Generally, the aim is to deliver 90Y microspheres as selectively as possible to treat the entire tumor while sparing as much normal liver as possible. A cone-beam CT catheter arteriogram is very helpful for identifying incomplete tumor perfusion or extrahepatic perfusion (42). Alternatively, if available in the angiography room, helical CT catheter angiography has better image quality than cone-beam CT. We typically administer 99mTc-labeled macroaggregated albumin (99mTc-MAA) to each artery in which we plan to administer 90Y microspheres. For example, if we are treating the entire liver, we deliver separate 99mTc-MAA doses to the right and left hepatic arteries. After the mapping angiogram is obtained, SPECT/CT is performed to ensure tumor coverage and evaluate for extrahepatic perfusion. Planar scintigraphy is typically used to calculate the LSF.
Treatment is typically performed 1–3 wk after mapping to allow time to order the 90Y dose, although mapping and treatment on the same day are possible (43). For bilobar disease, the right and left lobes are typically treated in separate sessions 4–8 wk apart. Treating the entire liver in a single session is associated with a higher rate of liver failure (44). After the 90Y microspheres are delivered, bremsstrahlung SPECT/CT is performed to confirm tumor coverage and evaluate for extrahepatic perfusion (45).
It is critical to avoid delivering 90Y microspheres to the stomach, duodenum, pancreas, or other nontarget organs because they could cause nonhealing ulcers or pancreatitis. In particular, the gastroduodenal artery, right gastric artery, dorsal pancreatic artery, accessory left gastric artery, cystic artery, and falciform artery should be identified. If there is a risk of extrahepatic perfusion, then off-target vessels are usually coil embolized during treatment instead of during mapping to reduce the risk of recanalization or formation of arterial collateral vessels in the interim (46). An antireflux catheter or sublobar superselective administration technique can be used to reduce reflux into off-target vessels, especially when coil embolization is not feasible or desirable (47).
There is a low rate of cholecystitis requiring cholecystectomy after coil embolization of the proximal cystic artery (48), and there is also a low rate of cholecystitis requiring cholecystectomy after radioembolization including the cystic artery (49). Ideally, 90Y microspheres should be delivered distal to the cystic artery. For example, for treatment of the right lobe, if the cystic artery arises from the right hepatic artery, then separate doses can be delivered into the right anterior and right posterior arteries to avoid delivery into the cystic artery. If this approach is not possible, then the cystic artery either can be coil embolized proximally or can be included in the treatment. We tend to prefer the latter option, acknowledging that cholecystitis is a possibility with either approach.
There is a low rate of serious complications from radioembolization involving nontarget microsphere delivery into the falciform artery, which supplies the anterior abdominal wall and skin (50). The falciform artery can be coil embolized before radioembolization. Alternatively, applying an ice pack to the skin may be sufficient to prevent radiation toxicity (51).
Glass microspheres are delivered in saline, precluding angiographic monitoring during infusion. However, at the beginning of the infusion, fluoroscopy can show the flow rate in the vessel while the catheter still contains contrast material. Glass microspheres are minimally embolic, so they rarely cause stasis. Resin microspheres are often delivered in sterile water or 5% dextrose. Many operators use 50% contrast material plus 50% saline in both the B line and the D line of the infusion set; this approach allows for continuous fluoroscopic monitoring of the flow rate during delivery and reduces the probability of terminating the infusion early because of stasis (37). However, this approach is not recommended by the manufacturer.
Preprocedure antibiotics (e.g., a single dose of cefazolin intravenously) are often given before radioembolization, although infectious complications are rare. For patients with a biliary anastomosis or incompetent sphincter, broad-spectrum antibiotics are recommended, starting before the procedure and continuing for 5 d after the procedure. To reduce the risk of gastric and duodenal ulcers, a proton-pump inhibitor should be administered before and for 1–4 wk after treatment. To reduce postradioembolization syndrome (nausea, fatigue, and pain), an antiemetic and a steroid can be given before the procedure, and the patient should go home with a steroid taper, antiemetic (as needed), and pain medications.
90Y ACTIVITY CALCULATION
90Y decays to 90Zr with a half-life of 2.7 d, emitting a high-energy electron (β– decay) with mean and maximum tissue penetration values of 2.5 and 11 mm, respectively. As the electron decelerates in tissue, it emits bremsstrahlung (German for “braking radiation”), which can be detected on SPECT. 90Y also emits a small fraction of positrons, which can be detected on PET, allowing for more accurate quantitation of the dose distribution—but with increased image noise due to the small number of positrons emitted (52).
