Abstract
68Ga-DOTATOC, a somatostatin receptor–targeted ligand, has been used clinically in Europe over the past decade for imaging neuroendocrine tumors (NETs). It appears to be quite sensitive and effective for clinical management decision making. This metaanalysis summarizes the efficacy of 68Ga-DOTATOC for several distinct indications and is intended to support approval of this agent by the U.S. Food and Drug Administration. Methods: The major electronic medical databases were searched for relevant papers over the period from January 2001 to November 2015. Papers were selected for review in 3 categories: clinical trials that reported sensitivity and specificity, comparison studies with 111In-octreotide, and change of management studies. All the eligible papers underwent Quality Assessment of Diagnostic Accuracy Studies (QUADAS) assessment, which was useful in the final selection of papers for review. Results: The initial search yielded 468 papers. After detailed evaluation, 17 papers were finally selected. Five types of studies emerged: workup of patients with symptoms and biomarker findings suggestive of NET, but with negative conventional imaging (3 papers, yield was only 13%); sensitivity (12 papers; sensitivity, 92%) and specificity (7 papers; specificity, 82%); identification of site of unknown primary in patients with metastatic NET (4 papers, yield was 44%); impact on subsequent NET patient management (4 papers, change in management in 51%); and comparison with 111In-octreotide (2 papers, sensitivity of DOTATOC on a per-lesion basis was 100%, for 111In-octreotide it was 78.2%; specificity was not available). Safety was not explicitly addressed in any study, but there were no reports of adverse events. Conclusion: 68Ga-DOTATOC is useful for evaluating the presence and extent in disease for staging and restaging and for assisting in treatment decision making for patients with NET. It is also effective in locating the site of an unknown primary in NET patients who present with metastatic NET, but no known primary tumor. It also appears to be more accurate than 111In-octreotide. Although 68Ga-DOTATOC would seem to be useful in evaluating patients with suggestive symptoms and biomarker findings, it does not perform well in this setting and has low yield. Overall, it appears to be an excellent imaging agent to assess patients with known NET and frequently leads to a change in management.
Keywords: neuroendocrine, DOTATOC, octreotide, pentetreotide, systematic review, metaanalysis
See an invited perspective on this article on page 1450.
Neuroendocrine tumors (NETs) are a class of slow-growing tumors that arise from cells distributed mainly in the lungs, gastrointestinal tract, or pancreas. NETs have been considered to be rare neoplasms, but an analysis from Surveillance, Epidemiology and End Results reports a 5-fold increase from 1973 (1.09/100,000) to 2004 (5.25/100,000) (1). This increase has been ascribed, in part, to improved methods of diagnosis and greater disease awareness. Overall 5-y survival is about 75% and is strongly dependent on stage and grade of the tumor (1). Surgery can be curative for early stage disease, but metastatic disease is often present at the time of presentation, precluding complete resection. Chemotherapy and hormonal blockade are effective in slowing progression but are rarely curative.
A unique feature of NETs is their overexpression of somatostatin receptors on the tumor cells, which has established the basis for both diagnostic imaging and peptide receptor radionuclide therapy. The first approved somatostatin receptor ligand for imaging NETs was octreotide, labeled with 111In. The agent is an 8-peptide sequence linked to diethylenetriaminepentaacetic acid, which is a chelator that binds the 111In. The 8-peptide sequence is a subset of the amino acids in somatostatin and has been demonstrated to avidly bind to the type 2 somatostatin receptor (2). The commercial radiopharmaceutical OctreoScan was approved on June 2, 1994.
Although 111In-octreotide has been successfully used in thousands of patients with NETs over the past 2 decades, it does have some limitations. Because of the relative high energy of the γ-rays from 111In (171 and 245 keV), a medium-energy collimator must be used and spatial resolution is degraded compared with 99mTc agents. The localization of 111In-octreotide is relatively slow, so that imaging is usually done at 4 and 18–24 h after injection.