90Y microspheres preferentially flow to tumors (Fig. 1), which have greater hepatic artery supply than the surrounding normal liver (13,14). Specificity can be further improved by selective catheter positioning. The microspheres are deposited in arterioles, preferentially at the periphery of the tumor (53). The microspheres are too large to pass through capillaries, but if there is a shunt from the hepatic artery to the hepatic vein, some microspheres can exit the liver and be deposited in the lungs.
For glass microspheres, the manufacturer recommends delivering a dose of 120 Gy (range, 80–150 Gy). One GBq of 90Y microspheres distributed uniformly in 1 kg of tissue results in an absorbed dose of 50 Gy. Thus, to achieve a dose of 120 Gy:
where treatment mass is in kilograms. If this activity is uniformly distributed throughout the treatment volume, then the dose will be 120 Gy. However, if there is preferential flow of microspheres to the tumor, then the tumor will receive a dose of greater than 120 Gy, and the background liver will receive a dose of less than 120 Gy. For glass microspheres, the maximum lung dose per treatment is 30 Gy, and the maximum total lung dose is 50 Gy.
For resin microspheres, the body surface area method is most commonly used for calculating activity:
where body surface area is in meters squared. The resin microspheres dose is reduced if the LSF is elevated, and the maximum allowed LSF is 20%. The maximum lung dose is 30 Gy.
The recommended activity for glass microspheres depends on the treatment volume (tumor + normal liver), whereas the recommended activity for resin microspheres depends on both tumor and treatment volumes. The optimal dose and number of particles for each patient remain unknown and are being investigated.
After radioembolization of metastatic CRC, tumors with a good metabolic response received a median dose of 46 Gy, and tumors with a poor metabolic response received a median dose of 20 Gy (54). On mapping 99mTc-MAA SPECT/CT, a tumor-to-normal liver uptake ratio of greater than 1 has been correlated with a good metabolic response (54). However, the correlation between tumor uptake seen on pretreatment 99mTc-MAA SPECT/CT and posttreatment 90Y PET/CT is low (55). Thus, accurately predicting the tumor dose on the basis of pretreatment imaging may be challenging.
FOLLOW-UP
For hypermetabolic liver tumors (such as colorectal metastases), we routinely perform 18F-FDG PET/CT before treatment and 1–2 mo after treatment to evaluate the response; this approach allows for an earlier evaluation of the response than CT or MRI. After radioembolization of CRC liver metastases, patients with an 18F-FDG metabolic response according to PERCIST guidelines have better overall survival (23,24,56–58). For bilobar treatments, 18F-FDG PET/CT can allow for an evaluation of the response of one lobe before a decision about whether to treat the other lobe is made.
A CT or MRI evaluation of the response after radioembolization can take 2–3 mo (59,60) because tumor necrosis and edema can result in increased tumor size at 1 mo, even when the tumor is responding to treatment. Thus, for tumors that are usually not hypermetabolic (such as HCC or neuroendocrine tumors), we perform follow-up CT or MRI 2–3 mo after treatment to evaluate the response.
OUTCOMES
In the SIRFLOX randomized controlled trial (19), radioembolization as an adjuvant to first-line chemotherapy for metastatic CRC was examined. Chemotherapy-naive patients with liver-dominant colorectal liver metastases (530 patients) were randomized to a group receiving FOLFOX with or without bevacizumab and a group receiving FOLFOX with or without bevacizumab and radioembolization. There was an improved objective response rate in the liver with radioembolization (79%) versus without radioembolization (69%). There was also an 8-mo increase in liver-progression–free survival in the radioembolization arm (21 vs. 13 mo). However, there was no difference in overall progression-free survival because of the high percentage of patients with progression of extrahepatic disease.
Combined data from the SIRFLOX, FOXFIRE, and FOXFIRE Global studies (1,075 patients) showed that despite an improved response in the liver and liver progression-free survival when radioembolization was added to first-line chemotherapy for metastatic CRC, there was no difference in overall survival, perhaps because of the progression of extrahepatic disease (22). On the basis of these data, radioembolization is currently not recommended as first-line therapy, especially in the presence of extrahepatic disease. Better patient selection may be required to identify a subset of patients who can benefit from radioembolization as an adjuvant to first-line chemotherapy.
A secondary analysis of the SIRFLOX and FOXFIRE Global trials suggested that patients with right-sided colon cancers had a 4.9-mo improvement in survival when radioembolization was added to first-line chemotherapy (61). Right-sided colon cancers tend to have different mutations (62) and worse survival and to be less responsive to epidermal growth factor receptor inhibitors (63) than left-sided colon cancers.
An earlier phase 2 randomized trial showed improved survival when radioembolization was combined with systemic chemotherapy (64). Another study showed that in 10% of cases, unresectable colorectal liver metastases could be downsized to resection after radioembolization and systemic FOLFOX (65).
Several factors are associated with shorter survival after radioembolization of colorectal liver metastases: an Eastern Cooperative Oncology Group performance status of greater than or equal to 1, prior chemotherapy, uncontrolled ascites, elevated liver function tests, low albumin level, LSF of greater than 10%, presence of extrahepatic metastases, lymphovascular invasion of the primary tumor, carcinoembryonic antigen level of greater than 62 ng/mL, KRAS mutant tumors, greater than 25% tumor involvement of the treated liver volume, and low apparent diffusion coefficient values on diffusion-weighted MRI (35,40,41,66–69). Many of these factors are simply markers of more aggressive tumors that would respond poorly to any treatment (10,70), and data on whether these factors can be used to choose one therapy option over another are limited.
Radioembolization can be safely and effectively performed in the extreme salvage setting, including in patients who have undergone liver resection, hepatic arterial infusion pump chemotherapy, or 3 or more lines of systemic chemotherapy (35,71). These patients had a complete or partial metabolic response rate of 45% and a median overall survival of 13 mo after radioembolization (35). In addition, extensively pretreated patients can still receive additional therapy after radioembolization, including ablation or systemic or intraarterial chemotherapy (35,72). Elderly patients (≥75 y old) can safely receive radioembolization, with the same overall survival and complications as younger patients (73).
Interestingly, colorectal liver metastases with increased arterial perfusion did not show a better response to radioembolization—a somewhat surprising result for an arterially directed therapy (14). However, a higher ratio of arterial to portal venous perfusion did predict a better response (14). This result suggests that portal flow plays an important role in determining the response to radioembolization.
COMPLICATIONS
Postradioembolization syndrome—including nausea, fatigue, pain, and low-grade fever—is very common after radioembolization and usually lasts for 1–2 wk but only rarely requires treatment or hospitalization. The most common severe adverse events (40) are hyperbilirubinemia (5.4%), ascites (3.6%), and gastrointestinal ulceration (1.7%). Rare but serious complications include pancreatitis, radiation pneumonitis, and liver abscess (74).
Radioembolization-induced liver disease (REILD) is characterized by elevated liver function tests (mostly bilirubin and alkaline phosphatase) and ascites in the absence of tumor progression. REILD can develop 4–8 wk after radioembolization, although delayed hepatotoxicity can also occur (75). Histology shows venoocclusive disease in severe cases (76). In 1 study, REILD developed in 20% of patients after radioembolization, and 11% of patients with REILD died of progressive liver failure (76). A much lower risk of REILD was reported in another study (77). Risk factors for REILD include prior chemotherapy, younger age, low body mass index, non-HCC pathology, low tumor volume, higher bilirubin level, and whole-liver radioembolization (76). There are limited data on the treatment of REILD, but potential treatment options include steroids, ursodeoxycholic acid, low-molecular-weight heparin, and pentoxifylline (78,79). Steroids can be routinely given before and after the procedure to reduce the risk of REILD.
SPECIAL SITUATIONS
High LSF
If the LSF is greater than 20%, then temporary balloon occlusion of the hepatic veins can reduce the LSF to allow safe radioembolization (80). Sorafenib can also reduce the LSF in patients with advanced HCC (81).
Arterioportal Shunting
HCC with a portal vein tumor and associated arterioportal shunting can still be treated with 90Y microspheres if the 99mTc-MAA scan shows good distribution in the tumor. Arterioportal shunting can sometimes be reduced with coil embolization of the shunt or selective catheter positioning beyond the shunt. Alternatively, HCC with a portal vein tumor can be safely treated by bland embolization (82).
Repeated Treatment
Data on the safety of repeated radioembolization in a territory that has already been treated are mixed (83,84). One study showed an elevated risk of REILD after repeated radioembolization, especially repeated whole-liver radioembolization (83). Another study showed acceptable toxicity when an average of 3 lobar treatments was performed, with 4–6 wk between sequential lobar treatments; when a bilirubin cutoff of 1.75 mg/dL was used for both initial and repeated treatments; and when repeated radioembolization was performed only for patients who initially demonstrated a response to radioembolization (6 wk after treatment) but then later showed disease progression (84).