PET has significantly higher spatial resolution than γ-camera imaging; in the late 1990s a new PET agent, 68Ga-DOTATOC, was shown to rapidly localize to NETs and imaging could be accomplished at 1 h after injection. The first paper on clinical imaging with 68Ga-DOTATOC was published in 2001 (3). Over the following few years, 68Ga-DOTATOC began to be widely used in Europe including The Netherlands, Germany, Austria, Italy, and several other countries to image NETs to assist in the identification of sites of disease and to help in the management of these patients. In recent years, all imaging has been done using PET/CT.
Most of the published medical literature derives from sites in Europe, with a few more recent papers from India, Korea, Taiwan, and Japan. Because of the different regulatory systems in these countries, almost all use of 68Ga-DOTATOC has been in the clinical management of NET patients and not in well-controlled clinical trials. Accordingly, in most of the papers the reference standard is suboptimal or biased and no rigorous safety studies have been done. Despite this, the results that have been reported are remarkably consistent and there is little question that this agent is safe, effective, and more accurate than 111In-octreotide, the standard for the past 22 y.
MATERIALS AND METHODS
Literature Search
A health sciences librarian performed literature searches in November 2015 for English-language studies from 2000 to 2015. The start date of 2000 was chosen because the first paper on the use of 68Ga-DOTATOC in humans was published in 2001. Databases searched included MEDLINE/PubMed, Embase.com, the Cochrane Register of Diagnostic Test Accuracy Studies, and the Cochrane Central Register of Controlled Trials. In all databases, the following search strategy was used without any search field tags: (68Ga OR Ga68 OR Ga-68 OR Ga OR gallium) AND DOTATOC. There is not a subject term for 68Ga-DOTATOC in either PubMed or Embase. One logical workaround would be using phrase searching and proximity searching to supplement subject term searching. But a search strategy simply using the Boolean operator < AND > works better in this case than other complex strategies, because DOTATOC is an exact subject term. Similarly, it is not necessary to include terms for NETS.
Study Selection
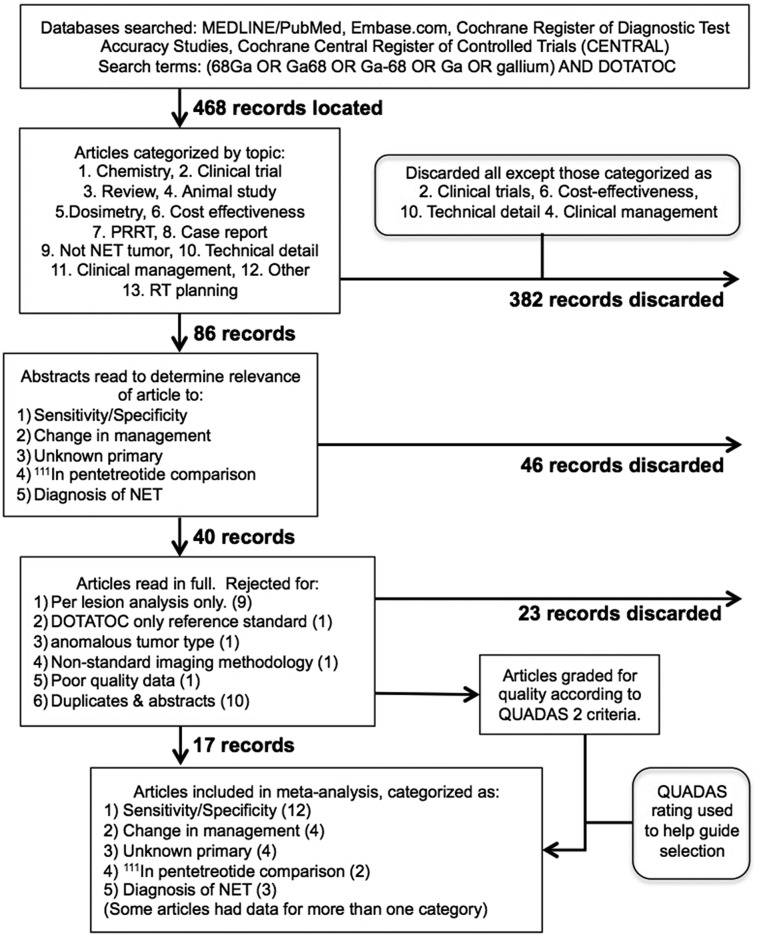
The studies identified from the literature search were evaluated for duplicates and were then categorized independently by 2 experts into 13 categories (Fig. 1). Many studies were not relevant for evaluation of clinical performance and were eliminated. After careful evaluation for relevance and quality, 3 categories of papers were identified that met all criteria. The 3 categories were clinical trial studies with information relevant to sensitivity and specificity, studies comparing 68Ga-DOTATOC with 111In-octreotide, and change of management studies.