Temporary Protection of Off-Target Branches
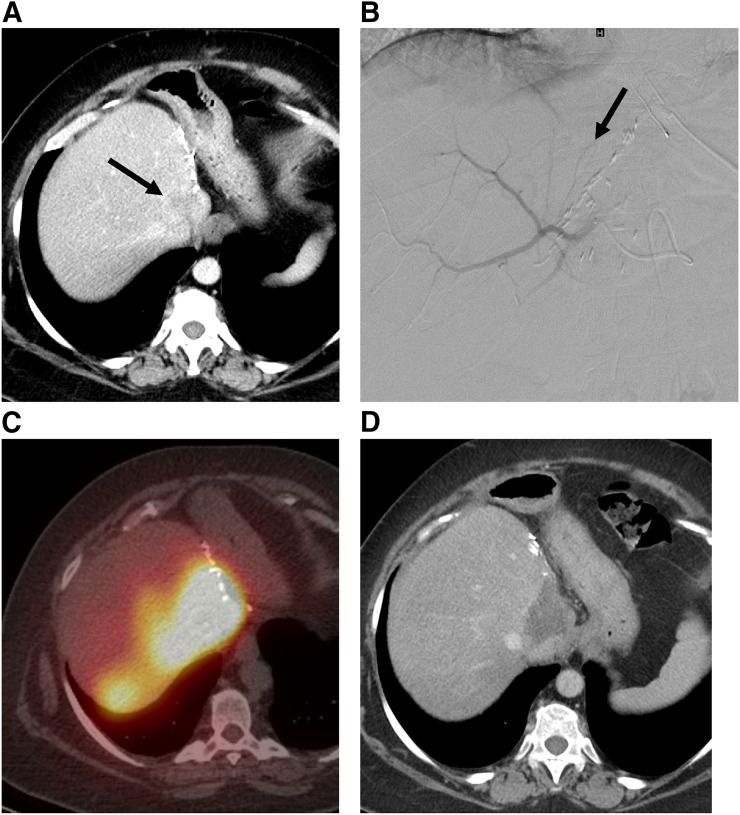
Gelatin foam (Gelfoam; Pfizer) or an autologous clot can be used to temporarily occlude and thus protect off-target branches before 90Y microspheres are delivered (Fig. 2); this approach allows for more selective delivery of 90Y.
FIGURE 2.
Radiation segmentectomy. (A) Patient with intrahepatic cholangiocarcinoma who developed a 3-cm recurrence at resection margin after left hepatectomy (arrow). (B) Entire right-lobe dose was delivered into tiny artery supplying tumor. This tiny artery (arrow) could not be selectively catheterized, so off-target vessels were protected with gelatin foam (Gelfoam; Pfizer) before dose was delivered into parent vessel. (C) Bremsstrahlung SPECT/CT after injection of 90Y microspheres shows selective delivery of particles into tumor. (D) CT at 2 mo after procedure shows lack of enhancement, corresponding to tumor necrosis.
Multiple Doses
Multiple doses can be delivered selectively during a single treatment session. This approach is helpful if the tumors to be treated are supplied by multiple vessels; it can also allow more selective catheter positioning to spare normal liver or avoid extrahepatic vessels.
Flow Redirection
Coil embolization of an artery supplying a tumor results in flow redistribution via intrahepatic collateral vessels, which can consolidate the vascular supply to the tumor into a smaller number of treatment locations (85). For comparison, embolization of a hepatic artery branch with gelatin foam (Gelfoam, Pfizer) tends to result in more distal embolization, which prevents 90Y microspheres from reaching that portion of the liver (see the earlier discussion about temporary protection of off-target branches). More proximal embolization with coils allows for flow redistribution via intrahepatic collateral vessels, thus allowing the coil-embolized territory to be treated with 90Y microspheres via an adjacent branch of the hepatic artery.
RADIATION SEGMENTECTOMY
Radiation segmentectomy (86) involves the delivery of a high dose of radiation selectively to treat a tumor that involves 1 or 2 segments of the liver (Fig. 2). The 90Y activity (Bq = decays/s) is calculated for a 120-Gy lobar treatment, but this is delivered selectively into a sublobar treatment volume, resulting in a higher absorbed tumor dose (Gy = J/kg). In one study, the median tumor dose was 1,200 Gy (86). The high absorbed dose effectively creates an “ablation” zone, with a high rate of tumor necrosis (87). This technique is suitable for tumors that are localized to 1 or 2 segments of the liver but are too large or are in an unsafe location for thermal ablation. The selective delivery allows the sparing of surrounding normal liver, reducing the risk of liver failure. Radiation segmentectomy using glass microspheres, which are less embolic, has been described; this approach allows the entire dose to be delivered to a small territory before stasis is reached.
DISCLOSURE
F. Edward Boas has received research supplies from Bayer. Constantinos T. Sofocleous received research support from Sirtex (2009–2012) and currently receives research support from TheraSphere/Biocompatibles. F. Edward Boas is a cofounder of Claripacs, LLC, and an investor in Labdoor, analyticsMD, CloudMedx, and Notable Labs. Lisa Bodei is a consultant for AAA and Ipsen. Constantinos T. Sofocleous is a consultant for Sirtex, owned Sirtex stock, and is a consultant for Neuwave/Johnson & Johnson. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol. 2014;21:501–506. [DOI] [PubMed] [Google Scholar]
- 2.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 3.Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. [DOI] [PubMed] [Google Scholar]
- 4.Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27:3465–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. [DOI] [PubMed] [Google Scholar]
- 6.Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg. 2003;90:1240–1243. [DOI] [PubMed] [Google Scholar]
- 7.Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc. 2011;81:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958–968. [DOI] [PubMed] [Google Scholar]
- 9.Sofocleous CT, Petre EN, Gonen M, et al. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22:755–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotirchos VS, Petrovic LM, Gonen M, et al. Colorectal cancer liver metastases: biopsy of the ablation zone and margins can be used to predict oncologic outcome. Radiology. 2016;280:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Livraghi T, Solbiati L, Meloni F, Ierace T, Goldberg SN, Gazelle GS. Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time approach.” Cancer. 2003;97:3027–3035. [DOI] [PubMed] [Google Scholar]
- 13.Boas FE, Kamaya A, Do B, et al. Classification of hypervascular liver lesions based on hepatic artery and portal vein blood supply coefficients calculated from triphasic CT scans. J Digit Imaging. 2015;28:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boas FE, Brody LA, Erinjeri JP, et al. Quantitative measurements of enhancement on preprocedure triphasic CT can predict response of colorectal liver metastases to radioembolization. AJR. 2016;207:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kemeny NE, Chou JF, Boucher TM, et al. Updated long-term survival for patients with metastatic colorectal cancer treated with liver resection followed by hepatic arterial infusion and systemic chemotherapy. J Surg Oncol. 2016;113:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschamps F, Elias D, Goere D, et al. Intra-arterial hepatic chemotherapy: a comparison of percutaneous versus surgical implantation of port-catheters. Cardiovasc Intervent Radiol. 2011;34:973–979. [DOI] [PubMed] [Google Scholar]
- 17.Fiorentini G, Aliberti C, Tilli M, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387–1395. [PubMed] [Google Scholar]
- 18.Martin RC, II, Scoggins CR, Schreeder M, et al. Randomized controlled trial of irinotecan drug-eluting beads with simultaneous FOLFOX and bevacizumab for patients with unresectable colorectal liver-limited metastasis. Cancer. 2015;121:3649–3658. [DOI] [PubMed] [Google Scholar]
- 19.van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: randomized phase III trial comparing first-line mFOLFOX6 (plus or minus bevacizumab) versus mFOLFOX6 (plus or minus bevacizumab) plus selective internal radiation therapy in patients with metastatic colorectal cancer. J Clin Oncol. 2016;34:1723–1731. [DOI] [PubMed] [Google Scholar]
- 20.Clinical practice guidelines in oncology: colon cancer. https://www.nccn.org/professionals/physician_gls/PDF/colon.pdf. Accessed June 26, 2017.