FIGURE 1.
Study flow diagram.
Data Abstraction
A data abstraction sheet was developed (supplemental materials [available at http://jnm.snmjournals.org]). Two reviewers independently assessed the collected data. A consensus was reached after discussion with a third reviewer. The key information that was abstracted included number of subjects, tumor type, reference standard, interpretation criteria, type of paper (the 5 groups of papers are discussed in the “Study Selection” section), and outcome (i.e., sensitivity, specificity, percentage change in management, yield in finding an unknown primary).
Quality Assessment of Individual Studies
A quality assessment sheet was developed based on QUADAS-2 (supplemental materials) (4). The quality elements determined for each paper were adequacy of blinding, reference standard, patient selection criteria, study design, and description of image interpretation criteria. The overall quality of each paper was determined and was used in selecting the final papers for review. However, as recommended by the developers of QUADAS we did not use a threshold applied to the sum QUADAS score for determination of paper acceptability.
Data Synthesis and Statistical Analysis
A bivariate normal random-effects model (5) for the joint metaanalysis of analyzing sensitivity and specificity was used to assess the effects of DOTATOC screening in the established literature. This method accounts for variation occurring between studies as well as the correlation between sensitivity and specificity. We also analyzed a measure of overall accuracy called the diagnostic odds ratio, defined as the ratio of the odds of a positive test for a diseased patient to the odds of a positive test for a nondiseased patient. The diagnostic odds ratio (6), a function of both sensitivity and specificity, was used to provide a univariate measure of accuracy. The DerSimonian–Laird random-effects model (7) was used to analyze diagnostic odds ratios on a log scale. Finally, change of management was calculated using a raw proportion of the number of patients whose management was changed divided by the total number of patients. The data were then analyzed on the log-odds scale to provide a normalizing transformation, with a gaussian random-effects model and residual maximum likelihood estimation used to perform the metaanalysis. All analyses were performed in R, and all confidence intervals are at the 95% significance level. The “madauni” and “reitsma” commands of the “mada” package in R were used for the diagnostic odds ratio and sensitivity/specificity estimates, respectively. The “rma.uni” command of the “metafor” package was used for the change in management proportion. Figures were created using SAS (version 9.4; SAS Institute).
RESULTS
Study Selection
A total of 634 references were found, with 227 from PubMed, 405 from EMBASE, and 2 from Cochrane. After the removal of duplicate references, a total of 468 were left. The details for selecting 17 suitable papers are detailed in Figure 1.
It was apparent from examination of the available published literature that there were several different indications or clinical settings in which 68Ga-DOTATOC was likely to be useful. We separated the papers into 6 different groups to address the different types of studies, and literature data attributed to each group are tabulated in Table 1: diagnosis of disease in patients with symptoms and blood chemistry strongly suggestive for NET (3 papers); sensitivity and specificity of 68Ga-DOTATOC (12 papers); identification of the site of an unknown primary in patients with metastatic NET, typically in the liver (4 papers); determination of the impact of 68Ga-DOTATOC imaging on subsequent patient management in patients with known NET (4 papers); and sensitivity and specificity of 68Ga-DOTATOC in comparison to 111In-octreotide (2 papers).
TABLE 1.
Qualified Literature Data Available and Used for Subanalysis of Each of the 6 Identified Indications or Clinical Settings
| Reference | Diagnosis | Sensitivity and specificity | Unknown primary | Change in management | Compared with OctreoScan | Carcinoid tumors |
| Hofmann et al. (3) | X | |||||
| Buchman et al. (24) | X | |||||
| Gabriel et al. (8) | X | X | ||||
| Frilling et al. (15) | X | X | X | |||
| Ruf et al. (21) | X | |||||
| Versari et al. (9) | X | |||||
| Jindal et al. (16) | X | X | ||||
| Kumar et al. (17) | X | |||||
| Poeppel et al. (19) | X | |||||
| Ruf et al. (10) | X | |||||
| Froeling et al. (20) | X | |||||
| Mayerhoefer et al. (11) | X | |||||
| Beiderwellen et al. (12) | X | |||||
| Schraml et al. (13) | X | X | ||||
| Schreiter et al. (22) | X | X | ||||
| Venkitaraman et al. (14) | X | X | ||||
| Nakamoto et al. (18) | X | X | X | |||
| Menda et al. (23) | X |
In addition, it also became clear that the sensitivity of 68Ga-DOTATOC for the detection of atypical carcinoid is much lower than for typical carcinoid tumors. Two papers explicitly looked at this characteristic and are reported separately: sensitivity and specificity of 68Ga-DOTATOC for the diagnosis of typical and atypical carcinoid tumor.
The sensitivity and specificity and the unknown primary literature were considered robust enough for formal metaanalysis. All other categories were deemed to not have sufficient data to warrant a true metaanalysis, so summary statistics are provided.
Sensitivity and Specificity (Metaanalysis)
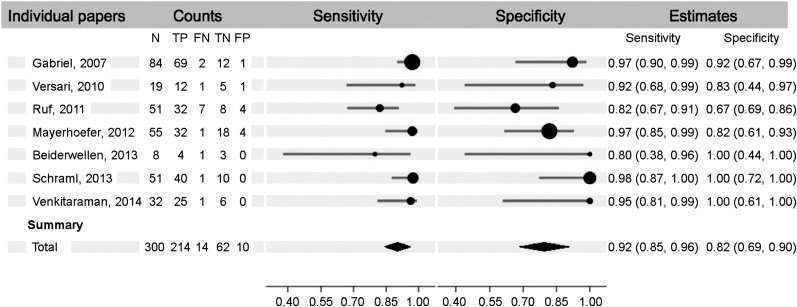
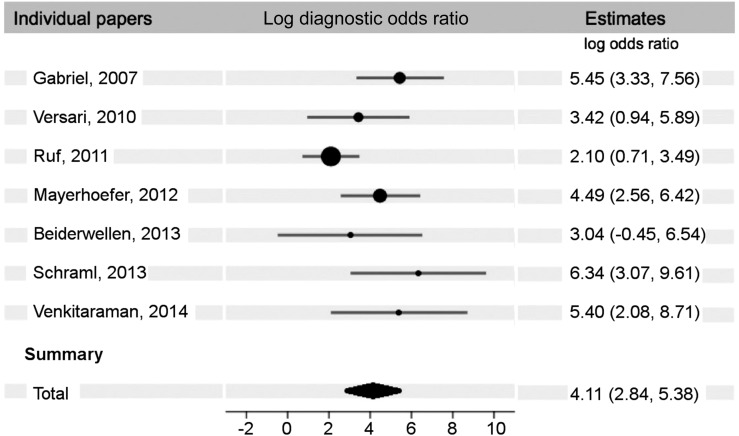
The findings of the metaanalysis on the first 7 papers (8–14), which reported true-positive, true-negative, false-positive, and false-negative results (n = 432), show an overall sensitivity and specificity of 92% (95% confidence interval [CI], 85%–96%) and 82% (95% CI, 69%–90%), respectively (Fig. 2). The diagnostic odds ratio for these papers was 61 (Fig. 3). When we included the 5 papers (15–19) that reported only true-positive and false-negative results (n = 214), the metaanalysis resulted in an overall sensitivity of 93% (95% CI, 87%–96%) (Table 2).
FIGURE 2.
Sensitivity and specificity forest plot.
FIGURE 3.
Diagnostic odds ratio forest plot. Total diagnostic odds ratio for these papers is 60.80 (17.07–216.60). Table lists log (base e) ratios.
TABLE 2.