- 21.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. [DOI] [PubMed] [Google Scholar]
- 22.Sharma RA, Wasan HS, Van Hazel GA, et al. Overall survival analysis of the FOXFIRE prospective randomized studies of first-line selective internal radiotherapy (SIRT) in patients with liver metastases from colorectal cancer [abstract]. J Clin Oncol. 2017;35(suppl):3507. [Google Scholar]
- 23.Shady W, Kishore S, Gavane S, et al. Metabolic tumor volume and total lesion glycolysis on FDG-PET/CT can predict overall survival after 90Y radioembolization of colorectal liver metastases: a comparison with SUVmax, SUVpeak, and RECIST 1.0. Eur J Radiol. 2016;85:1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shady W, Sotirchos VS, Do RK, et al. Surrogate imaging biomarkers of response of colorectal liver metastases after salvage radioembolization using 90Y-loaded resin microspheres. AJR. 2016;207:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolay NH, Berry DP, Sharma RA. Liver metastases from colorectal cancer: radioembolization with systemic therapy. Nat Rev Clin Oncol. 2009;6:687–697. [DOI] [PubMed] [Google Scholar]
- 26.Chua TC, Bester L, Saxena A, Morris DL. Radioembolization and systemic chemotherapy improves response and survival for unresectable colorectal liver metastases. J Cancer Res Clin Oncol. 2011;137:865–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erinjeri JP, Fong AJ, Kemeny NE, Brown KT, Getrajdman GI, Solomon SB. Timing of administration of bevacizumab chemotherapy affects wound healing after chest wall port placement. Cancer. 2011;117:1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown DB. Hepatic artery dissection in a patient on bevacizumab resulting in pseudoaneurysm formation. Semin Intervent Radiol. 2011;28:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam MG, Banerjee S, Louie JD, et al. Root cause analysis of gastroduodenal ulceration after yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36:1536–1547. [DOI] [PubMed] [Google Scholar]
- 30.Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594–601. [DOI] [PubMed] [Google Scholar]
- 31.Devcic Z, Rosenberg J, Braat AJ, et al. The efficacy of hepatic 90Y resin radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55:1404–1410. [DOI] [PubMed] [Google Scholar]
- 32.Cremonesi M, Chiesa C, Strigari L, et al. Radioembolization of hepatic lesions from a radiobiology and dosimetric perspective. Front Oncol. 2014;4:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–529. [DOI] [PubMed] [Google Scholar]
- 34.Piana PM, Bar V, Doyle L, et al. Early arterial stasis during resin-based yttrium-90 radioembolization: incidence and preliminary outcomes. HPB (Oxford). 2014;16:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sofocleous CT, Violari EG, Sotirchos VS, et al. Radioembolization as a salvage therapy for heavily pretreated patients with colorectal cancer liver metastases: factors that affect outcomes. Clin Colorectal Cancer. 2015;14:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadehfar H, Meyer C, Pieper CC, et al. Evaluation of the delivered activity of yttrium-90 resin microspheres using sterile water and 5 % glucose during administration. EJNMMI Res. 2015;5:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao C, Stavropoulos SW, Mondschein JI, et al. Effect of substituting 50% Isovue for sterile water as the delivery medium for SIR-Spheres: improved dose delivery and decreased incidence of stasis. Clin Nucl Med. 2017;42:176–179. [DOI] [PubMed] [Google Scholar]
- 38.Biederman DM, Titano JJ, Tabori NE, et al. Outcomes of radioembolization in the treatment of hepatocellular carcinoma with portal vein invasion: resin versus glass microspheres. J Vasc Interv Radiol. 2016;27:812–821.e2. [DOI] [PubMed] [Google Scholar]
- 39.Spreafico C, Maccauro M, Mazzaferro V, Chiesa C. The dosimetric importance of the number of 90Y microspheres in liver transarterial radioembolization (TARE). Eur J Nucl Med Mol Imaging. 2014;41:634–638. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy AS, Ball D, Cohen SJ, et al. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for 90Y resin microspheres. J Gastrointest Oncol. 2015;6:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y radioembolization of colorectal hepatic metastases using glass microspheres: safety and survival outcomes from a 531-patient multicenter study. J Nucl Med. 2016;57:665–671. [DOI] [PubMed] [Google Scholar]
- 42.Louie JD, Kothary N, Kuo WT, et al. Incorporating cone-beam CT into the treatment planning for yttrium-90 radioembolization. J Vasc Interv Radiol. 2009;20:606–613. [DOI] [PubMed] [Google Scholar]
- 43.Gabr A, Kallini JR, Gates VL, et al. Same-day 90Y radioembolization: implementing a new treatment paradigm. Eur J Nucl Med Mol Imaging. 2016;43:2353–2359. [DOI] [PubMed] [Google Scholar]
- 44.Seidensticker R, Seidensticker M, Damm R, et al. Hepatic toxicity after radioembolization of the liver using 90Y-microspheres: sequential lobar versus whole liver approach. Cardiovasc Intervent Radiol. 2012;35:1109–1118. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadzadehfar H, Duan H, Haug AR, Walrand S, Hoffmann M. The role of SPECT/CT in radioembolization of liver tumours. Eur J Nucl Med Mol Imaging. 2014;41(suppl 1):S115–S124. [DOI] [PubMed] [Google Scholar]
- 46.Enriquez J, Javadi S, Murthy R, et al. Gastroduodenal artery recanalization after transcatheter fibered coil embolization for prevention of hepaticoenteric flow: incidence and predisposing technical factors in 142 patients. Acta Radiol. 2013;54:790–794. [DOI] [PubMed] [Google Scholar]
- 47.Pasciak AS, McElmurray JH, Bourgeois AC, Heidel RE, Bradley YC. The impact of an antireflux catheter on target volume particulate distribution in liver-directed embolotherapy: a pilot study. J Vasc Interv Radiol. 2015;26:660–669. [DOI] [PubMed] [Google Scholar]
- 48.McWilliams JP, Kee ST, Loh CT, Lee EW, Liu DM. Prophylactic embolization of the cystic artery before radioembolization: feasibility, safety, and outcomes. Cardiovasc Intervent Radiol. 2011;34:786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewandowski R, Salem R. Incidence of radiation cholecystitis in patients receiving Y-90 treatment for unresectable liver malignancies [abstract]. J Vasc Interv Radiol. 2004;15(suppl):S162. [Google Scholar]
- 50.Ahmadzadehfar H, Mohlenbruch M, Sabet A, et al. Is prophylactic embolization of the hepatic falciform artery needed before radioembolization in patients with 99mTc-MAA accumulation in the anterior abdominal wall? Eur J Nucl Med Mol Imaging. 2011;38:1477–1484. [DOI] [PubMed] [Google Scholar]
- 51.Wang DS, Louie JD, Kothary N, Shah RP, Sze DY. Prophylactic topically applied ice to prevent cutaneous complications of nontarget chemoembolization and radioembolization. J Vasc Interv Radiol. 2013;24:596–600. [DOI] [PubMed] [Google Scholar]
- 52.Elschot M, Vermolen BJ, Lam MG, de Keizer B, van den Bosch MA, de Jong HW. Quantitative comparison of PET and bremsstrahlung SPECT for imaging the in vivo yttrium-90 microsphere distribution after liver radioembolization. PLoS One. 2013;8:e55742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kennedy AS, Nutting C, Coldwell D, Gaiser J, Drachenberg C. Pathologic response and microdosimetry of 90Y microspheres in man: review of four explanted whole livers. Int J Radiat Oncol Biol Phys. 2004;60:1552–1563. [DOI] [PubMed] [Google Scholar]
- 54.Flamen P, Vanderlinden B, Delatte P, et al. Multimodality imaging can predict the metabolic response of unresectable colorectal liver metastases to radioembolization therapy with yttrium-90 labeled resin microspheres. Phys Med Biol. 2008;53:6591–6603. [DOI] [PubMed] [Google Scholar]
- 55.Ilhan H, Goritschan A, Paprottka P, et al. Predictive value of 99mTc-MAA SPECT for 90Y-labeled resin microsphere distribution in radioembolization of primary and secondary hepatic tumors. J Nucl Med. 2015;56:1654–1660. [DOI] [PubMed] [Google Scholar]
- 56.Sabet A, Meyer C, Aouf A, et al. Early post-treatment FDG PET predicts survival after 90Y microsphere radioembolization in liver-dominant metastatic colorectal cancer. Eur J Nucl Med Mol Imaging. 2015;42:370–376. [DOI] [PubMed] [Google Scholar]
- 57.O JH, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee TK, Naik NK, Deng J, et al. Tumor response after yttrium-90 radioembolization for hepatocellular carcinoma: comparison of diffusion-weighted functional MR imaging with anatomic MR imaging. J Vasc Interv Radiol. 2008;19:1180–1186. [DOI] [PubMed] [Google Scholar]
- 60.Singh P, Anil G. Yttrium-90 radioembolization of liver tumors: what do the images tell us? Cancer Imaging. 2014;13:645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rabin K. Sirtex Medical Inc. provides update on SIR-Spheres® Y-90 resin microspheres in combination with first-line chemotherapy for patients with unresectable metastatic colorectal cancer (mCRC). BusinessWire website. http://www.businesswire.com/news/home/20170518005425/en/, 2017. Published May 18, 2017. Accessed June 26, 2017.