Sensitivity and Specificity
| Reference | n | True-positive | False-negative | True-negative | False-positive | Sensitivity | Specificity |
| Gabriel et al. (8) | 84 | 69 | 2 | 12 | 1 | 97.2% | 92.3% |
| Versari et al. (9) | 19 | 12 | 1 | 5 | 1 | 92.3% | 83.3% |
| Ruf et al. (10) | 51 | 32 | 7 | 8 | 4 | 82.1% | 66.7% |
| Mayerhoefer et al. (11) | 55 | 32 | 1 | 18 | 4 | 97.0% | 81.8% |
| Beiderwellen et al. (12) | 8 | 4 | 1 | 3 | 0 | 80.0% | 100.0% |
| Schraml et al. (13) | 51 | 40 | 1 | 10 | 0 | 97.6% | 100.0% |
| Venkitaraman et al. (14) | 32 | 25 | 1 | 6 | 0 | 96.2% | 100.0% |
| Frilling et al. (15) | 52 | 52 | 0 | 100.0% | |||
| Poeppel et al. (19) | 40 | 40 | 0 | 100.0% | |||
| Jindal et al. (16) | 13 | 13 | 0 | 100.0% | |||
| Kumar et al. (17) | 20 | 20 | 0 | 100.0% | |||
| Nakamoto et al. (18) | 46 | 6 | 1 | 85.7% |
Change of Management (Metaanalysis)
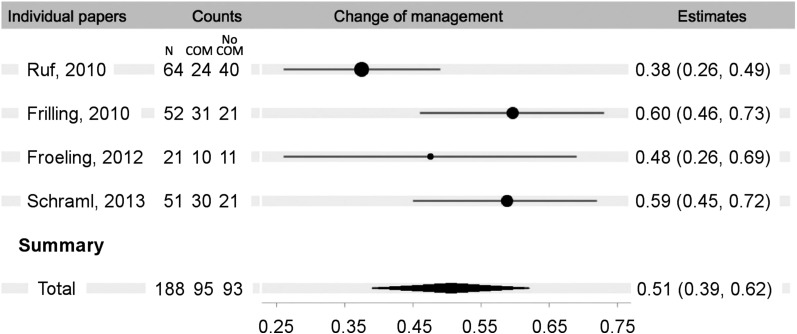
There were 4 eligible papers that reported change in management after 68Ga-DOTATOC PET/CT imaging (13,15,20,21). All the studies were retrospective, based on chart review. All patients had histologically confirmed or suspected multiple NETs. In the combined papers, 188 patients were imaged, with a reported change of management in 51% (95% CI, 39%–62%) (Fig. 4).
FIGURE 4.
Change of management forest plot.
Unknown Primary (Summary Statistics)
There were 4 eligible papers (Table 3) that reported on performance of 68Ga-DOTATOC PET/CT for detecting an unknown primary in patients with extensive metastatic disease (15,18,22,23). The overall success rate was 40 of 91 or 43.9%.
TABLE 3.
Identification of Unknown Primary
Diagnosis in Patients Suspected of Having NET (Summary Statistics)
There were 3 papers that reported results on 68Ga-DOTATOC imaging in patients with symptoms and elevated blood biomarkers strongly suggestive of NET but with no evidence of disease with conventional imaging (8,18,22). The biomarkers included chromogranin A, adrenocorticotropic hormone, gastrin, insulin, and 5-hydroxyindoleacetic acid. All of the papers also included patients with other indications. The reference standard was histology or follow-up with conventional imaging. Negative studies were scored as true-negative. Among the papers reviewed (Table 4), there were 57 patients studied with this indication with an overall yield of 7 true-positives (12%) and 1 false-positive.
TABLE 4.
Diagnosis in Patients Suspected of Having NET
Other Types of Papers
Comparison with OctreoScan and Typical and Atypical Carcinoids
These papers did not report true-negative or false-positive values, thus specificity and diagnostic odds ratio could not be calculated. Furthermore, with just 2 studies appearing in the literature, there were insufficient data to perform a reliable metaanalysis. Therefore, we present the data from these papers using only descriptive statistics in Tables 5 and 6.
TABLE 5.