- 62.Lee MS, Menter DG, Kopetz S. Right versus left colon cancer biology: integrating the consensus molecular subtypes. J Natl Compr Canc Netw. 2017;15:411–419. [DOI] [PubMed] [Google Scholar]
- 63.Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. April 12, 2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. [DOI] [PubMed] [Google Scholar]
- 65.Sharma RA, Van Hazel GA, Morgan B, et al. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. [DOI] [PubMed] [Google Scholar]
- 66.Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41:1861–1869. [DOI] [PubMed] [Google Scholar]
- 67.Narsinh KH, Van Buskirk M, Kennedy AS, et al. Hepatopulmonary shunting: a prognostic indicator of survival in patients with metastatic colorectal adenocarcinoma treated with 90Y radioembolization. Radiology. 2017;282:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janowski E, Timofeeva O, Chasovskikh S, et al. Yttrium-90 radioembolization for colorectal cancer liver metastases in KRAS wild-type and mutant patients: clinical and ccfDNA studies. Oncol Rep. 2017;37:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmeel FC, Simon B, Luetkens JA, et al. Prognostic value of pretreatment diffusion-weighted magnetic resonance imaging for outcome prediction of colorectal cancer liver metastases undergoing 90Y-microsphere radioembolization. J Cancer Res Clin Oncol. March 19, 2017 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer. 2010;103:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sofocleous CT, Garcia AR, Pandit-Taskar N, et al. Phase I trial of selective internal radiation therapy for chemorefractory colorectal cancer liver metastases progressing after hepatic arterial pump and systemic chemotherapy. Clin Colorectal Cancer. 2014;13:27–36. [DOI] [PubMed] [Google Scholar]
- 73.Kennedy AS, Ball DS, Cohen SJ, et al. Safety and efficacy of radioembolization in elderly (≥ 70 years) and younger patients with unresectable liver-dominant colorectal cancer. Clin Colorectal Cancer. 2016;15:141–151.e6. [DOI] [PubMed] [Google Scholar]
- 74.Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20:1121–1130. [DOI] [PubMed] [Google Scholar]
- 75.Barraza J, Currie B, Nadolski G, et al. Delayed hepatotoxicity of Y-90 radioembolization [abstract 378]. J Vasc Interv Radiol. 2017;28(suppl):S163–S164. [Google Scholar]
- 76.Sangro B, Gil-Alzugaray B, Rodriguez J, et al. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112:1538–1546. [DOI] [PubMed] [Google Scholar]
- 77.Sangha BS, Nimeiri H, Hickey R, Salem R, Lewandowski RJ. Radioembolization as a treatment strategy for metastatic colorectal cancer to the liver: what can we learn from the SIRFLOX trial? Curr Treat Options Oncol. 2016;17:26. [DOI] [PubMed] [Google Scholar]
- 78.Gil-Alzugaray B, Chopitea A, Inarrairaegui M, et al. Prognostic factors and prevention of radioembolization-induced liver disease. Hepatology. 2013;57:1078–1087. [DOI] [PubMed] [Google Scholar]
- 79.Seidensticker M, Seidensticker R, Damm R, et al. Prospective randomized trial of enoxaparin, pentoxifylline and ursodeoxycholic acid for prevention of radiation-induced liver toxicity. PLoS One. 2014;9:e112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bester L, Salem R. Reduction of arteriohepatovenous shunting by temporary balloon occlusion in patients undergoing radioembolization. J Vasc Interv Radiol. 2007;18:1310–1314. [DOI] [PubMed] [Google Scholar]
- 81.Theysohn JM, Schlaak JF, Muller S, et al. Selective internal radiation therapy of hepatocellular carcinoma: potential hepatopulmonary shunt reduction after sorafenib administration. J Vasc Interv Radiol. 2012;23:949–952. [DOI] [PubMed] [Google Scholar]
- 82.Borgheresi A, Brown K, Covey A, et al. Outcome following hepatic artery embolization for HCC in presence of portal vein tumor [abstract 284]. J Vasc Interv Radiol. 2017;28(suppl):S123. [Google Scholar]
- 83.Lam MG, Louie JD, Iagaru AH, Goris ML, Sze DY. Safety of repeated yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36:1320–1328. [DOI] [PubMed] [Google Scholar]
- 84.Zarva A, Mohnike K, Damm R, et al. Safety of repeated radioembolizations in patients with advanced primary and secondary liver tumors and progressive disease after first selective internal radiotherapy. J Nucl Med. 2014;55:360–366. [DOI] [PubMed] [Google Scholar]
- 85.Bilbao JI, Garrastachu P, Herraiz MJ, et al. Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Intervent Radiol. 2010;33:523–531. [DOI] [PubMed] [Google Scholar]
- 86.Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys. 2011;79:163–171. [DOI] [PubMed] [Google Scholar]
- 87.Vouche M, Habib A, Ward TJ, et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201. [DOI] [PubMed] [Google Scholar]