68Ga-DOTATOC Versus 111In-Octreotide
| DOTATOC |
Octreotide |
||||
| Reference | n | True-positive | False-negative | True-negative | False-positive |
| Hofmann et al. (3) | 8 | 40 | 0 | 34 | 6 |
| Buchman et al. (24) | 27 | 70 | 0 | 52 | 18 |
In both studies, reported evaluation is lesion by lesion.
TABLE 6.
Typical Versus Atypical Carcinoid Tumors
Comparison with 111In-Octreotide (OctreoScan) (Summary Statistics)
There were 2 eligible papers (Table 5) that reported comparison of 68Ga-DOTATOC PET/CT with 111In-octreotide SPECT imaging (3,24). All of the patients had known NET. Analysis in both papers was performed on a lesion-by-lesion basis. Sensitivity of 68Ga-DOTATOC on a per-lesion basis was 100% and for 111In-octreotide 78%.
Typical and Atypical Carcinoids (Summary Statistics)
There were 2 eligible papers (Table 6) that reported the performance of 68Ga-DOTATOC PET/CT in detecting pulmonary carcinoid tumors in 46 patients (14,16). This tumor is addressed separately because of the generally lower somatostatin receptor density on atypical (poorly differentiated) carcinoids. Grouped sensitivity was 100% for typical carcinoids and 83% for atypical.
Brief summaries of all the references are provided in the supplemental materials.
DISCUSSION
There was a remarkable breadth in the medical literature addressing the efficacy of 68Ga-DOTATOC PET in the evaluation of NETs. All of the papers were from outside the United States, because regulatory limitations are less restrictive elsewhere. Many of the studies were done retrospectively, and most of the papers had selection bias, often only imaging patients who had biopsy-proven NET. This bias was largely inconsequential as the population studied was representative of the population that will typically be clinically imaged. The reference standard across papers was variable and it unfortunately often includeed the results from the 68Ga-DOTATOC PET imaging. This is an inherent problem in the setting when the new agent is far better than any existing noninvasive test. The only reliable, objective reference standard is histopathology after biopsy. Several papers used limited biopsy information, but it is ethically and practically impossible to biopsy all the lesions that are seen.
Trial design in these papers was also widely varied. Some addressed the accuracy of the methodology, but most addressed other aspects, including effect on subsequent management, how the information could be combined with other imaging modalities (i.e., CT and MRI), and the utility of 68Ga-DOTATOC PET for diagnosing disease in patients with typical symptoms and biomarker findings. Many papers had multiple groups of patients with different indications.
Despite the broad variety, the results of the metaanalysis are generally consistent, showing excellent sensitivity and specificity, frequent change of management after the scanning, and a high success rate in finding unknown primaries in patients with metastatic disease.
The results of this metaanalysis show a pooled sensitivity for detection of NETS with 68Ga-DOTATOC of 92% and specificity of 82%. These values are similar to the results of an earlier, smaller metaanalysis (25), in which a pooled sensitivity of 93% and specificity of 85% was found. In addition, the sensitivity in this metaanalysis is identical to the value found in 2 meta-analyses of 68Ga somatostatin receptor ligands (DOTATOC, DOTATATE, and DOTANOC combined) (26,27). The specificity in the combined metaanalyses is somewhat higher, 96% in one and 93% in the other.
The sensitivity of 68Ga-DOTATOC PET is definitely better than 111In-octreotide SPECT imaging. In the 2 papers that directly compared the 2 approaches, the sensitivity for 68Ga-DOTATOC on a per-lesion basis was 100% and for 111In-octreotide 78%. In the package insert for 111In-pentetreotide, the reported sensitivity was 85.7% and specificity 50% (28).
Because determination of sensitivity and specificity is dependent on patient selection and the reference standard, it may be more appropriate to look at change in patient management as a more practical measure of efficacy than sensitivity and specificity. In this review, 3 papers were found that reported change in management after 68Ga-DOTATOC PET imaging. The pooled result reported change of management in 95 of 188 (51%), which clearly illustrates the clinical significance of 68Ga-DOTATOC imaging. A recent metaanalysis of the 3 common 68Ga somatostatin receptor ligands combined found an average of 44% change of management after imaging (29). This is somewhat higher than the results from the National Oncologic PET Registry study, which found that after 18F-FDG imaging there was a change in management an average of 36.5% (30).
A unique indication for 68Ga-DOTATOC PET imaging of NETs is in the setting in which a patient presents with multiple sites of metastatic NET, typically in the liver, and the site of the primary tumor is unknown. The issue of the need to resect the primary is controversial, but a systematic review of this issue found that 5-y survival was 72% for resected patients versus 35% for unresected (31). The 6 reviewed papers almost certainly suffered from patient selection bias, but the difference is so large that many surgeons feel that it is appropriate to resect the primary, if it can be found. In this review, there were 4 papers that reported on the success rate in finding unknown primaries with 68Ga-DOTATOC PET imaging, typically when other imaging approaches had failed. The overall success rate was 40 of 91 or 43.9%.
There are 2 settings in which 68Ga-DOTATOC PET imaging of NETs is less successful, for example, in atypical carcinoid and in patients who present with carcinoid syndrome symptoms (flushing and diarrhea) and elevated biomarkers. Atypical carcinoid tumors are more poorly differentiated than typical carcinoids and have significantly fewer somatostatin receptors. This is a well-known problem, and it is recognized that 18F-FDG imaging is more effective in this setting. Apparently, although many patients with NETs have typical carcinoid syndrome symptoms, the converse is not true. There are many other etiologies of such symptoms. In 3 papers with 53 patients, the overall yield was 7 true-positives (13%) and 1 false-positive.
CONCLUSION
68Ga-DOTATOC is useful for evaluating the presence and extent in disease for staging and restaging and assisting in treatment decision making for patients with NET. It is also effective in locating the site of an unknown primary in NET patients who present with metastatic NET but no known primary tumor. It also appears to be more accurate than 111In-octreotide. Although 68Ga-DOTATOC would seem to be useful in evaluating patients with suggestive symptoms and biomarker findings, it does not perform well in this setting and has low yield. Overall, it appears to be an excellent imaging agent to assess patients with known NET and frequently leads to a change in management.
DISCLOSURE
We thank the Petersen Foundation for financial support. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
We acknowledge Marin L. Schweizer for helping design the metaanalysis approach.
REFERENCES
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2.John M, Meyerhof W, Richter D, et al. Positive somatostatin receptor scintigraphy correlates with the presence of somatostatin receptor subtype 2. Gut. 1996;38:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann M, Maecke H, Borner R, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–1757. [DOI] [PubMed] [Google Scholar]
- 4.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. [DOI] [PubMed] [Google Scholar]
- 5.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–990. [DOI] [PubMed] [Google Scholar]
- 6.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. 2003;56:1129–1135. [DOI] [PubMed] [Google Scholar]
- 7.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 8.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–518. [DOI] [PubMed] [Google Scholar]
- 9.Versari A, Camellini L, Carlinfante G, et al. Ga-68 DOTATOC PET, endoscopic ultrasonography, and multidetector CT in the diagnosis of duodenopancreatic neuroendocrine tumors: a single-centre retrospective study. Clin Nucl Med. 2010;35:321–328. [DOI] [PubMed] [Google Scholar]
- 10.Ruf J, Schiefer J, Furth C, et al. 68Ga-DOTATOC PET/CT of neuroendocrine tumors: spotlight on the CT phases of a triple-phase protocol. J Nucl Med. 2011;52:697–704. [DOI] [PubMed] [Google Scholar]
- 11.Mayerhoefer ME, Schuetz M, Magnaldi S, Weber M, Trattnig S, Karanikas G. Are contrast media required for 68Ga-DOTATOC PET/CT in patients with neuroendocrine tumours of the abdomen? Eur Radiol. 2012;22:938–946. [DOI] [PubMed] [Google Scholar]
- 12.Beiderwellen KJ, Poeppel TD, Hartung-Knemeyer V, et al. Simultaneous 68Ga-DOTATOC PET/MRI in patients with gastroenteropancreatic neuroendocrine tumors: initial results. Invest Radiol. 2013;48:273–279. [DOI] [PubMed] [Google Scholar]
- 13.Schraml C, Schwenzer NF, Sperling O, et al. Staging of neuroendocrine tumours: comparison of [68Ga]DOTATOC multiphase PET/CT and whole-body MRI. Cancer Imaging. 2013;13:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkitaraman B, Karunanithi S, Kumar A, Khilnani GC, Kumar R. Role of 68Ga-DOTATOC PET/CT in initial evaluation of patients with suspected bronchopulmonary carcinoid. Eur J Nucl Med Mol Imaging. 2014;41:856–864. [DOI] [PubMed] [Google Scholar]
- 15.Frilling A, Sotiropoulos GC, Radtke A, et al. The impact of 68Ga-DOTATOC positron emission tomography/computed tomography on the multimodal management of patients with neuroendocrine tumors. Ann Surg. 2010;252:850–856. [DOI] [PubMed] [Google Scholar]
- 16.Jindal T, Kumar A, Venkitaraman B, Dutta R, Kumar R. Role of 68Ga-DOTATOC PET/CT in the evaluation of primary pulmonary carcinoids. Korean J Intern Med. 2010;25:386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar R, Sharma P, Garg P, et al. Role of 68Ga-DOTATOC PET-CT in the diagnosis and staging of pancreatic neuroendocrine tumours. Eur Radiol. 2011;21:2408–2416. [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto Y, Sano K, Ishimori T, et al. Additional information gained by positron emission tomography with 68Ga-DOTATOC for suspected unknown primary or recurrent neuroendocrine tumors. Ann Nucl Med. 2015;29:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poeppel TD, Binse I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52:1864–1870. [DOI] [PubMed] [Google Scholar]
- 20.Froeling V, Elgeti F, Maurer MH, et al. Impact of Ga-68 DOTATOC PET/CT on the diagnosis and treatment of patients with multiple endocrine neoplasia. Ann Nucl Med. 2012;26:738–743. [DOI] [PubMed] [Google Scholar]
- 21.Ruf J, Heuck F, Schiefer J, et al. Impact of multiphase 68Ga-DOTATOC-PET/CT on therapy management in patients with neuroendocrine tumors. Neuroendocrinology. 2010;91:101–109. [DOI] [PubMed] [Google Scholar]
- 22.Schreiter NF, Bartels AM, Froeling V, et al. Searching for primaries in patients with neuroendocrine tumors (NET) of unknown primary and clinically suspected NET: evaluation of Ga-68 DOTATOC PET/CT and In-111 DTPA octreotide SPECT/CT. Radiol Oncol. 2014;48:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menda Y, O’Dorisio TM, Howe JR. Localization of unknown primary site with 68Ga-DOTATOC PET/CT in patients with metastatic neuroendocrine tumor. J Nucl Med. 2017;58:1054–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. [DOI] [PubMed] [Google Scholar]
- 25.Yang J, Kan Y, Ge BH, Yuan L, Li C, Zhao W. Diagnostic role of gallium-68 DOTATOC and gallium-68 DOTATATE PET in patients with neuroendocrine tumors: a meta-analysis. Acta Radiol. 2014;55:389–398. [DOI] [PubMed] [Google Scholar]
- 26.Geijer H, Breimer LH. Somatostatin receptor PET/CT in neuroendocrine tumours: update on systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2013;40:1770–1780. [DOI] [PubMed] [Google Scholar]
- 27.Treglia G, Castaldi P, Rindi G, Giordano A, Rufini V. Diagnostic performance of gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. 2012;42:80–87. [DOI] [PubMed] [Google Scholar]
- 28. OCTREOSCAN: indium In-111 pentetreotide [package insert]. St. Louis, MO: Mallinckrodt Inc.; 2015.
- 29.Barrio M, Czernin J, Fanti S, et al. The impact of SSTR-directed PET/CT on the management of patients with neuroendocrine tumor: a systematic review and meta-analysis. J Nucl Med. January 12, 2017 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–2161. [DOI] [PubMed] [Google Scholar]
- 31.Capurso G, Rinzivillo M, Bettini R, Boninsegna L, Delle Fave G, Falconi M. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg. 2012;99:1480–1486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.